Abstract
In nerve cells the ubiquitous second messenger calcium regulates a variety of vitally important functions including neurotransmitter release, gene regulation, and neuronal plasticity. The entry of calcium into cells is tightly regulated by voltage-gated calcium channels, which consist of a heteromultimeric complex of a pore forming α1, and the auxiliary β and α2δ subunits. Four genes (Cacna2d1-4) encode for the extracellular membrane-attached α2δ subunits (α2δ-1 to α2δ-4), out of which three isoforms (α2δ-1 to -3) are strongly expressed in the central nervous system. Over the years a wealth of studies has demonstrated the classical role of α2δ subunits in channel trafficking and calcium current modulation. Recent studies in specialized neuronal cell systems propose roles of α2δ subunits beyond the classical view and implicate α2δ subunits as important regulators of synapse formation. These findings are supported by the identification of novel human disease mutations associated with α2δ subunits and by the fact that α2δ subunits are the target of the anti-epileptic and anti-allodynic drugs gabapentin and pregabalin. Here we review the recently emerging evidence for specific as well as redundant neuronal roles of α2δ subunits and discuss the mechanisms for establishing and maintaining specificity.
Keywords: Voltage-gated Ca2+ channels, Presynaptic, Postsynaptic, Synaptogenesis, CNS
1. Introduction
In the central nervous system (CNS) calcium entering nerve cells through voltage-gated calcium channels (CaVs) mediates and regulates a variety of neuronal functions ranging from neurotransmitter secretion and postsynaptic signal integration to gene regulation and neuronal plasticity. For example, presynaptic CaVs trigger neurotransmitter release (Stanley, 1993) and postsynaptic CaVs are involved in the transcriptional regulation of CREB (cAMP-responsive element-binding protein) and NFAT (nuclear factor of activated T cells) (Deisseroth et al. 2003; Dolmetsch 2003) and thus modulate the excitability and likely play a crucial part in the formation of new memory (Moosmang et al. 2005). Over the recent years a detailed picture on the distribution and function of pre- and postsynaptic calcium channel types has begun to emerge (Obermair and Flucher 2013) and the importance of CaVs is emphasized by the existence of channelopathies caused by loss-of-function as well as gain-of-function mutations (Pietrobon 2010; Striessnig et al. 2010). Calcium channels are organized in heteromultimeric complexes consisting of the pore-forming α1 subunit and the auxiliary β and α2δ subunits. The α1 subunit defines the basic biophysical, pharmacological and physiological properties of the channels while the β and α2δ subunits are involved in the localization, trafficking and stabilization of the channel complex (reviewed in Arikkath and Campbell 2003; Obermair et al. 2008; Dolphin 2009; Buraei and Yang 2010). The majority of today’s knowledge on the role of the individual channel subunits is based on studies in heterologous expression systems such as Xenopus laevis oocytes or human embryonic kidney (HEK) cells. However, the recent development of powerful neuronal expression systems and the characterization of CaV knockout and mutant animal models have provided novel insights into the physiological importance of auxiliary β and α2δ subunits in neuronal functions such as the regulation of gene expression as well as synaptic function. While these studies provide evidence for specific roles of individual CaV subunits, they also indicate that some of their basic functions may be rather common and redundantly shared between subunit isoforms. Therefore the challenge of ongoing and future investigations is to elucidate whether and how auxiliary CaV subunit isoforms and their respective splice variants contribute to the various neuronal functions. This is not merely relevant for our understanding of neuronal signaling, but may open up new therapeutic strategies for treating CNS diseases involving CaVs such as neurodegenerative diseases, epilepsy, or anxiety and mood disorders. Here we review recent evidence for both specific and redundant functions of auxiliary CaV subunits with a focus on the extracellular α2δ subunits.
2. Neuronal calcium channel complexes
2.1. Subunit composition
The core complex of neuronal voltage-gated calcium channels consists of an α1 subunit, which contains the ion-conducting pore, and the auxiliary β and α2δ subunits (Fig. 1A). In skeletal muscle also a γ subunit is part of the complex; however, in the nervous system various γ subunits act primarily as transmembrane AMPA receptor regulatory proteins (TARPs; Jackson and Nicoll 2011).
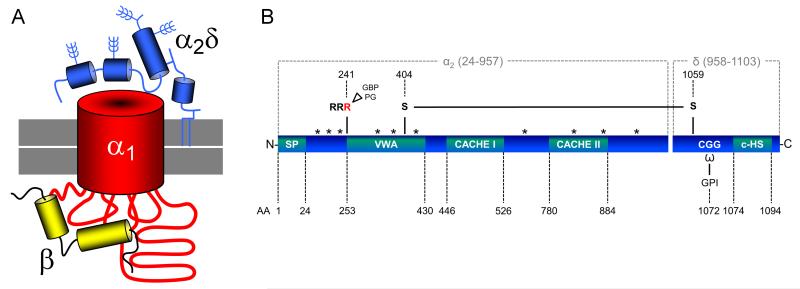
Figure 1.
A. The voltage-gated calcium channel complex. Voltage-gated calcium channels are composed of a transmembrane pore-forming α1 subunit (red) which forms a macromolecular complex together with extracellular α2δ (blue) and cytoplasmic β (yellow) subunits. The β subunit binds the intracellular I-II linker of α1 with high affinity. The α2δ subunit consists of posttranslationally cleaved highly glycosylated α2 and δ peptides, which are associated to each other by a disulfide bond and are most likely linked to the plasma membrane (indicated in grey) via a GPI-anchor. Note that the folding structure of the individual subunits is simplified. B. Domain structure of auxiliary α2δ subunits. Amino acid positions refer to the mouse α2δ-1 isoform [uniprot: O08532- CA2D1_MOUSE], however all isoforms have a similar topology. Amino acid residues 1—24 encode the signal peptide (SP). The α2 peptide contains a von Willebrand factor type A (VWA) domain and two sequence stretches homologous to extracellular domains of bacterial chemosensing proteins (Cache I and II). In α2δ-1 and α2δ-2 an arginine (RRR) motif proximal to the VWA domain represents the potential gabapentin (GBP) and pregabalin (PG) binding site. Two cysteine residues (AA 404 and 1059) were identified to be important for the formation of an intermolecular disulfide bond between α2 and δ. The δ peptide contains predicted ω amino acids, to which the GPI anchor can attach, and a C-terminal hydrophobic sequence (c-HS). Potential glycosylation sites are indicated by asterisks (*).
