Abstract
Study Design
Topic review
Objective
Describe value measurement in spine care and discuss the motivation for, methods for, and limitations of such measurement.
Summary of Background Data
Spinal disorders are common and are an important cause of pain and disability. Numerous complimentary and competing treatment strategies are used to treat spinal disorders and the costs of these treatments is substantial and continues to rise despite clear evidence of improved health status as a result of these expenditures.
Methods
The authors present the economic and legislative imperatives forcing the assessment of value in spine care. The definition of value in health care and methods to measure value specifically in spine care are presented. Limitations to the utility of value judgements and caveats to their use are presented.
Results
Examples of value calculations in spine care are presented and critiqued. Methods to improve and broaden the measurement of value across spine care are suggested and the role of prospective registries in measuring value is discussed.
Conclusions
Value can be measured in spine care through the use of appropriate economic measures and patient reported outcomes measures. Value must be interpreted in light of the perspective of the assessor, the duration of the assessment period, the degree of appropriate risk stratification, and the relative value of treatment alternatives.
Keywords: Value, QALY, Disability, Functional Outcome, Lumbar Fusion
Introduction
Spine care is a focus of cost and quality efforts in the United States and throughout the world for several important reasons. First, spinal disorders and disability due to spinal disorders are very common. In the 2010 Global Burden of Disease Study, of the 291 conditions studied, low back pain was ranked highest in terms of years lost to disability with 83 million disability adjusted life years lost attributed to low back pain in 2010 (1). Second, care of spinal disorders is very expensive. In the United States, 6% of adults see a physician for a back and neck complaint every year and the costs associated with these visits has doubled over the past decade, with physical therapy costs increasing most dramatically (2). Martin et al analyzed Medical Expenditure Panel Survey data from 22,258 respondents in 2005. Of these respondents, 3187 reported spine problems and those respondents who reported spine problems indicated that their medical costs were nearly twice that of respondents who did not report back or neck complaints ($4695 versus $2731) (3). These costs continue to increase despite a lack of information to indicate that these increased costs are associated with improved outcomes.
Complicating the issue is the fact that there are multiple different treatment strategies for spinal disorders. A quick literature search using the terms “low back pain” and “disability” performed in April, 2014 yielded papers describing the effectiveness and/or cost effectiveness of deep tissue massage, accupunture, whole body vibration exercise, manipulation under anesthesia, percutaneous adhesiolysis, core stability exercise, lumbar mobility exercise, pars interarticularis injections, epidural steroid injections, therapeutic ultrasound, microwave diathermy, cognitive therapy, balneotherapy, nucleoplasty, and the use of various surgically implanted non-fusion devices (4–16). These all appeared within the top 40 most recent references, and do not even include the commonly employed strategies for back pain such as chiropractic care, lumbar fusion, cognitive therapy, or medical management.
Clearly, there is a need to define the value of these different interventions in order to make judgements regarding the relative worth of these disparate treatments in different patient populations. The purpose of this paper is to explore the definitions of value, propose several mechanisms for the assessment of value, describe the limitations of these definitions and mechanisms, and to discuss some of the regulatory imperatives that are accelerating the pace of measurement despite acknowledged limitations in the ability of practitioners to define, let alone measure value in spine care. A glossary of commonly used terms is provided to help the clinician with unfamiliar terms.
Definitions of value in healthcare
For individuals affected by spine problems, the choice between operative and non-operative care is often viewed as a “preference-sensitive” decision —one that should hinge on how a patient feels about their current state of health relative to the risks and benefits of undergoing surgery. Informed decision making requires that patients fully understand the treatment alternatives and that they make choices that are aligned with their preferences for the benefits and harms of each possible treatment.
Preference for health may be formally measured using the concept of health utility where each health state is assigned a value or preference weight on a scale where 1 represents best imaginable health and 0 represents worst imaginable health or death. It is noteworthy that any two individuals with objectively equivalent health-related quality of life based on a health status measure such as the SF-36 may view their health quite differently and assign different utilities. There are a number of ways to elicit health utility ranging from direct utility assessment with a standard gamble, time tradeoff or visual analog scale, to preference-weighted health state classification systems such as the EuroQol EQ-5D or Health Utilities Index (HUI).
Valuing spine treatments requires measuring how care affects patients’ health-related quality of life. This is critically important because many spine conditions for which care is performed do not extend life. Instead, they improve patient’s health-related quality of life—their ability to function without pain in carrying out their daily activities. To assess the value of interventions that impact quality, not only length of life, it is critically important to have an outcome measure that characterizes the quality of life dimension.
