Abstract
Hormonal changes associated with the human menstrual cycle have been previously found to affect female mate preference, whereby women in the late follicular phase of their cycle (i.e., at higher risk of conception) prefer males displaying putative signals of underlying genetic fitness. Past research also suggests that romantic kissing is utilized in human mating contexts to assess potential mating partners. The current study examined whether women in their late follicular cycle phase place greater value on kissing at times when it might help serve mate assessment functions. Using an international online questionnaire, results showed that women in the follicular phase of their menstrual cycle felt that kissing was more important at initial stages of a relationship than women in the luteal phase of their cycle. Furthermore, it was found that estimated progesterone levels were a significant negative predictor for these ratings.
Keywords: Menstrual cycle, Romantic kissing, Mate assessment, Relationships
Hormonal changes associated with the human menstrual cycle have been found to have discernible effects on female sexual and mating behavior. While it has been known for some time that female sexual desire spikes in the days surrounding ovulation (Regan 1996), it has recently been discovered that preferences for certain types of mating partners also co-vary with phases of the menstrual cycle. For example, women in the late follicular phase of their cycle (in the days immediately preceding ovulation when risk of conception from a single act of intercourse is at its peak) report elevated levels of general attraction to men (Garver-Apgar et al. 2006) and show increased preferences for sexually dimorphic (i.e., “masculine”) faces, masculine body shapes (Little et al. 2007; Penton-Voak et al. 1999), “typical male behavioral displays” (Gangestad et al. 2004), for the scents of symmetrical men (Gangestad and Thornhill 1998) and of men who have greater genetic (MHC) compatibility (Wedekind et al. 1995), as well as for men who are socially dominant (Havlicek et al. 2005) and high in creative intelligence (Haselton and Miller 2006). Women in this cycle phase have also been found to be more accurate at judging male sexual orientation (Rule et al. 2011) and to pay closer attention to cues signaling status (Lens et al. 2011).
Many of the traits outlined above, including masculinity, dominance, and facial symmetry, are believed to be acting as signals of underlying genetic competence, which is in turn responsible for physical health, developmental stability and superior immune-responsivity (for reviews see Rhodes 2006; Roberts and Little 2008). Mating with a partner who possesses such a robust gene set increases the odds that resulting offspring will be endowed with similar genetic advantages. However, a negative relationship seems to exist between genetic fitness indicators such as these and behaviors associated with long-term parental investment. For example, higher testosterone levels in utero, responsible for the development of masculine features, higher fluctuating testosterone levels in adulthood, and various male-typical behaviors, have also been found to correlate with greater relationship infidelity, a larger number of lifetime sexual partners, higher chances of divorce, lower biological sympathy responses to crying infants, as well as compromised immune function (Booth and Dabbs 1993; Fleming et al. 2002; Folstad and Karter 1992; Manning et al. 2000; Pollet et al. 2011). Choosing a mate of superior genetic health, therefore, may necessitate a trade-off: genetically fit partners are likely to bequeath offspring with superior genes but at the same time are less likely to provide long-term parental investment and support, which has a significant effect on offspring survival (Hill and Hurtado 1996).
It has been proposed that, faced with this dilemma, women may seek to improve their overall long-term reproductive fitness by pursuing a mixed-mating strategy: preferring long-term partnerships with males willing to provide resources and parental support while being open to pursuing short-term sexual relationships with partners able to contribute superior genes to potential offspring (Baker and Bellis 1994; Foerster et al. 2003). Such a mating strategy explains the plethora of data found thus far on subconscious shifts in mate preference across the menstrual cycle, whereby genetically fit sexual partners are preferentially favored at a time of the cycle when copulation is most likely to lead to conception (i.e., the late follicular phase), while sexual partners offering the greatest long-term resource investment potential are favored at times less likely to lead to conception (i.e., the luteal phase).
