Abstract
Prolonged adaptation to delayed sensory feedback to a simple motor act (such as pressing a key) causes recalibration of sensory-motor synchronization, so instantaneous feedback appears to precede the motor act that caused it (Stetson, Cui, Montague & Eagleman, 2006). We investigated whether similar recalibration occurs in school-age children. Although plasticity may be expected to be even greater in children than in adults, we found no evidence of recalibration in children aged 8–11 years. Subjects adapted to delayed feedback for 100 trials, intermittently pressing a key that caused a tone to sound after a 200 ms delay. During the test phase, subjects responded to a visual cue by pressing a key, which triggered a tone to be played at variable intervals before or after the keypress. Subjects judged whether the tone preceded or followed the keypress, yielding psychometric functions estimating the delay when they perceived the tone to be synchronous with the action. The psychometric functions also gave an estimate of the precision of the temporal order judgment. In agreement with previous studies, adaptation caused a shift in perceived synchrony in adults, so the keypress appeared to trail behind the auditory feedback, implying sensory-motor recalibration. However, school children of 8 to 11 years showed no measureable adaptation of perceived simultaneity, even after adaptation with 500 ms lags. Importantly, precision in the simultaneity task also improved with age, and this developmental trend correlated strongly with the magnitude of recalibration. This suggests that lack of recalibration of sensory-motor simultaneity after adaptation in school-age children is related to their poor precision in temporal order judgments. To test this idea we measured recalibration in adult subjects with auditory noise added to the stimuli (which hampered temporal precision). Under these conditions, recalibration was greatly reduced, with the magnitude of recalibration strongly correlating with temporal precision.
Introduction
The temporal structure of a motor-sensory event shapes the causal relationship between action and perception. Only sensory events that occur within a consistent delay after a voluntary movement are considered to be consequences of the action, otherwise they seem attributable to external agents. Processing temporal order is therefore important for the brain, but challenging, because timing judgments have to be calibrated to take account of the various latencies involved in the physical propagation of external stimuli, and the internal temporal differences in the processing within the sensory pathways and between the sensory and the motor pathways (Eagleman, 2008; Stetson, Cui, Montague & Eagleman, 2006).
Recent research has investigated the perception of causality, but the mechanisms remain poorly understood. Haggard, Clark and Kalogeras (2002) have shown that the predictability of a sensory event leads to a temporal compression between action and sensation. They found that a delay between a voluntary movement and its sensory consequence goes unnoticed; the two events are perceived to be close together. A possible explanation for this phenomenon is a mechanism that binds together the intentional action and the expected feedback in order to preserve the experience of our own agency, despite the delay (Eagleman & Holcombe, 2002; Engbert, Wohlschlager & Haggard, 2008; Haggard et al., 2002; Sugano, Keetels & Vroomen, 2010). Indeed, the phenomenon of ‘intentional binding’ seems to be specific for voluntary movements (the effect disappears when the movement is induced through the use of Transcranial Magnetic Stimulation) and only occurs within a short temporal window.
Stetson et al. (2006) showed that adaptation to delayed sensory feedback from a simple motor act can change perceived motor-sensory synchronization. After adaptation, the perceived simultaneity between a voluntary action, such as a keypress, and a visual flash, is shifted in time in the direction of adaptation, to the point where a simultaneous flash can appear to precede the voluntary action (Stetson et al., 2006). The authors suggested that the brain adjusts the perceived time of the sensory event relative to the perceived time of action in a ‘motor-sensory recalibration process’ to keep causality assessment accurate. In other words, the delay in the sensory feedback violates the prediction of synchrony between the action and its sensory consequence, causing the brain to reorganize the temporal structure of the two events. This effect has been widely investigated in many different studies, which show that the temporal window for motor-sensory recalibration is constrained in a very short temporal range of about 150–250 ms (Keetels & Vroomen, 2012; Stetson et al., 2006), that the recalibration requires a voluntary action or an action observation to occur (Stetson et al., 2006; Watanabe, Shinohara & Shimojo, 2011) and that the effect on perceived simultaneity can be generalized to other sensory modalities, such as vision or audition, from the one used during the adaptation (Di Luca, Machulla & Ernst, 2009; Heron, Hanson & Whitaker, 2009; Sugano et al., 2010; Sugano, Keetels & Vroomen, 2012).
Recently, Cai, Stetson and Eagleman (2012) have proposed a model to explain motor-sensory recalibration, based on multisensory integration mechanisms found in the cat superior colliculus, which operate through a population of neurons sensitive to differences in timing bi-modal sensory inputs (Meredith, Nemitz & Stein, 1987). The authors speculate that there may exist a similar population of neurons selective for temporal disparities in neural activity in sensory pathways and in the motor efferent-copy. The perceptual judgments could therefore be based on the difference in the activity between these neurons (Cai et al., 2012). Like the renowned motion aftereffect (Addams, 1834), prolonged exposure to delayed sensory feedback makes simultaneous motor-sensory events appear to have the opposite lag. The general concept behind this model is a supramodal mechanism responsible for perceptual judgment about both space and time. This model is supported by two recent studies. The first shows that the recalibration of the motor-sensory simultaneity can be stored over time (Cai et al., 2012; Machulla, Di Luca, Froehlich & Ernst, 2012); the second shows that recalibration occurs in both directions, to sensory-led and to movement-led discrepancies (Rohde & Ernst, 2012).
