Abstract
Dietary lipids serve as important sources of energy and essential fatty acids for aquatic animals. Sources of animal and plant oils are increasingly limited as well as expensive, and dietary requirements associated with the inclusion of these oils must be carefully evaluated to facilitate sustainable and affordable formulations. In this study, we investigated quantities of menhaden oil (MO) with and without soybean lecithin or soybean oil (SO) to determine appropriate levels for optimal somatic growth for pre-gonadal juvenile Lytechinus variegatus. We prepared semi-purified diets that varied in neutral lipid content (0, 2, 4, or 8% dry matter) and soy lecithin (0 or 2%) and exchanged lipids reciprocally with purified starch while holding constant all other nutrients. We maintained laboratory-reared juvenile L. variegatus (average initial wet weight 82 ± 0.7 mg, mean ± SE , n = 9 treatment−1) in recirculating seawater systems and fed each daily a sub-satiation ration for five weeks. We assessed wet weights and test diameters every two weeks and at the end of the experiment (5 wk). Level of MO with or without soybean lecithin did not significantly affect wet weight gain; however, increasing levels of SO in the diet reduced wet weight gain and dry matter production efficiency and increased feed conversion ratio. Dry gut weight was positively correlated with level of MO. Lipid level in the gut increased with increasing dietary lipid level, regardless of source. These data suggest the composition of the SO is inhibitory for either nutrient absorption or metabolic processes associated with growth at this life stage. Diets containing total lipid levels of approximately 5 to 6% that include sources of n-3 fatty acids may support optimal growth for pre-gonadal juvenile L. variegatus.
Keywords: echinoid, nutrition, lipid, fish oil, soybean oil, production efficiency, Lytechinus variegates
1. Introduction
Feed costs can comprise 50 to 70% of the production costs of an aquaculture operation (Rana et al. 2009). With the cost of lipids (oils) increasing more than 250% over the past ten years due to rising fuel costs and decreased availability, lipids are now one of the most expensive feed ingredients used for aquafeeds (Shamshak and Anderson, 2008; Rana et al. 2009). Marine oils, which contain long chain polyunsaturated fatty acids (LC-PUFA), are commonly used in feeds for marine invertebrates such as crustaceans because their nutritive value is higher than that of n-6 polyunsaturated fatty acids (PUFAs) of vegetable oils (D’Abramo, 1997). Fish oil availability, however, is dependent on the success of marine fisheries that are unsustainable as historically utilized (Pauly et al. 2002; Shamshak and Anderson, 2008; Rana et al. 2009). Alternative plant lipid sources, including soybean oil, corn oil, linseed oil, rapeseed oil, and palm oil, are being investigated as potential replacements for fish oil, either partially or entirely, in aquafeed formulations (Rana et al. 2009; Gunstone, 2011). One of the most effective methods for reducing feed costs resides in minimizing the dietary lipid levels without compromising survival, growth, and normal physiological function of the animal.
Over the past twenty years, sea urchin culture technology (i.e., husbandry, reproduction, and diet formulation) has grown in an effort to improve the supply of sea urchin (Watts et al. 2013). For the sea urchin, Lytechinus variegatus, triacylglycerols and phospholipids are highly digestible components of the diet, and apparent lipid digestibilities can range from ca. 78 to more than 90% (Gibbs and Watts 2004; Hammer, 2006; Gibbs et al. 2009). Sea urchins are capable of storing nominal amounts of lipids in the gut, gonad, and body wall (Giese, 1966; reviewed by De Ridder and Jangoux, 1982; Gibbs et al. 2009). When starved, sea urchins can use these lipid stores (Lawrence et al. 1966), and quickly replenish these stores upon re-feeding (Bishop and Watts, 1992). While lipids may be important nutrients for sea urchins, dietary lipid levels > 6.3% (as fed) negatively affect growth in juvenile and adult L. variegatus (Gibbs et al. 2009; Hammer et al. 2010).
Minimizing the level of lipids in the diet may reduce feed costs, but the quality of dietary lipid must meet essential fatty acid requirements, which are not fully known for sea urchins. In the green sea urchin, Strongylocentrotus droebachiensis, dietary fatty acid profiles of 18:2n-6 and 18:3n-3 were observed in sea urchin tissues as 20:4n-6 and 20:5n-3 suggesting sea urchins may be capable of elongating dietary PUFAs to LC-PUFAs (Castell et al. 2004; Gonzalez-Duran et al. 2008). Hammer et al. (2010) reported marine oil (but not soybean oil) at a low level is required in the diet for optimal growth in small adult L. variegatus, suggesting the quality of dietary lipid (fatty acid profiles) may be more important for growth than overall dietary energy levels provided by lipids. Marsh et al. (2013) further confirmed the minimal requirements for lipids as an energy source, indicating carbohydrates are utilized primarily as the source of energy in sea urchins.
To date, dietary studies for sea urchins focus primarily on growth phases (small adult or adult stages) during which gonad production occurs. During pre-gonadal juvenile development, dry matter accumulates primarily in the calcified tissues of the test (body wall) and Aristotle’s lantern (the feeding apparatus), with lesser allocation to the gut; however, nutrient allocation to storage cells (nutritive phagocytes) and for gamete production in the gonads is not yet initiated. Previous work in juvenile sea urchins allowed overlap of the strictly somatic growth period with early production of gonad tissue (Kennedy et al. 2007; Gibbs et al. 2009). Consequently, the optimal quantity and quality of dietary lipids (including neutral lipids, phospholipids, cholesterol, and carotenoids) to be provided to pre-gonadal juvenile sea urchins in culture is very limited. In this study, we evaluated the contribution of dietary neutral lipids in the presence and absence of phospholipid in their ability to support growth and growth efficiencies in pre-gonadal juvenile L. variegatus. Menhaden oil is commonly provided as a source of n-3 fatty acids and has been used to support gonad production in adult L. variegatus (Hammer et al. 2010); however, its reduced availability and high cost relative to more available vegetable oils makes it unlikely as a sustainable source of dietary lipid. Soybean oil is a common alternative but varies in having higher portions of n-6 fatty acids and lower n-3 than menhaden oil. It is not known if soy oil can be used as a replacement for menhaden oil in sea urchins.
In addition to varying oils, phospholipid (soy lecithin) was added in some diets at a level shown previously to support juvenile growth of L. variegatus (Gibbs et al. 2009). Inclusion of dietary phospholipid has been suggested to improve growth and facilitate lipid transport in larval fish and the green sea urchin, Strongylocentrotus droebachiensis (Lu et al. 2008; Gonzalez-Duran et al. 2008). Supplementation of diets with soybean lecithin, primarily composed of phosphatidylcholine, may provide other nutrients such as phosphorous and choline in addition to fatty acids. To control for the contribution of fatty acids from lecithin in diets not receiving lecithin, soy oil was incorporated at levels reflecting the contribution of fatty acids from soy lecithin. Since multiple variables were altered among the various dietary comparisons, multi-variable general linear models were used to calculate parameter estimates to assess the contribution of the component oils to growth and body composition outcomes.
2. Materials and Methods
2.1 Collection of adults and culture of juveniles
Adult Lytechinus variegatus were collected in February 2009 from Saint Joseph Bay, FL (30°N, 85.5°W) and transported in aerated, insulated containers to the University of Alabama at Birmingham. Sea urchins were held in 80 L tanks within an ca. 4000 L recirculating, synthetic seawater system (Instant Ocean Sea Salt, 32 ± 1 ‰, mean ± SD) at constant photoperiod and temperature (12h light: 12h dark, 24 ± 1°C, mean ± SD). They were fed a formulated brood stock diet ad libitum (Table 1 and Suppl. Table 1). Adults were maintained for 12 wk prior to spawning. Spawning, fertilization, and larval culture procedures were conducted according to the methods described by Heflin et al. (2013). Approximately fifteen days post-fertilization, newly-metamorphosed sea urchins were transferred to a 160 L tank containing cultures of the benthic diatom Amphora helenensis, which supports growth and development in newly-metamorphosed L. variegatus (Powell et al. 2008). Metamorphosed sea urchins were maintained on the diatom culture until ca. 1 to 1.5 mm test diameter (ca. 4 wk). Juveniles were then transferred to 8 L aquaria and were fed a live, mixed-taxa algal biofilm (MTAB) that supports growth and survival of juvenile sea urchins (Taylor et al. 2009).
