Abstract
Mechanical circulatory support by a left ventricular assist device (LVAD) is used to bridge patients with advanced heart failure to transplant or as a definitive treatment. We retrospectively sought predictors of long-term outcome in a cohort of 83 patients who had undergone LVAD treatment. We subjected perioperative clinical data of patients to statistical analysis to establish parameters associated with all-cause mortality, and the cutoff values, sensitivity, and specificity of those that had a statistically significant relation with survival. Mean follow-up was 717 days (standard deviation, 334 days; range, 17–1,592 days). Fourteen patients (16.8%) died, but nine (10.8%) were weaned from support. Serum brain natriuretic peptide (BNP) concentration measured 60 days after implantation was significantly associated with all-cause mortality. The optimal BNP cutoff value to predict death during LVAD support was 322 pg/ml, with a sensitivity of 71.4% and specificity of 79.8%. Two-year survival was 92.0% in those with 60 days serum BNP concentration <322 pg/ml compared with 70.5% in those in whom it was ≥322 pg/ml (p = 0.003). The relation between BNP and survival likely reflects recovery of native myocardial function and improvements in global health and should assist clinicians in the on-going management of long-term LVAD therapy.
Keywords: natriuretic peptide, left ventricular assist device, heart failure, cardiac recovery, long-term prognosis
Heart transplantation (HTx) is reserved for patients with advanced heart failure refractory to conventional medical therapies1; however, the shortage of donor organs coupled with ever-growing waiting lists for HTx means that only a limited number of patients become HTx recipients. Against this background, the left ventricular assist device (LVAD) can be used to achieve sufficient hemodynamic stability2,3 and recovery of secondary organ function to act as a bridge to transplantation (BTT) in patients with advanced heart failure.4–6 The survival of patients undergoing LVAD treatment has significantly improved with advances in technology and greater clinical expertise; consequently, LVAD therapy has become a more routine part of clinical practice.7,8 These developments have been accompanied by a recognition that mechanical circulatory support can be used not only as a BTT but also as a destination therapy (DT) as the incidence of adverse events declines and the quality of life of patients with an implanted LVAD improves.9
Despite continuous improvements and refinements in LVAD technology and clinical outcomes, the life expectancy of patients with an LVAD remains impaired.10,11 Findings reported from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) clearly show that early postoperative outcome in advanced heart failure patients undergoing LVAD implantation is strongly influenced by their preoperative condition, and several studies have identified preoperative risk factors that predict early death after implantation.10,12–17 Although the postoperative survival of patients enrolled in the INTERMACS register shows that the risk of very early death after implantation is greatest in those with the least favorable preoperative profile, their mortality rate is broadly comparable with patients with more favorable preoperative profiles 2–3 months after implantation. Furthermore, studies designed to have stratified preoperative risk aimed to detect factors predicting early complications rather than long-term prognosis after LVAD implantation. As implantation of an LVAD is an increasingly common treatment for bridging and DT, it is important to identify predictors of long-term prognosis if possible. The aim of this study was to identify clinical predictors of long-term survival in patients receiving LVAD support regardless of preoperative factors.
Materials and Methods
Study Design
We retrospectively analyzed the records of 108 patients (82 men and 26 women) with advanced heart failure who had undergone LVAD implantation between May 2001 and July 2012 at the National Cerebral and Cardiovascular Center, Osaka, Japan. To elucidate the predictive factors for mid- to long-term prognosis more than 90 days after LVAD implantation, patients who died within 90 days of implantation (n = 5, 4.6%) were excluded from the analysis. Patients with biventricular support (n = 2, 1.8%), and those with incomplete data (n = 18, 16.7%), which mainly consist of patients receiving HTx at foreign country, were also excluded from the analysis. Ultimately, the data of 83 patients implanted with an LVAD were analyzed. All patients had undergone a detailed clinical evaluation before implantation, including clinical history, physical examination and evaluation of laboratory investigations, echocardiography, and cardiac catheterization. The study end-point was all-cause mortality after LVAD implantation. The institutional review board of the National Cerebral and Cardiovascular Center approved data collection, analysis and reporting.
Data Collection and Patient Follow-up
Baseline demographic data were collected retrospectively from the medical records, including age, sex, height, weight, underlying heart disease, duration of mechanical circulatory support, INTERMACS profile, and the manufacturer and model of the LVAD implanted. Baseline clinical data and laboratory evaluations had all been performed within 24 hours of surgery. Echocardiography was performed in all patients in the week before implantation. Conventional echocardiograms and laboratory investigations were subsequently undertaken 30, 60, and 90 days after implantation.
