Abstract
Purpose. The interaction of hormones of the pituitary-adrenal axis and adrenal cortex-associated circulating microRNAs is mostly unknown. We have studied the effects of dexamethasone and adrenocorticotropin on the expression of five circulating microRNAs (hsa-miR-27a, hsa-miR-200b, hsa-miR-214, hsa-miR-483-5p, and hsa-miR-503) reported to be related to the adrenal cortex in plasma samples. Methods. Expression of microRNAs was studied in plasma samples of 10 individuals examined by 1 mg dexamethasone suppression test and another 10 individuals stimulated by 250 μg tetracosactide (adrenocorticotropin). Total RNA was isolated and microRNA expression was analyzed by real-time reverse transcription quantitative polymerase chain reaction normalized to cel-miR-39 as reference. Results. Only circulating hsa-miR-27a proved to be significantly modulated in vivo by hormonal treatments: its expression was upregulated by dexamethasone whereas it was suppressed by adrenocorticotropin. Secreted hsa-miR-27a was significantly induced by dexamethasone in vitro in NCI-H295R cells, as well. The expression of hsa-miR-483-5p proposed as diagnostic marker for adrenocortical malignancy was not affected by dexamethasone or tetracosactide administration. Conclusions. hsa-miR-27a expression is modulated by hormones of the hypothalamic-pituitary-adrenal axis both in vitro and in vivo. The biological relevance of hsa-miR-27a modulation by hormones is unclear, but the responsiveness of circulating microRNAs to hormones of the pituitary-adrenal axis is noteworthy.
1. Introduction
MicroRNAs (miRNA, miR) are short nonprotein coding RNA molecules involved in the posttranscriptional regulation of gene expression as parts of the epigenetic machinery. MicroRNAs were shown to be implicated in the regulation of several basic homeostatic processes like cell proliferation, apoptosis, development, immune regulation, hormone secretion, and so forth, [1]. Alterations of tissue microRNA profiles have been described in a wide array of diseases, for example, atherosclerosis, inflammatory diseases, and tumors [1–3]. Beside tissue microRNAs, novel data show that microRNAs are released in the circulation by three main mechanisms: (i) passive release from damaged cells (inflammation, necrosis), or (ii) active release packed in membrane vesicles (microvesicles, exosomes, and apoptotic bodies), or (iii) active release in complex with macromolecules like high density lipoprotein or Argonaute proteins [4]. The physiological function of circulating microRNAs is mostly unknown, but it is hypothesized that they might act as hormones conveying epigenetic information to distant tissues [5].
There are some data that the expression of tissue microRNAs is affected by hormones. Tissue microRNA profiles of steroid-producing organs have been shown to be modulated by treatment with hormones, for example, adrenocorticotropin (ACTH), dexamethasone, and estradiol [6, 7]. There are also findings showing that circulating microRNA levels might also be influenced by hormone actions [8]. To the best of our knowledge, the circulating microRNA levels after administration of dexamethasone and ACTH affecting the hypothalamic-pituitary-adrenal axis have not been studied in humans in vivo, yet.
Adrenocortical cancer (ACC) is a rare tumor with an incidence of 0.5–2/million/year. The preoperative diagnosis of malignancy in adrenal tumors is very difficult. It is rather difficult to establish malignancy in small tumors and to exclude it in large adrenal tumors. Some circulating microRNA biomarkers, including hsa-miR-483-5p, have been proposed as promising markers of malignancy in ACC [9–11].
Keeping in mind that during the evaluation of an adrenal mass dexamethasone suppression and ACTH stimulation tests are widely used diagnostic approaches, the question might be raised whether the expression level of circulating microRNAs including proposed biomarkers for adrenocortical malignancies is affected during these functional endocrine tests.
Our objective has been to study whether the expression of selected circulating microRNAs is affected by dexamethasone and ACTH administration in vivo in plasma samples of humans. We have included microRNAs used in the diagnosis of adrenocortical cancer to assess whether their plasma levels are affected by these treatments.