Research on female mate preference shifts during luteal cycle phases goes some way to corroborating theories about mixed-mating strategies. When women are at the lowest risk of conception from a single sexual encounter, they have been found to show preferences for men displaying cues associated with kinship, such as pheromones indicating a similar MHC genetic makeup and faces with higher levels of self-resemblance, as well as for cues of present health (for a review see Jones et al. 2008). It has been proposed that because mating in the luteal phase of the menstrual cycle involves little risk of conception, women at this time pursue decision strategies geared towards affiliating with individuals likely to provide a supportive social environment, such as kin (DeBruine et al. 2005; Jones et al. 2005). However, it seems that mate-preference shifts driven by luteal phases of the cycle may not be as robust as shifts seen during late follicular phases. DeBruine et al. (2005) found that the cyclic shift for self-resemblance was considerably stronger for female faces than for male faces, while Jones et al. (2005) found that preference shifts for signs of health were stronger in pregnant women and women using hormonal contraception than in normally cycling women in the luteal phase, suggesting that similarity/kin affiliations might be strongest during hormonal profiles associated with pregnancy.
The drivers behind a behavioral adaptation such as this are likely to be related to the steroid hormones which fluctuate in tandem with the menstrual cycle, namely estrogen and progesterone. Indeed, part of the reason that mate preference shifts seem to be at their most pronounced between the late follicular and luteal phases of the menstrual cycle is due to the fact that the two phases are associated with the most prominent spikes in levels of estradiol (estrogen) and progesterone hormones, respectively (Durante and Li 2009; Jones et al. 2008; Meston and Frohlich 2000). Furthermore, menstrual cycle fluctuations in mate choice preferences are typically only observed among normally cycling women not using any kind of hormonal contraception (e.g., Penton-Voak et al. 1999), presumably owing to the moderating effects of such prophylaxes on the conventional fluctuation of estrogen and progesterone.
Since several studies have already demonstrated menstrual cycle effects on female preferences for various cues related to mate desirability, it seems worthwhile to examine whether these effects extend to other cues associated with mate choice. Previous research has found that the cross-culturally prevalent custom of romantic kissing is one mate cue that can been exploited in the assessment of potential mating partners (Hughes et al. 2007; Wlodarski and Dunbar 2013). This research also suggests that females are more reliant on kissing as a mate assessment device and are unwilling to have sexual intercourse with a partner without first kissing them; rate “bad kissers” as less attractive overall; and tend to base evaluations of kissing ability on chemical cues (such as breath and taste of mouth). Though the exact mechanism by which romantic kissing conveys mate information remains uncertain, these findings suggest that kissing might facilitate assessment of a mate’s genetic suitability either through the olfactory sampling of auxiliary gland pheromonal cues (e.g., Wedekind et al. 1995) or via the surreptitious olfactory/gustatory assessment of semiochemicals found in saliva or skin oils (Nicholson 1984).
Based on previous findings that romantic kissing can serve a mate assessment function, the present study was designed to explore whether menstrual cycle shifts can be seen in female attitudes towards romantic kissing at different stages of a relationship. It was predicted that normally cycling women in the late follicular (high risk of conception) phase of their menstrual cycle would place greater importance on kissing during the initial stages of a relationship, where it would be most useful as a preliminary mate assessment device, than women in the luteal (low risk of conception) phase of their cycle, with these shifts being driven by menstrual hormones. Furthermore, if this was the case, it was predicted that menstrual hormones would also affect women’s ratings of various factors associated with a romantic kiss, particularly kissing factors that might be effectively utilized in the process of mate assessment.
Methodology
Participant Recruitment
An online questionnaire, approved by Oxford University’s Research Ethics Committee, was distributed on several US and UK public online psychological testing repository websites, as well as through colleges at the University of Oxford. Participants were required to be over 18 years of age to complete the survey; no identifying personal data were collected and participants were informed that their responses were completely voluntary and anonymous. Participants were required to provide informed consent, and were offered the chance to enter a prize draw for an online shopping voucher upon completion of the questionnaire.
Questionnaire Design
Participants were informed that they were to be completing questions about their ‘attitudes towards kissing with romantic partners.’ In particular, participants were firstly asked “How important do you think kissing is … at the very initial stages of a relationship/during the established phases of a committed, long-term relationship?” They were also asked about various attributes of kissing: “In deciding whether someone is a ‘good kisser,’ how important are the following factors: How pleasant their breath is/The scent of their body/The taste of their lips/skin/How ‘wet’ the kiss is/How much touching/physical-contact/caressing is involved/How physically aroused it makes you/Whether their kissing style is the same as yours?” Responses to the two questions were collected using 5-point Likert-type scales, with endpoints ranging from “Not at all important” to “Extremely important” and presented using a grouped table layout for each of the sub-questions. For menstrual cycle phase estimation purposes, participants were asked whether they were currently taking any hormonal contraception, whether they experienced a “regular” menstrual cycle, the average length of their menstrual cycle, and the date of onset of their last menses. General demographic information was also collected.