The α1 subunit:
The α1 subunits of voltage-gated calcium channels are a family of 10 genes involving the high-voltage-activated classes CaV1 and CaV2 (α1A to α1F and α1S), and the low-voltage-activated class CaV3 (α1G to α1I) (Catterall 2011). Each α1 subunit consists of four homologous repeats (I to IV), each with six membrane spanning domains (S1 to S6). Specific functions have been assigned to particular structures within this sequence, like the voltage sensor in the four S4 segments and the ion selectivity filter of the conductance pore in the loops between S5 and S6 of each repeat. Depolarization of the neuronal plasma membrane leads to a conformational change within the α1 subunit that gates the channel and activates the calcium current. Domains involved in protein-protein interaction with the accessory channel subunits, regulatory proteins, and other channels have been localized in the large intracellular loops connecting the homologous repeats and in the long C-terminal tail. The α1 subunit also defines the basic biophysical and pharmacological properties due to important drug binding sites such as dihydropyridines, phenylalkylamines, as well as benzothiazepines (Catterall 2011). α1 subunit splicing increases the functional heterogeneity and the spectrum of pharmacological characteristics (Koschak 2010; Flucher and Tuluc 2011). Out of the four members of the dihydropyridine-sensitive L-type (CaV1) calcium channels, CaV1.2 and CaV1.3 are expressed in the CNS and play important roles in pace-making (CaV1.3), postsynaptic signal integration, and excitation-transcription coupling in neurons. L-type calcium channels have mainly been associated with postsynaptic functions with two notable exceptions. CaV1.3 and CaV1.4 regulate presynaptic glutamate release in two highly specialized synapses, namely auditory hair cells and retinal photoreceptor cells, respectively. The P/Q-, N-, and R-type calcium channels (CaV2.1, 2.2, and 2.3) can be specifically blocked with a number of invertebrate toxins (Catterall 2011), and their chief function is controlling neurotransmitter release in synapses (Nimmervoll et al. 2013). The members of the low-voltage activated, T-type calcium channels (CaV3 family) are highly expressed during early development of many cell types, can be blocked by Ni2+, and contribute to the excitability and pace-making in neurons (reviewed in Perez-Reyes 2003).
The β subunit:
The cytoplasmic β subunit consists of a conserved SH3 protein interaction domain and a nucleotide kinase-like domain (Chen et al. 2004; Opatowsky et al. 2004; Van Petegem et al. 2004) and thus resembles in structure the membrane-associated guanylate kinase proteins (Dolphin 2003; Takahashi et al. 2005). However, the SH3 domain of β subunits differs from that of canonical polyprolin-binding pockets and the guanylate kinase fold is modified so that it lacks kinase activity. Instead it binds the intracellular I-II linker of α1 subunits at the so-called α-interaction-domain (AID) with nanomolar affinity (De Waard et al. 1995; Van Petegem et al. 2008). The SH3 and the GK-like domain are highly conserved among the four genes encoding β subunits (Cacnb1-b4). The sequences connecting these domains as well as the N- and C-termini vary between isoforms and are subject to alternative splicing (Colecraft et al. 2002; Dolphin 2003). In the channel complex β subunits serve two roles: They have a chaperon function regulating the export of the calcium channel from the endoplasmic reticulum and thus membrane expression of functional channels (Obermair et al. 2010; Fang and Colecraft 2011). Moreover, they modulate gating properties of the channel directly as well as by interaction with other regulatory proteins like Rab binding proteins or G-proteins. β itself is subject to PKA mediated phosphorylation (reviewed in Buraei and Yang 2010). The β2a isoform is palmitoylated at two N-terminal cysteines and therefore membrane-associated even in the absence of an α1 subunit. Nevertheless, the association of β subunits with the channel complex entirely depends on their binding to the AID in the α1 subunit. This binding site in the cytoplasmic loop between repeats I and II of the α1 subunit is a unique feature of the CaV1 and CaV2 subclasses of CaVs. Accordingly, at least in heterologous expression systems all β subunits can associate with any of the CaV1 or CaV2 members but not with the low-voltage-activated calcium channels of the CaV3 subclass (Dolphin 2003). Because of their central role in regulating functional expression and biophysical properties of calcium channels, and because of the well-defined interaction site (see above), interference with the AID-β interaction is an attractive strategy for designing specific calcium channel antagonists. Indeed, a competing AID peptide induced functional uncoupling of CaV1.2 and β2a subunits (Hohaus et al. 2000). Nevertheless, until today no isoform-specific inhibitors could be validated.
The α2δ subunit:
Four genes (Cacna2d1-4) encode for α2δ subunits (α2δ-1 to α2δ-4). They display distinct tissue distribution and three isoforms (α2δ-1 to -3) are strongly expressed in the developing and mature CNS (Arikkath and Campbell 2003; Schlick et al. 2010). Mature α2δ subunits consist of posttranslationally cleaved highly glycosylated α2 and δ peptides (Fig. 1B), which are associated to each other by a disulfide bond (Calderon-Rivera et al. 2012). Glycosylation of α2δ subunits is required for the functional membrane expression of calcium channels, as deglycosylation and glycosylation-site-directed mutagenesis resulted in strongly reduced current densities without affecting the kinetic properties (Gurnett et al. 1996; Sandoval et al. 2004). α2δ subunits are most likely linked to the plasma membrane via GPI-anchors (Davies et al. 2010). Alternatively, the δ subunit may constitute a single-pass membrane protein (Robinson et al. 2011). The vast majority of the α2δ protein is extracellular, ideally situated to interact with constituents of the extracellular matrix or extracellularly exposed proteins. In the domain structure of α2 a von Willebrand factor type A (VWA) domain and two Cache domains were identified by sequence homology in all α2δ subunits (Fig. 1B; Anantharaman and Aravind 2000; Canti et al. 2005; Davies et al. 2007). VWA-domains are found in a variety of extracellular matrix proteins and integrin receptors and are well known for their role in cell-cell adhesion involving a metal ion-dependent adhesion site (MIDAS). The integrity of the MIDAS motif in α2δ-2 has been shown to be necessary for calcium current enhancement and CaV channel trafficking (Canti et al. 2005). It has been hypothesized that these domains may be regulated by small endogenous ligands, such as the amino acid isoleucine (reviewed in Dooley et al. 2007), and that they are involved in binding the anti-epileptic and anti-allodynic drugs gabapentin (GBP) and pregabalin (PG) (Davies et al. 2007), which have also proven clinical efficacy in the treatment of generalized anxiety disorders.
