Abstract
Hippocampal CA1 pyramidal cells, which receive γ-aminobutyric acid (GABA)ergic input from at least 18 types of presynaptic neuron, express 14 subunits of the pentameric GABAA receptor. The relative contribution of any subunit to synaptic and extrasynaptic receptors influences the dynamics of GABA and drug actions. Synaptic receptors mediate phasic GABA-evoked conductance and extrasynaptic receptors contribute to a tonic conductance. We used freeze-fracture replica-immunogold labelling, a sensitive quantitative immunocytochemical method, to detect synaptic and extrasynaptic pools of the alpha1, alpha2 and beta3 subunits. Antibodies to the cytoplasmic loop of the subunits showed immunogold particles concentrated on distinct clusters of intramembrane particles (IMPs) on the cytoplasmic face of the plasma membrane on the somata, dendrites and axon initial segments, with an abrupt decrease in labelling at the edge of the IMP cluster. Neuroligin-2, a GABAergic synapse-specific adhesion molecule, co-labels all beta3 subunit-rich IMP clusters, therefore we considered them synapses. Double-labelling for two subunits showed that virtually all somatic synapses contain the alpha1, alpha2 and beta3 subunits. The extrasynaptic plasma membrane of the somata, dendrites and dendritic spines showed low-density immunolabelling. Synaptic labelling densities on somata for the alpha1, alpha2 and beta3 subunits were 78–132, 94 and 79 times higher than on the extrasynaptic membranes, respectively. As GABAergic synapses occupy 0.72% of the soma surface, the fraction of synaptic labelling was 33–48 (alpha1), 40 (alpha2) and 36 (beta3)% of the total somatic surface immunolabelling. Assuming similar antibody access to all receptors, about 60% of these subunits are in extrasynaptic receptors.
Keywords: dendritic spine, electron microscope, GABAergic synapse, hippocampus, immunoreactivity, inhibition
Introduction
The activity of pyramidal cells in the cerebral cortex is governed by several differentially timed γ-aminobutyric acid (GABA)ergic inputs, acting via GABAA and GABAB receptors. In the CA1 area of the hippocampus, at least 18 distinct presynaptic GABAergic neurons release GABA to pyramidal cells (Klausberger & Somogyi, 2008). For example, the somata of pyramidal cells receive GABAergic input from three distinct basket cell populations expressing either parvalbumin, or cholecystokinin and vasoactive intestinal peptide (VIP), or cholecystokinin and vesicular glutamate transporter type 3 (VGLUT3), and they also show distinct firing behaviour during network oscillations (Klausberger et al., 2005). Such differences suggest different roles in the network supported by specialised molecular signalling machineries (Foldy et al., 2007).
The postsynaptic receptors activated by these GABAergic neurons may also be expressed in an input-dependent manner. Pyramidal cells express at least 14 different subunits of the GABAA receptor contributing to multiple pentameric, hetero-oligomeric receptor species (Persohn et al., 1992; Wisden et al., 1992; Sperk et al., 1997; Ogurusu et al., 1999). Most GABAA receptors include two alpha (alpha1–6), two beta (beta1–3), and either a gamma (gamma1–3), delta, epsilon or theta subunit (Sieghart & Sperk, 2002). The same alpha and/or beta subunit can have one or two copies in a receptor, increasing diversity, each receptor having a possible differential surface expression. Indeed, Thomson and colleagues have demonstrated differences in the pharmacology of unitary GABAergic inputs to CA1 pyramidal cells from identified interneurons (Pawelzik et al., 1999; Thomson et al., 2000; Ali & Thomson, 2008). Fast spiking basket cells activated receptors indicative of the alpha1 subunit, whereas regular spiking basket cells acted via receptors indicative of alpha2/3 subunits. Thus, pyramidal cells may target distinct receptor species to particular synaptic inputs. This was supported by the demonstration that the alpha1 subunit was enriched in synapses received from parvalbumin-immunopositive terminals (Klausberger et al., 2002), whereas the alpha2 subunit was enriched in synapses received from parvalbumin-negative GABAergic boutons, presumably originating from cholecystokinin-expressing basket cells (Nyiri et al., 2001). However, due to the lack of suitable antibodies, the relationship between synapses positive for the alpha1 or alpha2 subunit has not been evaluated directly. Therefore, in the present study, having raised the appropriate antibodies, we have used freeze-fracture replica immunogold labelling (FRIL), a sensitive immunocytochemical method (Fujimoto, 1995; Rash & Yasumura, 1999), to investigate the presence of both the alpha1 and alpha2 subunits in single synapses on the somata of pyramidal cells. The advantage of FRIL is that the plasma membrane distribution of proteins can be revealed en face, in two-dimensional membrane sheets in situ, at a lateral resolution of about 20 nm.
Correlated FRIL and electrophysiological recordings showed a single gold particle corresponding to each functional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor (Tanaka et al., 2005). Such sensitivity makes it possible to detect GABAA receptors expressed at low density, for example at extrasynaptic sites (Somogyi et al., 1989), which are responsible for a tonic current evoked by GABA in the extracellular spaces (Semyanov et al., 2004; Farrant & Nusser, 2005; Glykys & Mody, 2007; Belelli et al., 2009). These extrasynaptic receptors may be of the same subunit composition as synaptic receptors, which cycle in and out of the synapse by lateral diffusion (Bannai et al., 2009). Some extrasynaptic receptors may be of different subunit composition from synaptic receptors, as shown by electron microscopic immunolabelling (Nusser et al., 1998; Wei et al., 2003), and by the pharmacological characteristics of the tonic vs synaptic currents (Nusser & Mody, 2002; Caraiscos et al., 2004a; Belelli et al., 2005; Glykys et al., 2007). The gamma2 subunit is necessary for synaptic localisation (Essrich et al., 1998), and the replacement of the gamma by the delta subunit leads to the exclusion of the receptor from the synapse (Nusser et al., 1998). The relative participation of the other subunits in synaptic and extrasynaptic receptors is unclear, partly due to positional effects within the pentamer (Minier & Sigel, 2004) and the inclusion of two different alpha and/or beta subunits (Tretter et al., 1997). To test the relative contribution of the alpha1, alpha2 and beta3 GABAA receptor subunits to synaptic and extrasynaptic receptors, we have quantified their distribution using FRIL. Abstracts have been published (Somogyi et al., 2004; Kasugai et al., 2006).
Materials and methods
All procedures involving experimental animals used in this study in the UK were carried out under a Home Office project license in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated procedures. The experiments on animals in the laboratory at Okazaki, Japan, were conducted in accordance with the guidelines of the National Institute of Physiological Science’s Animal Care and Use Committee. A total of 21 adult rats (specified below), four guinea pigs, five wild-type and five gene-deleted mice were used (see below).
Sodium dodecyl sulphate (SDS)-digested freeze-fracture replica preparation
We followed the procedure developed by Fujimoto (1995), with recent modifications (Tanaka et al., 2005). Thirteen adult male Wistar rats were anaesthetised with Pentobarbital (50 mg/kg, i.p.), and perfused first for 3 min with 25 mm phosphate-buffered saline (PBS), then for 12 min with a fixative composed of 2% paraformaldehyde, ~0.2% w/v picric acid in 0.1 m sodium phosphate buffer (PB, pH 7.2–7.4), followed by PB for 3 min. The brains were dissected and sectioned coronally with a Lineaslicer PRO7 (Dosaka EM, Kyoto, Japan) at 120-μm thickness. The hippocampal CA1 area was dissected in PBS and cryoprotected with 30% glycerol in 0.1 m PB overnight at 4 °C. The sections were frozen quickly in a high-pressure freezing machine (HPM 010; Bal-Tec, Balzers, Liechtenstein). The frozen slices were then fractured at −120 °C and coated with carbon (5 nm), shadowed by platinum (2 nm) at 60°, and then coated with carbon (15 nm) again in a BAF 060 machine (Bal-Tec). After thawing, the tissue was removed from the replica by autoclaving for 15 min at 105 °C in a Tris buffer (15 mm, pH 8.3) containing 2.5% SDS and 20% sucrose. The specimens were removed from the autoclave after the temperature in the chamber had returned to room temperature in about 5 h, and were processed further or left in the same solution at room temperature until further processing.
Production of primary antibodies
Polyclonal antibodies were developed in guinea pig (beta3) and rat (alpha1) using glutathione-S-transferase (GST)-fusion proteins produced in Escherichia coli cells (BL21, Agilent Technologies, Santa Clara, CA, USA). The plasmid constructs that were used contained cDNA for GST fused with a portion of the intracellular loop corresponding to residues 328–382 of the alpha1 or residues 345–408 of the beta3 subunits of the GABAA receptor. The rat and mouse sequences at these positions are identical. For immunisation, these fusion proteins were purified by SDS–polyacrylamide gel electrophoresis (SDS–PAGE). After electrophoresis, the gels were washed briefly in several changes of deionised water and then transferred to 0.3 m CuCl2. The gels were shaken gently for 3 min, and the broad protein bands that appeared in the gel against a blue background were excised. The gel fragments containing the purified fusion protein were emulsified with Freund’s adjuvant (Nakalai tesque, Kyoto, Japan). Eight rats (Sprague–Dawley, female, 6 weeks old at first immunisation) and four guinea pigs (adult, female) were immunised by subcutaneous injections at several sites in the back with a total of 50–100 μg of the beta3 subunit fusion protein, or 20–50 μg of the alpha1 fusion protein, respectively. They received three sequential sets of injections at 2-week intervals. The first injection contained complete Freund’s adjuvant, the two later injections were done with incomplete Freund’s adjuvant. The antisera were collected 2 weeks after the last injection by exsanguination of the animals following anaesthesia with Pentobarbital.
The anti-alpha1 (328–382), anti-alpha2 (322–357), anti-alpha5 (337–388), anti-beta2 (351–405), anti-beta3 (345–408) and anti-gamma2 (319–366) antibodies were generated by immunising rabbits with a maltose-binding protein (MBP)-alpha1 (328–382)-7His, MBP-alpha2 (322–357)-7His, MBP-alpha5 (337–388)-7His, MBP-beta2 (351–405)-7His, MBP-beta3 (345–408)-7His or MBP-gamma2 (319–366)-7His fusion protein. Then the antibodies were purified by using the corresponding GST fusion protein coupled to affigel 10 (Bio-Rad, Hercules, CA, USA), as described earlier (Slany et al., 1995; Tretter et al., 1997; Jechlinger et al., 1998; Poltl et al., 2003; Table 1). The anti-alpha5 (337–388) antibody was described previously (Poltl et al., 2003; Ogris et al., 2006) as anti-alpha5 (337–380).
Table 1.