Changes occur continuously in the developing system and can affect many aspects of sensory and motor function. For example, the speed of processing of both the motor and the sensory pathways can change with the physical growth and continuous maturing of the sensory channels (Bremner, Lewkowicz & Spence, 2012). There is a constant need for ‘long term recalibration’ (Burr & Gori, 2012; Gori, Del Viva, Sandini & Burr, 2008) to take into account growing limbs, eye-length, inter-ocular distance, etc. In children younger than 8–10 years, there is little evidence for multisensory integration, suggesting that cross-sensory calibration may dominate this period (Gori et al., 2008; Gori, Tinelli, Sandini, Cioni & Burr, 2011; Nardini, Jones, Bedford & Braddick, 2008), and this process would preclude multisensory integration (see Burr & Gori, 2012; Murray & Wallace, 2012). Hillock-Dunn and Wallace (2012) have also shown that the temporal window for multisensory binding matures relatively late. In particular, they found that the sensitivity to audio-visual temporal asynchrony increases with age, with young and adolescent subjects binding more temporally disparate multisensory stimuli than adults.
Motor-sensory recalibration is fundamental for determining causality, despite the variability in temporal asynchrony between the sensory and the motor pathways. Is this mechanism functional in the developing system of young children? To address this question we studied sensory-motor adaptation in school-age children using the paradigm introduced by Stetson et al. (2006). Our results show that children less than 12 years of age do not show measurable cross-sensory recalibration. We suggest that the failure of recalibration may be related to reduced temporal sensitivity. In support of this idea, we found that in the adult population the effect of recalibration can also be reduced by external noise, suggesting that this is the explanation for the lack of recalibration in children.
Methods
Twenty-nine children and 10 adults participated in the first experiment. Children were divided by age into two different groups: 18 8-year-olds (average age and SD 7.7 ± 0.1) and 11 11-year-olds (11.3 ± 0.3). The adult group comprised two authors and eight students naïve to the purpose of the study (27.3 ± 1.4). All participants were right-handed with normal hearing and normal or corrected-to-normal sight. Adult subjects were recruited from the local university while children were recruited from elementary and intermediate schools in Genoa (GE, Italy).
The test was presented in the form of a game. During both experiments, subjects sat in a silent room, in front of a desktop (13.3” screen, TFT active matrix TFT 1280 × 800 WXGA) with their right index finger placed in a ring with a circular button (0.5 cm in diameter) on top. For the first experiment, subjects were instructed to perform a voluntary action pressing the button (Figure 1b). The motor action was extremely easy to perform since little force was required (0.2 N). To isolate the sound produced by the tap, the hand was set inside a box. To evaluate the effect of the adaptation to a delayed sensory feedback to this motor act we ran two trial blocks both with an adaptation phase followed by a test phase. Subjects first performed the ‘baseline block’, comprising 100 trials of adaptation to a sound simultaneous with their voluntary action and 80 or 60 trials (for adults and children, respectively) of the temporal order judgment task; then the ‘delayed feedback block’, comprising 100 trials of adaptation to delayed sensory feedback (usually 200 ms, but 500 ms in one session), and then the temporal order judgment task (similar to Stetson et al., 2006). We used more adapting trials than Stetson et al. (2006) to maximize the possibility of producing an effective adaptation. The total duration of the adaptation phase in each block was ~5 min. During both adaptation phases, participants were instructed to push one of the keyboard buttons at will, but trying to maintain a roughly constant inter-tap interval (they were given training in this before the beginning of the experiment). After each tap they received an acoustic feedback with a fixed delay (0, 200 or 500 ms, depending on the experimental condition). To keep attention maintained on the task, subjects had to verbally report the presence of a deviant stimulus (a higher tone of 1200 Hz), randomly delivered 18 times during each adaptation phase. To be sure that the effect of recalibration was not due to a change of criterion between the two experimental conditions we ran two trial blocks with adaptation in the baseline condition. In both blocks and phases of the blocks, the auditory stimulus comprised a 880 Hz tone of 25 ms duration presented through loudspeakers placed on either side of the screen. To accurately record the time of the voluntary action and sound delivery, both the speakers and the button were connected to a National Instrument NI USB-6008 Support interface, bypassing any delays that may have been introduced by the program.
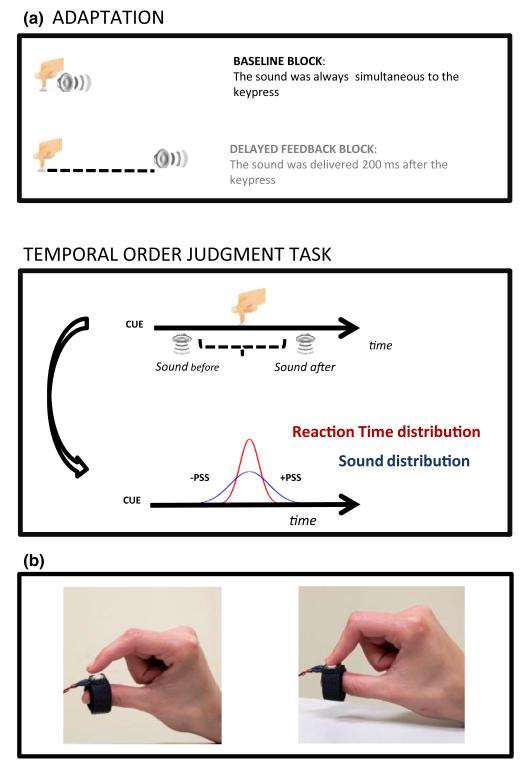
Figure 1.
(a) Adaptation and test trials. Each block started with 100 trials of adaptation to a synchronous sequence (‘baseline condition’), or to 200 ms or 500 ms delayed sensory feedback (‘delayed feedback condition’). After adaptation, subjects performed a temporal order judgment task, judging whether their own action (a keypress) or an acoustic stimulus appeared first.
(b) The button used during the test phase. The force required to press it was 0.2 N, and the distance to contact ~1 mm.