Estimations of Menstrual Cycle Phase and Risk of Conception
For all analyses, data were only used from women who reported having a regular menstrual cycle whose length fell within the normal ranges of 22 to 36 days (Chiazze et al. 1968), were not on any form of hormonal contraception, and provided the information required to make cycle phase calculations. To estimate the menstrual cycle phase at the time of answering the survey, information was used about the last date of menses onset and typical cycle length to estimate day of ovulation using the reverse cycle day method—approximated as 15 days prior to next estimated onset of menses (see Pillsworth et al. 2004; Thornhill and Gangestad 1999). This method is preferable to forward-counting methods because previous research has found that the follicular phase of the menstrual cycle accounts for much of the variation in average cycle length (Fehring et al. 2006; Lenton et al. 1984).
For analyses involving a binary measure of cycle phase, participants undertaking the experiment on the estimated day of ovulation or within 5 days prior to ovulation were classed as being in the late follicular phase of their cycle (i.e., at “high risk of conception”) and participants within 10 days of the next onset of menses were classed as being in the luteal phase (i.e., at “low risk of conception”) (Jones et al. 2005a; Pillsworth et al. 2004; Wilcox et al. 2001). These cycle days represent the largest differences in progesterone and estradiol levels throughout the menstrual cycle (Durante and Li 2009; Jones et al. 2005; 2008; Meston and Frohlich 2000).
For analyses investigating the hormonal mechanisms driving menstrual-cycle-shift behavior effects, estradiol and progesterone levels on any given day of the cycle were estimated using mean serum estradiol and progesterone reference values derived from normally cycling women within 15 days of ovulation (Stricker et al. 2006).
Participants
For analyses involving the binary variable of women in their late follicular and luteal cycle phases only, participants consisted of 50 women in the luteal phase and 34 women in the late follicular phase of their menstrual cycle at the time of completing the survey. These participants’ ages ranged from 18 to 47 (M=24.7, SD=0.6), their menstrual cycle length varied from 23 to 35 days (M=28.9, SD=2.2), and the sample was predominantly made up of North American (33%), British (28%), and Western (8%) and Eastern (6%) European nationals.
Analyses using the continuous variables of estimated estradiol and progesterone, which utilized data from women in all phases of their menstrual cycle, incorporated 173 women aged 18 to 51 (M=24.7, SD±6.5) with menstrual lengths varying between 23 to 36 days (M=28.9, SD±2.1) and included primarily North American (39%), British (25%), and Western (10%) and Eastern (5%) European nationalities.
Results
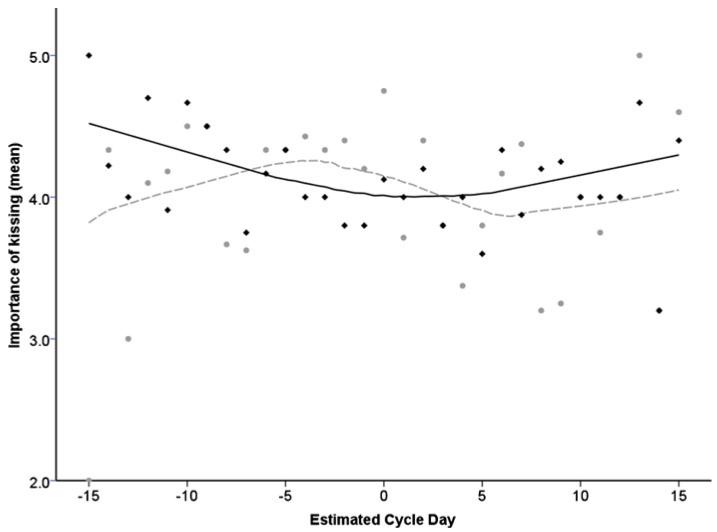
Participants’ responses to questions about the importance of kissing at both initial and established relationship stages can be seen plotted against the estimated day of their menstrual cycle in Fig. 1.