The QALY
The quality-adjusted life year (QALY) is the most widely used effectiveness measure that combines length and quality of life into a single number (17). QALYs were developed as a measure for use in economic evaluation in health and medicine more than 30 years ago (18, 19).
QALYs are typically estimated by multiplying the amount of time spent in each health state by each state’s health utility and summing up. For example, if one were to spend the next 10 years with 5 years in perfect health, 3 years with limited mobility and 2 years with limited mobility and moderate pain and these health states had respective utilities of 1.0, 0.85 and 0.7, this would be equivalent to 8.95 QALYs (5x1 + 3x0.85 + 2x0.7=8.95).
Societal Values and Cost-effectiveness Analysis
The Panel on Cost-effectiveness in Health and Medicine recommends that QALYs be used to measure the effectiveness in economic evaluations of health care interventions (20). Measuring effectiveness in terms of cost per QALY gained has the advantage of allowing comparisons of the value of healthcare interventions across a wide array of health domains. For example, the effectiveness and value of interventions for spinal disorders may be compared directly with cardiovascular disease interventions. The literature on the cost-effectiveness of diverse healthcare interventions is catalogued in the Tufts School of Medicine Cost-effectiveness Analysis Registry (https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx).
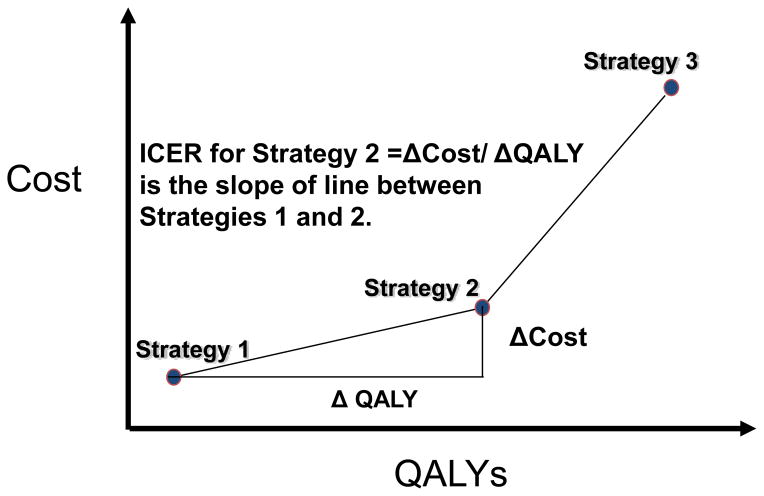
To evaluate the cost-effectiveness of competing health care interventions, an incremental cost-effectiveness analysis is required with the incremental cost-effectiveness ratio (ICER) serving as the cost-effectiveness measure. The ICER is defined as the change in cost divided by the change in effectiveness for each more costly alternative when they are ranked from lowest to highest cost ( ICER= (C2-C1)/ (QALY2-QALY1)). When the costs of alternative care strategies are plotted against their estimated effectiveness (Figure 1), the slope of the line between strategies is the ICER.
Figure 1.
Figure: Graphical representation of incremental cost-effectiveness ratio (ICER) for Strategy 2 relative to Strategy 1 when strategy costs are plotted against the QALYs associated with each strategy.
While it is widely accepted that the individual patient’s preferences and health utilities should guide his or her decision making, when assessing the cost-effectiveness of healthcare interventions, it is societal preferences for health outcomes rather than the preferences of those directly affected that are considered appropriate. Preference classification systems, such as EQ-5D and HUI, measure health status in individuals with the health condition of interest, but assign a societal health state value to each health state defined within the system on the basis of a scoring algorithm (20). This approach for valuing spine surgery was used in the Spine Patient Outcomes Research Trial (SPORT), which has assessed the cost-effectiveness of spine surgery relative to non-operative care for patients with intervertebral disc herniation, spinal stenosis and degenerative spondylolisthesis (21, 22, 23).
Economic analysis in Spine Outcomes Research
The importance of value when assessing spine treatments is becoming more important not only because of a need to control health care expenditures in general, but also because many recognize that higher quality might be associated with lower cost. Indeed comparative effectiveness research can not really be separated from cost-effectiveness research when it comes to spinal disorders. It is generally agreed that an economic analysis must compare costs per QALY gained for one treatment versus an alternative. While each society must ultimately decide on what they are willing to pay for an additional QALY for one individual, in the US a commonly used cost-effectiveness benchmark is $ 100,000/ QALY gained. (24).