2.2. Basic principles of neuronal functions mediated by voltage-gated calcium channels
The strong buffering of the second messenger calcium requires a close temporal and spatial vicinity of effector proteins to the calcium channel complex. Accordingly, neuronal CaVs are closely associated with upstream modulators, like protein kinases and phosphatases, and downstream effectors, as well as adapter and scaffold proteins. In neurons two highly specialized complexes have been intensively characterized: the synaptic vesicle fusion apparatus mediating excitation-secretion coupling and the postsynaptic calcium channel complex mediating excitation-transcription coupling (Fig. 2). At the synaptic vesicle fusion apparatus presynaptic calcium channels (mainly CaV2.1 and CaV2.2) are located in the close vicinity of the calcium sensor synaptotagmin, which triggers vesicle fusion and thereby neurotransmitter release. In the postsynaptic compartment activation of L-type channels (CaV1.2 and CaV1.3) initiates a signaling cascade to the nucleus that regulates gene expression. In both specialized compartments calcium channels need to be tightly organized in macromolecular complexes with upstream and downstream signaling molecules (Fig. 2B and C). The molecular organization of these signaling complexes is expected to be influenced by the subunit composition of the CaV complex in several ways. First, modulation of current properties (activation and inactivation kinetics, voltage-dependence) by auxiliary subunits will affect the local calcium transient and thereby downstream signaling. Second, a role of auxiliary CaV subunits in channel trafficking, targeting, and scaffolding will affect the composition of the entire complex and thereby determine specificity. Because different subunit isoforms and splice variants may differ with respect to their modulatory properties, protein-protein interactions, and subcellular targeting, the diversity of the auxiliary subunits may determine specific cellular functions in neurons. Therefore, in order to understand the role of the various distinct auxiliary CaV subunits in neurons it is necessary to address 1) which subunit isoforms and splice variants are actual components of specific signaling complexes, 2) whether different isoforms and splice variants serve distinct functions, 3) whether and to what degree can specific functions be compensated by other isoforms, and finally 4) do auxiliary subunits also act in a molecular context which is independent of the calcium channel complex.
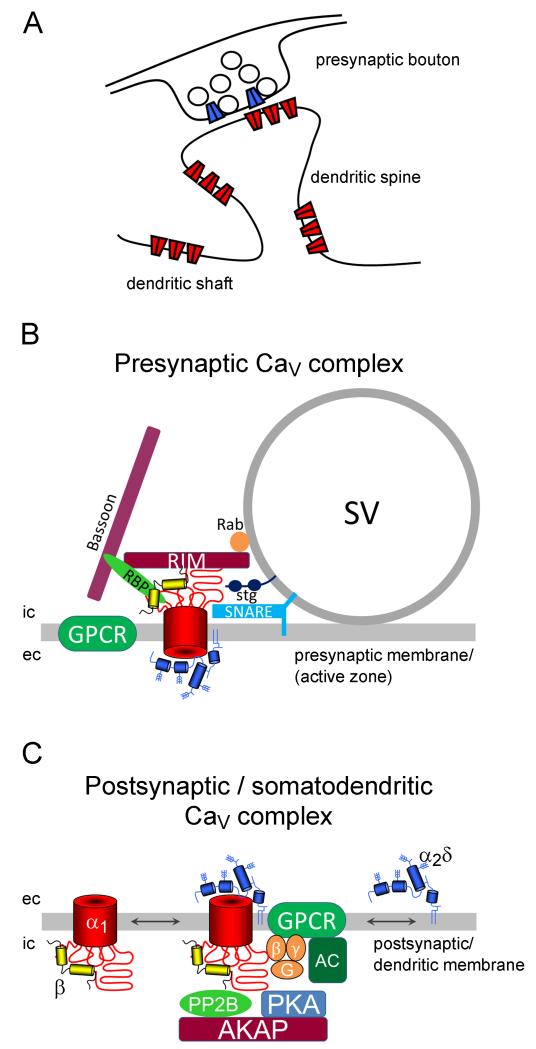
Figure 2.
Neuronal calcium channel complexes. A. In neurons voltage-gated calcium channels are located in presynaptic boutons, where they trigger neurotransmitter release, and in postsynaptic as well as extrasynaptic positions along dendrites and dendritic spines (Obermair et al. 2004; Jenkins et al. 2010). B. In the presynaptic active zone calcium channels are organized in a dense network of presynaptic proteins (depicted are RIM, RBP, Rab, bassoon, SNARE complex) and G-protein coupled receptors (GPCR) in the vicinity of the calcium sensor synaptotagmin (stg). Together this macromolecular complex orchestrates the tight regulation of synaptic vesicle (SV) fusion and thus neurotransmitter release (reviewed in Südhof 2012). C. In the postsynaptic/somato-dendritic compartment L-type channels can be found in complexes with G-protein coupled receptors (GPCR, e.g. the β2 adrenergic receptor), an adenylyl cyclase (AC), protein kinases (PKA) and phosphatases (PP2B), and scaffolding proteins (e.g. AKAP150) (reviewed in Dai et al. 2009). Downstream signaling initiates a signaling cascade which leads to the modulation of transcription such as CREB or NFATc4. Lateral mobility of L-type calcium channels in the plasma membrane also suggests regulation via the association or dissociation with distinct signaling complexes (Di Biase et al. 2011). Considering the membrane diffusion of GPI-anchored proteins, such a mechanism may also apply to α2δ subunits. ic, intracellular; ec, extracellular.
2.3. Establishing and maintaining signaling specificity
In order to elucidate the physiological neuronal functions of calcium channels and their auxiliary subunits, understanding the specificity of CaV subunit interactions and complex formation in native differentiated cell systems is a prerequisite. Three distinct mechanisms may contribute to the specificity of neuronal CaV complexes (Obermair and Flucher 2013). First, the calcium subunit complement in a specific cell type at a given time will yield the formation of particular complexes. Such preferential expression patterns of α1, β, and α2δ isoforms can indeed be observed in skeletal and cardiac muscle as well as highly specialized neuronal cell types like retina photoreceptor cells (Knoflach et al. 2013). In the CNS various brain regions including cortex, hippocampus, cerebellum, and even single cell types like cultured hippocampal pyramidal cells simultaneously express physiologically relevant mRNA levels of five out of seven high-voltage activated α1 subunits, all four β subunit isoforms, and three of four α2δ subunits (Schlick et al. 2010). The cerebellum, however, represents one notable exception as it preferentially expresses one set of calcium channel subunits (CaV2.1/β4/α2δ-2) (Ludwig et al. 1997; Brodbeck et al. 2002; Schlick et al. 2010). Altogether, in the majority of brain regions a restricted expression of auxiliary subunit isoforms may not be the prime strategy for establishing specific CaV complexes.
Second, distinct subcellular targeting properties of individual subunits or splice variants and/or possible interactions with other proteins may also yield specificity. We have recently identified such a mechanism for splice variants of the auxiliary β4 subunits. Whereas all β4 splice variants could increase the expression of presynaptic CaV2.1 by directly interacting with the channel, only β4 splice variants targeted into the nucleus (β4b, β4a) did specifically repress expression of synaptic proteins including CaV2.1 in a channel-independent manner (Etemad et al. 2014a, 2014b). Recently the differential up-regulation of an α2δ-1 splice variant in DRG neurons after spinal nerve ligation was identified (Lana et al. 2014). This finding supports a functional heterogeneity of splice variants also with α2δ subunits.