Table 1.
Calculated proximate nutrient levels on an “as fed” basis for the brood stock maintenance diet and the base experimental diet. Nutrient profiles for ingredients**, provided by vendors, were used to calculate nutrient levels of diets.
| Diet Nutrient Level | ||
|---|---|---|
| Nutrients | Brood Stock Maintenance Diet |
Base Experimental Diet |
| Crude Protein* | 28.53% | 27.75% |
| Carbohydrate | 26.76% | 31.34% |
| Crude Fiber* | 4.28% | 2.5% |
| Total Ash* | 26.99% | 23.53% |
| Crude Fat* | 7.72% | 3.78% |
| Cholesterol | 0.34% | 0.32% |
| Carotenoids | 1.70% | 1.70% |
| Mineral mix | 21.76% | 21.76% |
| Vitamin premix | 0.70% | 0.70% |
| Binder and antioxidant | 4.20% | 4.20% |
Empirically derived levels were determined by Eurofins Scientific, Inc. for the base experimental diet only.
Actual ingredients are proprietary, but all diets contain approximately 4% animal ingredients, 28% marine ingredients, and 29.1% plant ingredients.
2.2 Growth trial
2.2.1 Initial measurements and culture system
Several hundred juveniles were maintained in six 8 L, static renewal aquaria (20% water exchange week−1; 32 ± 1 ‰ salinity; 22 ± 1°C, means ± SD; 12h light:12h dark), until reaching a size appropriate for entry into the growth trial (ca. 5 mm test diameter, 70 – 100 mg wet weight). When individual juveniles reached the appropriate entry size, the individuals were randomly assigned to one of fifteen dietary treatments (n = 9 individuals dietary treatment−1).
Juveniles entering the study were placed individually into plastic, cylindrical cages (ca. 8.5 cm diameter, 25 cm high, with 3 mm open mesh on sides, a 3 mm open mesh bottom secured by plastic cable-ties, and a 2 mm open mesh circle over-laid on the 3 mm open mesh bottom). The mesh enclosures were fitted into 8.7 cm ID PVC couplings allowing the floor of the mesh enclosure to be ca. 5.5 cm from the bottom of the raceway. Each coupling was fitted on the bottom with three small Tygon® spacers (ca. 0.5 cm thick) to allow water circulation underneath the enclosures. Each of four raceways (235 cm × 53 cm × 31 cm, L × W × H) were filled to capacity with sixty cages to ensure similar water flow dynamics among raceways and throughout the study. Juveniles (n = 135) were placed in cages distributed randomly within the raceway. Cages were coded so that each individual could be tracked over the course of the study. A 160 × 23 cm (L × H) center baffle was fixed in the center of each raceway, and recirculating water flow was achieved by an in-line utility pump (Supreme® Mag Drive Utility Pump, 700 gallons of water hr−1) whereby saltwater on one side of the baffle passed through a mechanical and biological filter, respectively. The mechanically and biologically treated saltwater was then returned to the raceway on the opposite side of the baffle, creating a current with a flow rate of approximately 9.7 – 12.6 cm s−1 (system schematic provided in Heflin et al. 2013). Water depth was maintained at 15 cm. To minimize experimental variability associated with potential raceway effects, cages were transferred to a different raceway each week and relocated within the raceway to prevent bias due to cage position. Every 2 weeks, cages were cleaned to remove possible bacterial growth.
Uneaten feed, if present, was removed by siphon, and feces were removed by siphon each week. Water removed during siphoning was exchanged with fresh synthetic seawater at a rate of 10% of the volume week−1. Culture conditions were maintained at 32 ± 2 ‰, 22 ± 3°C, means ± SD and 12h light: 12h dark photoperiod throughout the duration of the experiment. Total ammonia nitrogen, nitrite, nitrate, and alkalinity levels were checked weekly using saltwater test kits from Aqua Pharmaceuticals, LLC (Malvern, PA, USA; ammonia, nitrogen, nitrite and nitrate) and La Motte Company (Chestertown, MD, USA; alkalinity). A YSI pH10 Pen (YSI Incorporated, Yellow Springs, Ohio, USA) was used to measure the pH level weekly.
2.2.2 Diet preparation and feeding
Using a base, semi-purified diet (Suppl. Table 1) containing no added oils (0% supplemented oil), we developed 14 additional experimental diets with different levels and sources of supplemental dietary oils with or without soy lecithin. In six diets, we added either 2, 4, or 8% supplemented menhaden oil (MO) or soybean oil (SO) to the base diet. We developed four diets that contained the four levels of MO (0, 2, 4, or 8% supplemented) plus 2% supplemented soybean lecithin. To evaluate the potential influence of the fatty acid complement of soybean lecithin on growth, we developed four diets that contained the four levels of MO (0, 2, 4, or 8% supplemented) plus 1.33% supplemented SO (equivalent to the fatty acid component of the supplemented lecithin). We prepared the diets as follows. Soybean lecithin (phospholipid) was added using Aqualipid 95 soybean lecithin (Central Soya, Fort Wayne, IN; analysis reported by Gibbs et al. 2009). Lipids were reciprocally exchanged (by weight) with purified starch; all other nutrients were held constant. Semi-purified and purified ingredients (Suppl. Table 1) were blended with a twin shell dry blender (Patterson-Kelley Co., East Stroudsburg, PA, USA) for 10 min and then mixed in a Hobart mixer (Model A-200, Hobart Corporation, Troy, OH, USA) for 40 min. De-ionized water (500 mL kg−1 dry feed) with alginate was then added to the dry ingredients and mixed an additional 10 min to achieve a mash consistency appropriate for extrusion. Diets were cold-pressed at room temperature (26 to 28°C) using a meat chopper attachment (Model A-200, Hobart Corporation, Troy, OH, USA) fitted with a 3.2 mm die. Moist, rod-like pellets were dried in a forced air oven at 35°C to a moisture content of 8–10%, placed in plastic bags and stored at 4°C. Proximate composition of the experimental diets was determined by Eurofins Scientific Inc. (Des Moines, Iowa, USA) (Table 1 and Suppl. Table 1), and total energy content of the feeds (cal g−1) was determined by micro-bomb calorimetry (Parr Instrument Company, Moline, IL, USA) (Table 2).
Table 2.
Calculated purified plant starch levels, on an “as fed” basis, of final diet treatments containing different lipid sources and levels. Total lipid (%) and total energy (kcal/g) mean values were empirically determined and protein: energy ratios were calculated based on empirical levels of protein and total energy.
| Lipid Source | Dietary Level (%) |
Purified Plant Starch (%) |
Total Lipid (%) |
Total Energy (kcal/g) |
Protein: Energy Ratio |
|---|---|---|---|---|---|
| No added oils | 0 | 19.1 | 3.78 | 3.45 | 80.4 |
| Menhaden Oil | 2 | 17.1 | 5.74 | 3.58 | 77.5 |
| 4 | 15.1 | 7.82 | 3.69 | 75.2 | |
| 8 | 11.1 | 11.6 | 3.94 | 70.4 | |
| Menhaden Oil + | 0 | 17.1 | 5.08 | 3.57 | 77.9 |
| 2% Phospholipid | 2 | 15.1 | 7.04 | 3.67 | 75.6 |
| 4 | 13.1 | 8.95 | 3.79 | 73.2 | |
| 8 | 9.1 | 12.8 | 4.04 | 68.8 | |
| Menhaden Oil+ | 0 | 17.8 | 5.09 | 3.60 | 77.1 |
| 1.33% Soy Oil | 2 | 15.8 | 7.16 | 3.72 | 74.5 |
| 4 | 13.8 | 9.07 | 3.85 | 72.2 | |
| 8 | 9.8 | 13.0 | 4.13 | 67.3 | |
| Soy Oil | 2 | 17.1 | 5.81 | 3.63 | 76.4 |
| 4 | 15.1 | 7.85 | 3.83 | 72.4 | |
| 8 | 11.1 | 11.8 | 4.10 | 67.7 |
Each individual sea urchin was fed a sub-satiation ration daily as a percentage of mean body weight to prevent compensatory feed intake. Providing a weighed ration also allowed feed intake to be quantified during the study. From pilot studies in our lab, we determined that a daily ration of 2.5 to 5% of the body weight using formulated diets sustained growth in juvenile sea urchins while preventing ad libitum feeding (unpublished data). The 0 to 2 wk ration was 5% of the mean stocking wet weight (mean juvenile weight = 85 mg) with a < 5% margin of error (4.3 ± 0.1 mg fed once day−1). The 2 to 4 wk ration was 5% of the mean wet weight of all individuals at the end of the first 2 wk period (18.2 ± 0.2 mg fed once day−1). The 4 to 5 wk ration was 2.5% of the mean wet weight of all individuals at the end of the first 4 wk period (34.7 ± 0.3 mg fed once day−1). Despite the increase in the daily ration at these time points, sub-satiation was maintained for a majority (> 90%) of individuals. Twenty-four hours after feeding, individual cages were checked for the presence or absence of food. If food was present, the size fraction of food remaining for each individual was estimated visually and recorded as estimated uneaten feed, and the uneaten food was then removed by siphon. Total feed intake (mg individual−1, as fed) over the 5 wk study period was calculated by total feed proffered (mg) – estimated total uneaten feed (mg).