Fig. 1.
Importance of kissing during initial relationship stages (grey markers, dashed line) versus established relationship phases (black markers, solid line) across the menstrual cycle. Day 0 represents reverse-day-count estimated day of ovulation, trend curves fitted using Loess curve estimation, 65% points fit, Epanechnikov kernel
A 2×2 mixed design ANOVA was carried out on answers to this question, with the binary variable of follicular/luteal cycle phase as a between-subject factor and stage of the relationship (initial stage/established phase) as a within-subject factor. Although no significant main effect of cycle phase on ratings of the importance of kissing (F1,82=3.55, p=0.063, partial η2=0.041) and no main effect of relationship stage on ratings of kissing importance (F1, 82=1.83, p=0.180, partial η2=0.022) were found, a significant interaction effect existed between cycle phase and relationship stage (F1, 82=5.58, p=0.021, partial η2=0.064). That is, during the initial stages of a relationship, participants in the follicular phase of their cycle (M=4.10, SD±0.97) believed that kissing was more important than participants in the luteal phase did (M=3.86, SD=1.03), while at established stages of a relationship both follicular and luteal phase participants stated that kissing was equally important (follicular phase M=4.00, SD±0.78, luteal phase M=3.98, SD±0.92).
To determine if key hormones associated with the menstrual cycle might be acting as underlying drivers of participant responses, linear regression analyses were carried out, regressing estimated levels of estradiol and progesterone onto answers to the same questions. As can be seen in Table 1, progesterone levels were found to be a significant negative predictor for ratings of the importance of kissing only during the initial stages of a relationship.
Table 1.
Linear regression analyses of estimated estradiol and progesterone levels regressed onto ratings of the importance of kissing at initial and established stages of a relationship
| B | SE B | β | |
|---|---|---|---|
| Importance of kissing at initial stages | |||
| R2=0.039 (p=0.033) | |||
| Constant | 4.021 | 0.150 | |
| Estimated Estradiol Levels | 0.001 | 0.000 | 0.120 |
| Estimated Progesterone Levels | −0.014 | 0.006 | −0.211** |
| Importance of kissing at established stages | |||
| R2=0.016 (p=0.262) | |||
| Constant | 4.274 | 0.150 | |
| Estimated Estradiol Levels | 0.000 | 0.000 | −0.115 |
| Estimated Progesterone Levels | −0.001 | −0.005 | −0.023 |
p<0.01
Next, answers given by women at different phases of their cycle to questions about the importance of factors contributing to a good kiss were examined. It was initially found that women in late follicular phases of the cycle believed that “pleasant breath” was a more important component of a good kiss than women in luteal cycle phases did (follicular M=4.15, SD±0.82, luteal M=3.70, SD±0.91, t82=2.34, p=0.022), with no significant differences found between participants ratings of other factors associated with kissing. Similarly, initial linear regression analyses found that estimated progesterone levels were a negative predictor when rating “pleasantness of breath” as an important component of a good kiss (constant B=3.867, SE B=0.131; Estradiol B=0.000, SE B=0.000; β=0.111 p=0.174; Progesterone B=−0.011, SE B=−0.005; β=−0.189, p=0.022; R2=0.032), whereas similar regression analyses did not find progesterone or estradiol levels to be significant predictors for importance ratings of other factors associated with kissing (scent of body, taste of their lips/skin, wetness of kiss, amount of touching, physical arousal, and similarity of kissing style) (0.29<t<1.91, 0.056<p<0.875). However, after these initial analyses were corrected for multiple comparisons, the two significant findings were edged out of the range of statistical significance.
A principal components analysis (PCA) with orthogonal (varimax) rotation was conducted on answers to the seven factors contributing to “good kissing.” Table 2 shows the results of the PCA, with factor loadings for the three main extracted factors shown after rotation, and coefficients over 0.5 displayed in bold. Linear regression analysis indicated that neither estimated estradiol nor progesterone levels were significant predictors for any of the three PCA extracted factors (0.01<t<1.80, 0.075<p<0.990).