During the test phases, subjects performed a temporal order judgment between a sound and their own motor action. Participants pressed the button attached to their finger as soon as possible in response to a visual cue (a small circle) that appeared on the screen while a sound was delivered before or after the key press. Subjects reported verbally whether the sound seemed to occur before or after the timing of their own action, thus making a temporal order judgment between the keypress and tone. The proportion of trials where the sound was judged as ‘after’ the keypress was computed for each keypress–sound time difference (SOA) and fitted with cumulative Gaussian error functions (see Figure 2), yielding PSS (Point of Subjective Simultaneity, corresponding to the mean) and threshold (the standard deviation). Standard errors for the PSS and threshold estimates were obtained with a bootstrap procedure (Efron & Tibshirani, 1993). To estimate the PSS we presented the auditory stimuli both before and after the tap event, based on the running average of each participant’s reaction time to the visual cue. The average reaction time was calculated every three trials, except for the first three trials where we assumed 300 ms. The minimum and maximum reaction times were 50 and 900 ms; trials outside those limits were discarded. The smallest interval between the onset of the visual cue and the delivery of the sound was 10 ms.
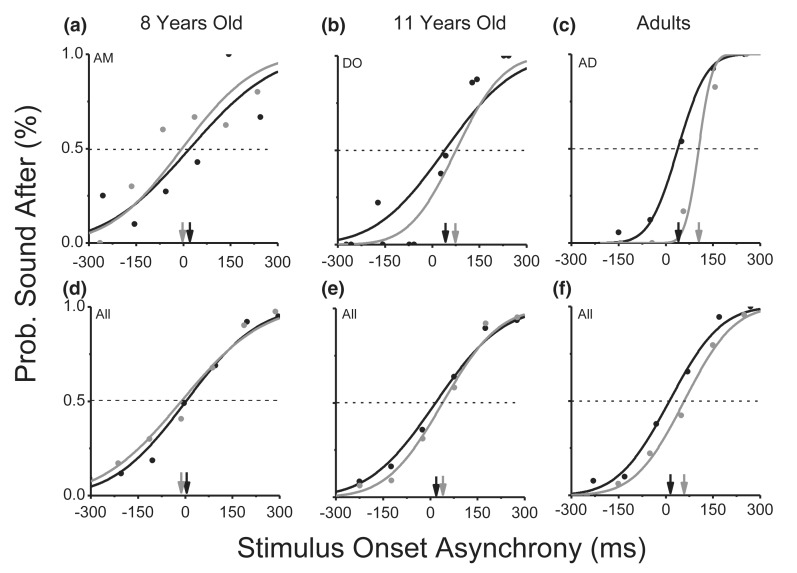
Figure 2.
Example psychometric functions for three representative subjects (a–c), and for data averaged over the three age groups (d–f). The curves plot the proportion of trials where the sound appeared to follow the movement as a function of stimulus offset asynchrony of the sound compared with the movement. The black curves and symbols plot data for the baseline condition, and grey the delayed feedback condition. Positive values of PSS mean that the sound occurs after the movement when they appeared to be simultaneous. While adults showed a positive shift in the perceived simultaneity (see grey and black arrows) in the ‘delayed feedback block’, for the 8-year-olds, the 11-year-olds and the adult group in the noise condition the two curves are fully overlapped, revealing no recalibration effect.
The presentation time of the sound was determined by an adaptive algorithm, with random scatter (Gaussian noise of 80 ms standard deviation). At the beginning of the session the mean of the distribution was determined by the estimate of reaction times, but this was then updated, every 20 trials, to correspond to the estimate of PSS. This ensured that the trials were placed efficiently to determine PSS (and threshold), and also that the responses tended to be equally distributed between alternatives.
Before the experiment, subjects were familiarized with the temporal order judgment task through a brief training session comprising 10 trials identical to the testing situation, except that the lower limit for the onset of the sound was set to ±50 ms with respect to the keypress. During training, participants received verbal feedback about the real time difference between the sound and their motor action after each trial. Afterwards, we asked subjects to perform another 10 trials of the temporal order judgment task (identical to the training). Subjects making 30% errors were not recruited for the experiment. In this way we were sure that participants, children in particular, were able to perform the task.
Trials of the training session were excluded from the analysis. The duration of each block of trials was about 15 minutes, while the duration of the training was about 10 minutes. The entire experiment lasted about 45 minutes.
For the second experiment a group of five adults (four new subjects plus a naïve subject who had participated in the first and second experiments) was tested. Methods and procedure of the Experiment 2 were the same as in Experiment 1, except that subjects were presented with white noise (65 dB; spectrum composed of frequencies ranging from 0 to 11 kHz) to headphones for the whole duration of the experiment. On the other hand, the auditory stimulus was presented via a loudspeaker placed on either side of the screen.
In a final control experiment we investigated whether a larger temporal delay between the motor action and the sensory feedback modifies the effect of recalibration in children. We replicated Experiment 1 in a new sample of 16 children (average age 8.9 ± 0.2) but this time the sound in the adaptation phase of the ‘delayed feedback block’ was released after 500 ms instead of 200 ms.
The experiment was approved by the local ethics committee (ASL3 of Genoa, Italy).
Results
Figures 2a–c show example psychometric functions for the main experiment, for three typical observers representing the three age groups. Each function plots the proportion of trials where the sound is perceived to come after the action, as a function of the physical delay of the sound. The black curves plot results for the ‘baseline block’ (adaptation to zero delay), the grey curves after the delayed feedback adaptation (see Methods for more details). The 50% point of each psychometric function (shown by the dashed lines) gives an estimate of the point of subjective simultaneity (PSS) between the sound and the subject’s action. A PSS of zero means that subjects perceived the sound to be synchronous with their keypress when they were physically aligned in time; negative values mean that the sound had to precede the keypress to be perceived as simultaneous, and positive values the converse.