2.2.3 Growth and production
The wet weight of each individual was determined at initial stocking (Week 0) and at 2, 4, and 5 wk. Individuals were blotted on paper towels for 10 to 20 s to remove excess water and were weighed on a Mettler Toledo PG503-S balance (Mettler-Toledo International, Inc., Columbus, OH, USA) to the nearest mg. Each individual was also photographed with a reference ruler using a Nikon® D70 digital camera. Images were analyzed using the image analysis software NIS Elements – 2.30 (Nikon Instruments Inc., Melville, NY, USA) to determine test diameters. Wet weight gain over the 5 wk study period was calculated by wet weight final (g) – wet weight initial (g). The feed conversion ratio (FCR) was calculated by total feed intake (mg, as fed)/ wet weight gain (mg).
At the end of 5 wk, each individual was weighed and photographed as described previously and six of the nine sea urchins treatment−1 were dissected. A circular incision around the peristomial membrane allowed the oral surface (including Aristotle’s lantern) of the sea urchin to be separated from the body. The esophagus was cut at the Aristotle’s lantern, and the lantern was separated from the oral surface of the test (body wall). The gut (esophagus, stomach, and intestine) was then removed from the coelomic cavity and rinsed in clean culture water to remove feed pellets. The coelomic cavity was investigated for the presence of measurable gonad tissue, but no notable gonad tissue was observed in any of the individuals or diet treatments. The complete test with spines, Aristotle’s lantern, and gut were dipped in de-ionized water to remove excess surface salt, blotted on paper towels, and each transferred to pre-weighed aluminum weigh pans. Organs were dried to constant dry weight (ca. 72 hr) in a forced air oven at 50°C.
A sub-sample of juveniles (n = 10) was randomly selected from the lab-reared population to serve as the initial dissection. Wet weights and test diameters were determined as described previously, and whole individuals were then dipped in de-ionized water to remove excess salt, placed in pre-weighed aluminum weigh pans, and dried in a forced air oven at 50°C to constant dry weight. The average total dry weight derived from the initial dissection was used for later calculations of dry matter production. Estimated total dry matter production was calculated by final dry weight (mg) – average initial dissection dry weight (mg). Dry matter production efficiency (PE) was calculated by estimated total dry matter production (mg)/ dry feed intake (mg) × 100%.
2.3 Lipid analysis
Total lipid content of the dry gut tissue was determined empirically using methods modified from Folch et al. (1957). To yield samples of sufficient weight for the lipid extraction procedure (ca. 50 mg), dry gut tissues were combined within a treatment. Gut tissues were placed in 25 mL of chloroform: methanol (2:1, v:v) and were left at room temperature for 24 hrs to facilitate extraction of lipid prior to heating in the 60°C water bath. After filtering through Whatman 541 filter paper, the crude lipid extracts were washed with 4 mL of 0.9% NaCl, centrifuged, and the 0.9% NaCl/methanol layer was removed. To ensure salts were sufficiently removed from the crude extract, an additional rinse with 4 mL of Milli Q filtered freshwater was conducted, samples centrifuged, and water layer removed. Samples were dried under a nitrogen stream while heated in a 50°C water bath. Samples were then dissolved in chloroform, transferred to pre-weighed shell vials and dried under a stream of air. Shell vials were placed in a vacuum desiccator overnight before weighing.
2.4 Statistics
Initial wet weights and diameters were compared using one-way analysis of variance (ANOVA). Due to the number of independent variables involved, General Linear Models were employed to mathematically model the information present in the dataset. As a result, information was maximized related to the effects of lipid sources on growth and production parameters and pair-wise comparisons among the 15 diet treatments were not necessary. The “glm” function was used in R version 2.12.1 (The R Foundation for Statistical Computing) to create general linear models to evaluate associations between dependent variables (outcomes) and independent variables (predictors). Each outcome associated with organismal growth, feed intake and efficiency, and organ weights was modeled with one or more of the following predictor variables: MO level, SO level, phospholipid level, total energy content, protein: energy ratio, and total lipid level. Given the strong effect that Age of the sea urchin when entered into the study has upon growth rates (Heflin et al. 2013), age as both a linear and quadratic effect were included as possible covariate for all outcomes except gut lipid level due to the pooling of samples. For outcomes dry test weight, dry lantern weight, and dry gut weight, total weight was always included as a covariate to correct for differences in total size. Non-significant predictor variables (those with a p-value > 0.1) were removed from the model to prevent overfitting. Parameter estimates were recorded for predictors with a p-value less than 0.1. The “glm.diag.plots” function in the library “boot” was used to confirm normality and homogeneity of variance of the residuals for all data. Variables were log transformed if necessary to ensure normality of residuals. Main effects plots of percent weight gain were generated to visualize potential trends related to lipid source (MO or SO) and supplemented lipid level.
3. Results
3.1 Water quality
Parameters of water quality were maintained as follows: salinity = 30.8 to 33.8‰, temperature = 19.7 to 25.2°C, pH = 8.2 to 9.1, alkalinity = 140 to 392 mg L−1, total ammonia nitrogen and nitrite = 0 to 0.02 mg L−1, nitrate = 10 to 80 mg L−1.