We have selected five microRNAs (hsa-miR-27a, hsa-miR-200b, hsa-miR-214, hsa-miR-483-5p, and hsa-miR-503) whose tissue counterparts were shown to be modulated by ACTH or dexamethasone in an animal model (hsa-miR-27a, hsa-miR-200b, hsa-miR-214, and hsa-miR-503) [7] and/or involved in the pathogenesis of ACC (hsa-miR-214, hsa-miR-483-5p, and hsa-miR-503) [9–11]. hsa-miR-483-5p is overexpressed not only in the tissue of adrenal cancer but also as a circulating microRNA in patient's blood [9–11]. We have studied these selected microRNAs in altogether 20 individuals examined for hypercortisolism (Cushing's syndrome) by low-dose dexamethasone test [12] and for adrenal insufficiency or late onset congenital adrenal hyperplasia (21-hydroxylase deficiency) by ACTH (tetracosactide) test [13].
2. Subjects and Methods
2.1. Patients
10 patients were tested for suspected hypercortisolism by low-dose overnight (1 mg) dexamethasone suppression test suffering from obesity, hirsutism, hypertension, and adrenal incidentaloma. Another 10 patients have been examined by 250 μg tetracosactide (Cosyntropin, Sandoz Inc.) for suspected Addison's disease or late onset congenital adrenal hyperplasia (deficiency of 21-hydroxylase) suffering from weakness, secondary oligomenorrhea, infertility, or hirsutism. Baseline cortisol was taken between 7.00 and 9.00 a.m. in fasting condition. Dexamethasone was taken at 11.00 pm, and blood was drawn the next morning between 7.00 and 9.00 a.m. Blood was taken one hour after tetracosactide administration. Patient data are included in Table 1. All tested individuals have been eventually found to be free from any functional disturbance of the hypothalamic-pituitary-adrenal axis. The study was approved by the Ethical Committee of the Hungarian Health Council and informed written consent was obtained from all patients involved.
Table 1.
Characteristics of patients.
(a) Dexamethasone test
| Patient number | Gender F/M |
Age (year) | Disease/indication for testing | Baseline plasma cortisol (μg/dL) | Cortisol after 1 mg Dex (μg/dL) |
|---|---|---|---|---|---|
| 1 | F | 73 | Obesity | 12.6 | 1.6 |
| 2 | F | 30 | Obesity, hirsutism | 19.5 | 0.5 |
| 3 | M | 65 | Obesity | 10.7 | 0.9 |
| 4 | M | 45 | Hypertension | 17.4 | 0.9 |
| 5 | F | 61 | Obesity, hypertension | 9.4 | 1.6 |
| 6 | F | 68 | Adrenal incidentaloma | 13.5 | 1.8 |
| 7 | M | 65 | Adrenal incidentaloma | 25.6 | 1.7 |
| 8 | M | 68 | Adrenal incidentaloma | 19.4 | 1.7 |
| 9 | M | 59 | Adrenal incidentaloma | 17.6 | 1.8 |
| 10 | F | 20 | Obesity | 20.0 | 1.3 |
(b) Tetracosactide test
| Patient number | Gender F/M |
Age (year) | Disease/indication for testing | Baseline plasma cortisol (μg/dL) | Cortisol after 250 μg tetracosactide (μg/dL) |
|---|---|---|---|---|---|
| 1 | F | 30 | Sec. amenorrhea | 14.4 | 35.8 |
| 2 | F | 46 | Suspicion for AI | 6.6 | 20.7 |
| 3 | F | 36 | Weakness | 13.4 | 31.4 |
| 4 | F | 23 | Raromenorrhoea | 16.3 | 33.9 |
| 5 | F | 36 | Infertility | 7.2 | 29.5 |
| 6 | F | 37 | Sec. amenorrhea | 14.2 | 35.4 |
| 7 | F | 34 | Raromenorrhoea | 26.0 | 35.7 |
| 8 | F | 23 | Hirsutism | 16.0 | 32.3 |
| 9 | M | 60 | Suspicion for AI | 15.4 | 28.0 |
| 10 | F | 23 | Infertility | 11.0 | 34.4 |
AI: adrenal insufficiency.
2.2. RNA Isolation and Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) from Plasma Samples
RNA isolation has been performed as described in our previous study [10]. Briefly, EDTA-anticoagulated blood was taken from patients and centrifuged at 3000 rpm for 20 minutes at 4°C. All extracted plasma samples were stored at −80°C until further processing.
Total RNA was isolated from 200 μL plasma with Qiagen miRNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol with minor modifications, as described earlier [10]. RNA concentration was measured by NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA), and the quality and quantity were determined by an Agilent 2100 Bioanalyzer (Agilent Tech. Inc., Santa Clara, CA, USA). RNA Integrity (RIN) numbers of RNA isolated from plasma samples were low (around 2.0), that is, similar to reported findings on RNA isolated from blood [14]. RNA was stored at −80°C until use.