The complexity comes from the calculation of cost and from what perspective. Healthcare charges are often used as a surrogate for healthcare costs because charges can readily be obtained from administrative databases. Charges from inpatient administrative databases such as the national inpatient sample are inflated and do not represent actual costs or even reimbursement. Applying cost-to-charge ratios (25) or calculating reimbursement using Medicare’s values (26) are commonly used techniques for estimating actual costs. Costs must be calculated not just for an episode of hospital care, but over a reasonable time horizon from a societal perspective and must include direct inpatient and outpatient health costs (hospital, pharmacy, professional, radiology, lab testing, etc.) and indirect health costs (e.g. lost productivity and missed work for the patient and his/her caregivers). These types of economic assessments generally require the expertise of healthcare economists.
The SPORT trials included an economic analysis that permitted the assessment of cost-effectiveness for surgical therapy versus non-operative therapy for lumbar disc disease, lumbar spinal stenosis, and lumbar spondylolisthesis. Two major concepts emerged from this analysis. Cost-effectiveness for lumbar discectomy versus non-operative treatment at 2 years was $ 69,403/ QALY gained (all payers), but a significantly different figure was generated ($34,355/ QALY gained) when Medicare-specific reimbursement costs were utilized (27). The critical importance of time horizon was also observed. For example, surgery versus non-operative treatment for lumbar spondylolisthesis was above the $ 100,000/ QALY threshold at 2 years ($ 115,600/ QALY) (28) but fell below the commonly accepted to pay threshold at 4 years ($ 64,300/ QALY)(29). Clearly, the choice of methodology affects the results of an economic analysis. Studies comparing more costly upfront treatments (e.g. surgery) can be compared to less costly upfront treatments over an appropriate time horizon that permits the durability of treatments to be compared.
The importance of time horizon is illustrated well in a study by Soegaard et al that compared circumferential lumbar fusion versus posterolateral lumbar fusion (30). The study found an incremental savings of $ 49,306/ QALY for patients treated with circumferential fusion versus posterolateral fusion over an 8 year time horizon. The circumferential fusion cohort had a higher fusion rate and had a 15% re-operation rate versus a 38% re-operation rate observed in the posterolateral cohort. Circumferential fusion was associated with superior functional outcomes but the long follow-up period demonstrated the overall cost-effectiveness because the circumferential fusion appeared to be more durable than the posterolateral only fusion cohort.
The increased utilization of recombinant human bone morphogenetic proteins (rhBMPs) as a fusion enhancer has attracted widespread attention for its potential risks and its costs. Administrative data using hospital charge data finds that spinal fusion with rhBMP is associated with an average hospital charge of $ 74,254 compared to $ 57,393 for spinal fusions without rhBMP (31). These data do not contain comparable populations nor do they include outcomes or QALY data. Many studies have compared the cost of iliac crest bone graft to rhBMP for lumbar fusion and have found similar actual hospital costs (32, 33); however, without comparing differences in overall outcome and including outpatient and societal costs, the cost-effectiveness of rhBMP is largely not known.
As more new implants and devices enter the spine market, it will be imperative that comparative outcomes research incorporate comprehensive cost evaluations. Calculating health costs from a societal perspective and comparing costs over time will enable investigators, payers, and policy makers to make rational decisions about the comparative cost-effectiveness of various options in spinal treatments going forward.
Legislative and Regulatory Imperatives
In the decade leading up to the signing of the Affordable Care Act (ACA), there was a growing sense of urgency within the public and private sector to create a more sustainable healthcare system. Some of this pressure came from documented shortfalls in U.S. health outcomes, which lagged sorely behind other developed nations. Other pressure came from groundbreaking estimates of medical error ubiquity and patient harm. (34, 35) But while these lapses in quality made headlines and caught the nation’s attention, the larger impetus for health reform was the growing disconnect between spending and quality. For over a decade leading up to the ACA, healthcare spending had been growing at a rate greater than the nation’s overall economy, (36) and by 2009, it was estimated that about 30 percent of healthcare spending was wasteful (37).
Responding to what was less of an opportunity and more of a mandate for change, the ACA, and other legislation leading up to it, presented concrete plans to leverage federal buying power and reduce expenditures through the pursuit of high-value care. In fact, the term “value” is used over 200 times throughout the text of the ACA (38) to characterize a range of provisions aimed at reducing waste on multiple fronts. Targets include spending on items and services that lack evidence of producing better outcomes; costs that result from avoidable medical injuries, such as preventable infections in hospitals; fraud and abuse; and overall inefficiencies in the provision of health care goods and services.