All further analyses were based on the PSS calculated individually for each subject, from curves like those in Figure 2. However, as a secondary analysis, we pooled the raw data from all subjects within a group to yield the averaged psychometric functions reported in Figures 2d–f. The values of PSS for these pooled functions are in line with the averages of the individual results (reported later). Adults showed an unbiased PSS in the baseline condition and a positive shift in PSS in the adapted condition, both in the example subject and in the pooled results. Thus, a tone presented less than 40 ms after the keypress appeared to precede the motor act after adaptation to a 200 ms delay, implying sensory-motor recalibration. However, the results with children were different. The 8-year-old average data show virtually no shift, and the 11-year-old a much-reduced shift compared with the adults.
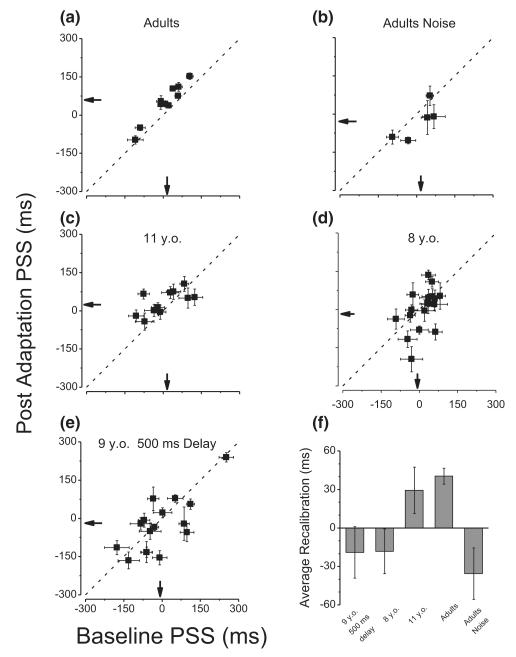
Figure 3 reports the PSSs of all subjects, plotting the value obtained after adaptation to a 200 ms delay against that with 0 ms adaptation. For the adults (standard condition: Figure 3a), all points lie above the equality line, showing that for every subject tested, delayed feedback caused a clear adaptation effect, so at the point of perceived simultaneity, the sound followed the keypress (and seemed to precede the keypress when they were physically simultaneous). The average difference in the two conditions was 40 ms, highly significant (one-tailed paired t-test, t9 = 6.53, p < .001). The data for the 11-year-olds (Figure 3c) followed the same trend as the adults, but less strongly, with only 9 out of 11 points above the equality line. The average difference was only 29 ms, only marginally significant (one-tailed paired t-test, t10 = 1.7, p = .059). For the 8-year-olds (Figure 3d), there was no significant difference between the two conditions (one-tailed paired t-test, t17 = 1.04, p = .84).
Figure 3.
Points of perceived simultaneity (PSS) after adaptation to delayed sensory feedback, plotted as a function of PSS in the baseline condition, for the various groups. (a) Adults: standard condition (adaptation at 200 ms delays). (b) Adults: adaptation to 200 ms delays, with auditory noise added to the stimulus (see Methods). (c) 11-year-old group (7.7 ± 0.1 years), standard adaption condition (200 ms adaptation); (d) 8-year-old group (11.3 ± 0.3 years), standard condition (200 ms adaptation); (e) Children group (9 ± 0.2 years) with 500 ms adaptation; (f) Bar graphs summarizing the average recalibration effects (difference in temporal order judgment task for the baseline and adaptation conditions), for the five different groups. Error bars show ±1 SEM.
Importantly, the PSSs after 0 ms adaptation were very similar for all age groups, in both mean and standard deviation: adults 7.2 ± 20.9 ms, 11-year-olds 4.3 ± 22.9 ms, 8-year-olds −4.24 ± 20.6 ms (one-way-ANOVA, between factor: age, F(2, 36) = 0.79; p = .92). This suggests that all groups use similar criteria in judging simultaneity. It would seem unlikely, for example, that differences in adaptation may result from younger children taking more time to press the button (in any event unlikely, given that very little force was required, and the excursion very short).
It may be argued that the young children show no calibration because they have longer time constants, so 200 ms may be insufficient to cause measurable adaptation. We therefore repeated the experiment with a new sample of children (average age 9 ± 0.2 years) using a 500 ms adaptation regime (all else ±remained as before). This condition (Figure 3e) also revealed no measurable effects (one-tailed paired t-test, t15 = 0.97, p = .17).
Figure 3f summarizes the results of all the groups with bar graphs plotting the difference in post-adaptation and baseline PSS, with error bars showing ± 1 SEM. It is clear that only the adult group shows ± a significant recalibration effect. The ‘adults noise’ condition (Figure 3b) is described later.
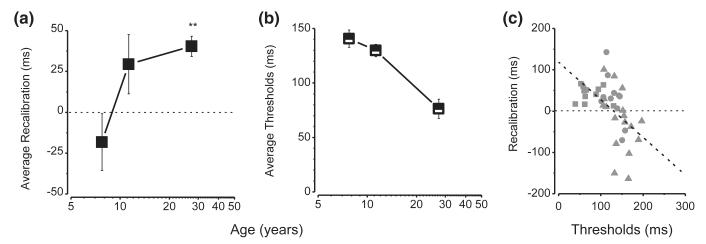
Figure 4a plots average recalibration (difference in PSS for the two adaptation conditions) as a function of age (same data as Figure 3f). The magnitude of recalibration clearly increases with age, with only the adult group showing a significant recalibration effect. The interaction between the recalibration effect and age was significant (mixed design ANOVA, between factor: age, within factor: delay; F(2, 36) = 3.85; p = .03).