3.2 Growth
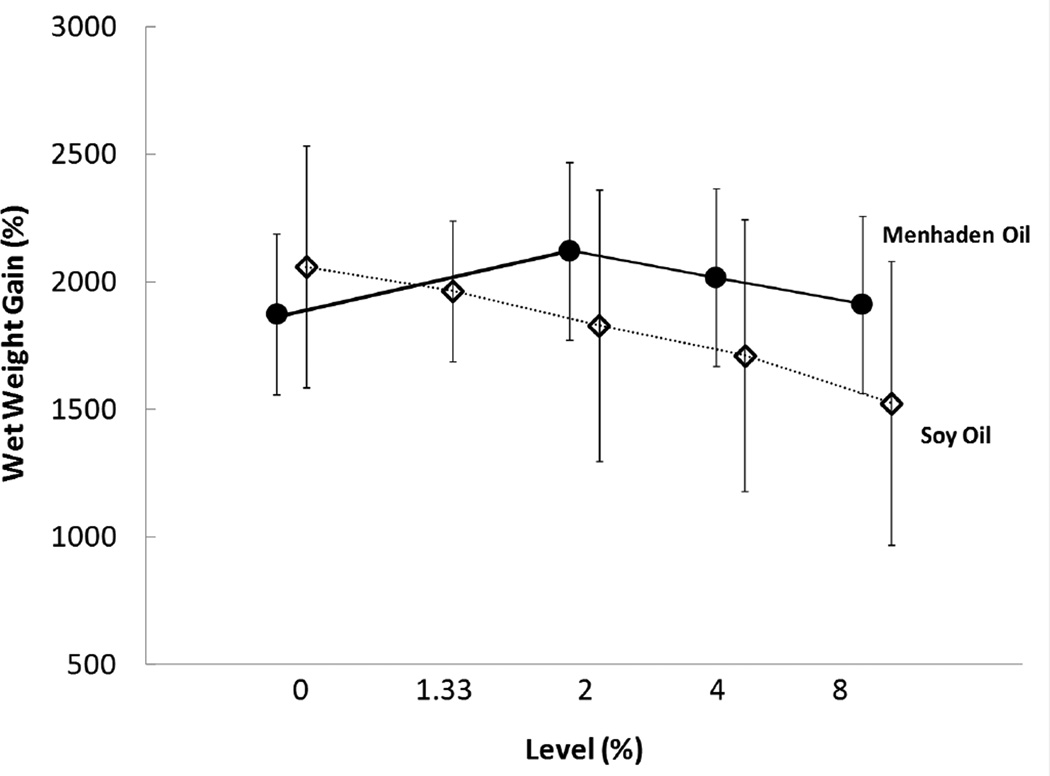
No significant differences were observed for initial wet weight (0.082 ± 0.01 g, mean ± SD, n = 9 treatment−1; F14, 120 = 0.47, p = 0.95) or initial test diameter (5.7 ± 0.3 mm, mean ± SD, n = 9 treatment−1; F14, 119 = 0.52, p = 0.92) among the treatment groups at Week 0 (Table 3). Wet weight gain exceeded 1400% among all treatments for the 5 wk study period (Figure 1) and survival for all treatments was 100%. As growth rates of newly-metamorphosed urchins are highly variable (Heflin et al. 2013), the time elapsed between the first individual and last individual entering the growth trial was 116 d. For simplicity, we termed day of entry in the study “age.” We found age of the individual was a significant predictor variable for nearly all outcome variables tested (Table 4). Thus, we included age in all applicable regression models. Neither supplemented MO nor the addition of phospholipid significantly affected weight gain. Supplemented SO negatively affected growth. Wet weight gain decreased as levels of dietary SO increased (Figure 1). Over the range of SO tested (0 to 8% of diet, as fed), model parameter estimates for growth indicated that for every 1% increase in dietary SO, wet weight gain decreased by 68 mg and test diameter gain decreased by 0.3 mm (Table 4).
Table 3.
Wet weights (g) and test diameters (mm) of juvenile sea urchins fed one of fifteen dietary treatments that differed in lipid source and level.
| Wet Weight (g) | Diameter (mm) | ||||
|---|---|---|---|---|---|
| Lipid Source | Dietary Level (%) |
Week 0 | Week 5 | Week 0 | Week 5 |
| No added oils | 0 |
0.079 ± 0.01 | 1.59 ± 0.50 | 5.61 ± 0.23 | 15.5 ± 1.98 |
| Menhaden Oil | 2 | 0.082 ± 0.01 | 1.92 ± 0.76 | 5.64 ± 0.26 | 16.4 ± 2.65 |
| 4 | 0.081 ± 0.01 | 1.76 ± 0.43 | 5.69 ± 0.20 | 15.8 ± 1.46 | |
| 8 | 0.082 ± 0.01 | 1.74 ± 0.74 | 5.73 ± 0.26 | 15.6 ± 2.54 | |
| Menhaden Oil + | 0 | 0.083 ± 0.01 | 1.81 ± 0.65 | 5.73 ± 0.35 | 15.6 ± 2.14 |
| 2% Phospholipid | 2 | 0.080 ± 0.01 | 1.81 ± 0.80 | 5.70 ± 0.21 | 15.8 ± 2.91 |
| 4 | 0.084 ± 0.01 | 1.86 ± 0.68 | 5.74 ± 0.23 | 16.2 ± 2.13 | |
| 8 | 0.081 ± 0.01 | 1.42 ± 0.67 | 5.71 ± 0.23 | 14.4 ± 2.59 | |
| Menhaden Oil + | 0 | 0.081 ± 0.01 | 1.72 ± 0.59 | 5.67 ± 0.23 | 15.6 ± 2.30 |
| 1.33% Soy Oil | 2 | 0.086 ± 0.01 | 1.73 ± 0.60 | 5.84 ± 0.26 | 15.8 ± 2.02 |
| 4 | 0.081 ± 0.01 | 1.59 ± 0.66 | 5.65 ± 0.33 | 15.1 ± 2.57 | |
| 8 | 0.084 ± 0.01 | 1.73 ± 0.69 | 5.79 ± 0.18 | 15.7 ± 2.25 | |
| Soy Oil | 2 | 0.082 ± 0.01 | 1.57 ± 0.66 | 5.65 ± 0.24 | 15.0 ± 2.33 |
| 4 | 0.084 ± 0.01 | 1.49 ± 0.54 | 5.78 ± 0.31 | 14.7 ± 2.11 | |
| 8* | 0.084 ± 0.01 | 1.33 ± 0.39 | 5.71 ± 0.24 | 14.1 ± 1.50 | |
Values represent means ± SD (n = 9; n = 8).
Figure 1.
Main effects plot of mean wet weight gain (%) for juvenile sea urchins fed diets containing menhaden oil (closed circles) or soy oil (open diamonds). Values represent means for all individuals fed a particular level of menhaden oil or soy oil; thus n = 8 to 64. Error bars represent 95% confidence intervals.
Table 4.
Associations between dependent variables (outcomes) and independent variables (predictors) were evaluated using general linear models: y = βX + e. Model parameter estimates (β) for the outcomes of growth, production, and efficiency measurements are presented.
| Menhaden Oil (%) |
Soy Oil (%) |
Phospholipid (%) |
Total Energy (kcal/g) |
Age (day) |
Age2 (day squared) |
|
|---|---|---|---|---|---|---|
| Wet Weight Gain (g) | - | −0.068*** | - | - | −0.00506 | −0.000066* |
| Diameter Increase (mm) | - | −0.300*** | - | - | −0.00119 | −0.0003903*** |
| Feed Intake, As Fed (mg) | - | - | - | - | 0.88645 | −0.010166* |
| Feed Conversion Ratio a | - | 0.0337** | - | 0. 23+ | −0.0012 | 0.000083*** |
| Dry Matter Production (g) | - | −0.0109*** | - | - | −0.00125+ | −0.000016** |
| Production Efficiency (%) | - | −3.2*** | −3.48* | - | −0.71*** | - |
| Dry Test Weight (g) b | 0.004325+ | 0.0121** | - | - | - | - |
| Dry Lantern Weight (g) b | - | - | - | - | −0.000023* | - |
| Dry Gut Weight (g) b | 0.00034*** | - | - | - | - | - |
| Gut Lipid Level (%) c | 1.6082 | 1.4577 | - | - | ND | ND |
Log Transformed
Corrected for total size
Effect of age could not be evaluated because samples were combined; ND = not determined.
Associated p values for model parameter estimates being significantly different than 0 are included as = p < 0.1,
= p < 0.05
= p < 0.01
= p < 0.001.