Identity, source and characterisation of antibodies
| Characterisation tests |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecule | Code# in original lab. or supplier |
Host animal |
Developer | Epitope, amino acid residues |
Protein concentration |
Optimal dil. FRIL |
IH, subunits expressed, HEK cells* |
FRIL, gene deleted & wild-type mice |
Western blot | Reference, production, characterisation |
| Neuroligin-2 | NL2B-KLH (bleed#l, rabbit CU904) | Rabbit | B. Chih N. Limthong P. Scheiffele |
750–767 | Purified ab., not known | 400× | Not known | No | Brain tested** | Budreck & Scheiffele (2007) |
| GABAA-R α1 | GABAa α1, GST-Rt2 | Rat | Y. Kasugai R. Shigemoto |
328–382 | Serum | 50× | Tested | Tested | Brain tested | This paper |
| GABAA-R α1 | α1(328–382), R11/12 | Rabbit | W. Sieghart | 328–382 | 1.2 mg/mL | 500× | Tested | Tested | Wt and gene-deleted mouse** | Tretter et al. (1997); Ogris et al. (2006) |
| GABAA-R α2 | α2(322–357), R18/19 | Rabbit | W. Sieghart | 322–357 | 991 μg/mL | 900× | Tested | Tested | Wt and gene-deleted mouse | Poltl et al. (2003); this paper |
| GABAA-R α5 | α5(337–388) R13/12 | Rabbit | W. Sieghart | 337–388 | 410 μg/mL | 50× | Tested | Tested | Brain tested** | Poltl et al. (2003); Ogris et al. (2006) |
| GABAA-R ß2 | ß2(351–405) R22/10 | Rabbit | W. Sieghart | 351–405 | 468 μg/mL | 200× | Tested | No | Purified receptor tested** | Jechlinger et al. (1998) |
| GABAA-R ß3 | GABAA ß3-Gp4 | Guinea pig | Y. Kasugai R. Shigemoto |
345–408 | Serum | 50× | Tested | Tested | Brain tested | This paper |
| GABAA-R ß3 | ß3(345–408) R14/22 | Rabbit | W. Sieghart | 345–408 | 325 μg/mL | 500× | Tested | Tested | Purified receptor tested**; brain tested | Slany et al. (1995); Poltl et al. (2003); this paper |
| GABAA-R γ2 | γ2(319–366) R12/20 | Rabbit | W. Sieghart | 319–366 | 264 μg/mL | 200× | Tested | No | Receptor in HEK cells tested** | Tretter et al. (1997) |
| Nav1.6 | K87A/10.2 (7/20/01) | Mouse monoclonal | J. S. Trimmer | 1904–1976 (C-t), rat Nav1.6 | 6.1 mg/mL | 500× | Not known | No | Brain tested** | Rasband et al. (2003) |
| PSD-95/SAP90 | CAT No 05–427 NeuroMab clone K28/43 | Mouse monoclonal | Upstate Biotech., now NeuroMab | 77–299 human PSD-95 | 1 mg/mL | 500× | Not known | No | Wt and gene-deleted mouse** | Beique et al. (2006) |
| Glutathione-S-transferase (GST) | CAT No G7781 | Rabbit | Sigma-Aldrich | Whole protein | 10 mg/mL | 5000× | Not known | No | Recombinant GST | Calon et al. (2006) |
see Table 2;
Data presented in cited publication. GABA, γ-aminobutyric acid; HEK, human embryonic kidney cells; IH, immimohistochemistry; FRIL, freeze-fracture replica immunolabelling; Wt, wild-type.
Tests for the specificity of antibodies
Proteins separated by SDS–PAGE (12%) from a crude membrane fraction of whole rat brains (Sprague–Dawley, male) were used to prepare Western blots, as described earlier (Notomi & Shigemoto, 2004). Both the rat antiserum to the alpha1 subunit and the guinea pig antiserum to the beta3 subunit were used at a dilution of 1: 500. These antisera also recognised GST, as seen in Western blots of SDS–PAGE-separated protein samples of lysed E. coli used for production of the fusion proteins and tested with rabbit antibodies to GST (Sigma-Aldrich, St Louis, MO, USA; Table 1). The purified rabbit anti-alpha1 (328–382) and anti-beta3 (345–408) antibodies were used at a dilution of 1: 1000. In addition, the purified rabbit anti-alpha2 (322–357) antibody was used here in a Western blot of membrane proteins from wild-type mice and alpha2 subunit gene-deleted mice, as described earlier for other antibodies (Ogris et al., 2006).
Transient transfection of GABAA receptor subunits in cultured cells
The cDNAs for the complete polypeptide sequence of the rat alpha1, alpha2 and alpha5, beta1, beta2, beta3 and gamma2 subunits of the GABAA receptor were cloned into a pCl plasmid vector, which uses the cytomegalovirus immediate-early enhancer/promoter region for strong constitutive expression. Human embryonic kidney cells (HEK-293) were subcultured in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum (GIBCO, Invitrogen, Paisley, UK), glutamine (0.29 g/L), sodium bicarbonate (2.2 g/L), penicillin (100 000 U/L) and streptomycin (10 mg/L) in an atmosphere of 5% CO2/95% air at 37 °C. The HEK-293 cells (0.5 × 106 cells per well) were grown for approximately 24 h in six-well plates on glass coverslips coated with poly-l-lysine. Transient transfection of the HEK-293 cells by the plasmids was carried out using FuGENE6 (Roche Applied Science, Indianapolis, USA) following the manufacturer’s recommended protocol. For a single transfection, 1 μg of plasmid and 3 μL of FuGENE6 were separately mixed in a volume of 50 μL OptiMEM (GIBCO). For double and triple transfections, the amount of DNA for each subunit was reduced, and the final ratio of FuGENE6 solution to plasmid was maintained at 1 μg DNA to 3 mL FuGENE6. After 5 min the plasmid was mixed with the diluted FuGENE6 and kept for 20 min at room temperature. Transfection was initiated by adding the entire 100 μL of each transfection mixture containing DNA to each well. The cells were grown for 48 h before fixation.
The cells were fixed with 4% paraformaldehyde and ~0.2% w/v picric acid dissolved in 0.1 m PB (pH 7.4) for 30 min at room temperature. The fixed cells were washed three times in 0.1 m PB for 5 min. Non-specific antibody binding was blocked with 20% normal horse serum (VECTOR, Burlingame, CA, USA) in 0.3% Triton X-100 and Tris[hydroxymethyl]amino-methane-buffered saline (TBS-T) for 1 h. Single antibodies, or mixtures of up to three primary antibodies, raised in different host species, were applied in 1% normal horse serum in TBS-T for 2 h at room temperature (Table 2). After washing, the cells were incubated with secondary antibodies. For simultaneous detection of different primary antibodies, Alexa Fluor-488 goat anti-rabbit IgG (1: 1000; Invitrogen, CA, USA), Cy3-coupled anti-guinea pig (1: 400; Jackson ImmunoResearch Europe, Cambridgeshire, UK) and Cy5-coupled anti-rabbit, anti-guinea pig or anti-rat (1: 400; Jackson ImmunoResearch Europe, Suffolk, UK) subtype- and species-specific secondary antibodies were used. Secondary antibodies were diluted with TBS-T, and reacted for 1 h at room temperature. After washing twice with TBS-T and once with TBS, the cells on glass coverslips were covered with Vectashield anti-fading medium (Vector, Burlingame, CA, USA). Epifluorescence images were captured with a Digital charge-coupled device (CCD) camera on a LEITZ DM RB microscope equipped with the following filter blocks A4, L5, Y3 and Y5, which ensured that only one fluorochrome was recorded in each channel. Image processing was carried out with the Openlab 4.0.4 software (Improvision, PerkinElmer Comp, Coventry, UK).
Table 2.
Immunofluorescence labelling tests of antibody specificity on transiently transfected HEK cells expressing subunits of the GABAA receptor
| Transfected GABAA receptor subunit | Primary antibodies (dilutions), applied mixed | Secondary antibodies (dilutions), applied mixed | Labelling |
|---|---|---|---|
| α1 | Rat anti-α1 (1 : 100) | Anti-rat Cy3 (1 : 400) | ++ |
| Rabbit anti-β3 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − | |
| α1 | Rabbit anti-α1 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | +++ |
| Guinea pig anti-β3 (1 : 100) | Anti-guinea pig Cy3 (1 : 400) | − | |
| α2 | Rabbit anti-α2 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | +++ |
| α2 | Guinea pig anti-β3 (1 : 100) | Anti-guinea pig Cy3 (1 : 400) | − |
| Rabbit anti-α1 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − | |
| α2 | Rat anti-α1 (1 : 100) | Anti-rat Cy3 (1 : 400) | − |
| Rabbit anti-β3 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − | |
| α5 | Rabbit anti-α5 (1 : 200) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | +++ |
| Rat anti-α1 (1 : 100) | Anti-rat Cy3 (1 : 400) | − | |
| α5 | Rabbit anti-α1 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − |
| Guinea pig anti-β3 (1 : 100) | Anti-guinea pig Cy3 (1 : 400) | − | |
| β3 | Guinea pig anti-β3 (1 : 100) | Anti-guinea pig Cy3 (1 : 400) | +++ |
| Rabbit anti-α1 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − | |
| β3 | Rat anti-α1 (1 : 100) | Anti-rat Cy3 (1 : 400) | − |
| Rabbit anti-β2 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | − | |
| β2 | Rabbit anti-β2 (1 : 500) | Anti-rabbit Alexa Fluor-488 (1 : 1000) | +++ |
| Rat anti-α1 (1 : 100) | Anti-rat Cy3 (1 : 400) | − | |
| α1 + β3 + γ2 | Rat anti-α1 (1 : 100) | Anti-rat Alexa Fluor-488 (1 : 1000) | ++ |
| Guinea pig anti-β3 (1 : 100) | Anti-guinea pig Cy5 (1 : 400) | ++ | |
| Rabbit anti-γ2 (1 : 300) | Anti-rabbit Cy3 (1 : 400) | ++ |
GABA, γ-aminobutyric acid.
GABAA receptor subunit-deficient mice
A further test of the specificity of the antibodies was carried out on SDS-digested freeze-fracture replica produced from two mice lacking the GABAA receptor alpha1 subunit (Sur et al., 2001), and two wild-type littermates (kindly provided by Drs D. Belleli and J. Lambert at the Neurosciences Institute, University of Dundee, and originated from Dr Thomas Rosahl, Merck Sharp & Dohme, UK). Brains from two mice each lacking the alpha2 subunit (Boehm et al., 2004) or the alpha5 subunit (used as positive controls for unaffected subunits), and wild-type littermates were kindly provided by Dr Thomas Rosahl (Merck Sharp & Dohme, UK). Two mouse brains lacking the beta3 subunits were obtained also from Dr G. Homanics, Dept Anesthesiology, Univ. Pittsburgh, School Med, PA, USA (Homanics et al., 1997).
Specificity of antibodies
Western blots of membrane preparations from rat brains showed a protein band at the expected molecular mass for the antibodies tested. Results from immunoblots were published previously for the affinity-purified rabbit antibodies to the alpha1 (Tretter et al., 1997; Ogris et al., 2006), alpha5 (Ogris et al., 2006), beta2 (Jechlinger et al., 1998), beta3 (Slany et al., 1995; Poltl et al., 2003) and gamma2 (Tretter et al., 1997) subunits, which in addition have been shown to precipitate recombinant receptors consisting of these subunits and did not have cross-reactivity with other subunits. The Western blot of rat brain membranes with the rat antiserum to the alpha1 subunit showed a major band at 51 kDa, as did the rabbit antibody in the same experiment. The antiserum raised in guinea pig and the purified rabbit antibodies to the beta3 subunit showed two strong bands at 53 and 56 kDa, as described previously (Todd et al., 1996), and some weaker bands. The Western blot of a wild-type mouse brain with the purified antibody alpha2 (322–357) showed a major protein band at approximately 53 kDa, and this was absent in the blot of brain membrane proteins from an alpha2 gene-disrupted mouse.
In addition, two immunohistochemical methods were used to assess the specificity of the antibodies on fixed cells for this study. Firstly, HEK cells transiently transfected with plasmids encoding cDNAs for different GABAA receptor subunits were tested by immunofluorescence labelling (Table 2). The antibodies for the alpha1 (rat, rabbit), alpha2 (rabbit) and beta3 (guinea pig, rabbit) subunits reliably labelled those cells transfected with the corresponding plasmids, and no non-specific cross-labelling was detected for any of the other tested subunits (Table 2). A second test of the specificity of the antibodies to the alpha1 (rat, rabbit), alpha2 (rabbit) and beta3 (guinea pig, rabbit) subunits was FRIL on hippocampi from wild-type and gene-deleted mice. In wild-type mice, we observed qualitatively similar immunogold distribution to that described in the rat below, but this was absent in the gene-deleted mice (not shown). We conclude that immunogold labelling on the protoplasmic-face (P-face) of cells in FRIL represents recognition of the respective subunits.
Replica immunolabelling
We followed recent modifications (Tanaka et al., 2005; Masugi-Tokita & Shigemoto, 2007; Tarusawa et al., 2009) of the technique developed by Fujimoto (1995). The replicas treated with the SDS solution were washed thrice in a washing buffer (WB) containing 0.05% bovine serum albumin (BSA; Nakalai tesque, Kyoto, Japan), 0.1% Tween 20 and 0.05% sodium azide dissolved in TBS. Non-specific binding was blocked by incubating the replicas for 2 h at room temperature in a blocking solution consisting of 5% BSA in WB. Replicas were then incubated overnight at 4 °C on a shaker while submerged in the primary antibodies, diluted in blocking solution. The source, testing of specificity and concentration of primary antibodies used are shown in Table 1. The following day, after three washes in the WB, the replicas were incubated with secondary antibodies conjugated to gold particles of either 5, 10 or 15 nm (British Bio-cell International, Cardiff, UK) for 2 h at 37 °C. For double-labelling, the various antibodies were applied sequentially as follows – the first primary antibody and then 5-nm gold-conjugated secondary antibody, followed by the second primary antibody and 10-nm gold-conjugated secondary antibody. The replicas were washed twice in WB and then in distilled water twice for 3 min. They were collected on 100-mesh copper grids and examined with an electron microscope (JEOL1010 or Philips CM100). Some images were taken on films and the negatives printed for measurement using a digitising pad. Most of the images were recorded using a Gatan UltraScan 1000 CCD camera (Gatan UK, Abingdon, UK) and measured directly on a computer screen. For the quantitative analysis of synaptic and extrasynaptic labelling, original images that included one or more synapses and covered an area of at least 1.4 × 1.4 μm, at 2048 × 2048 pixels of resolution, were used.