Figure 4.
Average recalibration effect for all groups measured as the differences in the PSS in the two experimental blocks as a function of age.
(a) Average thresholds (calculated from the individual standard deviations of each group) as a function of age.
(b) The recalibration effect as a function of thresholds, for individual subjects; squares represent adult subjects, circles 11-year-olds and triangles 8-year-olds. Recalibration magnitude correlates strongly with thresholds. The dashed line shows the linear regression (R2 = 0.25; p < .001, slope = −0.9).
The slopes of the psychometric functions, given by the standard deviations of the best-fitting Gaussian error function, give an estimate of the precision of the temporal judgments. From inspection of the functions in Figure 2, it is clear that they are steeper in adults than children. Measured thresholds in the baseline and in the adaptation condition were 82 ± 13 ms and 70 ± 10 ms for adults; 135 ± 10 ms and 124 ± 8 ms for 11-year-old children; 131.84 ± 20 ms and 149 ± 12 for 8-year-old children. We found no significant differences in precision between the two blocks in any of the groups (separate two-tailed paired t-test for each group: 8-year-olds: t17 = 0.9, p = .38; 11-year-olds: t10 = 0.76, p = .47; Adults: t9 = 0.77, p = .46). We calculated thresholds separately for all subjects, averaged between the two conditions and over individuals within a group. Thresholds show a clear developmental trend, decreasing with age from 141 ms in the 8-year-old group to 76 ms in the adult group, a factor of two (R2 = 0.52, p < .001). These differences suggest that children need a greater disparity between the on set of the stimuli to discriminate the temporal order between a motor and a sensory event.
We next plotted the magnitude of recalibration against thresholds for all individuals from the three age groups (Figure 4c). Recalibration is strongly correlated with thresholds (r = −0.5, R2 = 0.25, p < .001). The slope of the best-fitting regression is −0.9, suggesting a very strong association between threshold and recalibration.
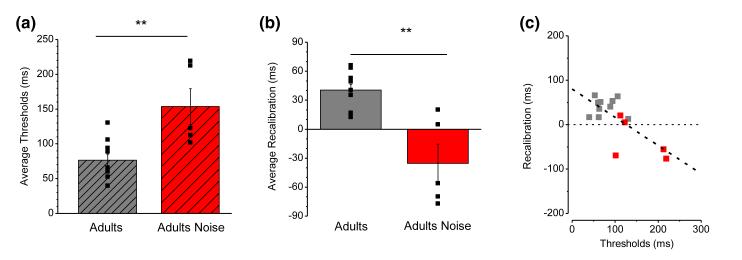
To study further the dependence of recalibration on thresholds, we tested adults in a condition with auditory noise, to increase artificially temporal judgment thresholds (see Methods). Figure 5a shows that the noise was effective in increasing thresholds, by more than a factor of two (two-tailed unpaired t-test, t13 = 3.6, p = .003). Under these conditions, very little temporal recalibration occurred in adults. Figures 3b and f show that PSSs after adaptation to a 200 ms delay were very similar to baseline (adaptation to 0 ms), if anything in the reversed direction (however, the difference was not significant: two-tailed paired t-test, t4 = 1.77, p = .15). The difference between the noise and no-noise conditions was highly significant (one-tailed, unpaired t-test, t13 = 4.66, p < .001). In Figure 5c we plot the recalibration effect against thresholds for all adults (those with noise shown in red). Again, the dependence was strong and significant (slope = −0.6, R2 = 0.48, p = .002).
Figure 5.
(a) Average thresholds for the two adult groups in the standard condition (grey) and in the noise condition (red). Black symbols represent individual data. Thresholds are significantly higher in the noise condition (153 ms) compared with the standard condition (76 ms).
(b) Average recalibration for the two adult groups in the standard noise conditions (color-coded as a), with symbols showing individual data. The noise causes a reduction of 53 ms in the average calibration.
(c) The recalibration effect as a function of thresholds. The grey symbols show data for the standard condition and red symbols the data for the adult noise group. The dashed line shows that the effect of recalibration linearly decreases with the increasing of thresholds (R2= 0.25; p < .005).
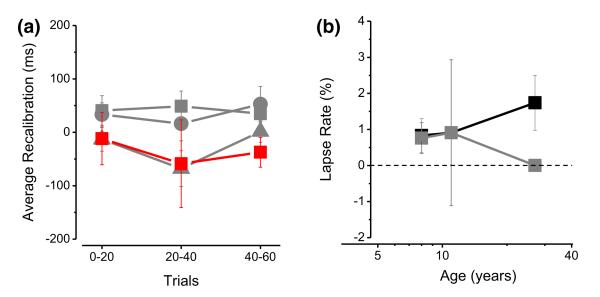
To be certain that the effects reported here are robust, we carried out two further analyses. The lack of recalibration in children may reflect a difference in decay time of adaptation. We therefore calculated the magnitude of the recalibration effect (difference between the two conditions) separately for the first, second and third 20-trial blocks (Figure 6a). There was no significant variance in the magnitude of recalibration over the 60-trial period, for any of the four groups (mixed design ANOVA, between factor: group, within factor: trial block; F(6, 80) = 0.53, p = .78). It would seem unlikely that the lack of recalibration results from stronger decay in young children.
Figure 6.
(a) The average recalibration effect calculated for each group of subjects separately for the first, second and third 20-trial blocks. Black symbols represent adults in the standard condition, red symbols adults in the noise condition and grey circles and squares represent 11- and 8-year-old children, respectively. There is no difference in the magnitude of recalibration between trial blocks in any of the group of participants.