10 ng of total RNA was reverse transcribed with TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and the specific looped RT primer. RT-qPCR was performed by TaqMan Fast Universal PCR Master Mix (2x) (Applied Biosystems) on a 7500 Fast Real-Time PCR System (Applied Biosystems) according to the manufacturer's protocol. The following probes have been used: hsa-miR-27a (000408), hsa-miR-200b (002251), hsa-miR-214 (002306), hsa-miR-483-5p (002338), hsa-miR-503 (001048), and cel-miR-39 (000200) as reference gene [15]. Samples were run in triplicate.
2.3. In Vitro Treatment of NCI-H295R Cells with Dexamethasone
The NCI-H295R adrenocortical carcinoma cell line was purchased from the American Type Culture Collection and maintained in the recommended media. For treatments, hormone-free fetal bovine serum (FBS) was prepared as follows: 0.1 g dextran coated charcoal (C6241, Sigma-Aldrich, St. Louis, MO) was added to 6 mL FBS and incubated for 24 h at 4°C. Then the mixture was centrifuged at 3000 ×g for 10 min and the supernatant was filtered through a 0.22 μm filter. Cells were seeded on 6-well plates as 106 cells/well using media containing 2.5% hormone-free FBS. Next day, cells were synchronized by serum starvation for 24 h. On the following day, 2.5% hormone-free FBS was added in the presence of 100 nM dexamethasone or vehicle (DMSO). After 8 h incubation, cells and supernatans were harvested and total RNA was extracted. Dexamethasone treatments were repeated four times.
Total RNA was extracted using miRNeasy Mini Kit (Qiagen) both from cells and culture medium according to the manufacturer's protocol with minor modifications, as described earlier [16]. RNA concentration was measured by NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific Inc.). RIN numbers determined by an Agilent 2100 Bioanalyzer (Agilent Tech. Inc., Santa Clara, CA, USA) varied between 9.0 and 10.0. RNA was stored at −80°C until use. RT-qPCR reactions were performed by Taqman miRNA Assays (Applied Biosystems) using specific primer/probe combinations: hsa-miR-27a (000408) and cel-miR-39 (000200) as reference gene [15].
2.4. Statistical Analysis
To identify microRNAs showing significant expression changes, Student's t-test or Mann-Whitney U test was used depending on the results of Shapiro-Wilks normality test [10]. Data were expressed as ΔCt; thus higher ΔCt indicates lower expression, whereas lower ΔCt shows higher expression. Statistical analysis of RT-qPCR data was done by Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA) software.
3. Results
3.1. Expression of Circulating MicroRNAs in Dexamethasone and ACTH Stimulation Tests In Vivo
From the five microRNAs selected, only one circulating microRNA, hsa-miR-27a, turned out to be significantly modulated by dexamethasone and tetracosactide treatment in our study. Most interestingly, dexamethasone and tetracosactide treatments resulted in opposite changes of hsa-miR-27a expression as dexamethasone upregulated its plasma level, whereas tetracosactide suppressed its expression (Figures 1 and 2).
Figure 1.
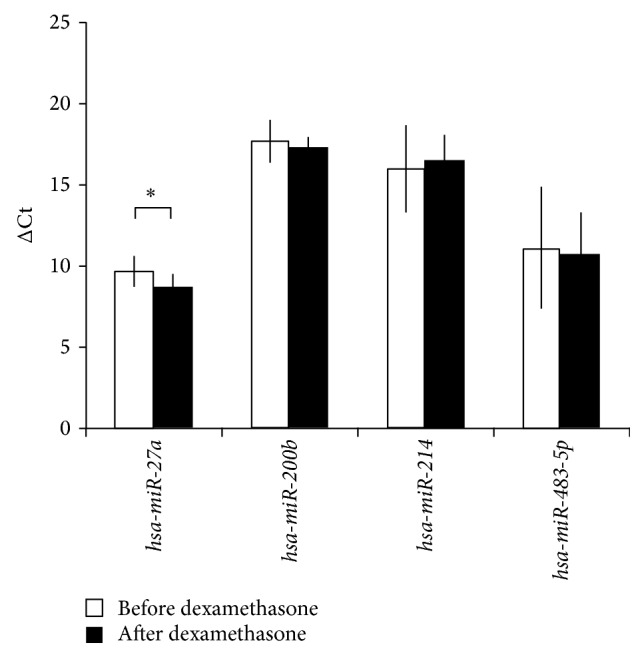
Expression change of microRNAs in plasma after 1 mg overnight dexamethasone test normalized to cel-mir-39. ΔCt values are represented: increased ΔCt indicates lower expression, whereas decreased ΔCt indicates higher expression (mean ± SD). ∗ p < 0.05, n = 10. t-test was performed following the Shapiro-Wilks normality test.