These new initiatives take aim at foundational features of the Medicare payment system that, to date, have discouraged efficiencies and posed as impediments to higher value care. A commonly critiqued feature is the fee-for-service approach to physician payment, which incentivizes volume over quality. While FFS still remains, programs such as the Physician Quality Reporting System (PQRS) and the more recent Physician Value-based Payment Modifier (VBM) represent a shifting paradigm under which physicians are increasingly scrutinized and held accountable for both the quality and cost of their care. Together, these programs could result in cuts to physician payments approaching −10% in future years. The severity of these cuts is compounded by the fact that these programs rely on arbitrary performance thresholds, rudimentary risk adjustments and attribution methodologies, and measures that are only tenuously relevant and meaningful to the spine surgeon.
Medicare’s inpatient hospital prospective payment system is another significant focus of value-based payment reforms. Although intended to promote efficiency by paying for pre-determined diagnosis-based bundles of services, it does little to discourage unnecessary admissions and readmissions. As such, hospitals now receive payment penalties based on what CMS deems as excess or unnecessary readmissions, as well as certain inappropriate hospital-acquired conditions, such as surgical site infections following certain orthopedic procedures. These penalties come on top of other value-based adjustments tied to hospital performance on a wide range of process-of-care, outcome, and patient experience metrics.
A broader effort to achieve higher value care aims to deconstruct the silos that exist between different care settings, resulting in fragmentation and duplication of services. This is particularly problematic at a time when individuals with five or more chronic conditions represent 22 percent of all Medicare beneficiaries, but 69 percent of all Medicare spending (http://health.usnews.com/health-news/best-practices-in-health/articles/2011/07/18/key-to-healthcare-costs-is-to-better-treat-chronically-ill). The ACA-authorized Center for Medicare & Medicaid Innovation (CMMI) continues to test and implement a growing portfolio of payment and service delivery models aimed at breaking down those silos and encouraging better care coordination. These include shared savings models for accountable care organizations that better manage care across multiple settings, bundled payments within and across care settings for pre-defined episodes of care, and initiatives that take additional steps to reduce preventable hospital conditions and readmissions through the promotion of team-based approaches to care and smoother care transitions across settings.
The pursuit of high value care also resulted in an unprecedented investment in comparative effectiveness research (CER). The ACA-authorized Patient-Centered Outcomes Research Institute (PCORI) aims to ensure that both physicians and patients have more meaningful and useful information regarding the comparative clinical effectiveness of different procedures and services and that these studies are conducted in a methodologically sound manner. This independent organization funded partially by the federal government and partially by private insurers is required to conduct research in an open and transparent manner, disseminate its findings rapidly, and ensure that the needs of individuals and special populations are taken into consideration. Although PCORI cannot, under statute, make recommendations to mandate or deny coverage based on cost-effectiveness findings, CMS is still free to take evidence from comparative effectiveness studies into consideration when making coverage and reimbursement decisions.
While much of the focus is on the entire value equation, other initiatives do not pretend to be about anything other than cost control. For example, beginning in 2015, if Medicare cost growth exceeds a certain rate, the ACA-authorized Independent Payment Advisory Board (IPAB) must make recommendations on how to reduce Medicare spending, and its proposals must be fast-tracked through Congress. Although concerns have been raised about the IPAB’s unrestricted power and its potential to arbitrarily limit patient access to necessary care, the President has yet to appoint members due to slower than expected spending growth.
The federal government’s unprecedented investment in achieving a high-performance health system will likely have a lasting effect on the way medical care is delivered, evaluated, and paid for in our nation. Nevertheless, the sustainable impact of these reforms will depend heavily on achieving a balance between the needs of the patient, preserving the autonomy of the healthcare provider, and promoting responsible stewardship of health care dollars.
Limitations of value calculations in the spine population
The discussion thus far has focused on the economic and legislative imperatives for the measurement of value in spine care. Definitions of value (such as cost/QALY) and potential mechanisms to measure value (such as prospective registries including health utility and cost information) have been described, and the rationale for these mechanisms has been explained. While these concepts are valid, there are important practical considerations to take into account when measuring value which can dramatically change the conclusions reached when making value judgements. Important considerations to consider are the perspective from which the value equation is being considered, the time frame of the value equation, the role of risk stratification, and the utility of value judgements in making payment and policy decisions.