(b) Lapse rate index (essentially the proportion of errors for very large asynchronies) plotted as a function of age. The black symbols represent the baseline condition while the grey symbols represent the adaptation condition. Each symbol represents the average lapse rate for each age group. The index does not change with age or with experimental condition. Bars show ±1 SEM.
Secondly, to test for inattentiveness, we measured the ‘lapse rate’ index (Figure 6b). This was calculated by fitting the curves with cumulative Gaussians with asymptotes of λ and 1–λ, where λ is a free parameter. High values of λ mean that subjects made errors even for easy conditions, suggesting inattention. As Figure 6b shows, average lapse was near zero for all the ages in both the experimental conditions, excluding inattention as a possible explanation for the lack of recalibration in children. A mixed design ANOVA confirmed that there are no differences in the lapse rate between groups (F(3, 40) = 0.72, p = .54), within the same group in the two conditions (ANOVA, F(1, 40) = 1.18, p = .28) and in the interaction between the two factors (F(3, 40) = 1.1974, p = .32).
Discussion
Stetson et al. (2006) have shown that adaptation to delayed sensory feedback to a simple motor act induces recalibration of sensory-motor synchronization, so that instantaneous feedback appears to precede the motor act that caused it. This motor-sensory recalibration is probably an important mechanism in attributing causality. Our study suggests that the mechanism develops late in humans, as children less than 12 years of age showed no evidence of motor-sensory recalibration. We also showed that the lack of recalibration is associated with poor temporal resolution in motor-sensory synchronization.
Temporal precision increased with age, from an average threshold of 140 ms in the 8-year-old group to 76 ms in the adult group, suggesting that children need a greater disparity between the onsets of the two events to discriminate their temporal order, a possible cause for the lack of recalibration in children. Indeed, as thresholds decrease the effect of recalibration becomes greater. Our results are in line with research from Hillock-Dunn and Wallace (2012) showing that sensitivity to audio-visual temporal asynchrony increases with age, with adults being less likely to bind more temporally disparate multisensory stimuli than younger participants.
There are several possible explanations for these results. One possibility is that the size of the motor-sensory temporal binding window is larger in children than in adults, which may account both for the poorer temporal discrimination and the lack of recalibration. However, this explanation seems unlikely, as there was no sign of adaptation in children even after adaptation to a 500 ms delay. It is also possible that the lack of recalibration may arise from a faster decay of adaptation in children; however, we found no differences in the magnitude of the effect over the 60-trial period, for any groups, suggesting that the adaptation remained stable over that period. Nor do the effects seem to reflect inattention in children, as lapse-rate analysis suggests that their attention was as effective as adults. As the responses in the baseline condition were very similar in all age groups (abscissae of Figure 3), it seems unlikely that the lack of adaptation results from differences in keypress of the different ages groups.
The lack of adaptation in young children probably reflects immaturity of the auditory and motor systems. Plastic changes occur continuously in the developing brain to initiate holistic perceptions and actions. A delay in development of these mechanisms could interfere with tasks for which fine acoustic and motor synchronization and perception is fundamental. As mentioned earlier, several studies have suggested that some auditory tasks, such as frequency discrimination (Allen, Wightman, Kistler & Dolan, 1989), discrimination of auditory spectra (Allen & Wightman, 1992), discrimination of low frequency tones (Maxon & Hochberg, 1982) and intensity coding (Buss, Hall & Grose, 2006), require several years to become adult-like. More relevant for our task is that children have poorer auditory temporal skills than adults (Wightman, Allen, Dolan, Kistler & Jamieson, 1989) and various psychophysical tasks suggest that their ability to distinguish rapidly presented auditory inputs continues to develop until early adolescence (Gori, Sandini & Burr, 2012; Hall & Grose, 1994; Hartley, Wright, Hogan & Moore, 2000; Irwin, Ball, Kay, Stillman & Rosser, 1985; Walker, Hall, Klein & Phillips, 2006). Experimental evidence suggests that motor coordination also develops late (Jansen-Osmann, Richter, Konczak & Kalveram, 2002; Konczak, Borutta, Topka & Dichgans, 1995; Konczak & Dichgans, 1997; von Hofsten, 1991). The poor temporal resolution in both the motor and sensory systems could make recalibration difficult, as a noisy estimate may interfere with the temporal synchronization between the two events.
To study further the effect of noise on recalibration, we repeated the experiment with adults with added auditory noise. Adding external noise increased average thresholds, and also strongly reduced the average recalibration effect. These results are in line with a recent study by Burge, Ernst and Banks (2008) showing that adaptation rate in human reaching is strongly reduced when the reliability of the visual feedback is blurred.
However, the results are not in immediate agreement with a recent study by Van der Burg, Alais and Cass (2013). They demonstrated a very rapid form of cross-modal adaptation, where the offset of the previous trial in a sequence of audio-visual presentations causes a negative aftereffect on the current trial. The effects are strong, and very rapid. However, the magnitude of the effects varied directly with audio-visual thresholds: the higher the audio-visual asynchrony thresholds, the greater the adaptation effect, the exact opposite of what we reported for sensory-motor adaption. However, as Van den Burg et al. report, the rapid adaptation they demonstrate is probably very different and independent from the long-term adaptation we are studying here, which does not decline in strength over 60 trials. In addition, the short-term adaptation effect does not occur with visual-tactile stimuli (Van der Burg, Orchard-Mills & Alais, 2014) and may well not occur with sensory-motor perceived simultaneity.