3.3 Feed intake, efficiencies, and production
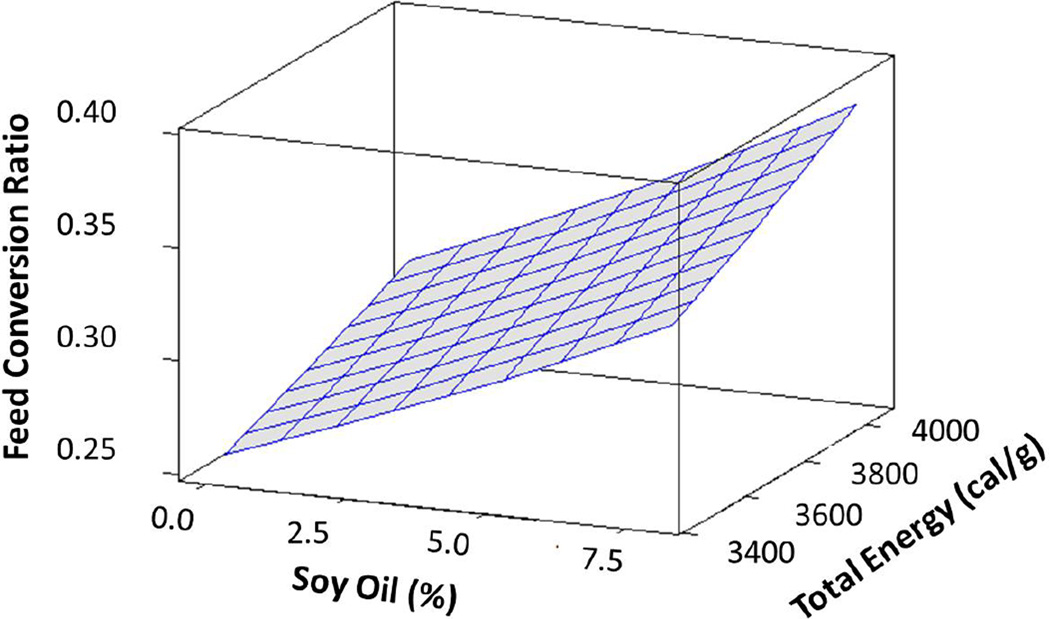
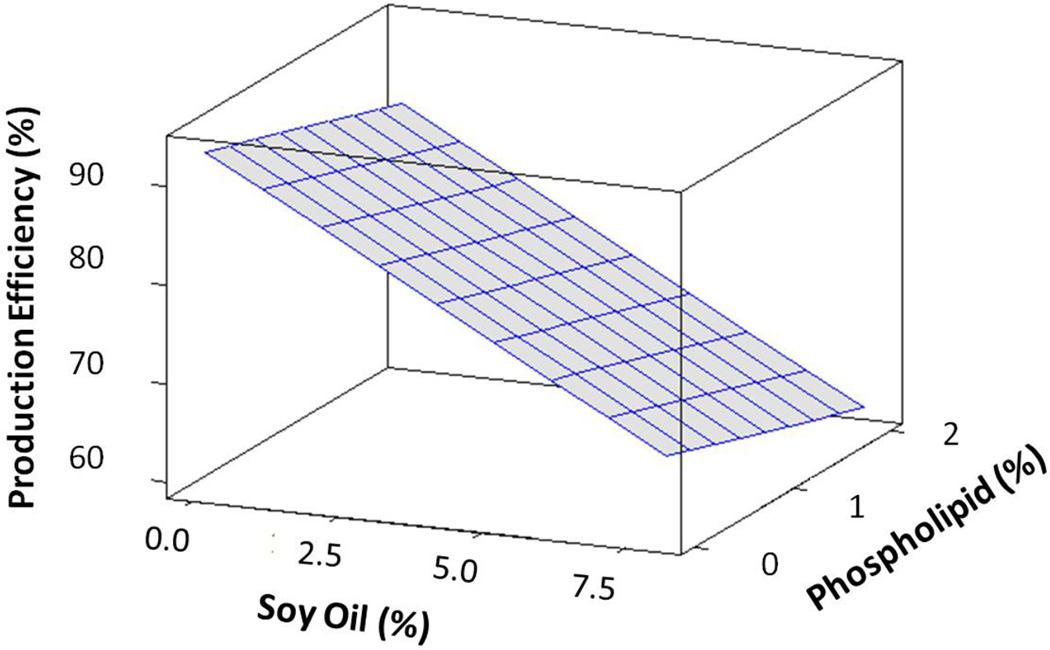
Feed intake (as fed) was similar among all dietary treatments (Suppl. Table 2), and instances where feed was remaining after 24 hr were fewer than 10%. Supplemented MO did not affect FCR or PE. Increasing the level of dietary SO and the associated increase in total energy level of the diet led to an increase in the FCR (Figure 2). Model parameter estimates indicated feed conversion ratio increased by 0.034 for a 1% increase in dietary SO, while a 1 kcal g−1 increase in total dietary energy increased feed conversion ratio by 0.23 (Table 4). Dietary SO negatively affected dry matter production, and model parameter estimates indicated a 1% increase in SO decreased dry matter production by 11 mg (Table 4). The presence of SO or phospholipid in the diet negatively affected PE (%) (Figure 3). Model parameter estimates indicated a 1% increase in dietary SO decreased PE by 3.2% and a 1% increase in dietary phospholipids decreased PE by 3.5% (Table 4).
Figure 2.
Surface plot of age-adjusted feed conversion ratios based on general linear models with dietary soy oil level (%) and total energy (cal/g) of the diet as predictor variables.
Figure 3.
Surface plot of age-adjusted production efficiencies based on general linear models with dietary soy oil level (%) and dietary phospholipid level (%) as predictor variables.
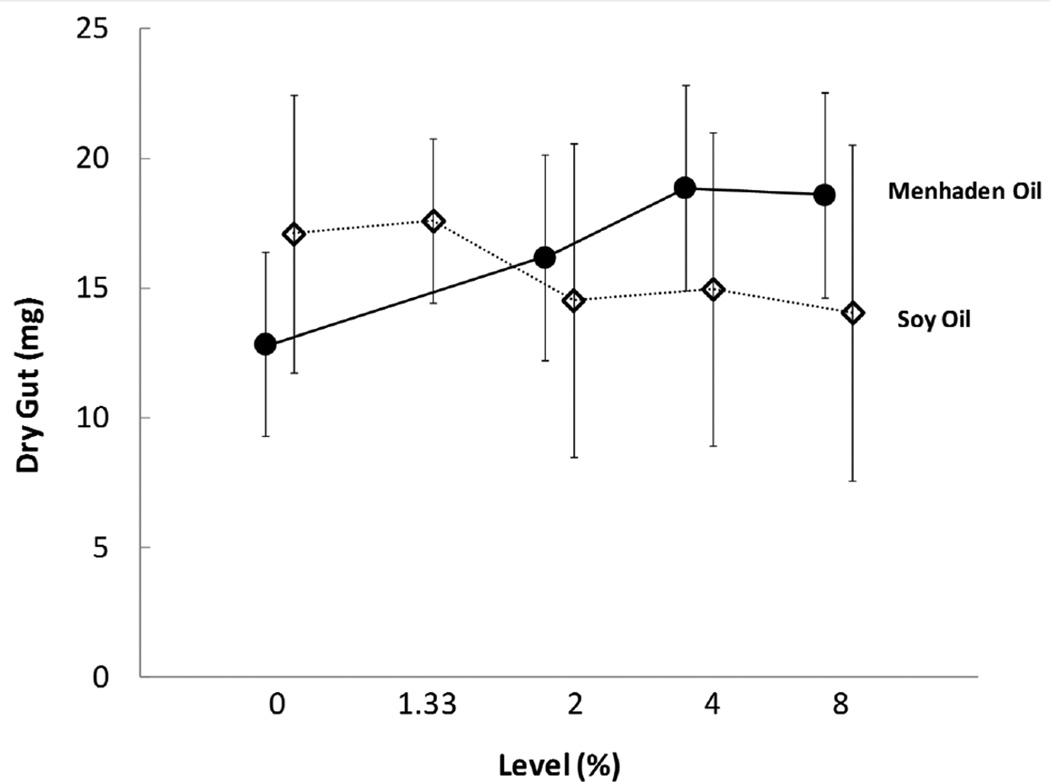
After adjusting dry test weight for total body size, both MO and SO were positively associated with dry test weight. Model parameter estimates indicated a 1% increase in dietary MO increased dry test weight by 4 mg, and a 1% increase in dietary SO increased dry test weight by 12 mg (Table 4). Aristotle’s lantern dry weights were similar for individuals among all diet treatments (Suppl. Table 3). Dietary MO but not SO affected dry gut weight (Figure 4) with a 1% increase in MO resulting in a 0.34 mg increase in gut dry weight (Table 4). Gut lipid level (%) increased with increasing levels of MO or SO (Suppl. Table 3). Based on model parameter estimates, a 1% increase in MO resulted in a 1.61% increase in gut lipid level while a 1% increase in SO resulted in a 1.46% increase in gut lipid level (Table 4).
Figure 4.
