Summary
Background
Stem-cell-based, tissue engineered transplants might offer new therapeutic options for patients, including children, with failing organs. The reported replacement of an adult airway using stem cells on a biological scaffold with good results at 6 months supports this view. We describe the case of a child who received a stem-cell-based tracheal replacement and report findings after 2 years of follow-up.
Methods
A 12-year-old boy was born with long-segment congenital tracheal stenosis and pulmonary sling. His airway had been maintained by metal stents, but, after failure, a cadaveric donor tracheal scaffold was decellularised. After a short course of granulocyte colony stimulating factor, bone marrow mesenchymal stem cells were retrieved preoperatively and seeded onto the scaffold, with patches of autologous epithelium. Topical human recombinant erythropoietin was applied to encourage angiogenesis, and transforming growth factor β to support chondrogenesis. Intravenous human recombinant erythropoietin was continued postoperatively. Outcomes were survival, morbidity, endoscopic appearance, cytology and proteomics of brushings, and peripheral blood counts.
Findings
The graft revascularised within 1 week after surgery. A strong neutrophil response was noted locally for the first 8 weeks after surgery, which generated luminal DNA neutrophil extracellular traps. Cytological evidence of restoration of the epithelium was not evident until 1 year. The graft did not have biomechanical strength focally until 18 months, but the patient has not needed any medical intervention since then. 18 months after surgery, he had a normal chest CT scan and ventilation-perfusion scan and had grown 11 cm in height since the operation. At 2 years follow-up, he had a functional airway and had returned to school.
Interpretation
Follow-up of the first paediatric, stem-cell-based, tissue-engineered transplant shows potential for this technology but also highlights the need for further research.
Funding
Great Ormond Street Hospital NHS Trust, The Royal Free Hampstead NHS Trust, University College Hospital NHS Foundation Trust, and Region of Tuscany.
Introduction
There are no universally effective solutions for the treatment of advanced structural disorders of the large airways in children. Such children need frequent stays in hospital. Although slide tracheoplasty is the primary treatment of choice for some children, others develop recurrent stenoses.1 Stent erosion and death can occur.2 Fetuses with laryngotracheal agenesis or severe stenosis identified before birth might be aborted because these abnormalities are regarded as fatal.3
In the past decade, tissue-engineered structures re-populated with cells or stem cells have been used clinically. Atala and colleagues4 used collagen scaffolds reseeded with urothelial and muscle cells to repair bladder defects in patients with myelomeningocele, and the successful clinical application of a stem-cell-based tracheal replacement in a woman with end-stage airway disease with 6 months follow-up has been reported.5 In 2011, good short-term (5 months) outcomes were reported in a patient who received a similar stem-cell-based tracheal graft, but in this study a nanocomposite trachea was used as the scaffold.6 However, the long-term outcomes of these and other patients who have received such grafts on compassionate grounds have yet to be published pending adequate follow-up, and there is no previous report of outcomes after a tracheal graft in a child.
The potential clinical advantages of autologous stem-cell-derived transplants are that patients who receive them would not need immunosuppression and that the transplants are hypothesised to be remodelled by local stroma to simulate native tissue.5 By contrast, allo-transplantation is associated with significant long-term mortality due to infection and immunosuppression, especially in the respiratory system.7 There is also a paucity of donors for transplantation. Thus, there is a significant unmet need for novel methods of replacing and regenerating human tissue.
The ideal endpoints for tracheal replacement in children are normal airway and lung function, appropriate growth, high quality of life, and the elimination of the need for repeated surgical interventions. Here, we describe the case of a child who received a stem-cell-based tracheal replacement as an urgent compassionate-use procedure and report findings after 2 years of follow-up.
Methods
The recipient
A child born with long-segment congenital tracheal stenosis and pulmonary sling underwent autologous patch tracheoplasty at Great Ormond Street Hospital NHS Trust (London, UK) at 6 days old. He could not be extubated after surgery because of collapse and scarring of the patch and severe bilateral bronchomalacia. Balloon-expandable stainless steel stents (Palmaz, Cordis, Miami Lakes, FL, USA) were implanted, with marked clinical improvement.
At 3 years old, the child had substantial bleeding into his airway. Emergency bronchoscopy and CT angiography revealed erosion by the stent into the aorta. Emergency aortic repair was done with a bovine pericardial patch (Synovis, St Paul, MN, USA), and the impacted stents and trachea were excised and replaced by a tracheal homograft.8 The homograft was replaced 1 week later by another (stented) homograft after mediastinitis occurred. After 3 months in hospital, the patient made an excellent recovery. Over the ensuing years, he needed occasional interventions, including further stents for recurrent stenosis.
At 10 years old, the patient suffered a second haemorrhage. Findings from bronchoscopy and CT scan suggested erosion of tracheal stents, creating a new aortotracheal fistula. Bleeding stopped spontaneously, which provided us with time to plan urgent reconstruction.