Measurement of synaptic area and quantification of the density of immunogold particles
The antibodies used in the study reliably showed labelling concentrated on the cytoplasmic face (P-face) of the plasma membrane, whereas the extracellular half of the plasma membrane (exoplasmic, E-face) had a very low level of labelling. Some of the P-face labelling was highly concentrated over small membrane patches rich in intramembrane particles (IMPs), which were also labelled for neuroligin-2 and considered to be synaptic junctions. All synapses on an analysed cell body that were present on the membrane close to perpendicular to the electron beam were collected. The specimens were not tilted, therefore synapses on areas of membrane with significant slope provide an area smaller than their real lateral extent, and were not included. The synaptic area was delineated manually using ImageJ (NIH, USA) or iTEM software (Olympus Soft Imaging Solutions GmbH, Munster, Germany) following the contour of the high IMP concentration, by two investigators independently for training sessions. If the difference in the measurement was larger than 20%, the area was re-measured by three investigators until an agreed area was arrived at. Gold particles within a synapse were counted to obtain the synaptic density, which is expressed as number of gold particles per square micrometre. Extrasynaptic immunolabelling density measurements were taken from the same images and cells from which synaptic measurements were obtained. Background labelling was measured on E-face membrane areas of the same cells from which P-face measurements were taken, or if they did not have E-face membrane areas, more often, from neighbouring cells. From each cell at least three images were taken, the E-face area was measured and the gold particles counted at high magnification.
The significance level for statistical tests was set at 0.05. Correlations were analysed by two-tailed Pearson correlation test. Results are expressed as mean ± SD.
Results
Distribution of GABAA receptor subunit immunogold labelling in rat CA1 area
Identification of replicated membranes, synapses and selection of antibodies
The somata of pyramidal cells are known to receive a high density of GABAergic synapses from several types of basket cell (Ramon y Cajal, 1893; Andersen et al., 1963; Buhl et al., 1994; Pawelzik et al., 2002) and provide a largely homogeneous population of cell bodies for testing the molecular composition of synapses. Interneurons represent only a few percent of the neuronal population in the pyramidal layer (Aika et al., 1994). The cell bodies of CA1 pyramidal cells are easily identifiable in freeze-fractured replicas of the hippocampus as a band of large elliptical or triangular structures located in the stratum pyramidale (Fig. 1A). In order to evaluate the antibodies and the membrane partitioning of receptors we studied these fractured somatic membranes. Preliminary screening of antibodies to the extracellular N- or C-terminals of subunits, which are accessible on the extracellular half (E-face) of the plasma membrane, and to the intracellular loop between transmembrane domains 3 and 4, which is accessible to antibodies on the cytoplasmic half (protoplasmic, P-face) of the fractured membrane, showed that a much higher level of labelling is obtained on the P-face than on the E-face (data not shown). These membrane domains can be identified by features such as the arrangements of IMPs (Rash & Yasumura, 1999). The E- and P-faces can be differentiated by the much higher density of IMPs in the P-face and the concavity of the structures. Nevertheless, there was significant specific labelling also on the E-face with antibodies to the C- or N-terminal of subunits, which are located on the extracellular surface of the membrane. Although the pattern of E-face labelling matched that of the P-face, it was not analysed, because the immunogold particle clusters that may represent synapses were not associated with any obvious structural feature (see below).
Fig. 1.
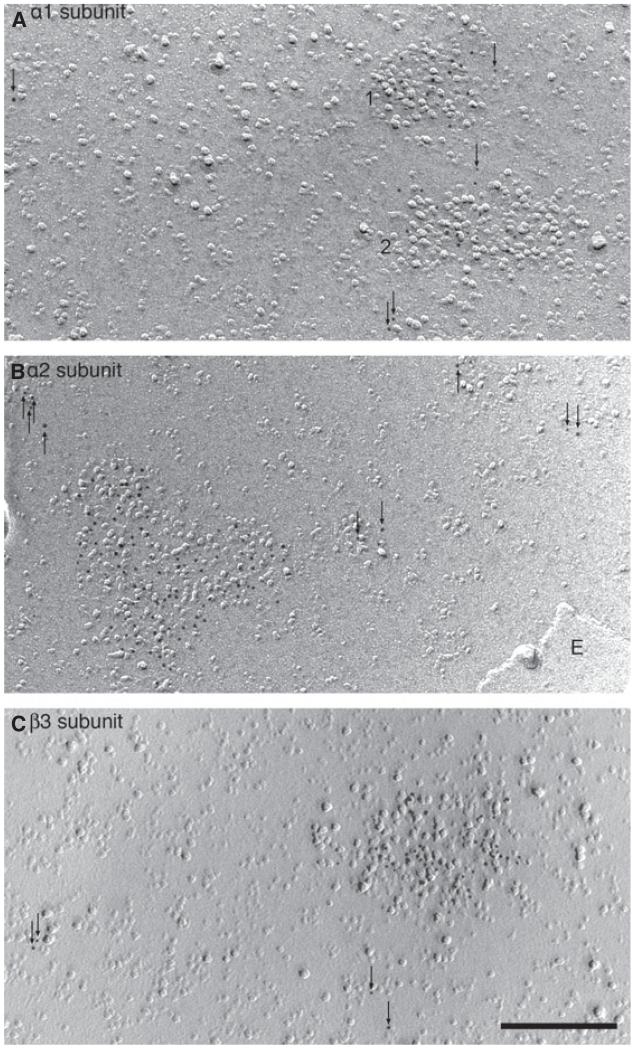
GABAergic synapses on the surface of CA1 pyramidal cell somata revealed by SDS-digested FRIL. (A) Low-magnification view of a replica of the soma surface of a pyramidal cell labelled with an antibody to the intracellular loop of the beta3 subunit of the GABAA receptor and 10-nm immunogold particles conjugated to secondary antibodies. The cytoplasmic face of the plasma membrane (protoplasmic; P-face) was replicated and the gold particles are concentrated on IMP clusters (arrows, framed area shown as inset), and also scattered at low density outside these clusters (arrows in inset). A medium sized area of exoplasmic (E)-face membrane replica and some smaller areas (E) of the membranes of neighbouring cells are attached to the P-face replica of the soma. (B) Immunolabelling for neuroligin-2 (5 nm gold, e.g. arrow). This is a protein specifically enriched in GABAergic synapses on the cell surface in the hippocampus; it is highly concentrated over an island of IMPs on the P-face of the replicated plasma membrane of another cell. (C) High-magnification image of the replicated plasma membrane of a pyramidal cell showing the co-localisation of neuroligin-2 (10 nm immunogold, arrowheads) and the beta3 subunit of GABAA receptors (5 nm immunogold, arrows) concentrated over an IMP cluster. (D) Delineation of the IMP cluster for measuring the synaptic area. Scale bars: 1 μm (A); 0.2 μm (inset A, B); 0.1 μm (C, D).
Using antibodies to the intracellular loop of the alpha1 (raised in rat or rabbit), alpha2 (rabbit) and beta3 (raised in rabbit or guinea pig) subunits, two distinct labelling patterns were observed on the P-face of somatic membrane replicas. Small (~0.02–0.05 μm2) areas had high immunogold density coinciding with a concentration of IMPs of a characteristic size and shape. The high immunogold density abruptly dropped at the edge of the IMP cluster (Fig. 1A, inset). Of all the IMPs in the P-face, those in the GABAA receptor subunit-dense areas were relatively uniform and of medium height and diameter as compared with other IMPs. Adjacent to these immunolabelled areas, larger diameter and higher IMPs were often present in small clumps, but were not immunolabelled. There were also unlabelled patches of high-density clusters of larger size IMPs distinct from the immunolabelled patches. The rest of the plasma membrane had scattered immunogold particles of varying density, depending on the antibody, and these particles could not be allocated to particular IMPs, although an association could not be excluded.
The size of the IMP clusters labelled for GABAA receptor subunits was similar to that of GABAergic synaptic areas reported from serial thin sections reconstructed on CA1 pyramidal cells (Nyiri et al., 2001). To further probe if the labelled IMP clusters are indeed synapses, we tested whether they also contained neuroligin-2 (n = 3, animals), a protein present apparently only in GABAergic and glycinergic synapses on the cell surface (Varoqueaux et al., 2004). In two animals, FRIL for neuroligin-2 (10-nm gold) resulted in the labelling of IMP-dense patches only on the P-face (Fig. 1B), which were similar in size (animal 1, n = 62, 0.053 ± 0.023 μm2; animal 2, n = 34, 0.047 ± 0.017 μm2) and shape to patches obtained with antibodies to GABAA receptor subunits (see below). In a third animal we used 5-nm particles for the beta3 subunit and 10-nm particles for neuroligin-2 immunolabelling, and found that every IMP cluster labelled for the beta3 subunit (guinea pig antibody, n = 26, 0.046 ± 0.019 μm2) was also labelled for neuroligin-2 (Fig. 1C and D). We concluded that P-face membrane patches densely labelled for GABAA receptor subunits are synaptic junctions. The size of neuroligin-2-labelled IMP-rich areas were not different in the three animals (Kruskal-Wallis test, P = 0.370), therefore they were pooled, resulting in a mean synaptic IMP cluster area of 0.050 ± 0.021 μm2 (range, 0.015–0.121 μm2).
Distribution of synaptic and non-synaptic GABAA receptor labelling on dendrites, spines and the axon initial segment
In general, it is not easy to establish the identity of the cell type relating to a particular freeze-fracture replicated membrane in the neuropil. However, due to the regular laminar organisation of the hippocampus and the characteristic shapes of pyramidal cells, some structures can be reliably identified. The main apical dendrites of pyramidal cells can be recognised on the basis of their radial origin from the cell body and/or their large diameter and perpendicular orientation to the layers. None of the GABAergic interneuron types have such large dendritic diameters. Synapses on main apical dendrites, as identified by receptor subunit-rich IMP clusters, had the same shape and IMP content (Fig. 2) as those synapses found on the oblique dendrites, which in fortuitous cases could be followed out from the apical dendrites (Fig. 3). The extrasynaptic labelling density did not appear to change along the apical and oblique dendrites at a distance of up to 150 μm from the soma. The GABAergic dendritic innervation of pyramidal cells is diverse, and in the region that we studied is supplied by at least 13 distinct interneuron types (Buhl et al., 1994; Pawelzik et al., 1999, 2002; Thomson et al., 2000; Klausberger & Somogyi, 2008). These synapses may be heterogeneous in terms of their subunit composition and it is uncertain if they all contain the beta3 subunit. Dendritic spines were identified by their size, shape and occasionally by the thin neck connecting them to the parent dendrite (Fig. 2), or by shape and co-labelling for PSD-95, a glutamatergic synaptic scaffolding protein (Fig. 2). Receptor subunit-rich IMP clusters were not encountered on dendritic spines, but the spines were diffusely labelled by extrasynaptic immunoparticles (Fig. 2).
Fig. 2.
Synaptic and extrasynaptic GABAA receptor subunit labelling on pyramidal cell apical dendrites and dendritic spines. (A) An apical dendrite in stratum radiatum shows beta3 subunit labelling in two synapses (1, 2) and scattered extrasynaptic labelling that includes the neck of a dendritic spine (s). The synapses are shown at higher magnification in (D). The amount of extrasynaptic labelling exceeds synaptic labelling in this area. Two small E-face plasma membrane patches (E) of adjacent cells remain attached. Note the lack of labelling on the E-face plasma membranes (top) and in other structures. (B) Extrasynaptic labelling for the beta3 subunit (e.g. arrows) on an apical dendrite and in an emerging spine (s, arrows). At the neck of the spine, the broken neck of another spine is seen. (C) Two dendritic spines (s) identified by their shape and PSD-95 immunolabelling in glutamatergic synapses. The larger one on the right shows extrasynaptic alpha2 subunit labelling (arrows). The glutamatergic synapse is partially covered by the E-face of the presynaptic nerve terminal (Et). (D) Higher-magnification view of the two synapses shown in (A). Scale bars: 0.2 μm (A–D).