Table 2.
Principal components analysis on factors contributing to a “good” kiss
| Components |
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| Pleasant breath | 0.783 | −0.058 | 0.050 |
| Scent of body | 0.843 | 0.097 | −0.112 |
| Taste of lips/skin | 0.704 | 0.112 | 0.210 |
| Wetness of kiss | 0.216 | 0.405 | 0.536 |
| Physical contact | 0.003 | 0.804 | 0.148 |
| Amount of arousal | 0.059 | 0.768 | −0.047 |
| Same kissing style | −0.023 | 0.045 | 0.886 |
Sampling adequacy verified by the Kaiser-Meyer-Olkin technique (KMO=0.585), with Bartlett’s test of sphericity indicating significantly large correlations (χ2 21=156.4, p<0.001). Inspection of the scree plot suggested a clear inflection point at 3 components, with all components displaying eigenvalues over 0.989 and combining to explain 63.6% of the variance
Discussion
This study found that when asked about the importance of kissing at initial stages of a relationship, women in the late follicular phase of their menstrual cycle (i.e., at high risk of conception) rated it as more important than women in the luteal (low conception risk) phase of their cycle. Furthermore, estimated levels of progesterone were found to be a significant negative predictor for women’s ratings of kissing importance at this relationship stage. When it came to kissing at the more established phases of a relationship, women in the late follicular and luteal phases of the menstrual cycle considered kissing equally important, and neither estimated estradiol nor estimated progesterone levels were associated with ratings of kissing importance.
Previous research indicates that women in late follicular phases of their menstrual cycle, who are at the highest risk of conception, show an increased preference for men possessing cues signaling underlying genetic superiority or compatibility, including masculinized faces, facial symmetry, social dominance, and MHC compatibility (Gangestad and Thornhill 1998; Havlicek et al. 2005; Penton-Voak et al. 1999; Wedekind et al. 1995). It has also previously been found that romantic kissing is used in assessing a mating partner’s desirability (Hughes et al. 2007; Wlodarski and Dunbar 2013). The results of the current study synthesize these findings by showing that attitudes towards romantic kissing vary across the menstrual cycle and are significantly associated with the fluctuation of menstrual hormones. Since women in the late follicular phase of their cycle are more attracted to, and motivated to find, genetically fit/compatible males, it follows that they would place greater value on devices which aid the assessment of genetic qualities—in this case, romantic kissing. That no cycle phase shift was found for ratings of kissing importance at established relationship phases, a situation in which the initial assessment of a mate is no longer pertinent, supports the premise that romantic kissing may play a particularly useful role in the earliest stages of a relationship, when preliminary partner evaluation is most likely to take place. The fact that progesterone was significantly negatively associated with ratings of kissing importance at initial relationship stages insinuates that it may be acting as a driver of this particular attitude shift. Progesterone is one of the candidate mechanisms thought to be responsible for various menstrual cycle behavioral changes (Haselton and Gangestad 2006) and has previously been implicated in cyclic shifts in mate preference for healthy faces (Jones et al. 2005), self-resemblance (DeBruine et al. 2005), feminine faces (Jones et al. 2005), and vocal masculinity (Puts 2005). Although our study design assesses temporary cognitive changes associated with variation in menstrual cycle hormone levels, tapping into “state” rather than “trait” effects, it seems that these state changes are substantial enough to influence participants’ attitudes towards kissing at other times.
Our results also suggest that menstrual cycle phase and estimated levels of progesterone across the cycle might be related to ratings of the importance of “pleasantness of breath” as a contributing factor to a “good kiss.” However our findings at this stage are only suggestive since adjustments for multiple comparisons nudged the observed differences out of the range of statistical significance. If one of the major functions of kissing is to assist individuals in assessing the quality of a mate via olfactory or gustatory cues, then women at high conception risk (and low progesterone levels) should place greater value on components of kissing which might aid them in assessing the genetic suitability of potential mates. Since it has been previously suggested that semiochemicals found in skin oils and pheromonal secretions might convey information about underlying health and genetic quality (Durham et al. 1993; Nicholson 1984; Wedekind et al. 1995), women at high risk of conception should be particularly interested in utilizing cues found in breath odor to assess mate quality. It is interesting to note than women in the late follicular phases of their cycle also show increased sensitivity to odors and display faster odor processing times (Doty et al. 1981; Pause et al. 1996). This increased sensitivity would be particularly useful in assessing mate olfactory cues at the most reproductively critical time of the menstrual cycle—when sexual intercourse is most likely to lead to conception.