Finally it is also possible that specific stable complexes may not exist in all neuronal compartments and calcium channels could be regulated by reversible interactions with pools of functionally diverse β or α2δ subunits. An elegant FRAP study in skeletal muscle cells recently confirmed the presence of a stable calcium channel complex between the homologous CaV1.1 and β1a isoforms, while the heterologous β2a and β4b subunits formed dynamic complexes with the channel (Campiglio et al. 2013). The existence of a mobile pool of the L-type channel CaV1.2 in hippocampal neurons may be a first hint towards regulation of neuronal CaVs by dynamic subunit interactions (Di Biase et al. 2011).
3. Neuronal roles of auxiliary α2δ subunits
3.1. Channel trafficking and current modulation
The classical roles of auxiliary α2δ subunits on voltage-gated calcium channels, namely regulating the functional membrane expression and modulating the calcium currents, are widely recognized (Fig. 3A and B). When heterologously expressed all α2δ isoforms can enhance the functional membrane expression of α1 subunits (reviewed in Arikkath and Campbell 2003; Davies et al. 2007; Obermair et al. 2008). α2δ-1 co-expression studies in tsA201 cells additionally identified a role in modulating the biophysical channel properties such as activation and inactivation kinetics as well as the voltage-dependence of activation (Felix et al. 1997). Similar to α2δ-1 also α2δ-3 increased the functional membrane expression and altered the biophysical properties of L-type calcium channels (Klugbauer et al. 1999). In contrast, expression of α2δ-2 in Xenopus oocytes did not reveal biophysical modulation of CaV1.2 and CaV2.2 channels, although it was also found to increase the channels’ current density (Gao et al. 2000; Brodbeck et al. 2002). α2δ-4 is the least well studied isoform among α2δ subunits with respect to its modulatory effects on CaVs. In HEK293 it has been shown to increase calcium influx via CaV1.2 channels (Qin et al. 2002); however, a detailed functional characterization is still missing. Altogether existing evidence indicates some isoform specificity in modulating the biophysical current parameters, while increasing the functional membrane expression of CaVs seems to be a rather general mechanism. On the other hand isoform specificity in channel trafficking has been described in non-neuronal excitable cells. In skeletal muscle cells were only one isoform (α2δ-1) is expressed, experiments utilizing an α2δ-1 shRNA approach demonstrated that this subunit is not essential for targeting of calcium channels or for their primary physiological role in activating skeletal muscle excitation-contraction coupling (Obermair et al. 2005). However, α2δ-1 was identified as the major determinant of the characteristic L-type calcium current kinetics (Obermair et al. 2005; Tuluc et al. 2007). Together this suggests that α2δ-1 does not regulate the membrane trafficking and targeting of L-type calcium channels in their native environment. In consistence with this hypothesis α2δ-1 had only a small trafficking effect on CaV1.2 in a model system for cardiac myocytes (Tuluc et al. 2007), whereas it strongly enhanced functional expression of neuronal N- (CaV2.2) and P/Q-type (CaV2.1) channels (Obermair et al. 2008). In neurons of the CNS, where three α2δ isoforms (α2δ-1, -2, -3) are expressed, co-expression of all three subunits with P/Q or N-type channels causes an increase in current density (Davies et al. 2007). Thus regulating membrane trafficking and thereby calcium channel abundance may be the primary mechanism for modulating specific neuronal functions, for example synaptic transmission, by α2δ subunits. This is supported by reduced presynaptic CaV2.1 targeting as well as reduced synaptic release probability in neurons were α2δ-1 was knocked down by shRNA (Hoppa et al. 2012). On the other hand, the largely mild CNS phenotypes of the individual knockout mice (see below) suggest compensatory actions of α2δ isoforms. The propensity of individual α2δ-isoforms to regulate channel targeting is one important aspect in controlling CaVs, the other aspect is their capacity to fine tune the current kinetics of individual calcium channels. This was not only observed for skeletal and cardiac muscle CaVs (see above). Constitutive overexpression of α2δ-1 in neuronal tissues, for instance, resulted in enhanced currents as well as altered kinetics and voltage-dependence of activation in sensory neurons (Li et al. 2006).
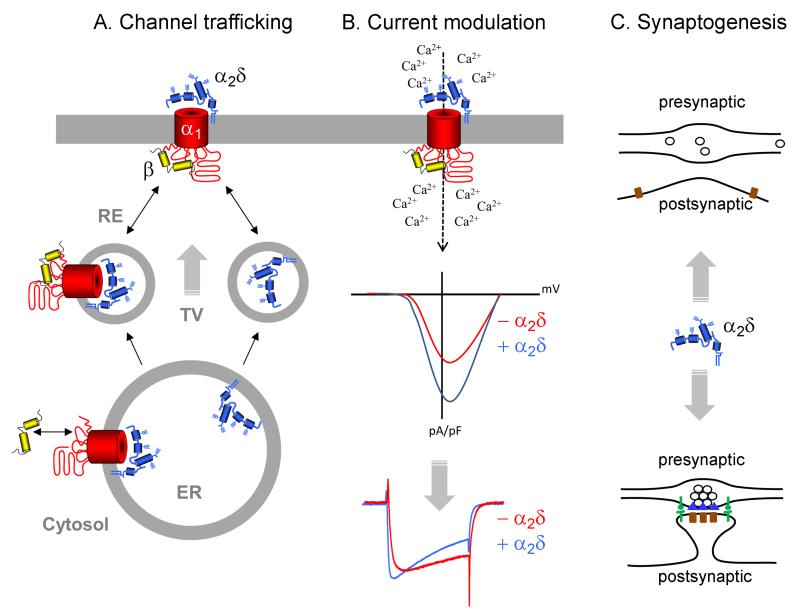
Figure 3.
Sketch summarizing the neuronal functions of auxiliary α2δ subunits. A. Channel trafficking: The export from the endoplasmic reticulum (ER) is mediated by β subunits; however, α2δ subunits can further enhance the trafficking. This may involve trafficking from the endoplasmic reticulum (ER) via the Golgi apparatus in transport vesicles (TV), recycling from recycling endosomes (RE), or stabilization of the channel complex at the plasma membrane. α2δ subunits can also be transported to the plasma membrane independent of the α1 subunit. B. Current modulation: Besides their role in channel trafficking α2δ subunits are important modulators of the calcium current. α2δ subunits can shift the voltage-dependence of channel activation to more negative potentials (IV curve, middle graph) and can modify the activation and inactivation kinetics (current traces, lower graph). This may be of particular relevance for somato-dendritic L-type calcium channels. C. Synaptogenesis: Accumulating evidence suggests an important role of neuronal α2δ subunits in synapse formation. Whether α2δs are necessary for the initial contact between the axon and the corresponding postsynaptic membrane (top) or for the differentiation into a fully aligned and mature synapse (bottom) remains to be answered.