Fig. 3.
Synaptic and extrasynaptic labelling for the beta3 subunit of the GABAA receptor on a main apical dendrite and a small oblique dendrite in stratum radiatum. (A) The oblique dendrite branches from the apical dendrite to the left and receives a synapse (s) identified by the high density of IMPs and immunogold particles with a sharp border. Both the main apical dendrite and the oblique dendrite have scattered immunolabelling (e.g. arrows) of extrasynaptic subunits. Note that there is no synapse in this membrane area on the main apical dendrite. There is no labelling on E-face (E) membranes. (B) High-magnification view showing the synaptic and extrasynaptic labelling. Note that the synapse is at a site where the oblique dendrite branches to the right (arrowhead). Scale bars: 0.2 μm (A, B).
We also investigated if the FRIL technique is suitable for the characterisation of GABAA receptor subunit composition of the axon initial segment of pyramidal cells, which receives GABAergic innervation exclusively from parvalbumin-positive axo-axonic cells (Somogyi et al., 1983). These synapses are particularly rich in the alpha2 subunit (Nusser et al., 1996), and are known to contain also the alpha1, beta2/3 and gamma2 subunits (Somogyi et al., 1996). We identified axon initial segments on the basis of selective labelling for the Nav1.6 alpha subunit of the sodium channel, as also reported recently (Lorincz & Nusser, 2010), and tested for GABAA receptor subunits with 5-nm particles. Because the membrane of the axon initial segment represents a very small fraction of all the membranes in strata pyramidale and oriens, axon initial segments were very rare, and we found only seven with GABAA receptor subunit-rich IMP clusters, three labelled for the alpha2 subunit (Fig. 4) and four labelled for the beta3 subunit. All these synapses were trans-fractured, i.e. the plane of membrane split jumped from the axon initial segment membrane to the presynaptic terminal membrane across the synaptic cleft, revealing the E-face of the latter (Fig. 4). Thus, only partial synapses were seen on axon initial segments having IMP organisation qualitatively similar to that of other GABAergic synapses on neurons. The density of extrasynaptic labelling on the axon initial segments could not be assessed quantitatively due to the small membrane areas and the low number of extrasynaptic particles.
Fig. 4.
Localisation of the alpha2 subunit of the GABAA receptor in a synapse on an axon initial segment. (A) The radially oriented axon initial segment is identified by immunolabelling for the Nav1.6 sodium channel alpha subunit (15-nm gold particles), which is highly expressed in the initial segment membrane of CA1 pyramidal cells. The alpha2 subunit is localised by small immunogold particles (nominally 5 nm), which are concentrated over a patch of IMPs, identifying it as a synapse, partially covered by the E-face (E) of the presynaptic terminal. There is also one low electron density particle on the E-face (double arrow). (B) To assist interpretation of the small gold particles, which are partially electron lucent and had low contrast, each was covered with a black marker dot. Note that at the boundary of the shift of the fracture plane from the membrane of the axon initial segment to the membrane of the presynaptic bouton, immunoparticles overlap the E-face, which is most likely due to a small overlap of undigested or trapped P-face membrane under the E-face membrane half, and is not the location of alpha2 subunit-containing receptors in the presynaptic terminal. Any intracellular protein domains in the E-face membrane half are covered with the carbon/platinum layer and are not accessible to antibodies. Scale bar: 0.1 μm (A).
Quantification of synaptic and extrasynaptic GABAA receptor subunit labelling on pyramidal cell somata
One of our aims was to quantify immunolabelling of synapses and of the extrasynaptic membrane, and this depends on labelling efficacy. We tested several of the antibodies using the same primary antibody dilution, and either 5-nm or 10-nm gold particles coated with secondary antibodies on separate replicas in the same experiment. The 5-nm particles resulted in 1.5–2 times higher synaptic immunogold densities than the 10-nm particles (data not shown). Therefore, we proceeded to analyse the densities of GABAA receptor labelling using 5-nm particles.
Synaptic areas and GABAA receptor subunits labelling densities
The distribution of GABAA receptor subunits was tested on pyramidal cell somata by the application of antibodies specific to the intracellular loop of the alpha1 (rat antiserum), alpha2 (rabbit antibody) and beta3 (guinea pig antiserum) subunits. A consistent labelling pattern was evident in all four rats in batch 1 (Fig. 5; Table 3). A total of 67 cells were measured, an average of 5.5 cells per animal per antibody (range 4–8). A synapse was considered immunopositive if it contained at least three gold particles, and the total area of each immunogold-labelled IMP cluster was measured, i.e. including the area of IMPs that had no overlying gold particles. All labelled synapses were recorded on the fractured part of the soma of each cell. The size of the smallest neuroligin-2-immunopositive IMP cluster was 0.0153 μm2, and this value was set as a lower limit for further analysis, which resulted in the exclusion of only a few labelled IMP clusters. The values of synaptic areas obtained for rats 1–4 labelled for the receptor subunits were (n, range) – 0.0502 ± 0.0261 μm2 (146, 0.0156–0.1536); 0.0513 ± 0.0254 μm2 (200, 0.0159–0.1719); 0.0472 ± 0.0252 μm2 (275, 0.0160–0.2710); 0.0535 ± 0.0230 μm2 (110, 0.0156–0.1407). There was no significant difference between the synaptic areas labelled for GABAA receptor subunits (n = 4 rats) or neuroligin-2 (n = 3 rats; Kruskal–Wallis test, P = 0.0758). The pooled mean synaptic area obtained from single receptor subunit immunolabelling was 0.0498 ± 0.0252 (n = 731). Pooled synaptic area size was not distributed normally (Kolmogorov–Smirnov, Z = 2.351, P = 0.00003), and showed a skewed distribution towards larger values (Fig. 6).
Fig. 5.
Synaptic and extrasynaptic localisation of GABAA receptor subunits on CA1 pyramidal cell somata. (A–C) Labelling for the alpha1, alpha2 and beta3 subunits, respectively (5-nm immunogold particles) is highly concentrated on clusters of IMPs on the P-face of the replicated plasma membrane with low-density scattered immunoparticles in the extrasynaptic regions. Note the high density of synaptic labelling in patches within the IMP clusters. Two synapses (1, 2) are labelled for the alpha1 subunit. (B) The extrasynaptic (E) face of the plasma membrane of a neighbouring cell is in the lower right corner. Scale bar: 0.2 μm (A–C).
Table 3.
Comparison of immunolabelling parameters for the alpha1 subunit using two different antisera, and the antiserum raised in rat on two different batches of rats
| Synapses |
Extrasynaptic |
Synaptic/extrasynaptic |
E-face (background) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density* (no/μm2) |
No. gold/ synapse |
Density* (no/μm2) |
Ratio | Density (no./μm2) |
% Density of | ||||||||
|
|
|
|
|
|
|||||||||
| Experi-mental animals |
Antisera raised in |
n | Mean ± SD | Range | Mean ± SD | Mean ± SD | Range | Mean ± SD | Range | % Synaptic labelling on soma |
Mean ± SD | Synaptic labelling |
Extrasynaptic labelling |
| Rats | |||||||||||||
| Batch 1 | Rat | 4 | 219 ± 30 | 192–261 | 11.5 ± 1.7 | 4.5 ± 3.9 | 1.8–10.2 | 78 ± 55 | 19–148 | 33 ± 17 | 0.99 ± 1.08 | 0.49 ± 0.58 | 20.0 ± 5.4 |
| Batch 2 | Rat | 3 | 237 ± 23 | 211–251 | 14.2 ± 1.0 | 3.3 ± 1.8 | 1.9–5.2 | 85 ± 34 | 47–114 | 37 ± 10 | 0.420 ± 0.220 | 0.175 ± 0.083 | 15.51 ± 9.54 |
| Rabbit | 443 ± 52 | 383–473 | 28.1 ± 1.3 | 3.5 ± 0.7 | 2.7–4.0 | 132 ± 39 | 102–176 | 48 ± 7 | 0.095 ± 0.068 | 0.022 ± 0.014 | 2.84 ± 1.68 | ||
| Cells | |||||||||||||
| Batch 2 | Rat | 17 | 234 ± 35 | 171–303 | 14.0 ± 2.8 | 3.1 ± 1.7 | 1.0–5.6 | 99 ± 53 | 40–229 | 39 ± 12 | |||
| Rabbit | 15 | 443 ± 84 | 332–638 | 28.1 ± 7.2 | 3.5 ± 1.3 | 1.7–6.3 | 141 ± 50 | 62–256 | 49 ± 9 | ||||
| Synapses | |||||||||||||
| Batch 1 | Rat | 249 | 226 ± 98 | 38–505 | 11.9 ± 6.6 | ||||||||
| Batch 2 | Rat | 181 | 232 ± 74 | 55–489 | 13.8 ± 6.7 | ||||||||
| Rabbit | 158 | 446 ± 137 | 130–833 | 28.5 ± 16.0 | |||||||||
Immunogold densities were corrected by subtracting the background [exoplasmic (E)-face density in the same specimen] from each synaptic and extrasynaptic density value.
Fig. 6.
Distribution of synaptic area values and GABAA receptor subunit immunolabelling. (A) Synaptic area size is not normally distributed, it is skewed towards larger values. (B) Average synaptic immunolabelling and variability in each of four rats shows consistent labelling. Horizontal lines show median, box indicates 50 percentile, whiskers show upper and lower 25%, and circles show outliers. (C, E, G) The distribution of immunolabelling strength (no. of immunogold particles per synapse) is skewed towards larger values for all subunits. (D, F, H) In contrast to the distributions in (C, E and G), the synaptic density value distributions are normal (see text), indicating uniform labelling density. Note that labelling intensity differences between antibodies do not represent differences in subunit abundance, because the degree of labelling is not comparable between antibodies due to the individual properties of each antiserum.
Synaptic and extrasynaptic immunolabelling densities were corrected in each animal and for each reaction, individually, by subtracting the labelling density measured on the somatic E-face membrane areas (see below) in the same replicas. The average synaptic labelling densities (mean ± sd, gold particles per μm2) of four rats for the alpha1 subunit were 219 ± 30 (range of animal mean, 192–261; range of synaptic densities, 38–505), for the alpha2 subunit 576 ± 64 (range of animals mean, 488–642; range of synaptic densities, 40–1582) and for the beta3 subunit 646 ± 109 (range of animals mean, 553–789; range of synaptic densities, 63–1688; Fig. 6B). The distribution of values representing the number of immunoparticles per synapse pooled across animals for each subunit was not normally distributed (Kolmogorov–Smirnov, two-tailed, alpha1, n = 249, Z = 2.198, P = 0.00013; alpha2, n = 257, Z = 2.365, P = 0.00003; beta3, n = 225, Z = 1.893, P = 0.00154), and showed a skewed distribution towards larger values (Fig. 6C, E and G).
There was a strong positive correlation between synapse size and number of immunoparticles (Pearson correlation test, two-tailed) in 11 of the 12 cases (four animals, three subunits each). The mean correlation coefficients were 0.544 ± 0.120 (n = 4, 0.014 ≥ P ≥ 8.05E-11), 0.725 ± 0.019 (n = 3, 2.47E-09 ≥ P ≥ 3.51E-19) and 0.731 ± 0.072 (n = 4, 2.01E-06 ≥ P ≥ 4.32E-14) for the alpha1, alpha2 and beta3 subunits, respectively. Rat 4 showed no correlation for the alpha 2 subunit (Pearson, r = 0.219, P = 0.192). In most cases, there was no or only very weak correlation between synapse size and the density of immunoparticles. The distribution of synapses according to labelling density was normal for the alpha2 (Kolmogorov–Smirnov, two-tailed, n = 257, Z = 0.754, P = 0.621), beta3 subunits (n = 225, Z = 0.583; P = 0.885), and if the two largest (values ≥ 500) outliers were omitted also for the alpha1 subunit (n = 247, Z = 1.331, P = 0.058). Accordingly, a main contributor to the skewed synaptic labelling strength distribution (particles per synapse) is the skewed synapse size distribution, as is also suggested by the correlation of synaptic area and particle number. On individual pyramidal cells, the density of immunogold particles per synapse could vary over a range of up to one order of magnitude for the same subunit (Fig. 7).