Figure 2.
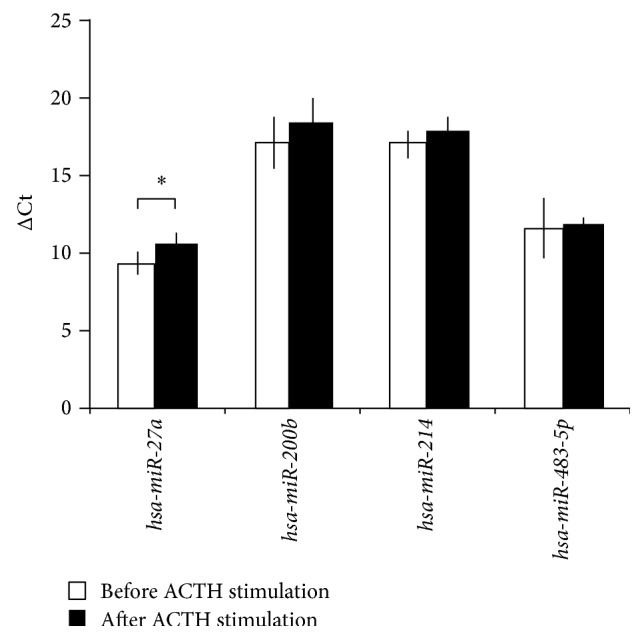
Expression change of microRNAs in plasma after 250 μg tetracosactide test, normalized to cel-mir-39. ΔCt values are represented: increased ΔCt indicates lower expression, whereas decreased ΔCt indicates higher expression (mean ± SD). ∗ p < 0.05, n = 10. Results of Mann-Whitney U test.
The expression of hsa-miR-503 proved to be so low in the plasma samples that we have decided to exclude it from further analysis (data not shown). We have not found any correlation between the changes of cortisol levels and circulating microRNAs neither in the dexamethasone nor in the tetracosactide tests (data not shown). There has been no correlation between basal hsa-miR-27 levels and body weight either.
To confirm the dexamethasone responsiveness of hsa-miR-27a, we have performed in vitro experiments on the adrenocortical NCI-H295R cell line. We have observed dexamethasone responsiveness in vitro, as well. Dexamethasone significantly induced secreted hsa-miR-27a expression in NCI-H295R culture medium (Figure 3). Dexamethasone induced intracellular hsa-miR-27a in NCI-H295R cells too, but this has not reached statistical significance (data not shown). These results demonstrate that hsa-miR-27a is secreted by NCI-H295R cells and the level of secreted hsa-miR-27a is induced by dexamethasone, as well.
Figure 3.
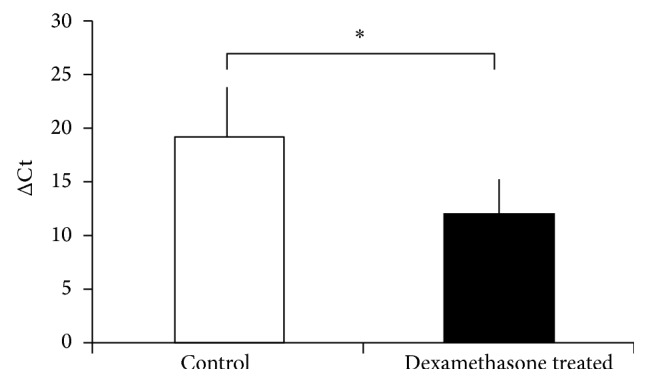
Expression change of secreted hsa-miR-27a after 100 nM dexamethasone treatment for 8 hours in the NCI-H295R adrenocortical cell line mediums normalized to cel-mir-39. ΔCt values are represented: increased ΔCt indicates lower expression, whereas decreased ΔCt indicates higher expression (mean ± SD). ∗ p < 0.05, n = 4. Results of t-test.