It has recently been shown that adult-like performance on visual-haptic size and orientation discrimination emerges around 8–10 years of age, after the development of both the visual and haptic systems (Gori et al., 2008). Some audio-visual integrative effects also do not develop until about 10 years (Gori et al., 2012; Hillock-Dunn & Wallace, 2012; Tremblay, Champoux, Voss, Bacon, Lepore & Theoret, 2007). The neural mechanisms responsible for the temporal binding seem to be similar for sensory-motor and multisensory associations (Heron et al., 2009; Sugano et al., 2010; Tsujita & Ichikawa, 2012; Watanabe et al., 2011). Recently, Cai et al. (2012) suggested an explanation for motor-sensory recalibration, based on a network of neurons selective for a limited range of different lags between the motor efferent copy and the sensory event. They suggested that this mechanism should work as a meta-modal operator, a network capable of performing the same processing whatever input it receives. The strong correlation between high thresholds and effect of recalibration that we found show that adaptation is not possible without accurate temporal discrimination of events in this short temporal range.
To conclude, we have shown that sensory-motor recalibration does not occur in young children. We suggest that this may result from the late maturation of mechanisms responsible for judging the temporal order between motor and sensory events.
Research highlights.
Sensory-motor recalibration does not occur in children aged 8–11 years.
Recalibration does not occur even after adaptation to a longer temporal delay.
Poor temporal resolution is associated with the lack of recalibration.
Recalibration is also reduced in adults under noisy conditions.
Acknowledgements
We would like to thank the ‘Contubernio D’Albertis’ School of Genova and all the children who participated at this study. We would also like to thank Stefania Saviotti and Marco Jacono for their important contribution to this work. The research presented here has been supported by the European ABBI project (FP7-ICT-2013-10-611452) and by the European Ecsplane project.
References
- Addams R. An account of a peculiar optical phaenomenon seen after having looked at a moving body. London and Edinburgh Philosophical Magazine and Journal of Science. 1834;5:373–374. [Google Scholar]
- Allen P, Wightman F. Spectral pattern-discrimination by children. Journal of Speech and Hearing Research. 1992;35(1):222–233. doi: 10.1044/jshr.3501.222. [DOI] [PubMed] [Google Scholar]
- Allen P, Wightman F, Kistler D, Dolan T. Frequency resolution in children. Journal of Speech and Hearing Research. 1989;32(2):317–322. doi: 10.1044/jshr.3202.317. [DOI] [PubMed] [Google Scholar]
- Bremner AJ, Lewkowicz DJ, Spence C. Multisensory development. Oxford University Press; Oxford: 2012. [Google Scholar]
- Burge J, Ernst MO, Banks MS. The statistical determinants of adaptation rate in human reaching. Journal of Vision. 2008;8(4):20, 1–19. doi: 10.1167/8.4.20. doi: 10.1167/8.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D, Gori M. Multisensory integration develops late in humans. In: Murray MM, Wallace MT, editors. The neural bases of multisensory processes (ch. 18) CRC Press; Boca Raton, FL: 2012. [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Development and the role of internal noise in detection and discrimination thresholds with narrow band stimuli. Journal of the Acoustical Society of America. 2006;120(5 Pt 1):2777–2788. doi: 10.1121/1.2354024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Stetson C, Eagleman DM. A neural model for temporal order judgments and their active recalibration: a common mechanism for space and time? Frontiers in Psychology. 2012;3:470. doi: 10.3389/fpsyg.2012.00470. doi:10.3389/fpsyg.2012.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luca M, Machulla TK, Ernst MO. Recalibration of multisensory simultaneity: cross-modal transfer coincides with a change in perceptual latency. Journal of Vision. 2009;9(12):7, 1–16. doi: 10.1167/9.12.7. doi: 10.1167/9.12.7. [DOI] [PubMed] [Google Scholar]
- Eagleman DM. Human time perception and its illusions. Current Opinion in Neurobiology. 2008;18(2):131–136. doi: 10.1016/j.conb.2008.06.002. doi:10.1016/j.conb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleman DM, Holcombe AO. Causality and the perception of time. Trends in Cognitive Sciences. 2002;6(8):323–325. doi: 10.1016/s1364-6613(02)01945-9. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall; New York: 1993. [Google Scholar]
- Engbert K, Wohlschlager A, Haggard P. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition. 2008;107(2):693–704. doi: 10.1016/j.cognition.2007.07.021. doi:10.1016/j.cognition.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Gori M, Del Viva M, Sandini G, Burr DC. Young children do not integrate visual and haptic form information. Current Biology. 2008;18(9):694–698. doi: 10.1016/j.cub.2008.04.036. doi:10.1016/j.cub.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Gori M, Sandini G, Burr D. Development of visuo-auditory integration in space and time. Frontiers in Integrative Neuroscience. 2012;6:77. doi: 10.3389/fnint.2012.00077. doi:10.3389/fnint.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori M, Tinelli F, Sandini G, Cioni G, Burr DC. Poor visual discrimination of size but not orientation in children with dyskinetic cerebral palsy show: failure to cross-calibrate between senses? Perception. 2011;40:43. [Google Scholar]
- Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nature Neuroscience. 2002;5(4):382–385. doi: 10.1038/nn827. doi:10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. Development of temporal resolution in children as measured by the temporal-modulation transfer-function. Journal of the Acoustical Society of America. 1994;96(1):150–154. doi: 10.1121/1.410474. doi:10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- Hartley DEH, Wright BA, Hogan SC, Moore DR. Age-related improvements in auditory backward and simultaneous masking in 6- to 10-year-old children. Journal of Speech, Language, and Hearing Research. 2000;43(6):1402–1415. doi: 10.1044/jslhr.4306.1402. [DOI] [PubMed] [Google Scholar]