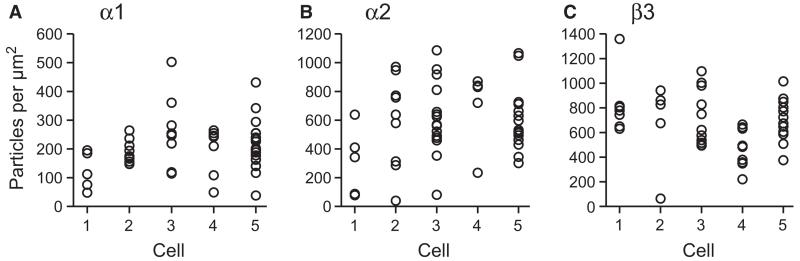
Fig. 7.
Variability of synaptic GABAA receptor labelling for the alpha 1, alpha2 and beta3 subunits on individual CA1 pyramidal cell somata. (A–C) All visible synapses were analysed on the somata of five pyramidal cells for each subunit. The cells for a given subunit were not different in synaptic particle density (pooled mean ± SD, particles/μm2; alpha1, 205 ± 88; alpha2 587 ± 261; beta3 675 ± 240). Synaptic immunolabelling density varied in a range of up to one order of magnitude amongst synapses for a given antibody. Note that labelling intensity differences between antibodies do not represent differences in subunit abundance, because the degree of labelling is not comparable between antibodies due to the individual properties of each antiserum.
Extrasynaptic immunolabelling density and background labelling measurements
Extrasynaptic immunolabelling was measured on the same micrographs taken for recording synapses. The synaptic areas and any attached E-face membrane fragment areas were measured and subtracted from the total somatic P-face membrane to arrive at the extrasynaptic membrane area. In the four animals, the average extrasynaptic particle density (corrected for background, gold particles/μm2) was 4.5 ± 3.9 (range 1.8–10.2) for the alpha1 subunit, 6.3 ± 1.7 (range 4.3–7.9) for the alpha2 subunit and 9.0 ± 4.4 (range 6.5–15.7) for the beta3 subunit.
The above data critically depend on the level of background labelling. Ideally this would be measured on the P-face membranes of cells that do not express the subunit in the same specimens, but such membranes cannot be identified. We used the labelling on the E-face membranes as these are also rich in proteins. For example, AMPA- and N-methyl-d-aspartate (NMDA)-type glutamate receptors are highly retained in the extracellular half of the plasma membrane replica (Tanaka et al., 2005; Tarusawa et al., 2009). E-face gold density was measured on large areas of individual cell bodies, and three–five random images were captured for each cell, corresponding to 14.2 ± 7.7 μm2 (n = 47) of somatic E-face membrane per cell. Any attached P-face membrane fragment areas were measured and subtracted from the cell surface. On average, four cells per animal per antibody were measured (range 2–5). In the four animals, the average E-face particle density (mean ± SD, n = 4, gold particles/μm2) was 0.99 ± 1.08 (range 0.36–2.60) for the alpha1 subunit, 0.71 ± 0.06 (range 0.65–0.80) for the alpha2 subunit and 0.91 ± 0.77 (range 0.49–2.06) for the beta3 subunit. These measurements show that, on average, the background labelling was 0.49% (range 0.14–1.35) of synaptic labelling for the alpha1 subunit, 0.13% (range 0.10–0.16) for the alpha2 subunit and 0.15% (range 0.07–0.36) for the beta3 subunit. For the background-corrected extrasynaptic labelling the proportion of background was inevitably higher. On average, the background labelling was 20.0% (range 12.7–25.6) of extrasynaptic labelling for the alpha1 subunit, 12.2% (range 8.8–18.8) for the alpha2 subunit and 12.0% (range 3.4–28.5) for the beta3 subunit.
From the density values corrected for background, a ratio of synaptic to extrasynaptic density was calculated for each animal and each antibody. The ratios were averaged across animals. On average, the ratio for the alpha1 subunit was 77.9 (Table 3), for the alpha2 subunit was 94.4 and for the beta3 subunit it was 78.6.
Somatic surface synaptic membrane and immunolabelling fraction
In order to calculate the total amount of subunit labelling in the synaptic and extrasynaptic membranes, we measured the plasma membrane area occupied by synaptic junctions on the somata in three additional rats (three cells in each). We used beta3 subunit labelling with an affinity-purified rabbit antibody as a GABAergic synaptic marker, and 10-nm gold particles, which are easily recognised (Fig. 1A). The three rats were not different (Kruskal–Wallis, P = 0.252) and were pooled, resulting in a mean proportion of somatic surface occupied by GABAergic synapses of 0.72 ± 0.11%. Using the mean synaptic and extrasynaptic densities for each subunit in each of the four animals, and taking into account the average synaptic fraction of soma surface, the proportions of synaptic and extrasynaptic labelling were calculated and averaged across animals. The proportion of the total labelling that was in synapses was 33 ± 17% for the alpha1 subunit, 40 ± 5% for the alpha2 subunit and 36 ± 7% for the beta3 subunit.
Estimation of alpha1 subunit distribution with two different antibodies
The above results were obtained with polyclonal antibodies, which are likely to contain several antibody species with different properties. Furthermore, even the same antibody species may lead to different labelling densities depending on how densely the epitopes are located in the membrane or in a single receptor channel. To test the effect of antibodies on synaptic vs extrasynaptic labelling, we compared the labelling for the alpha1 subunit obtained with the rat antiserum, which produced the lowest synaptic labelling signal (see above), to that obtained with affinity-purified rabbit antibodies. Because no tissue was available from the four animals (Batch 1, Table 3) analysed above, we used tissue from three further rats (Batch 2, Table 3) processed together under the same conditions and reacted together by the same person with both the rat and rabbit antibodies to the alpha1 subunit. The comparison is presented in Table 3. The non-parametric Mann–Whitney test was used for comparing variables.
The size of synapses was not different between the two batches of rats measured with either the rat antiserum (Z = −1.414, P = 0.229) or rabbit antibody (Z = −2.121, P = 0.057) to the alpha1 subunit. There was a strong positive correlation between the number of immunoparticles and synapse size (Pearson correlation test, two-tailed) in all three animals, confirming results obtained with the rat antiserum in Batch 1. For the rat antiserum, the mean correlation coefficient was 0.746 ± 0.029 (n = 3, 4.54E-09 ≥ P ≥ 4.06E-17). The distribution of labelling density values was normal (Kolmogorov–Smirnov, two-tailed, n = 181, Z = 0.817, P = 0.516), and there was no correlation between synapse size and the density of immunoparticles (Pearson correlation test, two-tailed, P > 0.65) in two animals, whereas the third animal (rat 2) showed a weak negative correlation (r = −0.290, P = 0.046). For the rabbit antibody, there was also a strong positive correlation between the number of immunoparticles and synapse size (Pearson correlation test, two-tailed) in all three animals. The mean correlation coefficient was 0.842 ± 0.064 (n = 3, 2.51E-11 ≥ P ≥ 5.2E-21). The distribution of labelling density values was normal (Kolmogorov–Smirnov, two-tailed, n = 158, Z = 0.797, P = 0.55), and there was no correlation between synapse size and the density of immunoparticles (Pearson correlation test, two-tailed, P > 0.20) in two animals, whereas the third animal (rat 2) showed a weak negative correlation (r = −0.269, P = 0.043).
Next, we tested if the rat antiserum produced similar or different results on the two batches, the four rats reacted individually, and the three rats reacted together. The number of immunogold particles per synapse (Z = −1.768, P = 0.114), the synaptic labelling density (Z = −0.707, P = 0.629), the extrasynaptic labelling density (Z = 0, P = 1) and the E-face labelling (Z = 0, P = 1) showed no difference between the two sets of rats.
Testing the same parameters using the rabbit antibody on specimens from batch 2 rats (n = 3) in comparison with rat antiserum showed no difference in the number of immunogold particles per synapse (Z = −1.964, P = 0.100), synaptic density (Z = −1.964, P = 0.100), extrasynaptic density (Z = −0.218, P = 1.000) or E-face labelling (Z = −1.528, P = 0.200). This was probably due to the low number of animals tested and the high level of variability between animals. For example, the mean number of gold particles per synapse was twice as high, on average, with the rabbit antibody than with the rat antiserum, and the density of synaptic labelling was 87% higher. Therefore, we also compared the parameters between the populations of pyramidal cells sampled from rats in batch 2 (n = 17, rat antiserum; n = 15, rabbit antibody; Table 3). In this comparison the use of the rabbit antibody resulted in a significantly higher number of gold particles per synapse (Z = −4.589, P = 1.10E-07) and synaptic labelling density (Z = −4.815, P = 3.50E-09). The extrasynaptic labelling densities were not different with the two antibodies (Z = −1.038, P = 0.313), but due to the higher synaptic labelling with the rabbit antibody the ratios of synaptic to extrasynaptic density per cell values were higher (Z = −2.398, P = 0.016) with the rabbit antibody. A significantly higher synaptic particle number (Z = −10.52, P = 7.00E-26) and synaptic immunolabelling density (Z = −13.44, P = 3.50E-41) was also obtained with the rabbit (n = 158 synapses) as compared with the rat antiserum (n = 181 synapses) when all synapses were pooled across animals. Accordingly, we concluded that the rabbit antibody provides higher labelling efficacy as compared with the rat antiserum in batch 2 rats.
Because the rat antiserum provides similar labelling in the two batches of rats, we pooled these data, and compared the resulting labelling parameters to those obtained with the rabbit antibody on batch 2 rats, for which both antibodies were tested. The number of gold particles per synapse (Z = −2.393, P = 0.017), the density of synaptic labelling (Z = −2.393, P = 0.017) and the E-face labelling (Z = −2.165, P = 0.033) were different for the two antibodies. The density of extrasynaptic labelling (Z = −0.342, P = 0.833) and the ratio of synaptic to extrasynaptic labelling (Z = −1.709, P = 0.117) were not different with the two antibodies. The ratio of synaptic to extrasynaptic labelling density (background corrected) was, on average, 131.6 ± 38.8 (n = 3) with the rabbit antibody, and that obtained with the rat antiserum was 80.8 ± 43.8 (n = 7).
Using the mean synaptic and extrasynaptic densities obtained with the rat antiserum for each of the seven animals, and with the rabbit antibody for each of the animals in batch 2, and taking into account the average fraction of synaptic soma surface obtained earlier (0.72%), the proportion of synaptic alpha1 subunit labelling 37 ± 10% (n = 3) obtained with the rat antiserum was not different (Z = −1.528, P = 0.200) from that obtained with the rabbit antibody 48 ± 7% (n = 3). Neither value was significantly different from that obtained with the rat antiserum in the first four rats (33 ± 17; Mann–Whitney, rat antiserum, Z = −0.354, P = 0.857; rabbit antibody, Z = −1.414, P = 0.229). In conclusion, 33–48% of the alpha1 subunit labelling on the soma surface was in synaptic junctions.
Co-localisation of two GABAA receptor subunits in individual synapses
Technical considerations
Previous studies suggested that GABAA receptors that contain different alpha subunits might be targeted to partially separate sets of synapses on CA1 pyramidal cells (Nusser et al., 1996; Thomson et al., 2000; Nyiri et al., 2001; Klausberger et al., 2002; Ali & Thomson, 2008). To assess the co-existence of multiple GABAA receptor subunits in individual synapses, we carried out double-labelling using antibodies raised in different host species and immunogold particles of different sizes (5 and 10 nm). Robust evidence for pair-wise co-existence of all three subunit combinations was observed in individual synapses on pyramidal cell somata (Fig. 8; Table 4). Simultaneous application of two primary antibodies to two different subunits reduces labelling intensities, due to steric hindrance if both subunits are present in the same channel. This can lead to false negative results for one or both subunits. Therefore, a sequential application of both primary and secondary antibodies was adopted. The first primary antibody (followed by the first secondary antibody) was predicted to represent the maximal population of labelled synapses, particularly when detected with 5-nm particles. Labelling intensity differences between antibodies cannot be taken as differences in subunit abundance, due to the individual properties of each antiserum. Due to the above factors, we did not calculate the ratios of labelling densities in individual synapses and we present the frequency of co-labelled synapses in two of the four rats in batch 1 (Table 4). First, we detected all synapses labelled with at least one 10-nm particle (applied second) and screened them for the presence of labelling by at least three 5-nm particles (applied first). The co-labelling threshold was set lower for the secondarily applied antibody because of the above-mentioned factors that reduce the probability of labelling. In order to test the effect of the reduced labelling probability by the second application of an antibody on the proportion of co-labelled synapses, we reversed the sequence of the two antibodies.