As alluded to above, value may be measured from the perspective of the payer, the individual, or the society. Differences in measured value may exist dependent solely upon differences in the perspective from which the value is measured. A simplistic example is illustrated by Angevine and McCormick in a discussion regarding the cost effectiveness of anterior cervical discectomy and fusion (ACDF) with and without plate fixation. The authors assumed (based on a literature review) that there were no significant long term differences in outcome between the ACDF and ACDF plus plate (ACDFP) groups. While both procedures were found to be cost effective (using hospital operative and peri-operative costs) in terms of cost per QALY, the ACDFP procedure was associated with a higher cost (the plate and its application cost approximately $1500 at their center). From a payer perspective then, it would seem that ACDF without plate would be the procedure with higher value-same outcome at a lower price. From a patient or societal perspective however, the story is not so clean cut. Based upon their institutional data and a literature review, the authors report that patients treated with ACDFP go back to work on average 3 weeks sooner than those treated with ACDF. It is assumed that this difference is due to the surgeon’s practice of requiring patients without plate fixation to wear a cervical collar for a period of time following the operation, prohibiting driving and work in most cases. If the lost income in those three weeks is more than $1500, then from a patient perspective, the ACDFP is the procedure with more “value.” Similarly, if the patient is able to produce more than $1500 in goods and or services during those three weeks, then society will also benefit greater and find greater value with the ACDFP option (39). Similar discussions now focus on minimally incisional spine surgery-particularly transforaminal interbody fusion techniques which generally require more OR time but are associated with faster recovery when compared to traditional open techniques (40). When making judgments regarding the value of an intervention, it is extremely important to use the appropriate perspective. How the question is framed has a direct influence on the answer obtained.
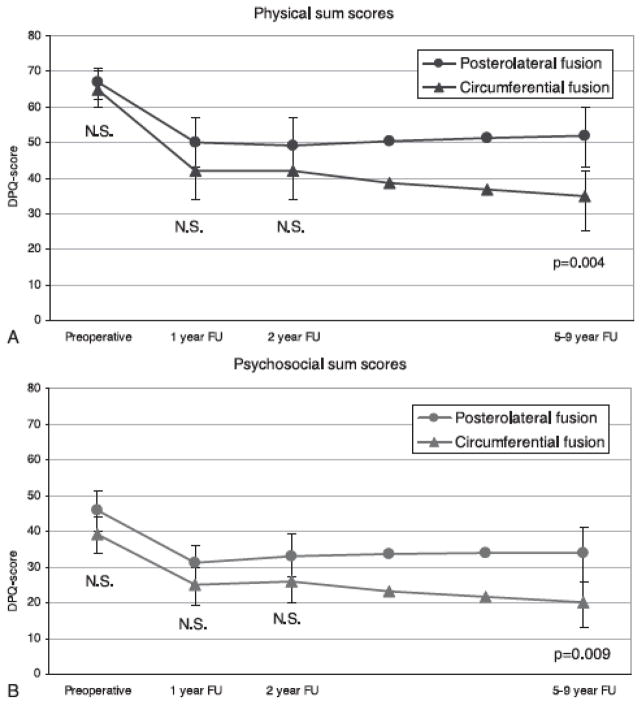
A second important consideration is the time frame used for the determination of “value.” An expensive technique that is effective and durable will not appear to have value compared to a cheaper but less durable alternative unless the treatment effect is measured over an appropriate period of time. For example, Soegaard et al performed a randomized controlled trial comparing postero-lateral fusion to circumferential fusion for axial back pain due to spondylolisthesis or degenerative disease (30). Both groups enjoyed substantial improvement in pain and functional outcome compared to their baseline measures. At two years, there were no significant differences between the groups in either physical (Figure 2A) or psychosocial (Fig. 2B) outcomes. Had the authors stopped their analysis at this point, they would have concluded that postero-lateral fusion was the more cost effective option for this patient population (similar beneficial effect for less upfront costs). The authors continued to follow these patients for a period of up to nine years. What they observed was a more durable improvement in the circumferential group which led to less health care costs and less societal costs-the exact opposite of what they would have concluded if they had stopped the project at two years. A major difference is illustrated in figure 2, taken from the Soegaard paper, demonstrating a significantly larger proportion of patients working in the circumferential group compared to the postero-lateral group (Figure 3). Therefore, it is important to determine the appropriate timeframe for study of competing techniques for similar disease states. In a non-lethal condition such as low back pain, longer term studies may make sense. A caveat to this general principle relates to opportunity cost of treatment. A therapy that results in a rapid improvement in health status (microdiscectomy for example) may have intrinsic value even if long term outcomes are similar to treatment alternatives which result in more gradual improvement. Getting a patient back to work in six weeks versus six months is certainly worth a substantial upfront investment.
Figure 2.
Figure 2 (from Soegaard, 2007):
Figure 3.