Use of tracheal homografts had been discontinued and so other options were discussed. Tracheal allografting9 was dismissed due to the prospect of lifelong immunosuppression. In view of our previous success with an autologous stem-cell-based tracheal replacement, the child’s parents were approached and asked to consider the use of a similar method for their child. The emergent nature of his disorder, unlike that of the adult recipient, meant that a more direct protocol for graft preparation was needed, and so the published technique was adapted using methods previously applied success fully to bone, skin, and nerve regeneration.10 After approval by the Medicines and Healthcare Products Regulatory Agency (MHRA) and the institutional Clinical Ethics Committee, his parents consented to the procedure. An appropriate scaffold was sought and the patient was prepared for surgery.
Pretransplant preparation
The patient received 10 mg/kg granulocyte colony stimulating factor (G-CSF; Chugai, London, UK) daily for 3 days before surgery to mobilise haemopoietic stem cells and endothelial progenitors11 and induce mesenchymal stem cell (MSC) proliferation.12 We measured leucocyte counts daily.
After general anaesthesia, 50 mL autologous bone marrow was aspirated into sterile, heparinised tubes, diluted 1:1, and mononuclear cells were isolated by discontinuous density gradient separation. Sterility testing was done on the washings. Cells were re-suspended, topped up, and counted. The total mononuclear cell count was 2·56×108, of which 5·51×106 were CD34+ or CD45wk haematopoietic stem cells and 1·68×106 were CD73+, CD90+, CD105+, CD117+, or CD45+ MSCs. The cell suspension was transferred into a 60 mL Cryocyte bag (Miltenyi, Bisley, UK) supplemented with 10 IU protamin (Wockhardt, Wrexham, UK) and shipped in a temperature-monitored container (4°C) to the operating room at Great Ormond Street Hospital NHS Trust.
A CT scan was done to identify the dimensions needed for the scaffold. An allogeneic trachea of appropriate size was retrieved by permission of the Tuscany regional authorities from a 30-year-old female donor. Infectious disease markers were negative. The scaffold was prepared in the Regenerative Surgery Laboratories of the University Hospital Careggi, Florence, Italy, using a published protocol,13 and was released by quality control after confirmation of sterility and absence of HLA-I+ cells. 3 days before surgery, the scaffold was imported (4°C) in phosphate-buffered saline supplemented with penicillin, streptomycin, and amphotericin B, in compliance with UK Human Tissue Authority codes and local licence number 11016. 10 000 IU erythropoietin (Roche, Welwyn, UK), 200 IU G-CSF (Neupogen, Cambridge, UK) and 50 μg transforming growth factor β (TGFβ; R&D Systems, Abingdon, UK) were contained in separate syringes and transported with the scaffold and cells.
Tracheal replacement surgery
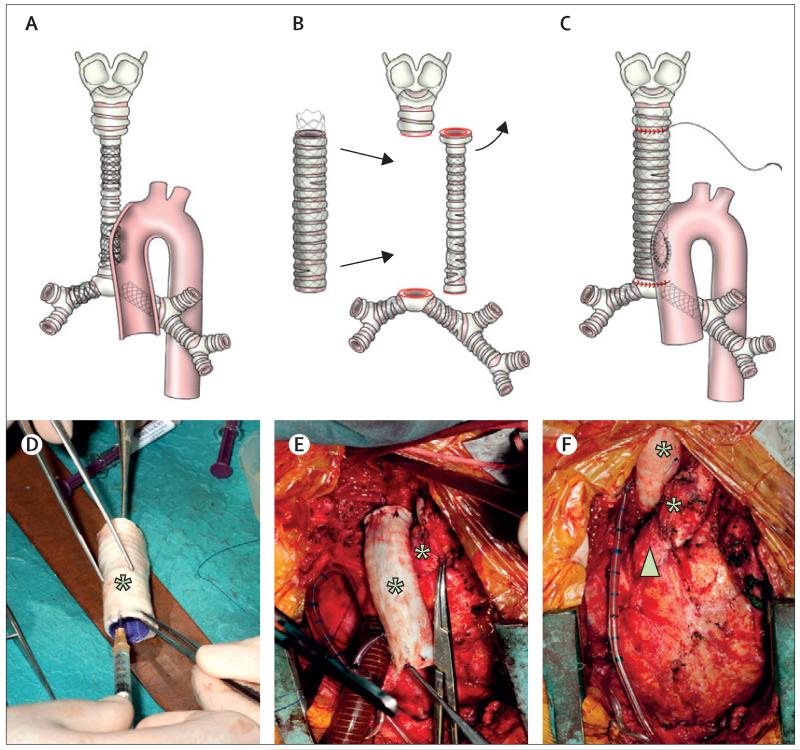
During surgery, the head-down tilt position, cardiopulmonary bypass, and progressive cooling to 18°C were used. With the heart decompressed, a resternotomy was done. Use of right atrium and superior vena cava venous lines permitted cardiac isolation and great vessels were mobilised. At 18°C, the aorta was cross-clamped, anterograde cardioplegia was instilled, head vessels were snared, and the circulation was stopped. After dissection, the stent that was entering the aorta was identified, as were others buried in the tracheal wall (figure 1A). The aortic defect was repaired with bovine pericardium. Circulation was resumed and re-warming commenced.