3.2. α2δ subunits as targets for gabapentinoid drugs
Although the chemical backbone of GBP and PG was derived from GABA the mechanism of action of both drugs was initially unclear. The only outcome was that they did not have any notable effect on GABA receptors, metabolism, or transport (reviewed in Taylor et al. 2007; Silverman 2008; Bauer et al. 2010). However, both drugs were shown to reduce the cellular influx of calcium via CaVs in synaptosome fractions and hippocampal synapses (Fink et al. 2002; van Hooft et al. 2002). Accordingly it was a surprising result as it turned out that the 3H-gabapentin binding site corresponded to α2δ-1 and finally a selective binding to α2δ-1 and α2δ-2 was confirmed by the availability of newly established mouse models (see below). Both GBP and PG were found to be effective treatments for various forms of neuropathic pain and are also used as an adjunctive therapy of certain forms of epilepsy. The effect of these drugs in neuropathic pain was found to be state-dependent in that they have little effect on acute pain perception in animals or humans, but they are effective in chronic neuropathic pain. In line with this long term effects GBP inhibits calcium currents when applied chronically, both in heterologous expression systems and in dorsal root ganglion neurons (e.g. Hendrich et al. 2008). The most likely explanation for this effect is that chronic GBP treatment interferes with CaV trafficking to the cell surface as well as to presynaptic terminals (Bauer et al. 2009; Tran-Van-Minh and Dolphin 2010). Besides affecting trafficking during chronic application, these drugs have also been shown to exert acute effects on the current amplitude and the biophysical properties of calcium currents as well as on CaV-dependent functions, such as synaptic transmission (e.g. Uchitel et al. 2010; Farrell et al. 2014). However, acute effects of GBP and PG have not been observed in all studies and may depend on the composition of the calcium channel complex (Martin et al. 2002).
3.3. α2δ subunits and synapse formation
Over the recent years accumulating evidence suggests an important role of α2δ subunits in synapse formation (Fig. 3C). α2δ-1 has been shown to act as a receptor for thrombospondin, an astrocyte-secreted protein that promotes CNS synaptogenesis (Eroglu et al. 2009). In this study overexpression of α2δ-1 strongly promoted, and shRNA knockdown inhibited excitatory synapse formation in cultured retinal ganglion cells. Drosophila mutants of the α2δ-3 homologue (straightjackt; stj) show defects in presynaptic calcium channel localization and synaptic function (Dickman et al. 2008), which also coincides with a failure in normal synapse development in motoneurons (Kurshan et al. 2009). Interestingly, this study provided evidence that the defect in synapse formation was independent from the Drosophila pore forming α1 subunit (cacophony), because in contrast to the stj mutants, cacophony null mutants did not show a defect in synapse formation. α2δ-2 mutant (ducky, ducky2J) and knockout mice display altered Purkinje cell morphology and reduced calcium currents (Barclay et al. 2001; Ivanov et al. 2004; Donato et al. 2006), which suggests a role in maintaining normal cellular physiology and likely also the morphology of synapses. A spontaneous mouse mutation of α2δ-4 (Cacna2d4) causes structural and functional abnormalities of retinal ribbon synapses associated with the loss of rods (Wycisk et al. 2006a). Unc-2 and unc-36, the C. elegans homologues of CaV2 and α2δ, respectively, were identfied as regulators of C. elegans synaptogenesis (Caylor et al. 2013). While for unc-2 the study revealed a role in regulating the dynamic changes in the size and morphology of synapses that occur during development, unc-36 was demonstrated to regulate presynaptic morphology. α2δ-1 upregulation in a nerve injury model for chronic pain induced abnormal excitatory synapse formation and enhanced neurotransmitter release (Li et al. 2014). Finally, α2δ-3 knockout mice have a defect in presynaptic CaV2.1 channel targeting and synapse formation of auditory nerve fiber terminals contacting cochlear nucleus bushy cells (Pirone et al. 2014).Taken together, evidence is accumulating suggesting a role of α2δ subunits in synapse formation, which may be in part or entirely independent of the calcium channel complex. The mechanistic background for an independent function of α2δ subunits could be that these proteins protrude far into the extracellular space and therefore have the potential to contribute to signaling events that coordinate synaptic development.
3.4. Insights from animal models and human disease mutations
Within the past years, there has been great progress in the generation and description of mouse models with targeted deletions and spontaneous mutations for all α2δ subunits. These knockout and mutant mice display various disease phenotypes, a part of which share similarities with recently identified human diseases linked to α2δ subunits. The involvement of α2δ subunits in disease gives important insight into possible functions of the distinct isoforms (summarized in Tab. 1).
Table 1.
α2δ subunit isoforms and mouse and human disease phenotypes
| Mouse | Human | |||
|---|---|---|---|---|
|
| ||||
| Isoform | Mouse models | Phenotype | Potential cause | Disease |
| α2δ-1 | knock-in1 (GBP insensitive) | no known CNS phenotype1 | aberrations of CACNA2D15 | epilepsy and intellectual disability5 |
| knockout2 | reduced pain sensitivity3, no known CNS phenotype1 | |||
| α2δ-1 over-expressing4 | hyperalgesia, tactile allodynia4 | |||
|
| ||||
| α2δ-2 | spontaneous loss-of-function mutations6,7,8,9 (ducky, ducky2j, entla), knockout10 | ataxia, epilepsy and paroxysmal dyskinesia6,7,8,9,10 | homozygous mutations (point11 and frameshift12) | epileptic encephalopathy11,12 |
| genomic deletion of CACNA2D213,14 | potential tumor suppressor gene13,14 | |||
| single-nucleotide polymorphisms (SNPs)15 | candidate gene in childhood absence epilepsy15 | |||
|
| ||||
| α2δ-3 | knockout16 | deficits in pain16 and auditory/acoustic startle processing17 | SNPs16 | reduced sensitivity to acute noxious heat and chronic back pain16 |
| splice site mutation in CACNA2D318 | autism spectrum disorders18 | |||
|
| ||||
| α2δ-4 | spontaneous mutation (premature stop)19 | structural and functional abnormalities in ribbon synapses, loss of rods19 | mutation (premature stop) of CACNA2D420 | slowly progressive cone dystrophy and night blindness20 |
| partial deletion of CACNA2D421 | psychiatric disorders21 | |||
α2δ-1:
As α2δ-1 (and α2δ-2) were identified targets for GBP and PG, the need for mouse models was soon recognized for studying the involvement of the distinct α2δs in neuropathic pain and epilepsy. By generating a knock-in mouse expressing a mutated α2δ-1, that is not able to bind GBP and PG, Field et al. (2006) revealed α2δ-1 being the in vivo target for gabapentinoid drugs. This was ultimately confirmed by the lack of high affinity GBP binding in the brain of a conventional α2δ-1 knockout mouse (Fuller-Bicer et al. 2009). As previously expected from studies in skeletal and cardiac myocytes (Obermair et al. 2005; Tuluc et al. 2007), these mice display decreased cardiac L-type currents with altered activation kinetics. In somatosensory neurons α2δ-1 null mice showed a reduced CaV2.2 level and calcium channel current density, which was associated with a deficit in mechanical and cold sensitivity (Patel et al. 2013). Nevertheless, despite the widespread and predominant expression of α2δ-1 in the brain, so far neither gross-anatomical alterations nor signs for neuronal disease have been identified. Very recently disruption and deletion of the CACNA2D1 gene in three human patients with epilepsy and intellectual disability has been described (Vergult et al. 2014). Interestingly similar gene aberrations have also been identified in healthy individuals; therefore the causal relation of α2δ-1 disruption and human disease is still elusive. Senatore et al. (2012) identified an interaction of α2δ-1 with mutant prion proteins, putting forward the idea that disrupted neurotransmission in prion diseases may be linked to impaired synaptic CaV trafficking via α2δ subunits. Taken together the role of α2δ-1 for CNS disease remains unclear and the resulting phenotypes and severities may be associated with alterations in the overall neuronal excitability, which can be influenced by a variety of other factors.