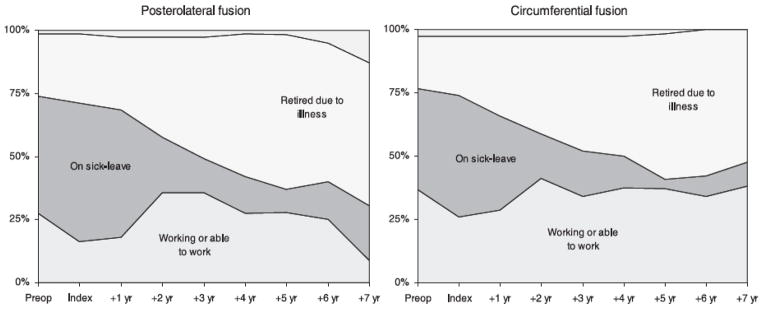
Figure 3 (from Soegaard, 2007)
The next issue to consider is the issue of risk stratification. Abdu et al performed a review of the surgical arm of the SPORT study looking at the results of different surgical strategies for stenosis associated with spondylolisthesis (41). When these authors compared the results of different surgical strategies-non-instrumented fusion, instrumented postero-lateral fusion, and interbody fusion techniques, they found no significant differences in outcome. An uninformed review of this data might lead a patient, payer, or policy maker to conclude that the least expensive option (non-instrumented fusion) provides the most value (same outcome for less cost). Such an interpretation is flawed however because no information is provided regarding the rationale for the selection of a given surgical procedure for a given patient. Patients with stenosis and spondylolisthesis are not homogeneous-they differ in age, lifestyle, medical comorbidities and anatomy. A 40 year old truck driver with a mobile slip, tall disc space, and a history of smoking is unlikely to do well with an un-instrumented fusion (42) whereas a 78 year old sedentary widow with a collapsed disc space may do quite well with such an approach. The best interpretation of the Abdu information is that when experienced surgeons present options to properly informed patients, the patient/surgeon team is able to choose appropriate procedures which are likely to work.
One of the most significant contributors to cost of a surgical procedure is a re-admission or re-operation for a medical or surgical complication. A treatment strategy with a lower morbidity is generally going to be more cost effective than one with high morbidity if the efficacy of the two strategies is similar. Retrospective analysis of administrative databases is not helpful for making these types of judgements because patient characteristics and patient-reported outcomes are not available. If more severely symptomatic patients tend to be offered more aggressive interventions, then comparing complication rates or costs between the groups is meaningless. One way to begin to address this issue is through the use of risk stratification.
Lee et al have developed a calculator (spinesage.com) that can predict the risk of medical complications following spine surgery based on information available pre-operatively. Using a prospective registry, they identified risk factors for medical complications based on demographic, medical history, and approach specific variables (43). Using such a calculator, it is possible to predict complications and costs for specific patient populations. This information, combined with patient reported outcomes, allows for the rational evaluation of true value.
Summary and Conclusion
“Value” is a popular buzzword in medicine and in spine care. Defining value is not an entirely straightforward exercise however and it is critical that the interpretation of value be done in context. The context must include multiple perspectives when it comes to cost and economic benefit, must include opportunity costs associated with the lack of treatment, and must include appropriate follow-up and risk stratification. Not every spine surgeon needs to be an economist or health policy analyst, however all spine surgeons can contribute to the definition of value in spine care through participation in prospective registries which incorporate adequate pre-operative risk assessment, accurate and complete complication reporting, and patient reported outcomes measures. Embracing what works and is valuable and discarding what is not effective or valuable is an easy concept to understand and an impossible one to argue with. It is our responsibility to establish the evidence base and to help payers and policy makers with rational interpretation of the evidence base.
Supplementary Material
Acknowledgments
Supported by AO Spine North America, Inc. Analytic support for this work was provided by Spectrum Research, Inc., with funding from the AO Spine North America. National Institute for Arthritis, Musculoskeletal and Skin Diseases (P60AR062799) grant funds were received in support of this work. Relevant financial activities outside the submitted work: board membership, grants, patents.