Figure 1. Surgical procedure.
(A) During surgery the airway was found to be severely stenotic with multiple stents including one entering the ascending aorta. (B and C) The old homograft trachea was removed and replaced by the engineered graft. (C) The aortic defect was closed with a bovine pericardial patch and air leaks sealed. (D) Transforming growth factor β was injected into tracheal rings in the operating theatre before (E) implantation of the recellularised graft. (F) Before closing, an omental wrap was brought up to cover the graft. The graft sits in the anatomical position to the right of the ascending aorta.
The trachea was transected above the upper metal stent and below the lower metal stent, leaving a 7 cm gap between the upper trachea and the carina (figure 1B). Patches of tracheal epithelium were removed from the excised trachea, cut into stamp grafts and retained. The stents in the main bronchi were trimmed back to provide cuffs of unstented bronchi for anastomosis. Bronchi were dilated with an 8 mm Hegar dilator (Lyall Willis, Hastings, UK).
In the operating room, the scaffold was saturated with the cell suspension. The mucosal stamps were placed as free grafts at regular intervals within the lumen. An absorbable polydioxanone (PDO) tracheal stent (Ella-Cs, Hradec Kralove, Czech Republic) measuring 12×72 mm was sutured in place (5/0 PDS, II, Ethicon, Edinburgh, UK). The construct was saturated with human recombinant erythropoietin (hrEPO) and G-CSF, and TGFβ was injected into the tracheal rings (figure 1D) to increase angiogenesis, improve autologous MSC recruitment, and induce chondrocyte differentiation. The construct was anastomosed superiorly and inferiorly using horizontal mattress interrupted sutures (4-0 PDS II, figure 1E). Before completing the anastomoses, a new trans-nasal endotracheal tube was placed under direct vision. Two small air leaks were sealed and the child was weaned from cardiopulmonary bypass. The omentum was mobilised and interposed between the trachea and heart to reduce the possibility of future fistulae and increase graft vascularity (figure 1F). On alternate days after surgery, hrEPO (10 000 IU for 2 weeks) and G-CSF (10 mg/kg for 1 week) were administered.
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Within 3 h of the surgery, ventilation became problematic and bilateral air trapping occurred. Fibreoptic bronchoscopy showed substantial narrowing of the origin of both bronchi due to the longitudinal rigidity of the absorbable stent. A temporary stent (Niti-S, Taewoong, Seoul, South Korea) was implanted in each bronchus under fluoroscopy, which resulted in an immediate improvement in ventilation. These stents were removed before extubation on day 26 after surgery. There was an initial increase in the number of circulating leucocytes (31·1×109/L [SD 6·6×109/L]) between days 2 and 8 after surgery, which corresponded to the period of application of G-CSF and hrEPO. This period was also the only time when circulating CD34+ cells (0·71×109/L [SD 0·05×109/L]) could be detected. Leucocyte counts normalised from day 9 (9·14 ×109/L [SD 1·18×109/L]; appendix).
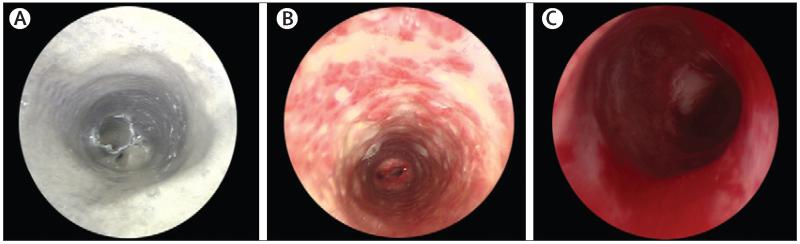
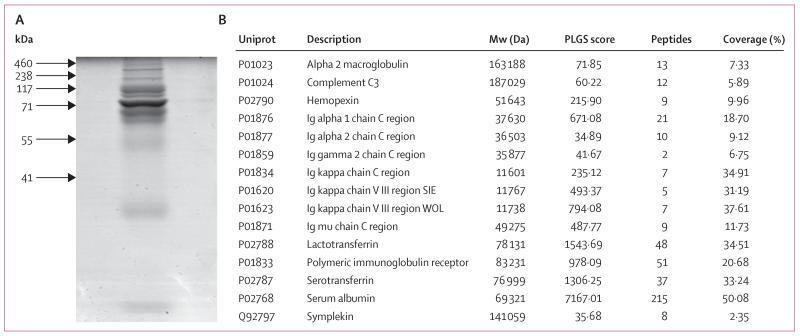
There was bleeding on contact from the internal lumen of the graft by 1 week after surgery, which proved that angiogenesis was occurring. The patient needed regular bronchoscopy for removal of dense secretions for 8 weeks (figure 2A). Assessment of the secretions showed that they included no cells, had a high DNA content, and had a net-like microscopic appearance. The features were identified as those of DNA neutrophil extracellular traps (NETs).14,15 SDS gel fractionation, tryptic digestion, and nano-liquid chromatography mass spectrometry identified a protein profile consistent with this diagnosis (figure 3).14 The secretions were treated with a combination of DNase and physiotherapy, and cleared as epithelialisation progressed. The patient was discharged on day 63.