4. Discussion
We have found that the expression of circulating hsa-miR-27a is modulated by hormonal treatments in vivo in humans, as its expression is induced by dexamethasone and suppressed by ACTH. Dexamethasone induced secreted hsa-miR-27a in vitro, as well. The expression of the most promising circulating microRNA marker of adrenocortical malignancy, hsa-miR-483-5p, was not affected by these treatments also used as diagnostic tests supporting its applicability as a biomarker.
Circulating microRNAs are promising biomarkers in several diseases including tumors and atherosclerosis [17]. There are some data that their levels might be affected by hormonal changes, for example, in patients suffering from polycystic ovarian syndrome, the serum concentration of four microRNAs appeared to be in part correlated with serum free testosterone concentration [8].
To the best of our knowledge, the association of circulating microRNAs and the hormonal actions affecting the hypothalamic-pituitary-adrenal axis in vivo has not been explored in humans, yet. Some circulating microRNAs have been proposed as useful biomarkers for prediction of malignancy of adrenocortical tumors [9–11]. Since adrenocortical cancer is frequently associated with adrenocortical hormone overproduction [18, 19], the potential association of hypothalamic-pituitary-adrenal axis functioning and circulating microRNA levels might also be of interest. However, no data about the expression changes of circulating microRNAs during dexamethasone or ACTH-tests have been presented to date.
We have selected five circulating microRNAs for studying their responsiveness to dexamethasone and ACTH administration in vivo. Among these, hsa-miR-214, hsa-miR-503, and hsa-miR-483-5p have been proposed as tissue biomarkers for adrenocortical malignancy [20–22], and hsa-miR-483-5p has been found to be significantly overexpressed in blood samples of adrenocortical cancer patients, as well [9–11]. Moreover, the tissue expression of miR-214 and miR-503 was downregulated by ACTH in a rat model [7]. Two further microRNAs reported to be responsive to hormonal treatments in a rat model were included in our study: the expression of both hsa-miR-27a and hsa-miR-200b was shown to be downregulated by dexamethasone, whereas ACTH also downregulated hsa-miR-27a in rat adrenals [7].
The expression of the five selected circulating microRNAs has been studied in vivo using plasma samples of ten patients before and after low-dose dexamethasone testing and samples from ten patients before and after tetracosactide administration. All individuals included turned out to be eventually free from any functional abnormality of the hypothalamic-pituitary-adrenal axis.
From the microRNAs selected, only hsa-miR-27 turned out to be significantly modulated by hormonal treatments in vivo. The expression of the other four circulating microRNAs that were shown to be associated with the adrenal was not affected by dexamethasone or ACTH-administration. The stable expression of circulating hsa-miR-483-5p not affected by these hormones supports its applicability as a biomarker of adrenocortical cancer. Our study has certainly limitations, since healthy individuals were tested, and a different response in ACC patients cannot be excluded.
Dexamethasone and tetracosactide treatments resulted in opposite changes of hsa-miR-27a expression as dexamethasone upregulated its plasma levels, whereas tetracosactide suppressed its expression (Figures 1 and 2). In addition, secreted hsa-miR-27a was significantly induced by dexamethasone treatment in vitro in the NCI-H295R adrenocortical cell line as well. Our findings in culture medium underline that dexamethasone induces the secretion of hsa-miR-27a from adrenocortical cells (Figure 3). The molecular mechanism of hsa-miR-27a secretion in NCI-H295R cells and its interaction with dexamethasone, however, awaits further studies. In a rat model, ACTH-treatment also suppressed tissue miR-27a expression, but dexamethasone did the same. This discrepancy might be related to species differences; moreover, the expression of tissue and circulating microRNAs can be different [11].
The in vitro and in vivo action of dexamethasone on hsa-miR-27a expression is similar, since it upregulated hsa-miR-27a expression in both NCI-H295R adrenocortical cells in vitro (both cellular and secreted) and circulating hsa-miR-27a in vivo. The cellular origin of circulating microRNAs is, however, unclear, but these parallel changes in expression might raise the possibility of its partial adrenocortical origin. There are several data underlining the relevance of hsa-miR-27a in muscles, angiogenesis, adipogenesis and obesity, inflammation, immune response, lipid metabolism, atherosclerosis, and metabolic syndrome [23]. Circulating hsa-miR-27a has been raised as a biomarker for left ventricular contractility after acute myocardial infarction [24] and hypertrophic cardiomyopathy [25], and it was found to be underexpressed in early stage non-small cell lung cancer [26].