Laboratory Investigations
Laboratory investigations, including white blood cell count, hemoglobin, platelet count, and the serum concentrations of bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, blood urea nitrogen, uric acid (UA), sodium, potassium, chloride, C-reactive protein (CRP), total protein, albumin, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, cholinesterase, lymphocytes, and brain natriuretic peptide (BNP) were recorded.
Echocardiography
A standard two-dimensional transthoracic echocardiogram was recorded, and left ventricular ejection fraction (LVEF) calculated by using Simpson’s method. The Teichholz M-method or visual estimates of LVEF were made when left ventricular (LV) tracing was not possible. Measurements of left ventricular end-diastolic diameter (LVDd) and left ventricular end-systolic diameter (LVDs) were obtained from the parasternal long-axis view and measured as the largest or smallest ventricular diameter, which did not necessarily coincide with electrocardiographic (ECG) diastole owing to the asynchronous relation between the cardiac and LVAD cycles. The severity of aortic valve regurgitation was semiquantitatively graded by using color-flow Doppler and characterized as none or trivial (0), mild (1), moderate (2), moderate to severe (3), or severe (4). Pulmonary artery systolic pressure was estimated from Doppler measurements of the tricuspid regurgitant jet, described by the tricuspid regurgitation pressure gradient (TRPG). All postoperative echocardiograms were performed under LVAD support.
Statistical Analysis
Patients were divided into two groups based on whether they survived or died during LVAD support. Data are presented as mean ± standard deviation. The clinical characteristics, laboratory data, and echocardiographic data of the groups before LVAD implantation were compared using the unpaired Student’s t-test or by the nonparametric Mann–Whitney test as appropriate. Univariate Cox proportional hazard analyses were performed with continuous variables, and a p value <0.15 was used as the threshold for inclusion in multivariate models to identify the risk of death during LVAD support. Receiver operating characteristic curves were plotted, and the areas under the curves calculated to assess the optimal cutoff values for factors that predicted all-cause mortality during LVAD support. The sensitivity, specificity, positive predictive value, and negative predictive value also were calculated. Patients were allocated into groups on the basis of the cutoff value of each parameter. Kaplan–Meier analysis was undertaken to estimate the overall survival rate, and the survival of different groups was compared using log-rank analysis. Patients were censored for HTx or if they were weaned from LVAD. Because BNP concentrations were markedly skewed, we assessed the logarithmic transformation (log10) of BNP concentration. All p values were two sided, and values <0.05 were considered be statistically significant. All data were analyzed using JMP version 10.0 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Of the 83 patients included in the analysis implanted with LVADs between May 2001 and July 2012 in our institution, 63 patients received pulsatile extracorporeal pumps (Toyobo-VAS; NIPRO, Tokyo, Japan), 2 received pulsatile implantable pumps (HeartMate XVE [Thoratec, Pleasanton, CA] or Novacor [WorldHeart, Oakland, CA]), and 18 received continuous-flow implantable pumps (EVAHEART [Sun Medical, Nagano, Japan]; DuraHeart [Terumo Heart, Ann Arbor, MI]; HeartMate II [Thoratec]; or Jarvik 2000 [Jarvik Heart, New York, NY]). The baseline clinical characteristics of all patients are summarized in Table 1. Their mean age was 39.3 ± 12.4 years, and 75.9% were men. The most common cause of heart failure was idiopathic dilated cardiomyopathy (74.7%). All patients had New York Heart Association class IV symptoms of congestive heart failure and had required inotropic support. All patients underwent LVAD implantation for BTT. Patients were followed for a mean 717 ± 334 days (range, 17–1,592 days). Thirty-eight patients (45.7%) underwent HTx after a mean 891 ± 329 days of LVAD support (range, 99–1,592 days). Fourteen patients (16.8%) died (five of sepsis, four of right heart failure, and five after a cerebrovascular event), whereas nine patients (10.8%) were successfully weaned from LVAD therapy, and 22 (26.5%) were awaiting HTx on LVAD support at the end of the study period.
Table 1.