Fig. 8.
Co-localisation of alpha1, alpha2 and beta3 subunits in individual synapses on CA1 pyramidal cell somata. Sequential applications of two antibodies with different size immunoparticles for the two subunits in each replica. The antibody labelled with 5-nm particles was applied first. Extrasynaptic subunit labelling is marked by vertical arrows for the 5-nm particles and horizontal arrows for the 10-nm particles. (A) Two synapses (1, 2) are labelled for both alpha1 (5 nm, e.g. arrowheads) and beta3 (10 nm, large vertical arrows) subunits. (B) Two synapses (1, 2) are labelled for both the alpha2 (5 nm, e.g. arrowheads) and beta3 (10 nm, large vertical arrows) subunits. (C) Two synapses (1, 2) are labelled for both the alpha1 (5 nm, e.g. arrowheads) and alpha2 (10 nm, large vertical arrows) subunits. The ratio of labelling for the two subunits is different in the two synapses (synapse 1, alpha1/alpha2, 2/12 particles; synapse 2, alpha1/alpha2, 14/16 particles). Note that labelling intensity differences between antibodies do not represent differences in subunit abundance, because the degree of labelling is not comparable between antibodies due to the individual properties of each antiserum. The application of a primary antibody applied second in the sequence and the use of larger particles both reduce labelling efficacy. Scale bar: 0.2 μm (A–C).
Table 4.
Frequency of co-localisation of different GABAA receptor submits in individual synapses
| Antibodies to subunit |
Proportion [% (total no. tested)] of double-labelled synapses of the synapses labelled by antibody applied second |
||
|---|---|---|---|
| First applied | Second applied | Rat 1 | Rat 2 |
| α1 | β3 | 81 (58) | 95 (49) |
| β3 | α1 | 96 (23) | 100 (48) |
| α2 | β3 | 85 (59) | 98 (50) |
| β3 | α2 | 100 (44) | 100 (50) |
| α1 | α2 | 98 (65) | 93 (20) |
| α2 | α1 | 87 (30) | 98 (29) |
Pairwise testing of individual synapses for the alpha1, apha2 and beta3 subunits
A total of 525 synapses (144–203 per antibody combination) were analysed (Table 4). The results were not different between the two animals (Chi square test, DF = 1, 0.000 < Chi-square < 0.268, Fisher’s exact 1 > P > 0.67) for any of the six combinations of the three subunits. Nevertheless, all but one of the proportion values representing the coexistence of two subunits were slightly higher for rat 2, indicating more favourable subunit recognition by all antibodies, probably due to technical factors. The mean number of gold particles per synapse for the first applied antibody was 16.8 ± 8.4 (n = 12 reactions, six in each of two animals, 5-nm gold, including immunonegative synapses) and for the secondarily applied antibody it was 7.8 ± 5.6 (10-nm gold). As expected, all but one of the alpha1 or alpha2 subunit-positive synapses (n = 165) were also positive for the beta3 subunit in the two animals. The vast majority (95%) of synapses tested (n = 144) were also labelled for both the alpha1 and alpha2 subunits, showing the presence of receptors including one or both of these subunits in most basket cell synapses. The degree of synaptic labelling in doubly-labelled synapses as measured by the number of immunoparticles per synapse was highly variable, for both the alpha1 subunit (5 nm, n = 83, range 3–28, median 6; 10 nm, n = 59, range 1–9, median 2) and the alpha2 subunit (5 nm, n = 54, range 3–44, median 22; 10 nm, n = 85, range 3–29, median 10). There was either no or only very weak positive correlation between the number of immunoparticles labelling these two subunits in individual synapses.
Discussion
The results show that GABAergic synapses can be recognised in freeze-fracture replicas in the cytoplasmic, P-face membrane halves as distinct IMP clusters. On somata of CA1 pyramidal cells, the total amount of alpha1, alpha2 and beta3 subunit expression in the synapses is similar to that in the extrasynaptic membrane. Synaptic receptor clusters end with a sharp decrease in labelling density, only occupy 0.72% of the soma surface and have 50–130 times higher immunolabelling densities than the extrasynaptic membrane. Double-labelling experiments with combinations of antibodies to these three subunits indicate that virtually all somatic synapses contain the alpha1, alpha2 and beta3 subunits at varying densities. Synaptic labelling density for a given subunit may vary up to an order of magnitude amongst synapses.
Technical considerations
The labelling efficacy of membrane molecules in immunohistochemistry in general as well as in FRIL depends on many factors that may influence our results. The current method, which evolved after many difficult approaches had been tried to identify proteins in freeze-fracture replicas (Rash & Yasumura, 1999), provides a two-dimensional landscape of the plasma membrane. The breakthrough came with the introduction of SDS by Fujimoto (1995, 1997) to wash the tissue components from the replica film, leaving only molecules, such as membrane-spanning proteins and lipids (Fujimoto et al., 1996), that are directly in contact and trapped by the initial carbon coating deposited over the middle of the split membrane. Subsequently, it was shown that by carefully adjusting the washing conditions, proteins associated with the membrane-spanning molecules, but not having intramembrane domains themselves, can be also retained and immunolabelled on the cytoplasmic P-face membrane leaflet (Hagiwara et al., 2005). This and other unpublished experiments indicate that the SDS washing conditions, such as temperature and duration, may influence the retention of the membrane protein including the GABAA receptor.
Immunolabelling may also be affected by factors that do not uniformly apply to all parts of the plasma membrane. Fixation may reduce the recognition of the epitopes differentially depending on the local protein environment. A reduction of immunoreactivity by fixation has been reported for GABAA receptors with antibodies recognising extracellular epitopes (Fritschy et al., 1998b).
We have detected immunoreactivity in both the E-face and the P-face of the split plasma membranes with different antibodies. On the P-face replica the IMPs represent the domains of the membrane proteins that span the outer leaflet of the lipid bilayer and include the extracellular domain as well. It is thought that the splitting of the membrane does not lead to the breaking of covalent bonds, and individual molecules are pulled to either one or the other membrane leaflet (Fisher, 1989). The partitioning of a molecule between the E-face and the P-face may depend on its subcellular location and could be influenced by the local macromolecular environment, particularly by interactions with cytoskeletal and other proteins. Therefore, further studies are needed with face-matched double replicas (Hagiwara et al., 2005; Masugi-Tokita & Shigemoto, 2007) in which the E-face and the P-face leaflets of the same membrane area are analysed with two different antibodies to either extracellular epitopes or intracellular epitopes, respectively, of the same subunit, in order to establish if the partitioning may be biased in different subcellular compartments.
The density of GABAA receptor channels has been estimated from combined immunogold labelling and recording quantal synaptic responses (miniature inhibitory postsynaptic currents; mIPSCs) in vitro to be about 1250 receptors per μm2 in cerebellar stellate cell synapses (Nusser et al., 1997), where a uniform receptor density distribution was found. In our measurements, the density showed a high variability for a given subunit with coefficients of variation ranging from 0.25 to 0.49 for synapses pooled across animals, and showing a Gaussian distribution. The variability in density may indicate that in the high-density location due to steric hindrance the true subunit abundance is under-reported by our method at the applied antibody concentrations. Using FRIL on unfixed tissue, ~1000 immunoparticles (~5 nm diameter) per μm2 were found, on average, for the AMPA-type glutamate receptor in developing climbing fibre to Purkinje cell synapses, which matched well the expected number of functional channels (Tanaka et al., 2005). In some synapses, up to 1460 particles per μm2 were recorded with antibodies that recognised all four different receptor subunit species. Because four subunits are present in an AMPA receptor, this indicates that, on average, only one of them per receptor may be reported by the immunogold particle. A similar under-reporting may also occur for the GABAA receptors, although the two alpha and two beta subunits are not directly next to each other in the pentameric channel (Minier & Sigel, 2004), and the cytoplasmic loops that are recognised may be more accessible than the extracellular epitopes detected on AMPA receptors. A similarly high density of immunolabelling has been achieved also in fixed tissue in adult retino-geniculate glutamatergic synapses (Tarusawa et al., 2009), and even higher densities, up to 3400 particles per μm2, were reported in the spinal cord (Antal et al., 2008). The highest labelling density of synaptic receptors in our study was also about 1000–1500 particles per μm2, lower than for the AMPA-type glutamate receptors cited above, indicating that steric hindrance between antibodies on neighbouring receptors did not reduce labelling.
It is not yet possible to compare labelling densities and subunit abundance across different antibodies, although such comparisons have been reported (Caruncho et al., 1995). Two immunised animals may produce antibodies of widely differing labelling efficacy even to the same immunogen. To relate labelling density to subunit and GABAA receptor number, independent measures of calibration are required as has been done in the GABAergic synapses on stellate cells (Nusser et al., 1997) or AMPA-type glutamate receptors (Tanaka et al., 2005; Tarusawa et al., 2009). Our comparison of antibodies raised in different species to the alpha1 subunit showed that a doubling of synaptic labelling density can be achieved in samples from the same animal. However, due to the small surface fraction of synaptic areas on the soma, the proportion of synaptic labelling for the subunit in synaptic vs extrasynaptic membrane changed only by 15%. Surprisingly, the extrasynaptic labelling density was not different between the two antibodies. One possible explanation is that in the case of the rat antiserum the extrasynaptic labelling density may contain some non-specific labelling for which the E-face measurement is not a full control. In future studies, the recognition of such P-face membranes, which contain no GABAA receptors, will provide a better control for non-specific labelling. The other possibility is that labelling with the purified rabbit antibody that provided a higher synaptic labelling density was amplified by the presence of anti-idiotypic antibodies, which may amplify labelling in an antibody density-dependent manner. The immunisation of the rabbit was carried out over a prolonged period, and we cannot exclude the presence of anti-idiotypic antibodies.
The relationship between subunit abundance and receptor abundance also requires further analysis. There are two alpha and two beta subunits, which may not be identical molecular species, in most GABAA receptors (Sieghart & Sperk, 2002). For example, pyramidal cells may express receptors with two alpha1 subunits or an alpha1 and an alpha2 subunit preferentially in different synapses, or selectively in the synaptic or extrasynaptic membrane. When two identical subunit species are present in a channel, the binding of an antibody to one of them may occlude the binding of a further antibody to the second subunit. However, in a receptor containing two different alpha or beta subunits, every subunit of a particular species may be labelled. The above difference could influence labelling density in particular molecular compartments.
Notwithstanding the above uncertainties, FRIL provides a highly sensitive method to compare GABAA receptor distribution in membrane microdomains such as synapses. The particular advantages and limitations have been discussed recently (Hagiwara et al., 2005; Masugi-Tokita & Shigemoto, 2007). Here we exploited the advantages of the method that shows the proteins in two-dimensional membrane sheets, which can be sampled uniformly.
Synaptic receptors and phasic inhibition
The presence of alpha1, alpha2 and beta3 subunit immunoreactivity in somatic synaptic junctions of CA1 pyramidal neurons is in line with results of previous immunocytochemical (Nusser et al., 1996; Somogyi et al., 1996; Crestani et al., 1999) and pharmacological (Pawelzik et al., 1999; Thomson et al., 2000) studies of the adult hippocampus in rat and mouse (Crestani et al., 1999; Prenosil et al., 2006).