Figure 2. Bronchoscopic appearances.
(A) Microlaryngobronchoscopy 15 days after the transplant showing a dense web covering the stent and partially occluding the lumen (A), which was cleared by regular bronchoscopies and DNAase. (B) Image at 6 months, showing that reabsorption of the stent (white areas) caused so-called cobblestones of granulation tissue with little normal epithelium. (C) At 15 months after surgery, the graft seemed to be patent, with healthy mucosa.
Figure 3. Identification of protein in the tracheal exudate.
Proteins in the tracheal exudate identified in the early weeks (sampled postoperative week 2) were separated using SDS-PAGE and stained with colloidal Coomassie Blue (A). Destained gel slices were digested with trypsin (Promega, Southampton, UK), fractionated by high-performance liquid chromatography (NanoAcquity, Waters, Manchester, UK), and analysed using an in-line Q-TOF mass spectrometer (Waters). (B) The table shows the proteins identified from at least two peptides and with a PLGS score greater than 10. PLGS=Protein Lynx Global Server.
6 weeks after surgery the stent had dissolved and there was mild collapse of the proximal graft. A shorter (10×45 mm) PDO stent was implanted under fluoroscopy. The patient underwent bronchoscopy or balloon dilatation under fluoroscopy, or both, regularly for 6 months (figure 2B). The major reason for further bronchoscopy or balloon dilation was mucus retention and crusting within the native bronchi in which there were still embedded metal stents. At 5 months, after dissolution of the latest stent, we remained concerned about the rigidity of the proximal graft, and so overlapping, self-expanding Nitinol stents (S.M.A.R.T. Control, Cordis, Waterloo, Belgium) were implanted into the trachea. At 6 months after the initial surgery the graft seemed stable, the patient’s airway was patent, and he returned to school.
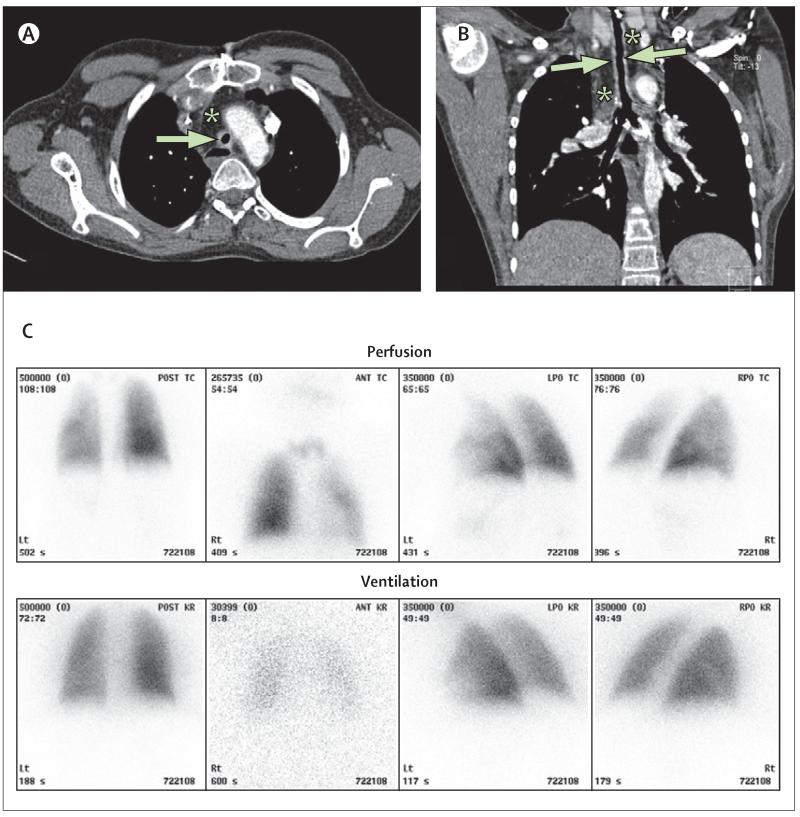
The patient’s last endoscopy (15 months after surgery) showed complete epithelialisation (figure 2C), and cytology of tracheal brushings showed healthy, ciliated respiratory epithelial cells (figure 4D). At 18 months, he had his last fluoroscopic balloon dilatation because the malacic segment had strengthened such that he had not needed any further admissions to hospital. As of May 7, 2012, he was well, active, and had grown 11 cm and 5 kg since graft implantation. His lungs appeared normal on CT scan, without bronchiectasis or air trapping, and a ventilation-perfusion scan at 12 months was normal (figure 5). As of May 13, 2012, there has been no serological or clinical evidence of rejection of the graft and a comprehensive screen of his serum at 15 months showed no anti-HLA antibodies.