Patients’ Baseline Characteristics
Cox Regression Analysis and Receiver Operating Characteristic Curve Analysis for Potential Predictors of All-Cause Mortality During LVAD Support
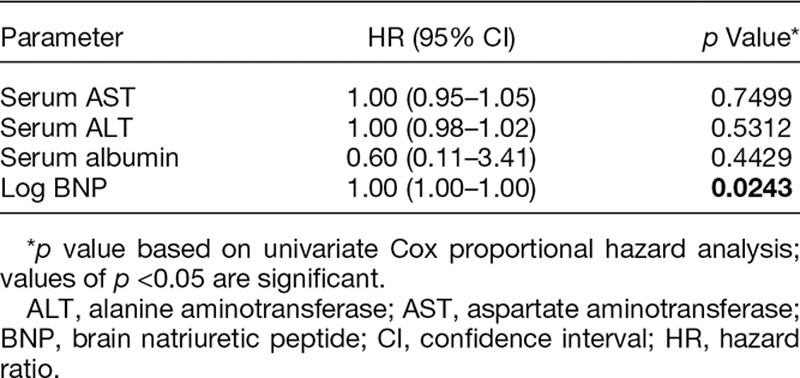
The results of univariate Cox proportional hazard analysis of clinical variables measured during treatment pathways of patients are shown in Table 2: preoperative CRP and TRPG showed statistical significance in the univariate analysis, but neither remained significant on multivariate analysis. None of the variables measured 30 days after implantation achieved statistical significance. At 60 days, serum AST, ALT, and albumin concentrations and log10 BNP concentration were significantly associated with all-cause mortality during LVAD support on univariate analysis, but only log10 BNP concentration remained significant on multivariate analysis (Table 3). Hemoglobin, serum AST, UA, chloride, CRP, and albumin concentrations and log10 BNP concentration at 90 days also showed significance in the univariate analysis, and hemoglobin, serum AST, UA, and serum albumin concentrations were found to be significant factors on multivariate analysis. Notably, echocardiographic measurements were not associated with overall mortality on univariate analysis at any time and thus were not included on multivariate analysis.
Table 2.
Univariate Cox Proportional Hazard Analysis of pre-LVAD and Postoperative Variables
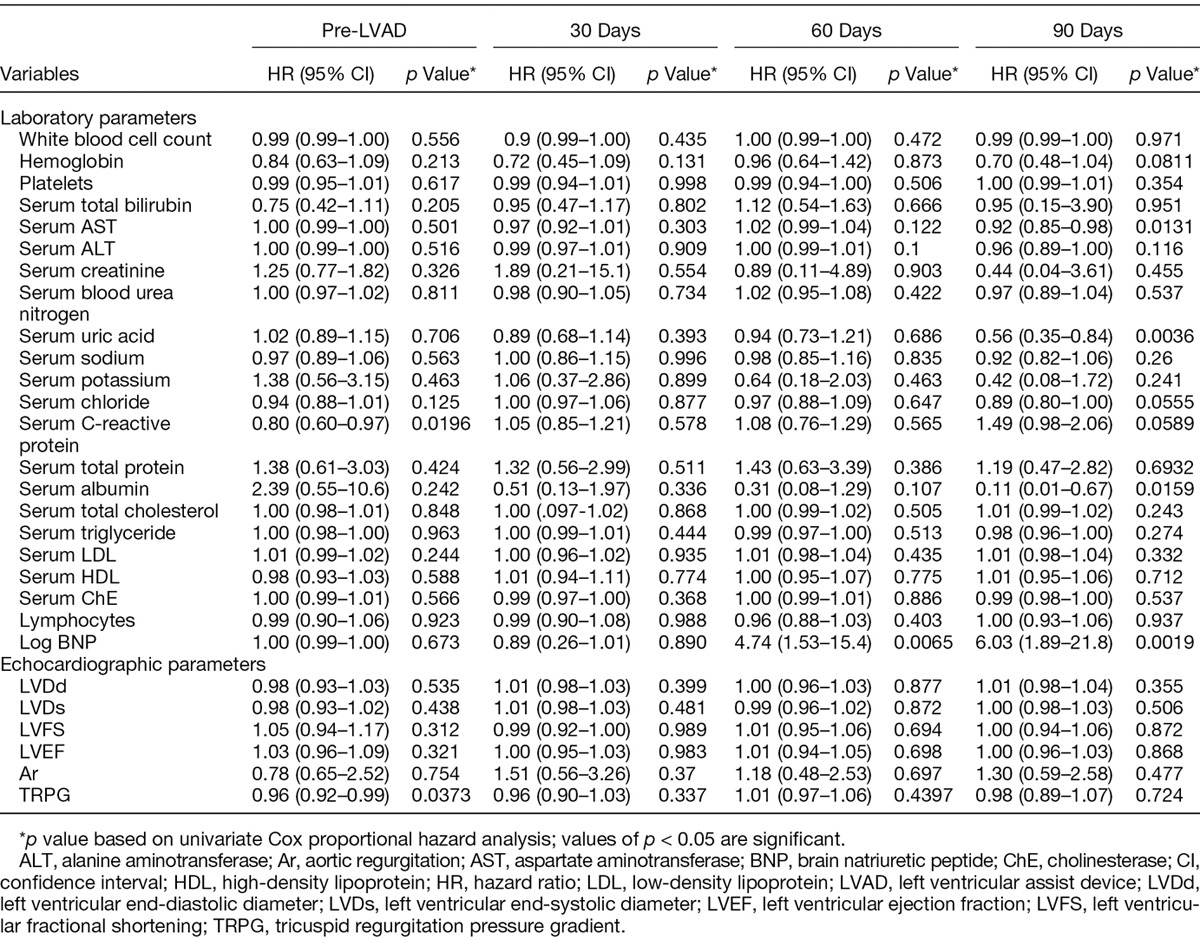
Table 3.