All these tissues are targets for glucocorticoid actions mediated via the glucocorticoid receptor. Since dexamethasone treatment also altered hsa-miR-27a expression in the NCI-H295R adrenocortical cell line, it might be hypothesized that this microRNA may be also regulated via the glucocorticoid receptor. As the transcription of hsa-miR-27a is made by RNA Polymerase II [27] it would be interesting to test whether a functional glucocorticoid response element is present in the hsa-miR-27a promoter (by in silico prediction, a glucocorticoid response element can be localized within the hsa-miR-27a promoter (data not shown)).
hsa-miR-27a has been shown to downregulate myostatin expression that is a major growth factor implicated in muscle development and muscle atrophy. Increased myostatin expression was associated with muscle wasting [28]. miR-27a and myostatin appear to be involved in an autoregulatory loop as myostatin increases miR-27a expression via SMAD3 and miR-27a in turn inhibits myostatin expression in a murine model [28]. As glucocorticoids inhibit the transcriptional activation of SMAD3 [29], administration of dexamethasone might interfere with the myostatin-SMAD3-miR-27a loop at multiple points. The ACTH-induced downregulation of circulating hsa-miR-27a might also be relevant, for example, in ACTH-dependent Cushing's syndrome. The overall effects of these actions on myostatin, miR-27a, and SMAD3 would be difficult to predict at present, but these findings might be implicated in the pathogenesis of glucocorticoid-induced muscle atrophy characteristic for hypercortisolism.
Levels of circulating hsa-miR-27a have been found to be strongly associated with fasting glucose levels and type 2 diabetes mellitus [30]. Since glucocorticoids are involved in the pathogenesis of insulin resistance [31], these findings raise the possibility that ACTH- and glucocorticoid-induced changes in hsa-miR-27a expression might be relevant in the pathogenesis of various diseases, and most of all in hypercortisolism, but further studies are needed to establish the pathological relevance of these alterations.
5. Conclusions
By analyzing the expression of selected microRNAs based on literature data, we have established that hsa-miR-27a is significantly downregulated by ACTH and induced by dexamethasone-treatment in vivo. We have also observed the in vitro induction of secreted hsa-miR-27a in adrenocortical NCI-H295R cells by dexamethasone. The expression of hsa-miR-483-5p proposed as a biomarker of ACC was not affected by hormonal treatments that underlines its applicability as a potential diagnostic test in the preoperative diagnosis of ACC. These data together highlight again that microRNAs are present in the circulation, and some of these are targets for hormone actions, and similarly to the hormone concentration measurement, strict preanalytical and analytical conditions should be followed before sampling.
Acknowledgments
This study has been funded by Hungarian Scientific Research Grant (OTKA K100295 to Peter Igaz; OTKA, PD100648 to Attila Patócs) and Technology Innovation Fund, National Developmental Agency (KTIA-AIK-2012-12-1-0010).
Conflict of Interests
The authors have no conflict of interests to report.