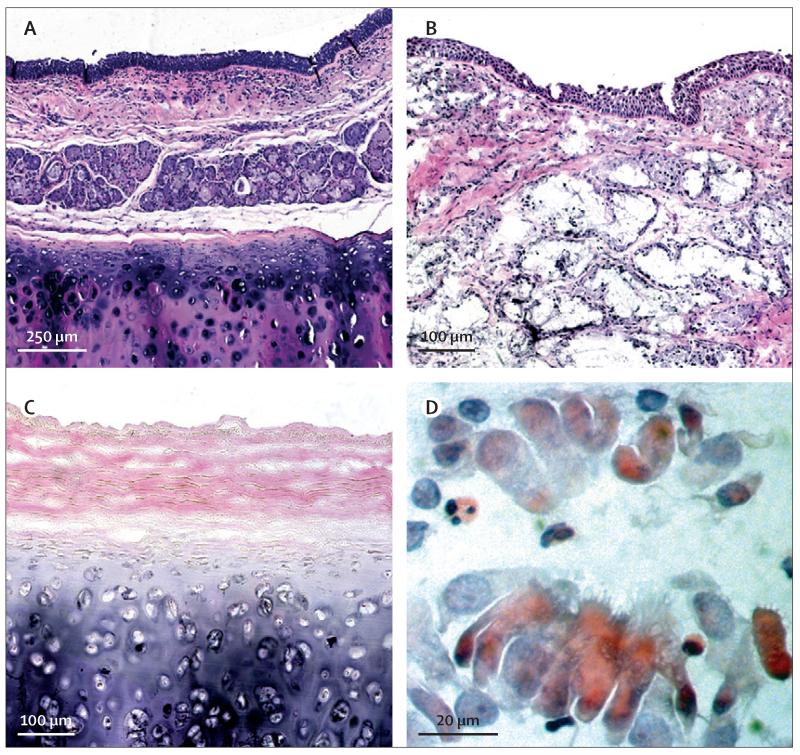
Figure 4. Findings on cytology.
Haematoxylin and eosin staining of (A) normal trachea compared with (B) the patient’s previous tracheal homograft removed at the time of surgery, which shows an epithelialised lining but atypical gland formation. (C) A sample of the decellularised tracheal graft used in this study shows loss of cells but preservation of normal architecture. (D) Bronchial brushing taken from the middle of the graft 1 year after surgery shows a cluster of ciliated cells.
Figure 5. Follow-up scans.
(A) CT axial scan and (B) coronal scan done 12 months after surgery show the tracheal graft (arrows) surrounded by omental fat (*). The lumen of the graft is narrow (6 mm) and its wall is thick (3-4 mm). Growth in length of the graft was not seen on serial images, possibly because growth in height of the child was not matched by lengthening of the chest. (C) A lung scan (ventilation-perfusion) at 18 months showed normal bilateral ventilation (the left lung is contributing 45% to the total ventilation and the right lung 55%). There is a slight reduction in perfusion in the left lung (receiving 37% of the right heart output) compared with the right lung (63%).
Histological assessment of the homograft trachea removed at the time of surgery showed an inflamed mucosa overlying dense fibrous tissue and islands of cartilage unlike normal tracheal architecture (figure 4B). Histology of the decellularised scaffold showed complete decellularisation with good retention of tracheal architecture (figure 4C) and absence of MHC expression (not shown). High-resolution proteomic analysis of the scaffold by ion-trap mass spectrometry identified 166 proteins, including several extracellular matrix components. Bioinformatic analysis (IPA, Ingenuity Systems, Redwood City, CA, USA) identified a broad range of potential biological roles for these proteins.
Discussion
We report a stem-cell-based tissue replacement in a child and long-term follow-up of a stem-cell-based tissue-engineered graft (panel). The child is well, growing, and had not needed medical intervention for 6 months by May 5, 2012.
Because the protocol used in this study was devised in an emergency, we applied empirically a new combination of technologies on the basis of previous clinical successes in non-airway settings (ie, bone, skin, and nerve regeneration). Thus, to minimise delays, there was no previous expansion of epithelial cells and MSCs, nor any chondrocytic differentiation of MSCs.5 Instead, we used an intraoperative protocol, which was similar to those used in clinical trials of MSCs for patients with myocardial infarction.17 Not undertaking long-term culture of MSCs also has the potential advantage of avoiding the risk of malignancy.18 We aimed to create an in-vivo microenvironment that represented some of the events that occur during the normal physiological response to injury. A similar method is in phase 2 clinical trials of bone, skin, and nerve regeneration.10 We hypothesise that this altered protocol, in addition to the length of the graft, the presence of an absorbable stent, and the underlying different physiology and regenerative potential of children’s compared with adults’ tissues, were responsible for the differences in clinical course and outcomes from the published adult case, at least at the 6-month timepoint.5 Specifically, the graft in the present study took longer to epithelialise and did not have proximal rigidity until almost 2 years. However, at last follow-up the boy was alive, growing, had normal lung function, and had returned to school.