Multivariate Cox Proportional Hazard Analysis of Parameters Measured 60 Days After Implantation
Quantitative Analysis of BNP Concentration
We further examined the relation between serum BNP concentration and prognosis after LVAD implantation. Serum log BNP concentration showed a continuous decrease from a mean of 3.00 ± 0.34 just before LVAD implantation to 2.46 ± 0.43 at 30 days, 2.21 ± 0.46 at 60 days, and 2.15 ± 0.51 at 90 days. The difference between preoperative and postoperative concentration became statistically significant at 60 days before a steady state was achieved. There was no statistically significant difference between BNP concentrations measured at 60 and 90 days (Figure 1).
Figure 1.
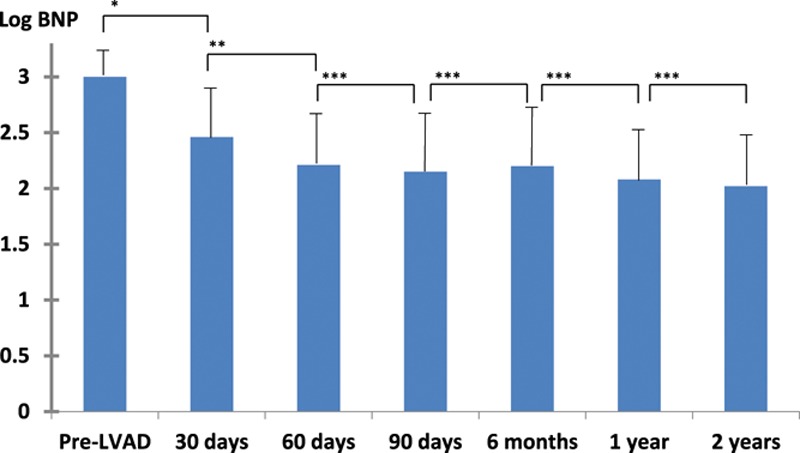
Serial serum brain natriuretic peptide (BNP) concentrations during left ventricular assist device (LVAD) support. There were significant reductions in BNP concentration 30 and 60 days after implantation compared with that of preoperative status; however, no significant difference in BNP concentration more than 60 days through 2 years after LVAD implantation. Data are presented as the mean, and error bars represent the standard deviation; *p < 0.0001, **p = 0.0001, and ***p > 0.05.
Survival After LVAD Implantation
Receiver operating characteristic curve analysis revealed that the optimal BNP cutoff value 60 days after implantation to predict all-cause mortality during LVAD support was 322 pg/ml, and the area under the curve was 0.779; this cutoff value had a sensitivity of 71.4% and specificity of 79.8% (Figure 2).
Figure 2.