It was predicted that hormones associated with the menstrual cycle may also be related to other facets of kissing which could be conveying mate quality information (such as scent of body or taste of lips). However, none of the other traits sampled suggested significant relationships, implying that kissing may be useful in assessing cues related primarily to breath. When responses to this question were analyzed using PCA, the three factors that were extracted coincided with several previously proposed theories on possible functions of romantic kissing. The first component, relating to “sensory factors,” implies that sensations involving smell contribute in a similar way to making someone a good kisser, possibly because these cues can be used to make inferences about the underlying mate quality or genetic suitability using pheromonal cues or on signals of general health (Durham et al. 1993; Wedekind et al. 1995). The second PCA factor related to contact/arousal themes, which have been proposed as another possible function of kissing promoting partner arousal and the initiation of sexual relations (Hughes et al. 2007). The final PCA factor appears to be related to kissing technique and may also be indicative of mate quality in certain situations, as research in related fields suggests that the quality of execution of complicated behavioral rituals (such as dancing) may be a cue to underlying genetic quality (Hugill et al. 2010). It might be that physical awkwardness or poor coordination during intimate courtship behaviors such as kissing may act as a signal of substandard fitness. None of these extracted factors, however, were significantly related to estimated levels of progesterone or estradiol, suggesting that they may not confer the same level of mate-quality information as pleasantness of breath alone does.
The findings from this study indicate that women in the late follicular stages of their menstrual cycles place greater value on kissing in the early stages of a relationship, and that this behavioral shift seems to be related to menstrual cycle fluctuations in the hormone progesterone. Furthermore, women with low estimated levels of serum progesterone seemed to state that pleasantness of breath was a more important component of kissing than did women with high estimated levels of progesterone. These findings extend previous research on shifts in mating partner preferences across the mating cycle, while also building on previous evidence that romantic kissing may be utilized in the process of assessing the suitability of potential mates. At this point, investigations into the menstrual cycle and romantic kissing as a mate assessment device are still in their infancy, and future research in this field would benefit from study designs based on more methodologically challenging experimental designs in which subjects rated actual kissing experiences, and where measures of fluctuating menstrual hormones were taken using more direct measures.
Acknowledgments
The authors would like to thank Eiluned Pearce for early document proofing. RW and RD are both supported by a European Research Council Advanced Grant to RD.
Biography
Rafael Wlodarski BBus, BA(Hon) is a PhD research student at Oxford University’s Department of Experimental Psychology. His research interests revolve around evolutionary approaches to the study of human behavior, particularly human courtship and pair-bonding.
Robin I. M. Dunbar MA, PhD, FRAI, FBA, is a professor of evolutionary psychology at the University of Oxford. His principal research interests are in social evolution in mammals, with particular reference to ungulates, primates and humans, and the ways in which ecology, behavior, cognition and neurobiology interact.