References
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2013. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 2.Davis MA, Onega T, Weeks WB, Lurie JD. Where the United States spends its spine dollars: expenditures on different ambulatory services for the management of back and neck conditions. Spine. 2012 Sep 1;37(19):1693–701. doi: 10.1097/BRS.0b013e3182541f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin BI1, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008 Feb 13;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 4.Strunk RG, Pfefer MT, Dube D. Multimodal chiropractic care of pain and disability for a patient diagnosed with benign joint hypermobility syndrome: a case report. J Chiropr Med. 2014 Mar;13(1):35–42. doi: 10.1016/j.jcm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taber DJ, James GD, Jacon A. Manipulation under anesthesia for lumbopelvic pain: a retrospective review of 18 cases. J Chiropr Med. 2014 Mar;13(1):28–34. doi: 10.1016/j.jcm.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majchrzycki M, Kocur P, Kotwicki T. Deep tissue massage and nonsteroidal anti-inflammatory drugs for low back pain: a prospective randomized trial. ScientificWorldJournal. 2014 Feb 23;2014:287597. doi: 10.1155/2014/287597. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XQ, Pi YL, Chen PJ, Chen BL, Liang LC, Li X, Wang X, Zhang J. Whole body vibration exercise for chronic low back pain: study protocol for a single-blind randomized controlled trial. Trials. 2014 Apr 2;15(1):104. doi: 10.1186/1745-6215-15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manchikanti L, Helm S, 2nd, Pampati V, Racz GB. Cost Utility Analysis of Percutaneous Adhesiolysis in Managing Pain of Post-lumbar Surgery Syndrome and Lumbar Central Spinal Stenosis. Pain Pract. 2014 Mar 26; doi: 10.1111/papr.12195. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Stuber KJ, Bruno P, Sajko S, Hayden JA. Core Stability Exercises for Low Back Pain in Athletes: A Systematic Review of the Literature. Clin J Sport Med. 2014 Mar 20; doi: 10.1097/JSM.0000000000000081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Wald JT, Geske JR, Diehn FE, Murthy NS, Kaufmann TJ, Thielen KR, Morris JM, Lehman V, Maus TP. A Practice Audit of CT-Guided Injections of Pars Interarticularis Defects in Patients with Axial Low Back Pain: A Primer for Further Investigation. Pain Med. 2014 Jan 21; doi: 10.1111/pme.12344. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Ebadi S, Henschke N, Nakhostin Ansari N, Fallah E, van Tulder MW. Therapeutic ultrasound for chronic low-back pain. Cochrane Database Syst Rev. 2014 Mar;14:3, CD009169. doi: 10.1002/14651858.CD009169.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Durmus D, Ulus Y, Alayli G, Akyol YC, Bilgici A, Yazicioglu K, Kuru O. Does microwave diathermy have an effect on clinical parameters in chronic low back pain? A randomized-controlled trial. J Back Musculoskelet Rehabil. 2014 Mar 10; doi: 10.3233/BMR-140464. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Onat SS, Taolu O, Güneri FD, Oziler Z, Safer VB, Ozgirgin N. The effectiveness of balneotherapy in chronic low back pain. Clin Rheumatol. 2014 Mar 6; doi: 10.1007/s10067-014-2545-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Eichen PM, Achilles N, Konig V, Mosges R, Hellmich M, Himpe B, Kirchner R. Nucleoplasty, a Minimally Invasive Procedure for Disc Decompression: A Systematic Review and Meta-analysis of Published Clinical Studies. Pain Physician. 2014 Mar-Apr;17(2):E149–73. [PubMed] [Google Scholar]
- 15.Ahmed R, Shakil-Ur-Rehman S, Sibtain F. Comparison between Specific Lumber Mobilization and Core-Stability Exercises with Core-Stability Exercises Alone in Mechanical low back pain. Pak J Med Sci. 2014 Jan;30(1):157–60. doi: 10.12669/pjms.301.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Seo JC, Kwak MA, Park SH, Min BM, Cho MS, Shin I, Jung JY, Roh WS. Acupuncture for low back pain due to spondylolisthesis: study protocol for a randomized controlled pilot trial. Trials. 2014 Apr 2;15(1):105. doi: 10.1186/1745-6215-15-105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller W, Robinson LA, Lawrence RS, editors. Committee to evaluate measures of health benefits for environmental, health and safety regulation. Board on Health Care Services. Institute of Medicine of the National Academies. The National Academies Press; Washington, DC: 2006. Valuing Health for regulatory cost-effectiveness analysis. [Google Scholar]
- 18.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 19.Pliskin JS, Shepard D, Weinstein MC. Utility functions for life years and health status. Operations Res. 1980;28:206–227. [Google Scholar]
- 20.Gold M, Siegel J, Russell L, et al. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 21.Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, Birkmeyer N, Herkowitz H, Longley M, Lenke L, Emery S, Hu SS. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009 Jun;91(6):1295–304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, Herkowitz H, Hilibrand A, Albert T, Fischgrund J. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008 Dec 1;33(25):2789–800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008 Feb 21;358(8):794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett MG1, Stein SC, Bartels RH. Cost-effectiveness of current treatment strategies for lumbar spinal stenosis: nonsurgical care, laminectomy, and X-STOP. J Neurosurg Spine. 2010 Jul;13(1):39–46. doi: 10.3171/2010.3.SPINE09552. [DOI] [PubMed] [Google Scholar]