Main effects plot of mean dry gut (mg) for juvenile sea urchins fed dietary treatments containing menhaden oil (closed circles) or soy oil (open diamonds). Values represent means for all individuals fed a particular level of menhaden oil or soy oil; thus n = 4 to 40. Error bars represent with 95% confidence intervals.
4. Discussion
4.1 Growth
Parameters of water quality were maintained within favorable ranges and within safe levels for nitrogen (no negative effect on growth) according to Basuyaux and Mathieu (1999). Growth rates observed in the current study were similar to those reported for juvenile Lytechinus variegatus fed formulated feeds (Taylor et al. 2009; Gibbs et al. 2009).
Under the conditions of this study, increasing levels of dietary MO, with or without 2% phospholipid, did not significantly alter organismal weight gain and related outcomes in juvenile Lytechinus variegatus. In contrast, supplemented dietary SO reduced outcomes related to growth. Increased levels of dietary lipids from SO (Hammer et al. 2010), soybean lecithin (Gibbs et al. 2009), or combinations of menhaden, corn and linseed oils (Kennedy et al. 2007) have been shown to reduce growth in small adult L. variegatus, juvenile L. variegatus, and juvenile Strongylocentrotus droebachiensis, respectively. Feeding rates in these studies were ad libitum and while not quantified in the studies with juvenile urchins (Gibbs et al. 2009; Kennedy et al. 2007), feed intake was not attributed to reduced growth. In the current study, juveniles were fed a sub-satiation ration, and overall feed intake was consequently similar across all treatments. Thus, differences in growth could not be attributed to differential feed intake. Consequently, these data suggest that the quality of the food oil, representing the fatty acid content within, affects growth outcomes.
The quality of food oil is substantially different between MO and SO. The fatty acid composition of MO contains long chain polyunsaturated fatty acids (20:5n-3 and 22:6n-3) while SO contains polyunsaturated fatty acids, 18:2n-6 (linoleic acid) with a much smaller fraction of 18:3n-3 (linolenic acid). The sea urchins Strongylocentrotus droebachiensis, Paracentrotus lividus, and Psammechinus miliaris are capable of synthesizing LC-PUFA from dietary PUFA precursors:18:2n-6 to 20:4n-6 (arachidonic acid) and 18:3n-3 to 20:5n-3 (eicosapentaenoic acid) (Bell et al. 2001; Castell et al. 2004; Liu et al. 2007a,b; Gonzalez-Duran et al. 2008), indicating the activity of Δ6 and Δ5 desaturases. The relation of growth outcomes to fatty acid composition in sea urchins requires further analysis.
When accounting for overall animal size, test dry weights increased as dietary lipid level increased, indicating continued growth of this calcified tissue. The test or body wall of the sea urchin can serve as a depot for lipid storage (Giese, 1966; reviewed by De Ridder and Jangoux, 1982). The relative increase in test dry weight most likely represents storage of dietary lipids in the body wall; however, further analysis of the body wall would be needed to verify this possibility. Lipid content of the test does increase with high protein intake in L. variegatus (Y. Yuan, unpub. data).
4.2 Efficiencies
Efficient feed use, as estimated by the feed conversion ratio (FCR), can reduce feed costs. Increasing dietary SO levels decreased the efficiency of feed conversion by juvenile L. variegatus in the current study. Additionally, high energy diets (≥ 3.7 kcal g−1, as represented in Figure 2), regardless of lipid source, contributed to reduced feed conversion. These data suggest that diets in which soybean oils are added and diets with high energy (associated with total lipid content) are not efficiently converted to body mass by juveniles, contributing to reduced growth outcomes. We hypothesize excessive levels of dietary fatty acids may inhibit processes related to digestion and/or absorption. Urchin egesta (pellets) increased in size in response to increased dietary lipid intake (Dennis, 2014), suggesting high levels of lipid may negatively affect the gut microbiota associated with the urchin digesta.
Dry matter production efficiency (PE) is considered an accurate determinant of growth efficiency in that dry matter production is determined relative to dry feed intake without the weight gain contribution of water. Since the sea urchin coelom contains relatively large volumes of fluid, dry matter production is an effective parameter for evaluating dry matter gain. Increasing dietary SO levels and to a lesser extent, the addition of dietary phospholipids reduced PE in juvenile L. variegatus. In small adult L. variegatus, PE was reduced for individuals fed diets containing levels ≥ 1% of supplemented SO alone or diets with 4% supplemented SO or MO (Hammer, 2006). In the current study, juveniles fed diets containing up to 4% MO had high PE, suggesting inclusion of dietary MO did not limit production. Addition of soybean lecithin to MO-supplemented diets provided no apparent benefit for growth outcomes and instead reduced PE.
Increased FCR and decreased PE observed for juveniles fed diets supplemented with SO and soybean lecithin may be due to differences in digestibility of nutrients, absorption, and/or assimilation. Apparent dry matter digestibilities were reduced for adult L. variegatus fed diets containing 4% MO or 4% SO, and apparent crude protein digestibilities decreased as lipid level increased (Hammer 2006). Van Barneveld et al. (1998) reported reduced N and amino acid digestibility by juvenile greenlip abalone fed diets containing > 3% olive oil or > 6% jack mackerel oil. Thus, the fatty acid composition of SO and soybean lecithin may influence digestibility of nutrients such as protein, contributing to both reduced weight gain and reduced PE for juvenile L. variegatus.
The mechanism by which a food oil reduced weight gain is not understood. We hypothesize the gut microbiota or microbiome of the sea urchin may be negatively affected by high levels of dietary fatty acids. Diet influences the composition of gut microbiota of many animals (Mai, 2004). In mice, changes to the microbiota have been reported to occur in as little as 24hrs in response to acute diet changes (i.e., low-fat, plant-polysaccharide rich to high-fat, high-sugar diets) (Turnbaugh et al. 2009). Historically, oleic, linoleic, and linolenic acids were found to inhibit growth of intestinal bacteria found in higher vertebrates and ruminants (Nieman, 1954; Maczulak et al. 1981). The gut microbiota of sea urchins have been shown to enhance amino acid uptake and aid nutrient digestion (Fong and Mann, 1980; Lawrence et al. 2013), and changes to or inhibition of beneficial bacteria may contribute to reduced growth.
PE for pre-gonadal juvenile L. variegatus have not been previously reported. In the current study, PE ranged from 72 to 98% across all dietary treatments. These values exceeded PE values reported for young L. variegatus at onset of gonadogenesis (12.4 to 32.8%), small adults (15.2 to 28.5%), and adults (19.6 to 26%) (Hammer et al. 2004; Hammer et al. 2006; Watts et al. 2011). Pre-gonadal juveniles may be able to utilize dissolved organic and inorganic matter, potentially available in the culture water, through epidermal absorption (Bamford, 1982). If dissolved nutrients absorbed through the epidermis contribute significantly to dry matter production for the fast-growing, pre-gonadal juvenile stage, PE values based on dry feed intake may be an over-estimate of the efficiency of feed utilization.
4.3 Gut tissue
The weight of gut tissues of juvenile sea urchins was significantly associated with the percent of MO in the diet. Increases in sea urchin gut size can be attributed to increases in cell number and/or cell size of the gut epithelium and also increasing levels of lipid stored in the gut tissue (Lawrence et al. 1965; Lawrence et al. 1966; Klinger et al. 1988; Bishop and Watts, 1992). Regardless of the lipid source, levels of lipid found in juvenile gut tissues increased with dietary lipid level, suggesting that increased gut size for juveniles fed the high MO diets was, in part, the result of increases in storage of nutrients in the gut tissues.