References
- Baker RR, Bellis MA. Human Sperm Competition: Copulation, Masturbation and Infidelity. Springer; 1994. [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men’s marriages. Social Forces. 1993;72(2):463. doi: 10.2307/2579857. [Google Scholar]
- Chiazze L, Brayer FT, Macisco JJ, Parker MP, Duffy BJ. The length and variability of the human menstrual cycle. Journal of the American Medical Association. 1968;203(6):89–92. [PubMed] [Google Scholar]
- DeBruine LM, Jones BC, Perrett DI. Women’s attractiveness judgments of self-resembling faces change across the menstrual cycle. Hormones and Behavior. 2005;47(4):379–83. doi: 10.1016/j.yhbeh.2004.11.006. doi: 10.1016/j.yhbeh.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Doty RL, Snyder PJ, Huggins GR, Lowry LD. Endocrine, cardiovascular, and psychological correlated of olfactory sensitivity changes during the human menstrual cycle. Journal of Comparative and Physiological Psychology. 1981;95(1):45–60. doi: 10.1037/h0077755. [DOI] [PubMed] [Google Scholar]
- Durante KM, Li NP. Oestradiol level and opportunistic mating in women. Biology Letters. 2009;5(2):179–82. doi: 10.1098/rsbl.2008.0709. doi: 10.1098/rsbl.2008.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham TM, Malloy T, Hodges ED. Halitosis: knowing when “bad breath” signals systemic disease. Geriatrics. 1993;48(8):55–9. doi: 10.1515/semi.1974.12.3.189. [PubMed] [Google Scholar]
- Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2006;35(3):376–84. doi: 10.1111/j.1552-6909.2006.00051.x. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42(4):399–413. doi: 10.1006/hbeh.2002.1840. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425(6959):714–7. doi: 10.1038/nature01969. doi: 10.1038/nature01969. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. American Naturalist. 1992;139(3):603–622. [Google Scholar]
- Gangestad SW, Thornhill R. Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society B: Biological Sciences. 1998;265(1399):927–933. doi: 10.1098/rspb.1998.0380. doi: 10.1098/rspb.1998.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA, Cousins AJ, Garver-Apgar CE, Christensen PN. Women’s preferences for male behavioral displays change across the menstrual cycle. Psychological Science. 2004;15(3):203–207. doi: 10.1111/j.0956-7976.2004.01503010.x. doi: 10.1111/j.0956-7976.2004.01503010.x. [DOI] [PubMed] [Google Scholar]
- Garver-Apgar CE, Gangestad SW, Thornhill R, Miller RD, Olp JJ. Major histocompatibility complex alleles, sexual responsivity, and unfaithfulness in romantic couples. Psychological Science. 2006;17(10):830–5. doi: 10.1111/j.1467-9280.2006.01789.x. doi: 10.1111/j.1467-9280.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Gangestad SW. Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior. 2006;49(4):509–18. doi: 10.1016/j.yhbeh.2005.10.006. doi: 10.1016/j.yhbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Miller GF. Women’s fertility across the cycle increases the short-term attractiveness of creative intelligence. Human Nature. 2006;17(1):50–73. doi: 10.1007/s12110-006-1020-0. doi: 10.1007/s12110-006-1020-0. [DOI] [PubMed] [Google Scholar]
- Havlicek J, Roberts SC, Flegr J. Women’s preference for dominant male odour: effects of menstrual cycle and relationship status. Biology Letters. 2005;1(3):256–9. doi: 10.1098/rsbl.2005.0332. doi: 10.1098/rsbl.2005.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. Aldine; New York: 1996. [Google Scholar]
- Hughes SM, Harrison MA, Gallup GGJ. Sex differences in romantic kissing among college students: An evolutionary perspective. Evolutionary Psychology. 2007;5(3):612–631. [Google Scholar]
- Hugill N, Fink B, Neave N. The role of human body movements in mate selection. Evolutionary Psychology. 2010;8(1):66–89. doi: 10.1177/147470491000800107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Little AC, Boothroyd L, Debruine LM, Feinberg DR, Smith MJL, Cornwell RE, et al. Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Hormones and Behavior. 2005a;48(3):283–90. doi: 10.1016/j.yhbeh.2005.03.010. doi: 10.1016/j.yhbeh.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jones BC, Perrett DI, Little AC, Boothroyd LG, Cornwell RE, Feinberg DR, Tiddeman BP, et al. Menstrual cycle, pregnancy and oral contraceptive use alter attraction to apparent health in faces. Proceedings of the Royal Society B: Biological Sciences. 2005b;272(1561):347–54. doi: 10.1098/rspb.2004.2962. doi: 10.1098/rspb.2004.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, DeBruine LM, Perrett DI, Little AC, Feinberg DR, Law Smith MJ. Effects of menstrual cycle phase on face preferences. Archives of Sexual Behavior. 2008;37(1):78–84. doi: 10.1007/s10508-007-9268-y. doi: 10.1007/s10508-007-9268-y. [DOI] [PubMed] [Google Scholar]