References
- 1.Alvarez-Garcia I., Miska E. A. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 2.Malumbres M. MiRNAs and cancer: an epigenetics view. Molecular Aspects of Medicine. 2013;34(4):863–874. doi: 10.1016/j.mam.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siasos G., Kollia C., Tsigkou V., et al. MicroRNAs: novel diagnostic and prognostic biomarkers in atherosclerosis. Current Topics in Medicinal Chemistry. 2013;13(13):1503–1517. doi: 10.2174/15680266113139990099. [DOI] [PubMed] [Google Scholar]
- 4.Redis R. S., Calin S., Yang Y., You M. J., Calin G. A. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacology and Therapeutics. 2012;136(2):169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortez M. A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A. K., Calin G. A. MicroRNAs in body fluids-the mix of hormones and biomarkers. Nature Reviews Clinical Oncology. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riester A., Issler O., Spyroglou A., Rodrig S. H., Chen A., Beuschlein F. ACTH-dependent regulation of MicroRNA as endogenous modulators of glucocorticoid receptor expression in the adrenal gland. Endocrinology. 2012;153(1):212–222. doi: 10.1210/en.2011-1285. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z., Shen W.-J., Cortez Y., et al. Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0078040.e78040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murri M., Insenser M., Fernández-Durán E., San-Millán J. L., Escobar-Morreale H. F. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. Journal of Clinical Endocrinology and Metabolism. 2013;98(11):E1835–E1844. doi: 10.1210/jc.2013-2218. [DOI] [PubMed] [Google Scholar]
- 9.Chabre O., Libé R., Assie G., et al. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocrine-Related Cancer. 2013;20(4):579–594. doi: 10.1530/erc-13-0051. [DOI] [PubMed] [Google Scholar]
- 10.Szabó D. R., Luconi M., Szabó P. M., et al. Analysis of circulating microRNAs in adrenocortical tumors. Laboratory Investigation. 2014;94(3):331–339. doi: 10.1038/labinvest.2013.148. [DOI] [PubMed] [Google Scholar]
- 11.Patel D., Boufraqech M., Jain M., et al. MiR-34a and miR-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery. 2013;154(6):1224–1229. doi: 10.1016/j.surg.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandraki K. I., Grossman A. B. Novel insights in the diagnosis of Cushing's syndrome. Neuroendocrinology. 2010;92(1):35–43. doi: 10.1159/000314295. [DOI] [PubMed] [Google Scholar]
- 13.Husebye E. S., Allolio B., Arlt W., et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. Journal of Internal Medicine. 2014;275(2):104–115. doi: 10.1111/joim.12162. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. H., Jin H. O., Park J. A., Chang Y. H., Hong Y. J., Lee J. K. Comparison of three different kits for extraction of high-quality RNA from frozen blood. SpringerPlus. 2014;3(1, article 76):5. doi: 10.1186/2193-1801-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell P. S., Parkin R. K., Kroh E. M., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butz H., Likó I., Czirják S., et al. MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary. 2011;14(2):112–124. doi: 10.1007/s11102-010-0268-x. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzenbach H., Nishida N., Calin G. A., Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nature Reviews Clinical Oncology. 2014;11(3):145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 18.Fassnacht M., Kroiss M., Allolio B. Update in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism. 2013;98(12):4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 19.Terzolo M., Daffara F., Ardito A., et al. Management of adrenal cancer: a 2013 update. Journal of Endocrinological Investigation. 2014;37(3):207–217. doi: 10.1007/s40618-013-0049-2. [DOI] [PubMed] [Google Scholar]
- 20.Tömböl Z., Szabó P. M., Molnár V., et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocrine-Related Cancer. 2009;16(3):895–906. doi: 10.1677/erc-09-0096. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E. E., Holloway A. K., Weng J., Fojo T., Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117(8):1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soon P. S. H., Tacon L. J., Gill A. J., et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clinical Cancer Research. 2009;15(24):7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 23.Chen W.-J., Yin K., Zhao G.-J., Fu Y.-C., Tang C.-K. The magic and mystery of microRNA-27 in atherosclerosis. Atherosclerosis. 2012;222(2):314–323. doi: 10.1016/j.atherosclerosis.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Devaux Y., Vausort M., McCann G. P., et al. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070644.e70644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncarati R., Anselmi C. V., Losi M. A., et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2014;63(9):920–927. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Heegaard N. H. H., Schetter A. J., Welsh J. A., Yoneda M., Bowman E. D., Harris C. C. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. International Journal of Cancer. 2012;130(6):1378–1386. doi: 10.1002/ijc.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y., Kim M., Han J., et al. MicroRNA genes are transcribed by RNA polymerase II. The EMBO Journal. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarlane C., Vajjala A., Arigela H., et al. Negative auto-regulation of myostatin expression is mediated by Smad3 and MicroRNA-27. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0087687.e87687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song C.-Z., Tian X., Gelehrter T. D. Glucocorticoid receptor inhibits transforming growth factor-β signaling by directly targeting the transcriptional activation function of Smad3. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):11776–11781. doi: 10.1073/pnas.96.21.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karolina D. S., Tavintharan S., Armugam A., et al. Circulating miRNA profiles in patients with metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism. 2012;97(12):E2271–E2276. doi: 10.1210/jc.2012-1996. [DOI] [PubMed] [Google Scholar]
- 31.Geer E. B., Islam J., Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinology and Metabolism Clinics of North America. 2014;43(1):75–102. doi: 10.1016/j.ecl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