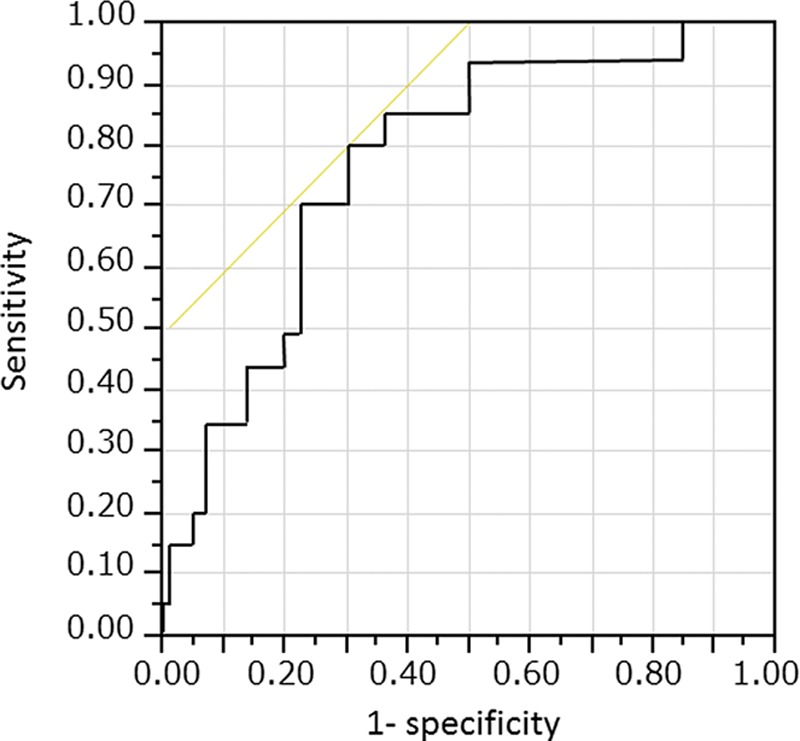
A receiver operating characteristic curve plot showing an optimal cutoff value of brain natriuretic peptide (BNP) concentration >322 pg/ml 60 days after implantation for all-cause mortality, with a sensitivity of 71.4% and specificity of 79.8%. The area under the curve is 0.779.
Comparison of the Kaplan–Meier curves of patients with an LVAD with BNP concentration <322 pg/ml and those with a BNP concentration ≥322 pg/ml 60 days after implantation showed that a significantly greater proportion of the group with the lower BNP concentration were alive 2 years after surgery. At 6 months, survival was 100% in both groups. At 1 year, survival was 98.5% and 96% in the low and high BNP groups, respectively. By 2 years, however, survival was 92.0% in the BNP <322 pg/ml group compared with 70.5% in the BNP ≥322 pg/ml group (p = 0.003; Figure 3).
Figure 3.
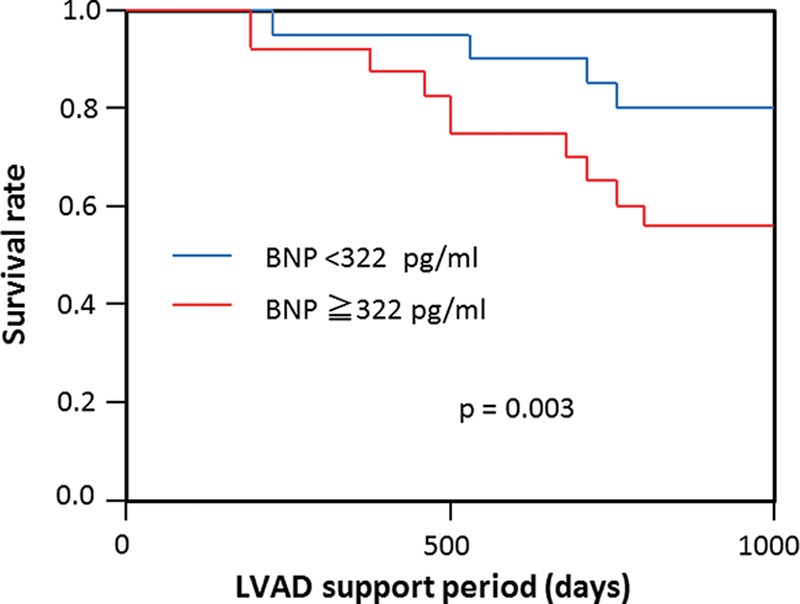
Patient survival after left ventricular assist device (LVAD) implantation. Red, patients with 60 days postoperative brain natriuretic peptide (BNP) concentration ≥322 pg/ml; blue, patients with 60 days postoperative BNP concentration <322 pg/ml. Survival was significantly better in those patients with 60 days postoperative BNP concentration <322 pg/ml (p = 0.003).