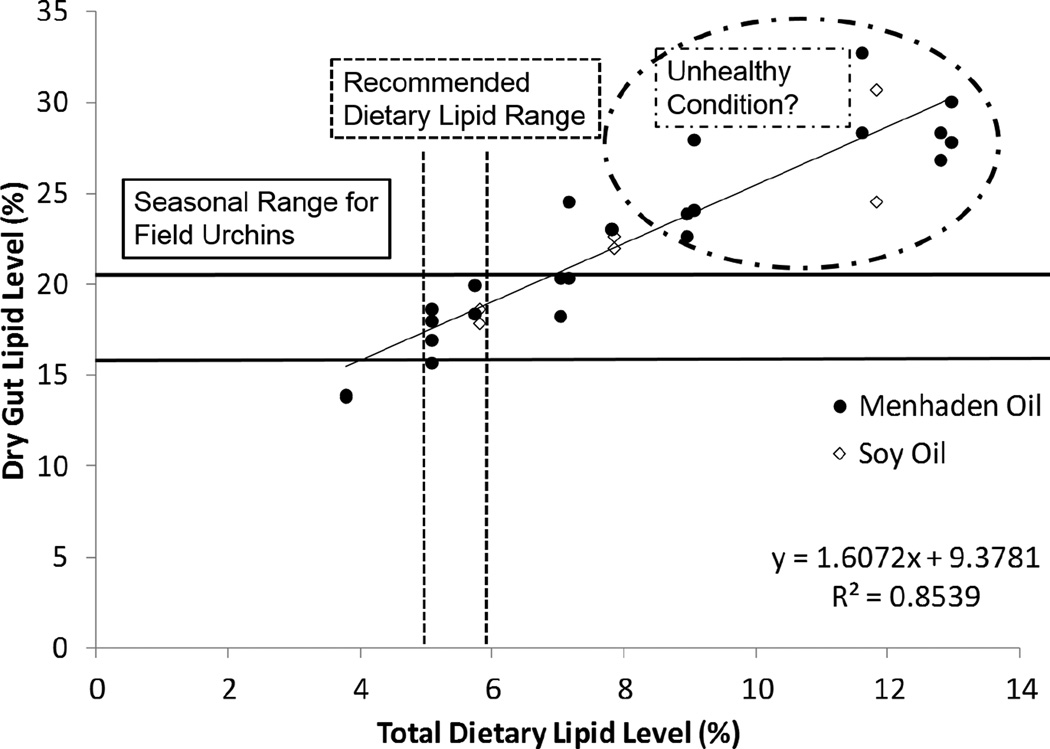
In wild populations of L. variegatus, the level of lipid observed in the gut tissues of adults ranged from 16.8 to 20.2% seasonally (Gibbs et al. 2008). Juveniles in the current study fed diets containing oils supplemented at levels > 2% (total dietary lipid > 5%) may be accumulating lipid in the gut tissues at higher than normal levels observed in wild populations. L. variegatus with excess fat in the gut may be considered steototic, or even obese, and may be more susceptible to those factors that cause inflammation and disease (Figure 5). Further histological evaluation of cellular ultrastructure of the gut is warranted. The lipid levels in gut tissues of juveniles from the current study ranged from 13.8 to 30.5% suggesting that diets with oils supplemented at levels > 2% (total lipid > 6%) are excessive.
Figure 5.
Linear regression of dry gut lipid level (%) relative to the total dietary lipid level (%). Each point represents the value for an individual pre-gonadal juvenile from the current study. The solid black horizontal lines represent the range of dry gut lipid levels observed seasonally (16.8 to 20.2%) for adult Lytechinus variegatus from Saint Joseph Bay, FL. The dashed vertical lines represent the range of dietary lipid levels that supported high growth outcomes for juveniles in this study and similar dry gut lipid levels to adult urchins in the field. The dashed circle highlights those individuals fed diets containing > 8% total dietary lipid having dry gut lipid levels exceeding those observed in the field. Excessive lipid accumulation in tissues is associated with steatosis which can lead to organ dysfunction and an unhealthy condition for most organisms. The potential effects of steatosis in sea urchins are not known.
Growth performance of juvenile L. variegatus fed semi-purified diets may be best when individuals are fed diets with ≤ 6% total lipid containing n-3 fatty acids. Based on the growth and efficiency data from this study, SO is not an appropriate source of dietary lipid. The dietary lipid requirements in young L. variegatus at the onset of gonadogenesis or in adults (Gibbs et al. 2010) may differ from those of pre-gonadal juveniles. Thus, dietary lipid requirements of later life stages of L. variegatus require further study.
Supplementary Material
Highlights.
We estimated the optimal dietary inclusion of lipids for growth in pre-gonadal sea urchins.
Level of menhaden oil with or without soybean lecithin did not affect weight gain.
Increasing soybean oil reduced weight gain by reducing production efficiency.
Total lipid > 6% may be excessive as evidenced by high lipid storage in the gut.
Acknowledgments
We would like to thank undergraduates Lacey Dennis, Matthew Snead, and Shara Legg of the Watts lab for their technical support with this experiment. We also thank Jeff Barry and the staff at the Texas AgriLife Research Mariculture Lab at Port Aransas, Texas for technical support. Approval for this study was granted by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. This publication was supported by the National Sea Grant College Program of the U.S. Department of Commerce’s National Oceanic and Atmospheric Administration under NOAA Grant # NA07OAR4170449 and the University of Alabama at Birmingham. RM was supported by NIH training grant T32HL072757 UAB Statistical Genetics Post-doctoral Training Program. The views expressed herein do not necessarily reflect the views of any of those organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bamford D. Epithelial absorption. In: Jangoux M, Lawrence JM, editors. Echinoderm Nutrition. Rotterdam: A.A. Balkema; 1982. pp. 317–330. [Google Scholar]
- Basuyaux O, Mathieu M. Inorganic nitrogen and its effect on growth of the abalone Haliotis tuberculata Linnaeus and the sea urchin Paracentrotus lividus Lamarck. Aquaculture. 1999;174:95–107. [Google Scholar]
- Bell M, Dick J, Kelly M. Biosynthesis of eicosapentaenoic acid in the sea urchin Psammechinus miliaris . Lipids. 2001;36:79–82. doi: 10.1007/s11745-001-0671-2. [DOI] [PubMed] [Google Scholar]
- Bell MV, Tocher DR. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new directions. In: Arts Michael T, Brett Michael T, Kainz Martin, editors. Lipids in Aquatic Ecosystems. New York: Springer; 2008. pp. 211–236. [Google Scholar]
- Bishop CD, Watts SA. Biochemical and morphometric study of growth in the stomach and intestine of the echinoid Lytechinus variegatus (Echinodermata) Marine Biology. 1992;114:459–467. [Google Scholar]
- Castell JD, Kennedy EJ, Robinson SMC, Parsons GJ, Blair TJ, Gonzalez-Duran E. Effect of dietary lipids on fatty acid composition and metabolism in juvenile green sea urchins (Strongylocentrotus droebachiensis) Aquaculture. 2004;242:417–435. [Google Scholar]
- D’Abramo LR. Triacylglycerols and fatty acids. In: D’Abramo Louis R, Conklin DE, Akiyama Dean M., editors. Crustacean nutrition: Advances in world aquaculture. Baton Rouge: World Aquaculture Society; 1997. pp. 71–84. [Google Scholar]
- Dennis L. MS Thesis. University of Alabama at Birmingham; 2014. Effects of biological, environmental, and nutritional factors on fecal pellets size and morphology in the sea urchin Lytechinus variegatus. Biology. [Google Scholar]
- De Ridder C, Jangoux M. Digestive systems: Echinoidea. In: Jangoux Michel, Lawrence John M., editors. Echinoderm Nutrition. Rotterdam, The Netherlands: A.A. Balkema; 1982. pp. 213–234. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Fong W, Mann KH. Role of gut flora in the transfer of amino acids through a marine food chain. Can. J. Fish. Aquat. Sci. 1980;37:88–96. [Google Scholar]
- Gibbs VK, Watts SA. In: Heinzeller T, Nebelsick JH, editors. Exposure temperature affects nutrient absorption in the regular sea urchin Lytechinus variegatus; Echinoderms: Müchen, Proceedings of the 11 th International Echinoderm Conference; A.A. Balkema, Leiden. 2004. pp. 187–192. [Google Scholar]
- Gibbs VK, Cunningham A, Watts SA. Annual cycle of the gut of the variegated sea urchin Lytechinus variegatus from the Northern Gulf of Mexico. Gulf of Mexico Sci; Melbourne, FL. Fifth North American Echinoderm Conference; 2008. p. 148. [Google Scholar]
- Gibbs VK, Watts SA, Lawrence AL, Lawrence JM. Dietary phospholipids affect growth and production of juvenile sea urchin Lytechinus variegatus. Aquaculture. 2009;292:95–103. [Google Scholar]
- Gibbs VK, Powell ML, Hammer HS, Jones WT, Watts SA, Lawrence AL, Lawrence JM. In: Harris LG, Boettger SA, Walker CW, Lesser MP, editors. Effects of dietary phospholipids and cholesterol on growth and organ production in the sea urchin, Lytechinus variegatus; Proceedings of the 12th International Echinoderm Conference; Durham, New Hampshire. CRC Press/Balkema; 2010. pp. 369–375. [Google Scholar]
- Giese AC. Lipids in the economy of marine invertebrates. Physiol. Rev. 1966;46:244–298. doi: 10.1152/physrev.1966.46.2.244. [DOI] [PubMed] [Google Scholar]