- Lens I, Driesmans K, Pandelaere M, Janssens K. Would male conspicuous consumption capture the female eye? Menstrual cycle effects on women’s attention to status products. Journal of Experimental Social Psychology. 2011:4–7. doi: 10.1016/j.jesp.2011.06.004. [Google Scholar]
- Lenton EA, Landgren B, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. BJOG: An International Journal of Obstetrics and Gynaecology. 1984;91(7):685–689. doi: 10.1111/j.1471-0528.1984.tb04831.x. doi: 10.1111/j.1471-0528.1984.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Little AC, Jones BC, Burriss RP. Preferences for masculinity in male bodies change across the menstrual cycle. Hormones and Behavior. 2007;51(5):633–9. doi: 10.1016/j.yhbeh.2007.03.006. doi: 10.1016/j.yhbeh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Manning JT, Barley L, Walton JC, Lewis-Jones DI, Trivers RL, Singh D, Thornhill R, et al. The 2nd:4th digit ratio, sexual dimorphism, population differences, and reproductive success. evidence for sexually antagonistic genes? Evolution and Human Behavior. 2000;21(3):163–183. doi: 10.1016/s1090-5138(00)00029-5. [DOI] [PubMed] [Google Scholar]
- Meston CM, Frohlich PF. The neurobiology of sexual function. Archives of General Psychiatry. 2000;57(11):1012–30. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- Nicholson B. Does kissing aid human bonding by semiochemical addiction? British Journal of Dermatology. 1984;3:623–627. doi: 10.1111/j.1365-2133.1984.tb06635.x. [DOI] [PubMed] [Google Scholar]
- Pause BM, Sojka B, Krauel K, Fehm-Wolfsdorf G, Ferstl R. Olfactory information processing during the course of the menstrual cycle. Biological Psychology. 1996;44(1):31–54. doi: 10.1016/s0301-0511(96)05207-6. [DOI] [PubMed] [Google Scholar]
- Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. Menstrual cycle alters face preference. Nature. 1999;399(6738):741–2. doi: 10.1038/21557. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- Pillsworth EG, Haselton MG, Buss DM. Ovulatory shifts in female sexual desire. Journal of Sex Research. 2004;41(1):55–65. doi: 10.1080/00224490409552213. doi: 10.1080/00224490409552213. [DOI] [PubMed] [Google Scholar]
- Pollet TV, van der Meij L, Cobey KD, Buunk AP. Testosterone levels and their associations with lifetime number of opposite sex partners and remarriage in a large sample of American elderly men and women. Hormones and Behavior. 2011;60(1):72–77. doi: 10.1016/j.yhbeh.2011.03.005. doi: 10.1016/j.yhbeh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Puts DA. Mating context and menstrual phase affect women’s preferences for male voice pitch. Evolution and Human Behavior. 2005;26(5):388–397. doi: 10.1016/j.evolhumbehav.2005.03.001. [Google Scholar]
- Regan PC. Rhythms of desire: The association between menstrual cycle phases and female sexual desire. Canadian Journal of Human Sexuality. 1996;5:145–156. [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Little AC. Good genes, complementary genes and human mate preferences. Genetica. 2008;134(1):31–43. doi: 10.1007/s10709-008-9254-x. doi: 10.1007/s10709-008-9254-x. [DOI] [PubMed] [Google Scholar]
- Rule NO, Rosen KS, Slepian ML, Ambady N. Mating interest improves women’s accuracy in judging male sexual orientation. Psychological Science. 2011 doi: 10.1177/0956797611412394. doi: 10.1177/0956797611412394. [DOI] [PubMed] [Google Scholar]
- Stricker R, Eberhart R, Chevailler M-C, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clinical Chemistry and Laboratory Medicine. 2006;44(7):883–7. doi: 10.1515/CCLM.2006.160. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. The scent of symmetry: A human sex pheromone that signals fitness? Evolution and Human Behavior. 1999;20(3):175–201. doi: 10.1016/S1090-5138(99)00005-7. [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proceedings of the Royal Society B: Biological Sciences. 1995;260(1359):245–9. doi: 10.1098/rspb.1995.0087. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63(4):211–5. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Wlodarski R, Dunbar RIM. Examining the possible functions of kissing in romantic relationships. Archives of Sexual Behavior. 2013 doi: 10.1007/s10508-013-0190-1. doi: 10.1007/s10508-013-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]