α2δ-2:
There are several strains of naturally occurring mice with mutations in the Cacna2d2 gene (ducky, ducky2j, and entla) (Barclay et al. 2001; Brodbeck et al. 2002; Brill et al. 2004; Donato et al. 2006) as well as a targeted α2δ-2 knockout mouse (Ivanov et al. 2004), which result in a loss of the full-length α2δ-2 protein (ducky, ducky2j, knockout) or a structurally altered protein (entla). Affected homozygous mice show a decreased life span, infertility, and reduced body size when compared to heterozygous or wildtype littermates. The mice suffer from ataxia, paroxysmal dyskinesia, as well as epilepsy. α2δ-2 null mice (ducky, ducky2j, knockout) also exhibit altered cerebellar purkinje cell morphology and reduced calcium currents, as well as brainstem dysgenesis and spinal cord and myelination defects. In humans CACNA2D2 has been discussed as a potential tumor suppressor gene (Hesson et al. 2007) and a single nucleotide polymorphism (SNP)-based study identified CACNA2D2 as a candidate gene in childhood absence epilepsy (Chioza et al. 2009). Indeed a point mutation in CACNA2D2 resulting in reduced current density and slowed inactivation in neuronal calcium channels has recently been associated with early infantile epileptic encephalopathy (Edvardson et al. 2013). Pippucci et al. (2013) identified a frameshift mutation in CACNA2D2 in a patient with epilepsy, dyskinesia, cerebellar atrophy, psychomotor delay and dysmorphic features, a disease strikingly similar to the above-mentioned phenotypes of α2δ-2 mouse models.
α2δ-3:
Mice with a targeted deletion of the Cacna2d3 gene and the concomitant insertion of a bacterial β-galactosidase under its promoter were generated by Deltagen, Inc. These mice exhibit deficits in nociceptive pain processing and a delay in inflammatory heat hyperalgesia (Neely et al. 2010). Interestingly, α2δ-3 knockout mice display a reduced acoustic startle reflex and distorted auditory brainstem responses, which are likely caused by defects in presynaptic CaV2.1 channel targeting and synapse formation of auditory nerve fiber terminals contacting cochlear nucleus bushy cells (Pirone et al. 2014). Drosophila embryos lacking the α2δ-3 homologue straightjacket display altered heat nociception, a decrease in synaptic transmission and CaV2 channel abundance, as well as morphologically altered synaptic structure (Dickman et al. 2008; Kurshan et al. 2009; Neely et al. 2010). The C. elegans α2δ-3 mutant unc-36 revealed a role in CaV2 (unc-2) channel trafficking (Saheki and Bargmann 2009) which is associated with altered presynaptic morphology (Caylor et al. 2013). However, so far the only link between α2δ-3 and human disease is the identification of SNPs within the large genomic region of CACNA2D3 in patients with reduced sensitivity to acute noxious heat and chronic back pain (Neely et al. 2010).
α2δ-4:
Although expression of α2δ-4 is negligible in CNS neurons (Schlick et al. 2010), α2δ-4 is the major α2δ subunit in retinal photoreceptor cells (Knoflach et al. 2013). Mice with a spontaneous mutation of Cacna2d4 resulting in a truncated α2δ-4 protein show structural and functional abnormalities in mouse ribbon synapses associated with the loss of rods (Wycisk et al. 2006a). A similar phenotype was reported in humans, where a comparable mutation of the CACNA2D4 gene causes slowly progressing cone dystrophy associated with night blindness (Wycisk et al. 2006b). In a genome-wide association study a rare partial deletion in CACNA2D4 in two patients with late onset bipolar disorder was identified and also other genetic studies provide links between the α2δ-4 gene and psychiatric disorders (Van Den Bossche et al. 2012; see discussion therein). Any causal and mechanistic explanations for these associations remain as for now speculative, but may involve the expression of α2δ-4 in a specific and low abundant CNS cell type. Other causes could be provided by the role of α2δ-4 in vision or the close genetic proximity to the CACNA1C gene encoding for CaV1.2, which was shown to be associated with psychiatric disorders (Splawski et al. 2004).
4. Conclusion and outlook
Over the last years progress in unraveling the specific neuronal functions of neuronal α2δ subunits has been made at several levels. The availability of novel knock-out and mutant animals together with sophisticated heterologous expression studies in a variety of cell systems opened the possibility studying the specific roles of α2δ subunit isoforms. The findings showed that all α2δ subunits have classical roles dependent on the CaV complex, such as the modulation of membrane trafficking or current properties. Interestingly, more recent studies suggested functions of α2δ subunits in synapse formation, which may be in part or entirely independent of the CaV complex. Because, however, any defect or loss-of-function of α2δ subunits will inevitably affect the entire calcium channel complex, a clear distinction between independent or dependent functions is inherently difficult.