As virtually all somatic synapses defined by IMP clusters contained beta3 subunit immunoreactivity, it is likely that synapses innervated by both cholecystokinin- and parvalbumin-expressing basket cells contained this subunit, but our results shed no light on the subunit partners in single-receptor pentamers. Even in double-labelled synapses the lateral resolution of about 20 nm does not allow any two immunogold particles to be assigned to a single receptor. In CA1 pyramidal neurons of beta3 gene-deleted mice, a selective loss of slow GABAA receptor-mediated synaptic responses has been reported (Hentschke et al., 2009), and these are probably of dendritic origin in contrast to the fast IPSCs, many of which are mediated by somatic synapses. The fast IPSCs had only slightly faster decays in beta3 gene-deleted pyramidal cells (Hentschke et al., 2009), indicating that either in the mouse the beta3 subunit makes little contribution to somatic synapses, or other subunit(s) compensated for the loss.
The presence of both alpha1 and alpha2 subunits in virtually all somatic synapses in our study of the rat may appear at odds with conclusions obtained in mice (Prenosil et al., 2006). Making alpha2-containing receptors diazepam insensitive was reported to abolish selectively the diazepam enhancement of synaptic currents in pyramidal cells evoked by extracellular stimulation in the soma layer. Synaptic currents in mice in which alpha1 subunit-containing receptors were rendered diazepam insensitive responded with enhancement to the same stimulus (Prenosil et al., 2006). Although the subcellular source of the currents cannot be identified with extracellular stimulation, these results would predict a predominance of the alpha2 subunits in somatic synapses in the mouse. Apart from species differences between rat and mouse, another explanation may be the different position of the alpha1 and alpha2 subunits in the same single-receptor pentamer; the alpha2, but not the alpha1, subunit being next to the gamma2 subunit and thereby contributing to the benzodiazepine-binding pocket (see below). Although the subunit composition of receptors containing alpha1 or alpha2 subunits have not been defined in hippocampal pyramidal cells, receptors containing both alpha1 and alpha2 subunits are almost as abundant in the mouse forebrain as those containing two alpha2 subunits (Benke et al., 2004).
Is there an input-specific sorting of synaptic GABAA receptors?
Previous studies of somatic synapses suggested an enrichment of alpha1 or alpha2 subunit-containing GABAA receptors in synapses established, respectively by parvalbumin- or presumed cholecystokinin-expressing basket cells. First, zolpidem, a benzodiazepine receptor agonist, which shows some selectivity for alpha1 subunit-containing receptors, enhanced IPSPs evoked by fast-spiking (presumed parvalbumin-expressing) basket cells more than IPSPs evoked by regular firing (presumed cholecystokinin-expressing) basket cells (Thomson et al., 2000). Second, a higher level of alpha2 subunit immunoreactivity was found in synapses made by parvalbumin-negative boutons (Nyiri et al., 2001), which were suggested to originate from cholecystokinin-expressing basket cells, than in synapses made by parvalbumin-positive boutons. In a complementary study (Klausberger et al., 2002), the synapses made by parvalbumin-positive boutons had higher alpha1 subunit immunoreactivity than those made by parvalbumin-negative boutons. These quantitative studies used the postembedding immunolabelling technique that has lower sensitivity than the FRIL method.
Immunoreactivity for both alpha1 and alpha2 subunits in virtually all synapses on somata found here appears to contradict these previous conclusions. However, we have not been able to establish the relative abundance of these two subunits in individual synapses, because the recognition of two molecules on the same membrane replica face requires the use of two different sizes of gold particles, the larger one having a lower sensitivity. In addition, in order to produce results comparable to the single antibody labelling used in quantification, the two antibodies need to be applied sequentially leading to a reduced density of labelling for the antibody applied second. Therefore, a potential negative correlation between the levels of the alpha1 and alpha2 subunits, which might follow from the results obtained with the postembedding immunogold method, remains an open question. Because both the E-face and the P-face replicas of the two membrane halves of a single synapse can be immunoreacted with different antibodies (Hagiwara et al., 2005; Shinohara et al., 2008), future studies could address the relative abundance of different receptor subunits in individual synapses using antibodies recognising extracellular epitopes in one subunit on the E-face replica and intracellular epitopes in another subunit on the P-face replica.
Tonic GABAA receptor-mediated inhibition and extrasynaptic receptor subunits
Following the immunohistochemical demonstration of extrasynaptic GABAA receptors (Somogyi et al., 1989; Soltesz et al., 1990; Nusser et al., 1995) and the pioneering electrophysiological observations on hippocampal granule cells (Otis et al., 1991), a tonic GABAA receptor-mediated current was discovered in cerebellar granule cells (Kaneda et al., 1995; Brickley et al., 1996; Wall & Usowicz, 1997). These cells have a particularly high level of alpha6 and delta subunit-containing extrasynaptic receptors (Nusser et al., 1996, 1998). Subsequent recordings in tissue slices in vitro suggested that GABAA receptors also mediate a tonic current in hippocampal granule cells (Nusser & Mody, 2002), CA1 pyramidal cells (Bai et al., 2001; Caraiscos et al., 2004a), thalamic relay cells (Porcello et al., 2003; Belelli et al., 2005; Cope et al., 2005) and hippocampal interneurons (Semyanov et al., 2003; Glykys et al., 2007; Mann & Mody, 2010), among others. Because the delta subunit has not been found in synapses (Nusser et al., 1998; Wei et al., 2003), its involvement in the tonic currents supports its extrasynaptic origin. Low concentrations of the naturally occurring neurosteroid 3α,21-dihydroxy-5α-pregnan-20-one (allotetrahydrodeoxycorticosterone) selectively enhance the tonic current in mouse dentate granule cells, but not in CA1 pyramidal cells, in a delta subunit-dependent manner (Stell et al., 2003). In the mouse CA1 area the tonic current is accounted for by alpha5 subunit-containing receptors, and in the absence of the alpha5 subunit also by delta subunit-containing receptors (Caraiscos et al., 2004a; Glykys & Mody, 2006). There may be species differences between the mouse and rat. It was proposed (Scimemi et al., 2005) that in rat CA1 pyramidal neurons the tonic current is mostly mediated by alpha4/delta subunit-containing receptors at low concentrations of GABA, but not by alpha5 subunit-containing receptors, whereas at higher GABA concentration alpha5 subunit-containing receptors, which probably do not include the delta subunit, carry much of the tonic current in vitro. In the mouse, deleting genes coding for the alpha5 subunit (Caraiscos et al., 2004a; Glykys & Mody, 2006) significantly reduces the tonic current in hippocampal pyramidal cells, and other neurons (Glykys et al., 2008). Although receptors containing the alpha5 subunit are thought to be predominantly in the extrasynaptic membrane, synaptic localisation (Fritschy et al., 1998a; Christie & deBlas, 2002) and the involvement in synaptic responses (Ali & Thomson, 2008; Zarnowska et al., 2008) of the alpha5 subunit have also been reported. Pharmacological studies using a low dose of the benzodiazepine inverse agonist, L-655,708, selective for alpha5 subunit-containing receptors, also showed the involvement of this subunit in mediating tonic current (Caraiscos et al., 2004a; Scimemi et al., 2005; Glykys et al., 2008). In mice, deficient in both the delta and alpha5 subunits, virtually all of the tonic current was abolished in hippocampal pyramidal and dentate granule cells in vitro (Glykys et al., 2008). However, the above results do not exclude the presence of additional subunits, including the alpha1 and alpha2 subunits, in the receptors mediating the tonic current under normal conditions, but show that in the absence of the delta and/or alpha5 and/or alpha4 subunits, other subunit-containing receptors do not compensate for their absence in mediating a tonic current. Neither the delta nor the alpha5 subunit (when next to the gamma subunit)-containing receptors would be sensitive to zolpidem, which enhances the tonic current in rat CA1 pyramidal cells (Liang et al., 2004). Therefore, the extrasynaptic receptor composition may be different in the rat.
As 50–67% of the alpha1 and alpha2 subunit immunoreactivity is outside synaptic junctions, these subunits may be part of receptors contributing to tonic inhibitory currents with particular pharmacological sensitivity (Belelli et al., 2009). One of the two alpha subunits is positioned between two beta subunits and is not part of the benzodiazepine-binding site. The other alpha subunit is situated between a beta and the gamma2 subunit, and contributes to the benzodiazepine-binding pocket at the interface with the latter (Baumann et al., 2003; Minier & Sigel, 2004; Baur et al., 2006). Although the precise subunit composition of native GABAA receptors on CA1 pyramidal cells is unknown, some may contain two different alpha subunits.
A large variety of immunoprecipitated receptors have been reported that contained more than one alpha subunit species (Duggan et al., 1991; McKernan et al., 1991; Pollard et al., 1993; Jechlinger et al., 1998; Sieghart & Sperk, 2002; Poltl et al., 2003; Benke et al., 2004). In particular, antibodies specific for the alpha4 subunit, which is often associated with the tonic current, also precipitated alpha1/2/3, but not alpha5 subunits (Benke et al., 1997; Bencsits et al., 1999) from rat forebrain. Accordingly, the alpha1 and alpha2 subunits detected in the extrasynaptic membrane in our study could be part of high-affinity alpha4 subunit-containing receptors, which exhibit a pharmacological profile characteristic of alpha4 subunit-containing receptors. The existence of a specific class of alpha1 subunit-containing, potentially extrasynaptic, receptors has also been suggested on the basis of ligand-binding autoradiographic experiments involving several lines of subunit-deficient mice, including delta+alpha4 double knockout mice (Halonen et al., 2009). The alpha1 subunit has also been shown to participate in mediating tonic current in partnership with the delta subunit in presumed extrasynaptic receptors on some GABAergic interneurons in the dentate gyrus of the mouse (Glykys et al., 2007). These neurons, unlike CA1 pyramidal cells, do not appear to express a significant level of other alpha subunits. Accordingly, it cannot be excluded that some of the alpha1 subunits that we found in the extrasynaptic membrane of pyramidal cells in the rat participate in the same alpha1/delta subunit combination reported by Glykys et al. (2007).
The beta3 subunit in extrasynaptic receptors
Both in situ hybridisation and immunohistochemical data suggest that the beta3 subunit is a major contributor to GABAA receptors in CA1 pyramidal cells (Persohn et al., 1992; Wisden et al., 1992; Pirker et al., 2000), but its role in extrasynaptic receptors has not been evaluated. In whole-brain extracts, the beta3 subunit is thought to assemble more with the alpha2 subunit, and the beta2 subunit more with the alpha1 subunit (Benke et al., 1994). The alpha1 and alpha2 subunits in the extrasynaptic membrane may be associated with different beta subunits, but the abundance of the beta2 subunit in the extrasynaptic membrane remains to be evaluated. In dentate granule cells, the beta2 subunit may contribute more to the tonic current suggested to be mediated by extrasynaptic receptors, than the beta3 subunit, which appears to be more involved in synaptic responses (Herd et al., 2008). Our finding that about 64% of the beta3 subunit immunoreactivity is in the extrasynaptic membrane shows that it is a major contributor to extrasynaptic receptors, but whether it is present in receptors responsible for the tonic current in pyramidal cells remains to be determined.
The two alpha subunits and the beta3 subunit may be present in extrasynaptic receptors composed of only alpha and beta subunits, which have been shown on cultured hippocampal neurons (Mortensen & Smart, 2006). But the enhancement of the tonic current by both diazepam and zolpidem in CA1 pyramidal cells in adult hippocampal slices suggests that at least some of the receptors also contain a gamma subunit and next to it an alpha subunit, which is not an alpha5 subunit (Liang et al., 2004), as the alpha5/gamma interface is insensitive to zolpidem.
Receptor trafficking and extrasynaptic receptors
Some of the receptors detected in the extrasynaptic membrane may have properties identical to synaptic receptors, presumably containing a gamma2 subunit, but forming a pool of receptor transiently outside the synapse, as the receptors move in and out of synapses (Bannai et al., 2009). Receptors destined for synapses may be incorporated outside the synaptic junction and move by lateral diffusion. Indeed, single tagged receptors have been shown to move from synapse to synapse within the extrasynaptic plasma membrane in vitro (Bannai et al., 2009). The synaptic receptors are thought to have lower affinity for GABA than extrasynaptic delta subunit-containing receptors, therefore at physiological extrasynaptic GABA concentrations it is uncertain whether receptors with subunit composition identical to synaptic receptors contribute to the tonic current when outside the synapse. Nevertheless, the fact that some hippocampal GABAergic neurons, such as the parvalbumin-expressing fast-spiking cells, have very high level of the alpha1 subunits in their extrasynaptic plasma membrane (Baude et al., 2007) suggests that such receptors contribute to the action of GABA in the extrasynaptic space. A different type of interneuron showing a large tonic current with a likely receptor subunit composition including the alpha1 and delta subunits showed a high density of extrasynaptic immunolabelling (Glykys et al., 2007). These neurons very likely correspond to the neurogliaform cells (Olah et al., 2009).