A key criterion for paediatric implants is that of growth potential. In this study, although we were unable to measure graft length, there was no CT evidence of shortening of the graft, as has been previously described, for example, with an alternative aortic allo graft approach.19 At age 13 years the child’s torso is not expected to elongate much further as his height increases and so the growth demands on this graft are limited. However, experimental evidence of graft growth is crucial for the clinical use of similar protocols of transplantation for children of all ages. Equally crucial is rapid vascularisation. As with the adult case,5 touch bleeding on the internal graft surface was visible by 1 week, which proved that rapid angiogenesis was occurring.
In both this study and a previous case,5 a cadaveric donor trachea was decellularised, with successful removal of cellular components including MHCs. Neither patient had developed rejection by May, 2012, and the child had not developed anti-donor antibodies by 20 months. These findings, in addition to reports of preclinical success with similar methodologies for heart and lung grafts,20,21 suggest that decellularised scaffold-based technologies could be an immunosuppression-free alternative to conventional transplantation.
In the UK, patients operated upon under a Hospitals Exemption Certificate on compassionate grounds, as was the case with the patient in this study, are not treated as research patients. Thus, we did not label the applied cells and so cannot comment on whether the eventual stromal and epithelial cells originated from those implanted or from cells recruited from neighbouring tissues. Future preclinical and clinical trials should incorporate markers that will answer the question of the exact contribution of applied cells to the final result.
Many clinicians assume that decellularised scaffolds are inert composites of structural proteins. Proteomic measurement of non-structural, or minor structural, proteins has been difficult because of the dominance of collagen and elastin in protein preparations. In this study, with new techniques we identified 166 proteins with diverse functions relevant to regenerative medicine (eg, angiogenesis and immunity) that were preserved despite decellularisation, although no MHC molecules were found. We hypothesise that many of these proteins are crucial to revascularisation, cell migration, and differentiation in tissue-engineered organs and represent a major difference from synthetic scaffolds. Therefore, proteomic analysis might be a valuable addition to release criteria for biological scaffolds.
TGFβ was added to the scaffold to induce chondrocytic differentiation, G-CSF to boost autologous MSC recruitment, and hrEPO to increase angiogenesis. G-CSF is used to mobilise bone marrow progenitor production before haemopoietic cell transplantation.22 Although some studies report a beneficial effect of G-CSF on MSC mobilisation,12,23 others suggest the opposite effect.17 Identification of the contribution of G-CSF to the survival and function of the graft in one patient in the short and long term is not possible. However, we hypothesise that systemic application of G-CSF increased leucocyte counts in week 1 and contributed to NET accumulation in the trachea in the first 6 weeks after surgery.23,24
hrEPO is used clinically to support erythropoiesis in patients with cancer and renal disease.25 Pretreatment with erythropoietin might improve the survival of cells within tissue where angiogenesis is not yet adequate to fully support respiration, by a mechanism mediated by nitric oxide and vascular endothelial growth factor.26 Angiogenesis, measured by appearance, contact bleeding, and laser doppler fluxmetry, was equally fast in the previously reported adult patient5 as in the child in the present study. Despite the substantial increase in graft mass in the child, we can only speculate about the added angiogenic effect of hrEPO. Findings are further confounded by the use of an omental flap, because the purpose of it is to provide an improved vascular bed for the graft. More research into angiogenic mechanisms in recellularised regenerative constructs is needed.
We hypothesised that TGFβ, a key signal for chondrocytic differentiation of MSCs,27 would enable repopulation of the preserved scaffold cartilage niche and provide adequate biomechanical support in the long term. However, TGFβ is also a powerful promoter of myofibroblasts and scar tissue28 and restricts epithelial cell survival and migration,29 both of which are undesirable actions during the regeneration of tissue-engineered trachea. The absence of rigidity in the proximal trachea suggests that TGFβ did not support adequate cartilage regeneration throughout the graft, although the length of the graft, presence of PDO stents, or the absence of a preoperative chondrocytic differentiation step in the process5 might also have been responsible.
PDO stents30,31 have been used in six lung transplant patients who needed multiple insertions,31 which was also the case with the child in this study. All six patients were free of stenosis at a median of 24 months (range 7–44). Recent experience in children with airway stenosis is similar.32 The PDO stents were quick to apply and provided circumferential support for 8 weeks, but the absence of vertical elasticity was a problem and they might have contributed to NET formation.