Discussion
The outcomes of patients receiving mechanical circulatory support have gradually improved over time owing to improvements in device technology, appropriate patient selection, and refinements in postoperative patient care. In a clinical context, after technological advances, judicious patient selection is thought to have had the next greatest impact on outcome. To date, multiple organ dysfunction caused by cardiogenic shock and severe right heart failure manifested by increased serum bilirubin concentration have been reported to predict postoperative prognosis, informing the selection of suitable candidates for LVAD implantation. However, risk indices based on these preoperative factors have only been shown to predict outcome in the first 30–90 postoperative days.
The technology underpinning LVAD therapy is evolving steadily. Until recently, the primary indication for LVAD implantation was as a BTT, and LVADs were expected to be needed for at most a couple of months until HTx could be undertaken. Therefore, previous studies that aimed to stratify the risks of LVAD implantation focused on very short-term outcomes. Since 2010, when the implantable continuous-flow LVAD HeartMate II became available as a DT, the number of patients receiving long-term mechanical circulatory support has exploded; INTERMACS registry data show that DT became the most common indication for implantation in 2012.5 Furthermore, even in BTT, most countries share the common problem of the discrepancy between the availability of donor organs and the ever-increasing number of patients with advanced heart failure, which has led to the use of LVADs for BTT for periods much longer than a year.
Against this background, we examined the serial changes in a variety of preoperative and postoperative clinical parameters to establish whether they had prognostic value, particularly for late outcomes more than 90 days after LVAD implantation. We found that serum BNP concentration measured 60 days after implantation significantly and independently predicted overall long-term survival in patients receiving LVAD therapy. Brain natriuretic peptide is a cardiac neurohormone secreted from the cardiac ventricles in response to the stress and stretching of cardiac myocytes. Serum BNP concentration correlates with dilatation of the left ventricle, decreased left ventricular contractility, and ventricular stiffness; consequently, it is used as a valuable diagnostic tool in patients with heart disease.18 The outlook for patients with advanced heart failure has been drastically improved by LVAD therapy: implantation of an LVAD immediately improves the decompensated hemodynamic status of patients with advanced heart failure by assisting ejection of blood from the left ventricle, which in turn offloads left ventricular myocytes—reflected in a reduction in serum BNP concentration.19
Recent reports of the neurohormonal changes in patients implanted with an LVAD have suggested that BNP concentration falls to a steady state between 30 and 60 days after implantation; this early fall in BNP concentration is proposed to be indicative of the recovery of native ventricular function.20,21 A previous retrospective study also reported that maximal improvement in myocyte diameter and collagen deposition had occurred after 40 days of LVAD support19 and that structural reverse remodeling had peaked and was complete within 60 days of implantation.22
In our cohort, changes in postimplantation BNP concentration occurred over a similar time course to those documented by other investigators, and they achieved a steady state by 60 days after implantation, despite LVAD support being provided for much longer. This raises the question as to why BNP concentration measured at 60 days should be an early and independent predictor of long-term prognosis in patients implanted with LVADs. In our opinion, BNP concentration at 60 days likely reflects the extent of native cardiac recovery and that patients with the lowest BNP concentrations will likely have received the greatest benefit and recovery of native cardiac function as a result of the mechanical circulatory support provided. This in turn will likely improve prognosis by reducing the incidence of post-LVAD complications such as right heart failure,23 pump thrombosis,24 and de novo aortic insufficiency,25,26 and the adverse events most likely to be fatal later in the postoperative period. It should be noted that BNP may also be influenced by confounding factors such as central nervous system disease27,28 and sepsis,29 which suggest that other mechanisms may also contribute to an elevated serum BNP concentration. Serum BNP concentration measured at 60 days may be a systemic biomarker of a patient’s more general health, with cardiac and noncardiac influences. In our study, such influential factors as administration of NH blockage, right heart function estimated by right heart catheter, serum creatinine, serum CRP, serum albumin, and serum total bilirubin did not show substantially different between the two groups (BNP concentration<322 pg/ml and ≥322 pg/ml). Although other factors may play less influential roles, this does not diminish the clinical use of BNP concentration as an independent long-term prognostic indicator—and indeed may help to explain it.
Conclusions
Serum BNP concentration 60 days after LVAD implantation independently predicted long-term survival of patients receiving LVAD support. As the need for long-term BTT and DT will likely grow further, the ability to predict long-term prognosis during LVAD support will have substantial clinical benefit. More data from larger cohorts are needed to substantiate our findings.