Roles of synaptic and extrasynaptic GABAA receptors
Synaptic and extrasynaptic GABAA receptors have been shown to be differentially regulated in physiological and pathological conditions (Mody & Pearce, 2004; Farrant & Nusser, 2005; Belelli et al., 2009; Skilbeck et al., 2010). Synaptic GABAA receptors mediate phasic inhibition that influences which postsynaptic neurons participate in information processing and also governs the spike timing of the postsynaptic neurons (Klausberger & Somogyi, 2008). Extrasynaptic receptors, in addition to supplying synapses through receptor trafficking, may also contribute to setting the gain of the input–output function and/or the threshold of firing (Mitchell & Silver, 2003; Semyanov et al., 2004). The strong outward rectification of extrasynaptic GABAA receptors on CA1 pyramidal cells predicts that they carry most charge near firing threshold and therefore regulate firing threshold rather than gain in vitro in juvenile rats (Pavlov et al., 2009). The method that we applied to quantify GABAA receptors containing selected subunits or their combinations offers a strategy to assess physiological and pathological changes in receptor pools in different parts of neurons and in distinct membrane compartments.
Acknowledgements
The authors are grateful to the late Prof. Kazushi Fujimoto for training, advice, encouragement and initial pilot experiments. P.S. and D.R. thank Dr Etsuko Tarusawa for training and help with replica preparation. We thank Dr Jeff McIlhinney for expressing different receptor subunits in HEK cells for antibody specificity tests, Peter Scheifelle for antibody to neuroligin-2, Ms Sanae Hara for some replica preparation and labelling, Ben Micklem for assistance with electron microscopy and Philip Cobden for help with immunofluorescence experiments. We thank the following scientists for brains of mice with disrupted GABAA receptor subunit genes for testing the specificity of antibodies: Dr Thomas Rosahl, Merck Sharp & Dohme, UK, alpha1 or alpha2 or alpha5 or beta3 subunits and wild-type littermates; Drs Delia Belelli and John Lambert, Neurosciences Institute, Univ. Dundee, UK, alpha1 subunit and wild-type littermates; Dr Gregg Homanics, Dept. Anesthesiology, Univ. Pittsburgh, School Med, PA, USA, beta3 subunit. We thank Drs Francesco Ferraguti, Gabor Nyiri and Alex Thomson for their critical comments on an earlier version of the manuscript.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BSA
bovine serum albumin
- CCD
charge-coupled device
- E-face
exoplasmic-face
- FRIL
freeze-fracture replica immunolabelling
- GABA
γ-aminobutyric acid
- GST
glutathione-S-transferase
- HEK
human embryonic kidney cells
- IMP
intramembrane particle
- MBP
maltose-binding protein
- mIPSC
miniature inhibitory postsynaptic potential
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- P-face
protoplasmic-face
- SDS
sodium dodecyl sulphate
- SDS–PAGE
SDS–polyacrylamide gel electrophoresis
- TBS
Tris[hydroxymethyl]amino-methane-buffered saline
- TBS-T
TBS-Triton
- VGLUT3
vesicular glutamate transporter type 3
- WB
washing buffer
References
- Aika Y, Ren JQ, Kosaka K, Kosaka T. Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the disector. Exp. Brain Res. 1994;99:267–276. doi: 10.1007/BF00239593. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Synaptic α5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb. Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Antal M, Fukazawa Y, Eördögh M, Muszil D, Molnár E, Itakura M, Takahashi M, Shigemoto R. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J. Neurosci. 2006;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by -aminobutyric acidA receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor α1 subunit, somatostatin and connexin36 distinguishes axoaxonic, basket and bistratified interneurons of the rat hippocampal. Cereb. Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABAA receptors. J. Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Minier F, Sigel E. A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. doi: 10.1016/j.febslet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl. Acad. Sci. USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABA(A) receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J. Biol. Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Benke D, Fritschy J-M, Trzeciak A, Bannwarth W, Mohler H. Distribution, prevalence, and drug binding profile of g-aminobutyric acid type A receptor subtypes differing in the b-subunit variant. J. Biol. Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- Benke D, Michel C, Mohler H. GABAA receptors containing the a4-subunit:0000 Prevalence, distribution, pharmacology, and subunit architecture in situ. J. Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H. Analysis of the presence and abundance of GABAA receptors containing two different types of α subunits in murine brain using point-mutated α subunits. J. Biol. Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. γ-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem. Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. (Lond.) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Calon A, Gross I, Davidson I, Kedinger M, Duluc I, Domon-Dell C, Freund JN. Functional interaction between the homeoprotein CDX1 and the transcriptional machinery containing the TATA-binding protein. Nucleic. Acids. Res. 2007;35:175–185. doi: 10.1093/nar/gkl1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, YouTen KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing, γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA. 2004a;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruncho HJ, Puia G, Mohler H, Costa E. The density and distribution of six GABAA receptor subunits in primary cultures of rat cerebellar granule cells. Neuroscience. 1995;67:583–593. doi: 10.1016/0306-4522(95)00065-q. [DOI] [PubMed] [Google Scholar]
- Christie SB, deBlas AL. α5 Subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABA(A) receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy J-M, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Duggan MJ, Pollard S, Stephenson A. Immunoaffinity purification of GABAA receptor α-subunit iso-oligomers. J. Biol. Chem. 1991;266:24778–24784. [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy J-M, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the g2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisher KA. Membrane-splitting analyses of membrane-spanning proteins. In: Hui SW, editor. Freeze-fracture studies of membranes. CRC Press, Inc.; Boca Raton, Florida: 1989. pp. 11–39. [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat. Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci. Lett. 1998a;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J. Comp. Neurol. 1998b;390:194–210. [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. SDS-digested freeze-fracture replica labeling electron microscopy to study the two-dimensional distribution of integral membrane proteins and phospholipids in biomembranes: practical procedure, interpretation and application. Histochem. Cell Biol. 1997;107:87–96. doi: 10.1007/s004180050092. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Umeda M, Fujimoto T. Transmembrane phospholipid distribution revealed by freeze-fracture replica labeling. J. Cell Sci. 1996;109:2453–2460. doi: 10.1242/jcs.109.10.2453. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor alpha 5 subunit-deficient mice. J. Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, DeguchiTawarada M, Ohtsuka T, Shigemoto R. Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J. Comp. Neurol. 2005;489:195–216. doi: 10.1002/cne.20633. [DOI] [PubMed] [Google Scholar]
- Halonen LM, Sinkkonen ST, Chandra D, Homanics GE, Korpi ER. Brain regional distribution of GABAA receptors exhibiting atypical GABA agonism: roles of receptor subunits. Neurochem. Int. 2009;55:389–396. doi: 10.1016/j.neuint.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke H, Benkwitz C, Banks MI, Perkins MG, Homanics GE, Pearce RA. Altered GABAA,slow inhibition and network oscillations in mice lacking the GABAA receptor β3 subunit. J. Neurophysiol. 2009;102:3643–3655. doi: 10.1152/jn.00651.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA βsubunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J. Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CEM, Korpi ER, Makela R, Brilliant MH, Hagiwara N, Ferguson C, Snyder K, Olsen RW. Mice devoid of g-aminobutyrate type A receptor b3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl Acad. Sci. USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing a6 subunits. J. Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J. Physiol. (Lond.) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasugai Y, Fukazawa Y, Roberts JDB, Somogyi P, Sieghart W, Shigemoto R. Localization of GABA-A receptor subunits on rat hippocampal pyramidal cells by freeze-francture replica immunolabelling. Soc. Neurosci. Abs. Program No. 2006;527:18. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JDB, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J. Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JHJ, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J. Pharmacol. Exp. Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:14329–14340. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat. Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Shigemoto R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr. Opin. Neurobiol. 2007;17:387–393. doi: 10.1016/j.conb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Quirk K, Prince R, Cox PA, Gillard NP, Ragan CI, Whiting P. GABAA receptor subtypes immunopurified from rat brain with a subunit-specific antibodies have unique pharmacological properties. Neuron. 1991;7:667–676. doi: 10.1016/0896-6273(91)90379-e. [DOI] [PubMed] [Google Scholar]
- Minier F, Sigel E. Positioning of the α-subunit isoforms confers a functional signature to γ-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA. 2004;101:7769–7774. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neuosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. (Lond.) 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 2002;87:2624–2648. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J. Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major g-aminobutyric acid type A receptor a subunits on hippocampal pyramidal cells. Proc. Natl Acad. Sci. USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of a2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur. J. Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Ogris W, Lehner R, Fuchs K, Furtmuller B, Hoger H, Homanics GE, Sieghart W. Investigation of the abundance and subunit composition of GABA(A) receptor subtypes in the cerebellum of alpha 1-subunit-deficient mice. J. Neurochem. 2006;96:136–147. doi: 10.1111/j.1471-4159.2005.03509.x. [DOI] [PubMed] [Google Scholar]
- Ogurusu T, Yanagi K, Watanabe M, Fukaya M, Shingai R. Localization of GABA receptor r2 and r3 subunits in rat brain and functional expression of homooligomeric r3 receptors and heterooligomeric r2 r3 receptors. Receptors Channels. 1999;6:463–475. [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J. Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelzik H, Bannister AP, Deuchars J, Ilia M, Thomson AM. Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur. J. Neurosci. 1999;11:3552–3564. doi: 10.1046/j.1460-9568.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J. Comp. Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J. Comp. Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pollard S, Duggan MJ, Stephenson FA. Further evidence for the existence of a subunit heterogeneity within discrete g-aminobutyric acidA receptor subpopulations. J. Biol. Chem. 1993;268:3753–3757. [PubMed] [Google Scholar]
- Poltl A, Hauer B, Fuchs K, Tretter V, Sieghart W. Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. J. Neurochem. 2003;87:1444–1455. doi: 10.1046/j.1471-4159.2003.02135.x. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J. Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J. Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Estructura del asta de ammon y fascia dentata. Anal. Soc. Espan. Historia Natural. 1893;22:53–114. [Google Scholar]
- Rasband MN, Kagawa T, Park EW, Ikenaka K, Trimmer JS. Dysregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J. Neurosci. Res. 2003;73:465–470. doi: 10.1002/jnr.10675. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Direct immunogold labeling of connexins and aquaporin-4 in freeze-fracture replicas of liver, brain, and spinal cord: factors limiting quantitative analysis. Cell Tissue Res. 1999;296:307–321. doi: 10.1007/s004410051291. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABA(A) receptor currents in the hippocampus. J. Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat. Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc. Natl Acad. Sci. USA. 2008;105:19498–19503. doi: 10.1073/pnas.0807461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. J. Neurochem. 2010;112:1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- Slany A, Zezula J, Tretter V, Sieghart W. Rat beta 3 subunits expressed in human embryonic kidney 293 cells form high affinity [35S]t-butylbicyclophosphorothionate binding sites modulated by several allosteric ligands of gamma-aminobutyric acid type A receptors. Mol. Pharmacol. 1995;48:385–391. [PubMed] [Google Scholar]
- Soltesz I, Roberts JDB, Takagi H, Richards JG, Mohler H, Somogyi P. Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/Cl- channel complex using monoclonal antibodies in the dorsal lateral geniculate nucleus of the cat. Eur. J. Neurosci. 1990;2:414–429. doi: 10.1111/j.1460-9568.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Nunzi MG, Gorio A, Smith AD. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983;259:137–142. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Takagi H, Richards JG, Mohler H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J. Neurosci. 1989;9:2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Fritschy J-M, Benke D, Roberts JDB, Sieghart W. The g2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the a1 and b2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Roberts JDB, Sieghart W, Momiyama T, Kasugai Y, Fukazawa Y, Shigemoto R. High-resolution visualization of synaptic and extrasynaptic GABA-A receptors on hippocampal pyramidal cells. Soc. Neurosci. Abs. Program No. 2004;625:7. [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus. I. Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J. Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J. Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J. Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur. J. Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of recombinant GABAA receptor subtype. J. Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur. J. Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Wei WZ, Zhang NH, Peng ZC, Houser CR, Mody I. Perisynaptic localization of d subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor α5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. J. Neurophysiol. 2008;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]