Analysis suggested that the problematic tracheal exudate in this study was DNA NETs.14,15 The macroscopic appearance of NETs is poorly described in man. Their perceived role is to prevent bacterial colonisation and dissemination, but formation can cause tissue damage.15 We hypothesise that neutrophil recruitment induced by the graft and stent plus G-CSF treatment were causative, and that NET resolution parallels the development of new epithelium.
The epithelium was patchy by 2 months, although stents caused discontinuity. The presence of viable, ciliated epithelial cells was confirmed on cytology at 1 year, when mucosal continuity was noted throughout. Epithelialisation occurred later than in the previous adult case,5 where mucosal coverage was achieved at 1 month and mucociliary clearance by 6 months.5 The need for early mucosal coverage and mucociliary clearance for airway grafts in patients, many of whom have compromised bronchial or lung function, or both, means that research into mechanisms of regeneration of the respiratory mucosa is crucial, as is identification of key stem or progenitor cells and migration and differentiation factors.
This report should be compared with other published case reports of tissue-engineered airways, described briefly earlier5,6,13 and reviewed in more detail elsewhere.3 Substantial areas for improvement in outcomes were identified by this experience of a stem-cell-based, paediatric tracheal replacement; specifically, the need for biomechanical strength throughout the graft and speedy, efficient restoration of the mucosa. The response of children to implants will probably differ from that of adults in important ways, including the need to accommodate growth. Urgent research is needed to convert one-off, compassionate-use successes, such as the one described in this study, into more widely applicable clinical treatments for the thousands of children with tracheal stenosis and malacia worldwide.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed for all publications, including clinical trials, meta-analyses, and reviews, with the terms “graft” and “short-term” or “long-term”. However, we did not restrict our searches to only papers that included the phrase “stem cells” and identified only two similar case reports, both in adults and with 6 and 5 months’ follow-up respectively.5,6 Although several conventional treatments are available for the treatment of congenital tracheal stenosis, no proven treatments exist for patients with end-stage disease.1,16 Evidence from studies in animals suggests that stem-cell-based tissue engineered tracheal implants could be useful as part of new treatment strategies for incurable tracheal stenosis or malacia in children.
Interpretation
This study describes a stem-cell-based organ transplant in a child and is the first in either adults or children to report long-term follow-up (2 years).
Acknowledgments
This work was supported by Great Ormond Street Hospital NHS Trust, The Royal Free Hampstead NHS Trust, and University College Hospital NHS Foundation Trust (all London, England), and by a grant (pd 239-28/04/2009, delib. GRT 1210/08) from the Region of Tuscany (Italy) entitled “Clinical laboratory for complex thoracic respiratory and vascular diseases and alternatives to pulmonary transplantation”. Both Great Ormond Street Hospital and University College Hospital receive translational research funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centres scheme (MJE, PDC, SJ, and MAB). MJE is Director of the Service for Severe Tracheal Disease in Children, funded by the National Health Service National Commissioning Mechanisms. SJ is a recipient of a Wellcome Trust Senior Fellowship in Clinical Science. Some laboratory work was supported by a Medical Research Council Translational Stem Cell Research Committee grant to MB (G1001539) and a Great Ormond Street Hospital Charity grant to PDC. We thank Caroline Doyle, whose superb administrative skills were essential to the co-ordination of this procedure. We also thank the anaesthetic, technical, paramedical, and nursing staff in operating theatres, intensive care units, and wards at Great Ormond Street Hospital for Children National Health Service Trust, as well as the senior management of the Trust who approved the internal funding. We thank staff at the Royal Victoria Hospital NHS Trust in Belfast who saved the child’s life at first presentation and cared for him on several occasions over the past 11 years. All the staff of the Paul O’Gorman Laboratories for Cell Therapy at the Royal Free Hospital Hampstead NHS Trust contributed to cell, cytokine, and graft preparation, as did many members of the Nanotechnology and Surgical Sciences Laboratories at the same institution. We thank our patient’s parents, who were an essential and supportive part of the team and decision-making processes. We also thank the Tuscany Transplant Authority, the Thoracic Surgeons, General and Medical Directors of the University Hospital Careggi in Florence (Italy), staff at the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA), especially Ian Rees, who gave timely and free advice on regulatory aspects of this case. The Chairman and members of the Clinical Ethics Committee at Great Ormond Street Hospital constructively considered all aspects to the case and helped with design of the parent’s information sheet. We finally pay particular tribute to the courageous and inspiring young man himself.