An emerging common feature in loss-of-function models of single α2δ isoforms is that the loss of one isoform only induces a strong phenotype in cells or tissues expressing predominantly this respective isoform. Such a pattern can be observed in the retina (α2δ-4; Wycisk et al. 2006a), in retinal ganglion cells (α2δ-1; Eroglu et al. 2009), in auditory nerves (α2δ-3; Pirone et al. 2014), and is also supported by studies in invertebrates (Kurshan et al. 2009; Caylor et al. 2013). Quite contrary, in CNS brain regions or neurons which simultaneously express three different α2δ isoforms the consequences of loss-of-function of a particular isoform may be compensated by the other isoforms thereby obscuring any potential phenotype. Thus, the severe consequences of α2δ-2 mutations in animal models and human disease are likely caused by the predominant expression of this particular isoform in the cerebellum. There the low protein levels of α2δ-1 and α2δ-3 do not suffice to compensate the loss of α2δ-2. Alternatively, CaV2.1 may exclusively form a complex with the auxiliary α2δ-2 and β4 subunits. Another possibility is that α2δ-2 itself mediates unique channel-independent functions, which cannot be compensated by the other isoforms. This possibility can be tested by analyzing calcium channels and CaV-dependent neuronal functions in cortical neurons from α2δ-2 knockout mice, as these neurons predominantly express α2δ-1 and α2δ-3 (Schlick et al. 2010). This endeavor is, however, complicated by the severe overall phenotype of α2δ-2 knockout and mutant mice. In a nutshell the available animal models, experimental co-expression studies, as well as identified human disease mutations at the moment do not provide a clear cut answer as to whether the individual α2δ isoforms have specific roles in CNS neurons.
Taken together, for the future the main challenge in elucidating the roles of neuronal α2δ subunits will be sorting out the functions of individual isoforms in cells and tissues simultaneously expressing more than one isoform. In order to clearly distinguish between potential redundant and specific functions it will be necessary to generate knockout mouse models lacking different combinations of α2δ isoforms (e.g. double knockout models). Such mouse models need to be complemented by native neuronal expression systems lacking two if not all three α2δ isoforms for studying the consequences on pre- and postsynaptic CaV-dependent functions. Characterization of the behavioral phenotype and the brain anatomy of such mouse models as well as analyzing neuronal functions could strongly contribute to a better understanding of the molecular mechanism of α2δ signaling and potentially uncover novel pathophysiological mechanisms.
Acknowledgements
We thank our colleagues and collaborators for helpful discussions. This work was supported by grants from the Austrian Science Fund (FWF): P24079-B21 and F4406. S.G. and C.L.S. contributed equally to this work which is part of their PhD thesis.
Footnotes
The authors declare no competing financial interests.
References
- Anantharaman V, Aravind L. Cache - a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem. Sci. 2000;25:535–537. doi: 10.1016/s0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Tran-Van-Minh A, Kadurin I, Dolphin AC. A new look at calcium channel alpha2delta subunits. Curr. Opin. Neurobiol. 2010;20:563–571. doi: 10.1016/j.conb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Brill J, Klocke R, Paul D, Boison D, Gouder N, Klugbauer N, Hofmann F, Becker CM, Becker K. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J. Biol. Chem. 2004;279:7322–7330. doi: 10.1074/jbc.M308778200. [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Davies A, Courtney JM, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, et al. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J. Biol. Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiological reviews. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Rivera A, Andrade A, Hernandez-Hernandez O, Gonzalez-Ramirez R, Sandoval A, Rivera M, Gomora JC, Felix R. Identification of a disulfide bridge essential for structure and function of the voltage-gated Ca(2+) channel alpha(2)delta-1 auxiliary subunit. Cell Calcium. 2012;51:22–30. doi: 10.1016/j.ceca.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglio M, Di Biase V, Tuluc P, Flucher BE. Stable incorporation versus dynamic exchange of beta subunits in a native Ca2+ channel complex. J. Cell. Sci. 2013;126:2092–2101. doi: 10.1242/jcs.jcs124537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni GL, Gao B, Nishizaki M, Xu K, Minna JD, Roth JA, Ji L. CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signaling and disruption of mitochondria membrane integrity. Oncogene. 2003;22:615–626. doi: 10.1038/sj.onc.1206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harbor perspectives in biology. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caylor RC, Jin Y, Ackley BD. The Caenorhabditis elegans voltage-gated calcium channel subunits UNC-2 and UNC-36 and the calcium-dependent kinase UNC-43/CaMKII regulate neuromuscular junction morphology. Neural. Dev. 2013;8:10. doi: 10.1186/1749-8104-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- Chioza BA, Aicardi J, Aschauer H, Brouwer O, Callenbach P, Covanis A, Dooley JM, Dulac O, Durner M, Eeg-Olofsson O, et al. Genome wide high density SNP-based linkage analysis of childhood absence epilepsy identifies a susceptibility locus on chromosome 3p23-p14. Epilepsy research. 2009;87:247–255. doi: 10.1016/j.eplepsyres.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J. Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh A. T., Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the alpha 1-beta anchoring site in voltage-dependent Ca2+ channels. J. Biol. Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opin. Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE. Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J. Neurosci. 2011;31:13682–13694. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J. Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci. STKE. 2003;2003:PE4. doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Donato R, Page KM, Koch D, Nieto-Rostro M, Foucault I, Davies A, Wilkinson T, Rees M, Edwards FA, Dolphin AC. The ducky(2J) mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. J. Neurosci. 2006;26:12576–12586. doi: 10.1523/JNEUROSCI.3080-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol. Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Oz S, Abulhijaa FA, Taher FB, Shaag A, Zenvirt S, Dascal N, Elpeleg O. Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J. Med. Genet. 2013;50:118–123. doi: 10.1136/jmedgenet-2012-101223. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad S, Campiglio M, Obermair GJ, Flucher BE. The juvenile myoclonic epilepsy mutant of the calcium channel beta subunit displays normal nuclear targeting in nerve and muscle cells. Channels (Austin) 2014a;8:334–343. doi: 10.4161/chan.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad S, Obermair GJ, Bindreither D, Benedetti A, Stanika R, Di Biase V, Burtscher V, Koschak A, Kofler R, Geley S, et al. Differential neuronal targeting of a new and two known calcium channel beta4 subunit splice variants correlates with their regulation of gene expression. J. Neurosci. 2014b;34:1446–1461. doi: 10.1523/JNEUROSCI.3935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K, Colecraft HM. Mechanism of auxiliary beta-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J. Physiol. 2011;589:4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell SR, Sargoy A, Brecha NC, Barnes S. Modulation of voltage-gated Ca2+ channels in rat retinal ganglion cells by gabapentin. Vis. Neurosci. 2014;31:47–55. doi: 10.1017/S0952523813000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J. Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. USA. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Tuluc P. A new L-type calcium channel isoform required for normal patterning of the developing neuromuscular junction. Channels (Austin) 2011;5:518–524. doi: 10.4161/chan.5.6.17951. [DOI] [PubMed] [Google Scholar]
- Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, et al. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H117–124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2) J. Biol. Chem. 2000;275:12237–12242. doi: 10.1074/jbc.275.16.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesson LB, Cooper WN, Latif F. Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene. 2007;26:7283–7301. doi: 10.1038/sj.onc.1210547. [DOI] [PubMed] [Google Scholar]
- Hohaus A, Poteser M, Romanin C, Klugbauer N, Hofmann F, Morano I, Haase H, Groschner K. Modulation of the smooth-muscle L-type Ca2+ channel α1 subunit (α1C-b) by the β2a subunit: a peptide which inhibits binding of β to the I—II linker of α1 induces functional uncoupling. Biochem. J. 2000;348:657–665. [PMC free article] [PubMed] [Google Scholar]
- Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov SV, Ward JM, Tessarollo L, McAreavey D, Sachdev V, Fananapazir L, Banks MK, Morris N, Djurickovic D, Devor-Henneman DE, et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am. J. Pathol. 2004;165:1007–1018. doi: 10.1016/S0002-9440(10)63362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 2010;30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach D, Kerov V, Sartori SB, Obermair GJ, Schmuckermair C, Liu X, Sothilingam V, Garrido MG, Baker SA, Glösmann M, et al. Cav1.4 IT mouse as model for vision impairment in human congenital stationary night blindness type 2. Channels (Austin) 2013;7:503–513. doi: 10.4161/chan.26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A. Impact of gating modulation in CaV1.3 L-type calcium channels. Channels (Austin) 2010;4:523–525. doi: 10.4161/chan.4.6.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat. Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana B, Schlick B, Martin S, Pratt WS, Page KM, Goncalves L, Rahman W, Dickenson AH, Bauer CS, Dolphin AC. Differential upregulation in DRG neurons of an alpha2delta-1 splice variant with a lower affinity for gabapentin after peripheral sensory nerve injury. Pain. 2014;155:522–533. doi: 10.1016/j.pain.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KW, Yu YP, Zhou C, Kim DS, Lin B, Sharp K, Steward O, Luo ZD. Calcium channel alpha2delta1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J. Biol. Chem. 2014;289:7025–7037. doi: 10.1074/jbc.M114.548990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–366. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmervoll B, Flucher BE, Obermair GJ. Dominance of P/Q-type calcium channels in depolarization-induced presynaptic FM dye release in cultured hippocampal neurons. Neuroscience. 2013;253:330–340. doi: 10.1016/j.neuroscience.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Flucher BE. Neuronal functions of auxiliary calcium channel subunits. In: Stephens G, Mochida S, editors. Modulation of Presynaptic Calcium Channels. Springer; London: 2013. pp. 29–60. [Google Scholar]
- Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J. Biol. Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Schlick B, Di Biase V, Subramanyam P, Gebhart M, Baumgartner S, Flucher BE. Reciprocal interactions regulate targeting of calcium channel beta subunits and membrane expression of alpha1 subunits in cultured hippocampal neurons. J. Biol. Chem. 2010;285:5776–5791. doi: 10.1074/jbc.M109.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur. J. Neurosci. 2004;19:2109–2122. doi: 10.1111/j.0953-816X.2004.03272.x. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Tuluc P, Flucher BE. Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol. 2008;8:311–318. doi: 10.1016/j.coph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- Patel R, Bauer CS, Nieto-Rostro M, Margas W, Ferron L, Chaggar K, Crews K, Ramirez JD, Bennett DL, Schwartz A, Dickenson AH, Dolphin AC. alpha2delta-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J. Neurosci. 2013;33:16412–16426. doi: 10.1523/JNEUROSCI.1026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- Pippucci T, Parmeggiani A, Palombo F, Maresca A, Angius A, Crisponi L, Cucca F, Liguori R, Valentino ML, Seri M, Carelli V. A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS One. 2013;8:e82154. doi: 10.1371/journal.pone.0082154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone A, Kurt S, Zuccotti A, Ruttiger L, Pilz P, Brown DH, Franz C, Schweizer M, Rust MB, Rubsamen R, et al. alpha2delta3 is essential for normal structure and function of auditory nerve synapses and is a novel candidate for auditory processing disorders. J. Neurosci. 2014;34:434–445. doi: 10.1523/JNEUROSCI.3085-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Yagel S, Momplaisir ML, Codd EE, D’Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol. Pharmacol. 2002;62:485–496. doi: 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- Robinson P, Etheridge S, Song L, Shah R, Fitzgerald EM, Jones OT. Targeting of voltage-gated calcium channel alpha2delta-1 subunit to lipid rafts is independent from a GPI-anchoring motif. PLoS One. 2011;6:e19802. doi: 10.1371/journal.pone.0019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, Bargmann CI. Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat. Neurosci. 2009;12:1257–1265. doi: 10.1038/nn.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval A, Oviedo N, Andrade A, Felix R. Glycosylation of asparagines 136 and 184 is necessary for the α2δ subunit-mediated regulation of voltage-gated Ca2+ channels. FEBS Lett. 2004;576:21–26. doi: 10.1016/j.febslet.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. doi: 10.1016/j.neuroscience.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore A, Colleoni S, Verderio C, Restelli E, Morini R, Condliffe SB, Bertani I, Mantovani S, Canovi M, Micotti E, et al. Mutant PrP suppresses glutamatergic neurotransmission in cerebellar granule neurons by impairing membrane delivery of VGCC alpha(2)delta-1 Subunit. Neuron. 2012;74:300–313. doi: 10.1016/j.neuron.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RB. From basic science to blockbuster drug: the discovery of Lyrica. Angew Chem. Int. Ed. Engl. 2008;47:3500–3504. doi: 10.1002/anie.200704280. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi SX, Miriyala J, Tay LH, Yue DT, Colecraft HM. A CaVbeta SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. J. Gen. Physiol. 2005;126:365–377. doi: 10.1085/jgp.200509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel OD, Di Guilmi MN, Urbano FJ, Gonzalez-Inchauspe C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels. 2010;4:490–496. doi: 10.4161/chan.4.6.12864. [DOI] [PubMed] [Google Scholar]
- Van Den Bossche MJ, Strazisar M, De Bruyne S, Bervoets C, Lenaerts AS, De Zutter S, Nordin A, Norrback KF, Goossens D, De Rijk P, et al. Identification of a CACNA2D4 deletion in late onset bipolar disorder patients and implications for the involvement of voltage-dependent calcium channels in psychiatric disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B:465–475. doi: 10.1002/ajmg.b.32053. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur. J. Pharmacol. 2002;449:221–228. doi: 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr. Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaValpha1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergult S, Dheedene A, Meurs A, Faes F, Isidor B, Janssens S, Gautier A, Le Caignec C, Menten B. Genomic aberrations of the CACNA2D1 gene in three patients with epilepsy and intellectual disability. Eur. J. Hum. Genet. 2014 doi: 10.1038/ejhg.2014.141. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nurnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest. Ophthalmol. Vis. Sci. 2006a;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, Berger W. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 2006b;79:973–977. doi: 10.1086/508944. [DOI] [PMC free article] [PubMed] [Google Scholar]