- González-Durán E, Castell JD, Robinson SMC, Blair TJ. Effects of dietary lipids on the fatty acid composition and lipid metabolism of the green sea urchin Strongylocentrotus droebachiensis . Aquaculture. 2008;276:120–129. [Google Scholar]
- Gunstone FD. The world's oils and fats. In: Turchini GM, Ng W-K, Tocher DR, editors. Fish oil replacement and alternative lipid sources in aquaculture feeds. Boca Raton, FL: CRC Press; 2011. pp. 61–98. [Google Scholar]
- Hammer BW, Hammer HS, Watts SA, Desmond RA, Lawrence JM, Lawrence AL. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea) Marine Biology. 2004;145:1143–1157. [Google Scholar]
- Hammer HS. Ph.D. Dissertation. University of Alabama at Birmingham; 2006. Determination of dietary protein, carbohydrate, and lipid requirements for the sea urchin Lytechinus variegatus fed semi-purified feeds. Biology. [Google Scholar]
- Hammer HS, Powell MS, Gibbs VK, Jones WT, Watts SA, Lawrence AL, Lawrence JM, D’Abramo LR. In: Harris LG, Boettger SA, Walker CW, Lesser MP, editors. Effect of dietary menhaden oil and soy oil on consumption, somatic growth and gonad production of the sea urchin, Lytechinus variegatus; Durham, New Hampshire. Proceedings of the 12th International Echinoderm Conference; 2010. pp. 377–383. [Google Scholar]
- Hammer HS, Watts SA, Desmond R, Lawrence AL, Lawrence JM. The effect of dietary protein concentration on the consumption, survival, growth and production of the sea urchin Lytechinus variegatus. Aquaculture. 2006;254:483–495. [Google Scholar]
- Heflin LE, Gibbs VK, Jones WT, Makowsky R, Lawrence AL, Watts SA. Growth rates are related to production efficiencies in juveniles of the sea urchin Lytechinus variegatus. Journal of the Marine Biological Association of the United Kingdom. 2013;93:1673–1683. doi: 10.1017/S0025315412001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EJ, Robinson SMC, Parsons GJ, Castell JD. Effect of lipid source and concentration on somatic growth of juvenile green sea urchins, Strongylocentrotus droebachiensis. Journal of the World Aquaculture Society. 2007;38:335–352. [Google Scholar]
- Klinger TS, Watts SA, Forcucci D. Effect of short-term feeding and starvation on storage and synthetic capacities of gut tissues of Lytechinus variegatus (Lamarck) (Echinodermata: Echinoidea) Journal of Experimental Marine Biology and Ecology. 1988;117:187–195. [Google Scholar]
- Lawrence JM, Lawrence AL, Watts SA. Feeding, digestion, and digestibility. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology, 3rd edition. Developments in Aquaculture and Fisheries Science. Vol. 38. The Netherlands: Elsevier, Amsterdam; 2013. pp. 135–154. [Google Scholar]
- Lawrence JM, Lawrence AL, Giese AC. Role of the gut as a nutrient-storage organ in the purple sea urchin (Strongylocentrotus purpuratus) Physiological Zoology. 1966;39:281–290. [Google Scholar]
- Lawrence JM, Lawrence AL, Holland ND. Annual cycle in the size of the gut of the purple sea urchin, Strongylocentrotus purpuratus (Stimpson) Nature. 1965;205:1238–1239. [Google Scholar]
- Liu H, Kelly MS, Cook EJ, Black K, Orr H, Zhu JX, Dong SL. The effect of diet type on growth and fatty-acid composition of sea urchin larvae, I. Paracentrotus lividus (Lamarck, 1816) (Echinodermata) Aquaculture. 2007a;264:247–262. [Google Scholar]
- Liu H, Kelly MS, Cook EJ, Black K, Orr H, Zhu JX, Dong SL. The effect of diet type on growth and fatty acid composition of the sea urchin larvae, II. Psammechinus miliaris (Gmelin) Aquaculture. 2007b;264:263–278. [Google Scholar]
- Lu S, Zhao N, Zhao A, He R. Effect of soybean phospholipid supplementation in formulated microdiets and live food on foregut and liver histological changes of Pelteobagrus fulvidraco larvae. Aquaculture. 2008;278:119–127. [Google Scholar]
- Maczulak AE, Dehority BA, Palmquist DL. Effects of long-chain fatty acids on growth of rumen bacteria. Appl. Environ. Microbiol. 1981;42:856–862. doi: 10.1128/aem.42.5.856-862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai V. Dietary modification of the intestinal microbiota. Nutrition Reviews. 2004;62:235–242. doi: 10.1301/nr2004.jun235-242. [DOI] [PubMed] [Google Scholar]
- Marsh A, Watts SA, Powell ML. Energy metabolism and reproduction. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology, 3rd edition. Developments in Aquaculture and Fisheries Science. Vol. 38. The Netherlands: Elsevier, Amsterdam; 2013. pp. 45–58. [Google Scholar]
- Nieman C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol. Rev. 1954;18:147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Guenette S, Pitcher TJ, Sumaila UR, Walters CJ, Watson R, Zeller D. Towards sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Powell ML, Watts SA, Lawrence AL. Researchers overcoming learning curve for production of sea urchin seedstock. Global Aquaculture Advocate. 2008 Sep-Oct;:103–104. [Google Scholar]
- Rana KJ, Siriwardena S, Hasan MR. Impact of rising feed ingredient prices on aquafeeds and aquaculture production. Fisheries and Aquaculture Technical Paper. Food and Agriculture Organization of the United Nations. 2009:541. [Google Scholar]
- Shamshak GL, Anderson JL. Offshore aquaculture in the United States: Economic considerations, implications, and opportunities. Silver Spring, MD: National Oceanic and Atmospheric Administration National Aquaculture Program; 2008. Chapter 4: Future aquaculture feeds and feed costs: The role of fish meal and fish oil; pp. 73–96. [Google Scholar]
- Shyu AB, Raff RA, Blumenthal T. Expression of the vitellogenin gene in female and male sea urchin. Proceedings of the National Academy of Sciences. 1986;83:3865–3869. doi: 10.1073/pnas.83.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Powell ML, Watts SA, Lawrence AL. Formulated feed supports weight gain and survivorship in juvenile sea urchins Lytechinus variegatus. Journal of the World Aquaculture Society. 2009;40:780–787. [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Science Translational Medicine. 2009;1 doi: 10.1126/scitranslmed.3000322. 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Barneveld RJ, Fleming AE, Vandepeer ME, Kruk JA, Hone PW. Influence of dietary oil type and oil inclusion level in manufactured feeds on the digestibility of nutrients by juvenile greenlip abalone (Haliotis laevigata) Journal of Shellfish Research. 1998;17:649–655. [Google Scholar]
- Watts SA, Hofer SC, Desmond RA, Lawrence AL, Lawrence JM. The effect of temperature on feeding and growth characteristics of the sea urchin Lytechinus variegatus fed a formulated feed. Journal of Experimental Marine Biology and Ecology. 2011;397:188–195. [Google Scholar]
- Watts SW, Lawrence AL, Lawrence JM. Nutrition. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology, 3rd edition. Developments in Aquaculture and Fisheries Science. Vol. 38. The Netherlands: Elsevier, Amsterdam; 2013. pp. 155–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.