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
See Online for a video interview with Martin Birchall
References
- 1.Kocyildirim E, Kanani M, Roebuck D, et al. Long-segment tracheal stenosis: slide tracheoplasty and a multidisciplinary approach improve outcomes and reduce costs. J Thorac Cardiovasc Surg. 2004;128:876–82. doi: 10.1016/j.jtcvs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs JP, Quintessenza JA, Botero LM, et al. The role of airway stents in the management of pediatric tracheal, carinal and bronchial disease. Eur J Cardiothor Surg. 2000;18:505–12. doi: 10.1016/s1010-7940(00)00534-0. [DOI] [PubMed] [Google Scholar]
- 3.Lange P, Fishman JM, Elliott MJ, De Coppi P, Birchall MA. What can regenerative medicine offer for infants with laryngotracheal agenesis? Otolaryngol Head Neck Surg. 2011;145:544–50. doi: 10.1177/0194599811419083. [DOI] [PubMed] [Google Scholar]
- 4.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–46. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 5.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 6.Jungebluth P, Evren A, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartifical nanocomposite: a proof-of-concept study. Lancet. 2011;6736:61715–17. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services [accessed Nov 13, 2011];Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: transplant data 1999–2008. Table 12.7: patients and annual death rates per 1000 patient-years at risk, 1999 to 2007 rate for recipients during first year after deceased donor lung transplantation. 2009 http://optn.transplant.hrsa.gov/ar2009/1207_lu.pdf.
- 8.Jacobs JP, Quintessenza JA, Andrews T, et al. Tracheal allograft reconstruction: the total North American and worldwide pediatric experiences. Ann Thorac Surg. 1999;68:1043–51. doi: 10.1016/s0003-4975(99)00878-4. [DOI] [PubMed] [Google Scholar]
- 9.Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–45. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 10.Bader A, Lorenz K, Richter A, et al. Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation Res. 2011;14:57–66. doi: 10.1089/rej.2010.1050. [DOI] [PubMed] [Google Scholar]
- 11.Engelmann MG, Theiss HD, Hennig-Theiss C, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–21. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Brouard N, Driessen R, Short B, Simmons PJ. G-CSF increases mesenchymal precursor cell numbers in the bone marrow via an indirect mechanism involving osteoclast-mediated bone resorption. Stem Cell Res. 2010;5:65–75. doi: 10.1016/j.scr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Baiguera S, Jungebluth P, Burns A, et al. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010;31:8931–38. doi: 10.1016/j.biomaterials.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–35. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Lögters T, Margraf S, Altrichter J, et al. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol. 2009;198:211–19. doi: 10.1007/s00430-009-0121-x. [DOI] [PubMed] [Google Scholar]
- 16.Elliott M, Roebuck D, Noctor C, et al. The management of congenital tracheal stenosis. Int J Pediatr Otorhinolaryngol. 2003;67(suppl 1):S183–92. doi: 10.1016/j.ijporl.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Ripa RS, Haack-Sørensen M, Wang Y, et al. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(11 suppl):I24–30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JO, Han JW, Kim JM, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–47. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wurtz A, Porte H, Conti M, et al. Surgical technique and results of trachea and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Surg. 2010;140:387–93. doi: 10.1016/j.jtcvs.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 21.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiq S, Pamphilon D, Brunskill S, Doree C, Hyde C, Stanworth S. Bone marrow harvest versus peripheral stem cell collection for haemopoietic stem cell donation in healthy donors. Cochrane Database Syst Rev. 2009;1:CD006406. doi: 10.1002/14651858.CD006406.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi K, Otani H, Sato D, et al. Granulocyte-colony stimulating factor increases donor mesenchymal stem cells in bone marrow and their mobilization into peripheral circulation but does not repair dystrophic heart after bone marrow transplantation. Circ J. 2008;72:1351–58. doi: 10.1253/circj.72.1351. [DOI] [PubMed] [Google Scholar]
- 24.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–76. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo JD, Brouwers M, Hurley P, et al. for the American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–59. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 26.Rezaeian F, Wettstein R, Amon M, et al. Erythropoietin protects critically perfused flap tissue. Ann Surg. 2008;248:919–29. doi: 10.1097/SLA.0b013e31818f678e. [DOI] [PubMed] [Google Scholar]
- 27.Moretti M, Wendt D, Dickinson SC, et al. Effects of in vitro preculture on in vivo development of human engineered cartilage in an ectopic model. Tissue Eng. 2005;11:1421–28. doi: 10.1089/ten.2005.11.1421. [DOI] [PubMed] [Google Scholar]
- 28.Antoshina E, Ostrowski LE. TGF beta 1 induces growth arrest and apoptosis but not ciliated cell differentiation in rat tracheal epithelial cell cultures. In Vitro Cell Dev Biol Anim. 1997;33:212–17. doi: 10.1007/s11626-997-0144-9. [DOI] [PubMed] [Google Scholar]
- 29.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novotny L, Crha M, Rauser P, et al. Novel biodegradable polydioxanone stents in a rabbit airway model. J Thorac Cardiovasc Surg. 2012;143:437–44. doi: 10.1016/j.jtcvs.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Lischke R, Pozniak J, Vondrys D, Elliott MJ. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg. 2011;40:619–24. doi: 10.1016/j.ejcts.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Vondrys D, Elliott MJ, McLaren CA, Noctor C, Roebuck DJ. First experience with biodegradable airway stents in children. Ann Thorac Surg. 2011;92:1870–74. doi: 10.1016/j.athoracsur.2011.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.