Limitations
Our study had several limitations. First, it was retrospective and conducted in a single center and, consequently, included a relatively small number of patients. Second, the etiology of heart failure in our patient cohort varied widely. Third, only 18 patients with a continuous-flow LVAD were included in the analysis, and only one patient have died during this study period; as these devices were not available in Japan before april 2011, we were unable to draw any firm conclusions about the influence of different devices on outcomes. As there appears to be a worldwide trend to implant continuous-flow LVADS, there is an urgent need to study patients who have been implanted with these devices.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Dang NC, Topkara VK, Kim BT, Mercando ML, Kay J, Naka Y. Clinical outcomes in patients with chronic congestive heart failure who undergo left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2005;130:1302–1309. doi: 10.1016/j.jtcvs.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Hiestand BC. Circulatory assist devices in heart failure patients. Heart Fail Clin. 2009;5:55–62, vi. doi: 10.1016/j.hfc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JG, Aaronson KD, Boyle AJ, et al. HeartMate II Investigators. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: A prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 9.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: The evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: Implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 11.Sabashnikov A, Mohite PN, Zych B, et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J. 2014;60:162–169. doi: 10.1097/MAT.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: The HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Klotz S, Vahlhaus C, Riehl C, Reitz C, Sindermann JR, Scheld HH. Pre-operative prediction of post-VAD implant mortality using easily accessible clinical parameters. J Heart Lung Transplant. 2010;29:45–52. doi: 10.1016/j.healun.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail. 2010;12:434–443. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 16.Miller LW, Guglin M. Patient selection for ventricular assist devices: A moving target. J Am Coll Cardiol. 2013;61:1209–1221. doi: 10.1016/j.jacc.2012.08.1029. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: The current picture. J Heart Lung Transplant. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 19.Madigan JD, Barbone A, Choudhri AF, et al. Time course of reverse remodeling of the left ventricle during support with a left ventricular assist device. J Thorac Cardiovasc Surg. 2001;121:902–908. doi: 10.1067/mtc.2001.112632. [DOI] [PubMed] [Google Scholar]
- 20.Milting H, EL Banayosy A, Kassner A, et al. The time course of natriuretic hormones as plasma markers of myocardial recovery in heart transplant candidates during ventricular assist device support reveals differences among device types. J Heart Lung Transplant. 2001;20:949–955. doi: 10.1016/s1053-2498(01)00289-3. [DOI] [PubMed] [Google Scholar]
- 21.Sodian R, Loebe M, Schmitt C, et al. Decreased plasma concentration of brain natriuretic peptide as a potential indicator of cardiac recovery in patients supported by mechanical circulatory assist systems. J Am Coll Cardiol. 2001;38:1942–1949. doi: 10.1016/s0735-1097(01)01677-1. [DOI] [PubMed] [Google Scholar]
- 22.Xydas S, Rosen RS, Ng C, et al. Mechanical unloading leads to echocardiographic, electrocardiographic, neurohormonal, and histologic recovery. J Heart Lung Transplant. 2006;25:7–15. doi: 10.1016/j.healun.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi JR, Sobieski MA, Schwartz S, Williams ML, Slaughter MS. Novel thrombosis risk index as predictor of left ventricular assist device thrombosis. ASAIO J. 2013;59:380–383. doi: 10.1097/MAT.0b013e318291d0c1. [DOI] [PubMed] [Google Scholar]
- 25.Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol. 2011;58:1705–1714. doi: 10.1016/j.jacc.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Toda K, Fujita T, Domae K, Shimahara Y, Kobayashi J, Nakatani T. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg. 2011;92:929–934. doi: 10.1016/j.athoracsur.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 27.Tomita H, Metoki N, Saitoh G, et al. Elevated plasma brain natriuretic peptide levels independent of heart disease in acute ischemic stroke: Correlation with stroke severity. Hypertens Res. 2008;31:1695–1702. doi: 10.1291/hypres.31.1695. [DOI] [PubMed] [Google Scholar]
- 28.Mäkikallio AM, Mäkikallio TH, Korpelainen JT, et al. Natriuretic peptides and mortality after stroke. Stroke. 2005;36:1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 29.McLean AS, Huang SJ, Hyams S, et al. Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35:1019–1026. doi: 10.1097/01.CCM.0000259469.24364.31. [DOI] [PubMed] [Google Scholar]