- Heron J, Hanson JVM, Whitaker D. Effect before cause: supramodal recalibration of sensorimotor timing. PLoS ONE. 2009;4(11):e7681. doi: 10.1371/journal.pone.0007681. doi:10.1371/Journal.Pone.0007681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillock-Dunn A, Wallace MT. Developmental changes in the multisensory temporal binding window persist into adolescence. Developmental Science. 2012;15(5):688–696. doi: 10.1111/j.1467-7687.2012.01171.x. doi:10.1111/j.1467-7687.2012.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RJ, Ball AKR, Kay N, Stillman JA, Rosser J. The development of auditory temporal acuity in children. Child Development. 1985;56(3):614–620. doi:10.1111/j.1467-8624.1985.tb00135.x. [PubMed] [Google Scholar]
- Jansen-Osmann P, Richter S, Konczak J, Kalveram KT. Force adaptation transfers to untrained workspace regions in children: evidence for developing inverse dynamic motor models. Experimental Brain Research. 2002;143(2):212–220. doi: 10.1007/s00221-001-0982-8. doi:10.1007/s00221-001-0982-8. [DOI] [PubMed] [Google Scholar]
- Keetels M, Vroomen J. Exposure to delayed visual feedback of the hand changes motor-sensory synchrony perception. Experimental Brain Research. 2012;219(4):431–440. doi: 10.1007/s00221-012-3081-0. doi:10.1007/s00221-012-3081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Borutta M, Topka H, Dichgans J. The development of goal-directed reaching in infants: hand trajectory formation and joint torque control. Experimental Brain Research. 1995;106(1):156–168. doi: 10.1007/BF00241365. [DOI] [PubMed] [Google Scholar]
- Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Experimental Brain Research. 1997;117(2):346–354. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- Machulla TK, Di Luca M, Froehlich E, Ernst MO. Multisensory simultaneity recalibration: storage of the aftereffect in the absence of counterevidence. Experimental Brain Research. 2012;217(1):89–97. doi: 10.1007/s00221-011-2976-5. doi:10.1007/s00221-011-2976-5. [DOI] [PubMed] [Google Scholar]
- Maxon AB, Hochberg I. Development of psychoacoustic behavior – sensitivity and discrimination. Ear and Hearing. 1982;3(6):301–308. doi: 10.1097/00003446-198211000-00003. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. Temporal factors. Journal of Neuroscience. 1987;7(10):3215–3229. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Wallace MT. The neural bases of multisensory processes. CRC Press; Boca Raton, FL: 2012. [PubMed] [Google Scholar]
- Nardini M, Jones P, Bedford R, Braddick O. Development of cue integration in human navigation. Current Biology. 2008;18(9):689–693. doi: 10.1016/j.cub.2008.04.021. doi:10.1016/j.cub.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Rohde M, Ernst MO. To lead and to lag – forward and backward recalibration of perceived visuo-motor simultaneity. Frontiers in Psychology. 2012;3:599. doi: 10.3389/fpsyg.2012.00599. doi:10.3389/fpsyg.2012.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson C, Cui X, Montague PR, Eagleman DM. Motor-sensory recalibration leads to an illusory reversal of action and sensation. Neuron. 2006;51(5):651–659. doi: 10.1016/j.neuron.2006.08.006. doi:10.1016/j.neuron.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Sugano Y, Keetels M, Vroomen J. Adaptation to motor-visual and motor-auditory temporal lags transfer across modalities. Experimental Brain Research. 2010;201(3):393–399. doi: 10.1007/s00221-009-2047-3. doi:10.1007/s00221-009-2047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano Y, Keetels M, Vroomen J. The build-up and transfer of sensorimotor temporal recalibration measured via a synchronization task. Frontiers in Psychology. 2012;3:246. doi: 10.3389/fpsyg.2012.00246. doi:10.3389/fpsyg.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Champoux F, Voss P, Bacon BA, Lepore F, Theoret H. Speech and non-speech audio-visual illusions: a developmental study. PLoS ONE. 2007;2(8):e742. doi: 10.1371/journal.pone.0000742. doi:10.1371/journal.pone.0000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita M, Ichikawa M. Non-retinotopic motor-visual recalibration to temporal lag. Frontiers in Psychology. 2012;3:487. doi: 10.3389/fpsyg.2012.00487. doi:10.3389/fpsyg.2012.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Burg E, Alais D, Cass J. Rapid recalibration to audiovisual asynchrony. Journal of Neuroscience. 2013;33(37):14633–14637. doi: 10.1523/JNEUROSCI.1182-13.2013. doi:10.1523/JNEUROSCI.1182-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Burg E, Orchard-Mills E, Alais D. Rapid temporal recalibration is unique to audiovisual stimuli: no effects for audiotactile or visuotactile stimuli. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-4085-8. (in press) [DOI] [PubMed] [Google Scholar]
- von Hofsten C. Structuring of early reaching movements: a longitudinal study. Journal of Motor Behavior. 1991;23(4):280–292. doi: 10.1080/00222895.1991.9942039. [DOI] [PubMed] [Google Scholar]
- Walker KMM, Hall SE, Klein RM, Phillips DP. Development of perceptual correlates of reading performance. Brain Research. 2006;1124:126–141. doi: 10.1016/j.brainres.2006.09.080. doi:10.1016/j.brainres.2006.09.080. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shinohara S, Shimojo S. Mirror adaptation in sensory-motor simultaneity. PLoS ONE. 2011;6(12):e28080. doi: 10.1371/journal.pone.0028080. doi:10.1371/journal.pone.0028080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Development. 1989;60(3):611–624. doi:10.1111/j.1467-8624.1989.tb02742.x. [PubMed] [Google Scholar]