- 25.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009 Jul;47(7 Suppl 1):S51–5. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 26.Whitmore RG, Schwartz JS, Simmons S, et al. Performing a Cost Analysis in Spine Outcomes Research: Comparing Ventral and Dorsal Approaches for Cervical Spondylotic Myelopathy. Neurosurgery. 2012;70:860–867. doi: 10.1227/NEU.0b013e3182367272. [DOI] [PubMed] [Google Scholar]
- 27.Tosteson AN, Skinner JS, Tosteson TD, Lurie JD, Andersson GB, Berven S, Grove MR, Hanscom B, Blood EA, Weinstein JN. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008 Sep 1;33(19):2108–15. doi: 10.1097/brs.0b013e318182e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tosteson AN, Lurie JD, Tosteson TD, Skinner JS, Herkowitz H, Albert T, Boden SD, Bridwell K, Longley M, Andersson GB, Blood EA, Grove MR, Weinstein JN SPORT Investigators. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008 Dec 16;149(12):845–53. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosteson AN1, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976) 2011 Nov 15;36(24):2061–8. doi: 10.1097/BRS.0b013e318235457b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soegaard R, Bünger CE, Christiansen T, Høy K, Eiskjaer SP, Christensen FB. Circumferential fusion is dominant over posterolateral fusion in a long-term perspective: cost-utility evaluation of a randomized controlled trial in severe, chronic low back pain. Spine (Phila Pa 1976) 2007 Oct 15;32(22):2405–14. doi: 10.1097/BRS.0b013e3181573b2d. [DOI] [PubMed] [Google Scholar]
- 31.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009 Jul 1;302(1):58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 32.Glassman SD, Carreon LY, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, Dimar JR. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008 Dec 15;33(26):2843–9. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 33.Carreon LY, Glassman SD, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, Dimar JR., 2nd RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost-utility study. Spine (Phila Pa 1976) 2009 Feb 1;34(3):238–43. doi: 10.1097/BRS.0b013e31818ffabe. [DOI] [PubMed] [Google Scholar]
- 34.Kohn Linda T, Corrigan Janet M, Donaldson Molla S. To Err Is Human: Building a Safer Health System. Vol. 1. Washington, DC: Institute of Medicine & Committee on Quality of Health Care in America; Nov 1, 1999. [Google Scholar]
- 35.New Study Estimates Eight Million American Families Experienced a Serious Medical or Drug Error. The Commonwealth Fund; 2002. [Google Scholar]
- 36.Holahan J, Blumberg LJ, McMorrow S, Zuckerman S, Waidmann T, Stockley K. Health Policy Center Occasional Paper. Washington, DC: The Urban Institute; 2011. Containing the Growth of Spending in the U.S. Health System. http://www.urban.org/url.cfm?ID=412419. [Google Scholar]
- 37.Institute of Medicine. The healthcare imperative: Lowering costs and improving outcomes. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 38.Keckley PH, Coughlin S, Gupta S. Value-based purchasing: a strategic overview for health care industry stakeholders. [Accessed October 16, 2012];Deloitte Center for Health Solutions. ( http://www.deloitte.com/assets/Dcom-UnitedStates/Local%20Assets/Documents/Health%20Reform%20Issues%20Briefs/US_CHS_ValueBasedPurchasing_031811.pdf)
- 39.Angevine PD, Zivin JG, McCormick PC. Cost-effectiveness of single-level anterior cervical discectomy and fusion for cervical spondylosis. Spine (Phila Pa 1976) 2005 Sep 1;30(17):1989–97. doi: 10.1097/01.brs.0000176332.67849.ea. [DOI] [PubMed] [Google Scholar]
- 40.Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ, Oglesby M, Pelton MA, Andersson GB, Isayeva D, Jegier BJ, Phillips FM. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 2013 Nov 16; doi: 10.1016/j.spinee.2013.10.053. pii: S1529-9430(13)01723-3. [DOI] [PubMed] [Google Scholar]
- 41.Abdu WA, Lurie JD, Spratt KF, Tosteson AN, Zhao W, Tosteson TD, Herkowitz H, Longely M, Boden SD, Emery S, Weinstein JN. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976) 2009 Oct 1;34(21):2351–60. doi: 10.1097/BRS.0b013e3181b8a829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, Mummaneni P, Watters WC, 3rd, Wang J, Walters BC, Hadley MN American Association of Neurological Surgeons/Congress of Neurological Surgeons. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005 Jun;2(6):679–85. doi: 10.3171/spi.2005.2.6.0679. [DOI] [PubMed] [Google Scholar]
- 43.Lee MJ1, Cizik AM2, Hamilton D2, Chapman JR. Predicting medical complications after spine surgery: a validated model using a prospective surgical registry. Spine J. 2014 Feb 1;14(2):291–9. doi: 10.1016/j.spinee.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.