Abstract
Background:
Adrenocortical cancer (ACC) is a rare disease that is difficult to treat. Laparoscopic adrenalectomy (LA) is performed, even for large adrenocortical carcinomas. However, the oncological effectiveness of LA remains unclear. This review presents the current knowledge of the feasibility and oncological effectiveness of laparoscopic surgery for ACC, with an analysis of data for outcomes and other parameters.
Database:
A systematic review of the literature was performed by searching the PubMed and Medline databases for all relevant articles in English, published between January 1992 and August 2014 on LA for adrenocortical carcinoma.
Discussion:
The search resulted in retrieval of 29 studies, of which 10 addressed the outcome of LA versus open adrenalectomy (OA) and included 844 patients eligible for this review. Among these, 206 patients had undergone LA approaches, and 638 patients had undergone OA. Among the 10 studies that compared the outcomes obtained with LA and OA for ACC, 5 noted no statistically significant difference between the 2 groups in the oncological outcomes of recurrence and disease-free survival, whereas the remaining 5 reported inferior outcomes in the LA group. Using a paired t test for statistical analysis, except for tumor size, we found no significant difference in local recurrence, peritoneal carcinomatosis, positive resection margin, and time to recurrence between the LA and OA groups. The overall mean tumor size in patients undergoing LA and OA was 7.1 and 11.2 cm, respectively (P = .0003), and the mean overall recurrence was 61.5 and 57.9%, respectively. The outcome of LA is believed to depend to a large extent on the size and stage of the lesion (I and II being favorable) and the surgical expertise in the center where the patient undergoes the operation. However, the present review shows no difference in the outcome between the 2 approaches across all stages. A poor outcome is likely to result from inadequate surgery, irrespective of whether the approach is open or laparoscopic.
Keywords: Adrenocortical carcinoma, Laparoscopic adrenalectomy, Peritoneal carcinomatosis
INTRODUCTION
Adrenocortical carcinoma (ACC) is a rare malignancy that frequently presents as a large retroperitoneal tumor. It often occurs in young patients who present at advanced stages of the disease.1,2 The tumor is usually associated with poor prognosis because of its high rate of recurrence, even after complete resection.3–15 The 5-year survival rate ranges from 15 to 60%, which correlates with the stage of disease and continues to be disappointing.3–8 The incidence of ACC is estimated to be 2 per million, and it is responsible for 0.2% of all cancer deaths.16,17 Unfortunately, most of these patients present with large tumors with the likelihood that invasion into adjacent organs will be detected at diagnosis. The primary determinant of survival of patients with ACC depends on complete resection with negative margins.3–8 The overall recurrence rate for all disease stages is 17–85%, with R0 resection being associated with a recurrence of 23% versus 51% for R1 and R2.18 The role of radiation and chemotherapy is limited, and the effect of adjuvant mitotane is unproven.4 For patients with localized disease at presentation, oncologic outcome and the success of surgical therapy are dependent on the completeness of resection of the primary tumor, the surrounding retroperitoneal tissue, and the regional lymph nodes.15 In view of the fragility of these tumors, it is prudent for the surgeon to use an approach that provides adequate exposure and access to the surrounding tissue planes and structures.19 The suitability of the laparoscopic approach for treating ACC remains a topic of debate.20 Guidelines for minimally invasive treatment for adrenal pathology by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) do not recommend laparoscopic resection for tumors suspected to be malignant, particularly when they are more than 6 cm in size.21 This advice is based on concerns that the dissection plane is indistinct, making a laparoscopic en bloc resection oncologically inadequate, and heightens the risk of breach of the tumor capsule and cell dissemination, during excision of a large tumor. Several reports of experiences with laparoscopic adrenalectomy (LA) have shown inconsistent outcomes.3–15,20–59 However, some of the recent reports have shown comparable results between the laparoscopic and open approaches in patients after resection of large tumors (≤10 cm).6–8,10,12 Based on these reports, the European Society of Endocrine Surgeons has revisited its guidelines with the suggestion that LA can be considered for stage I and II ACC with tumors <10 cm in size.60 Are the limits of the envelope being stretched and new guidelines for resectability being drawn?
LA, which was initially described in 1992, has rapidly become the gold standard of treatment for benign adrenal tumors.51 It is associated with significantly decreased morbidity, shorter length of hospital stay, more rapid convalescence, and improved cosmesis compared with open resection.9,61,62 The favorable outcomes have led surgeons to expand their criteria for elective adrenal resection. Technological advances in laparoscopic surgery and significant surgical experience gained by surgeons over the years have, to a large extent, made this feasible. Hence, the indication for LA has been successfully expanded from benign lesions to large nonfunctioning malignant lesions. Nevertheless, the role of LA remains controversial for the treatment of ACC, a rare but highly aggressive neoplasm. Although there are several studies reporting favorable oncological outcome,6–8,11,13 there are others in which the authors have questioned the ability to perform complete tumor resection (R0) by LA, which is detrimental for a long-term cure of ACC.4,10,14–16 Early reports warned of tumor fragmentation and port site or peritoneal recurrence of carcinomatosis, which were related to technical problems in the laparoscopic approach.40,63 To investigate these concerns, we conducted a systematic review of the available literature. The relevant literature included articles that dealt with LA for ACC and those that compared the outcome between LA and open adrenalectomy (OA).
LITERATURE SEARCH
A systematic review of the literature was performed by searching the PubMed and Medline databases for all relevant articles in English published between 1992 and August 2014. All articles were related to patients older than 16 years and were extracted by using the MeSH (Medical Subject Headings) terms “adrenal gland neoplasm” and “laparoscopy.” Duplication of data in multiple publications by the same authors, letters, and review articles were excluded. Primary adrenal tumors other than carcinoma and LA for metastatic tumors were excluded. For comparing observations of the studied parameters for the LA and OA approaches, the median score was taken as the single representative value of the related study, and the LA and OA observations were considered as a paired sample for the variable under study. Application of the Kolmogorov-Smirnov test showed that all samples followed a normal distribution pattern, and consequently, the paired t test was used to test the significance of differences between the LA and OA groups for the studied parameters, which included local recurrence rate, positive resection margin, peritoneal carcinomatosis, and time to recurrence
RESULTS
The search and the selection process led to retrieval of 29 studies, of which 10 articles addressed LA versus OA as the major topic (Table 1) and included 844 patients eligible for this review. Of these, 206 had undergone LA, and the remaining 638 had undergone OA. The mean size of tumors in patients who underwent LA (7.1 cm) was significantly smaller than those in patients who underwent OA (11.2 cm) (P = .0003). The mean overall recurrence rate observed in the LA and OA groups was 61.5 and 57.9% (P = .574), respectively, during a median follow-up of 35.5 months. The site of recurrence between LA and OA included peritoneal carcinomatosis (29.0% vs 9.3%; P = .066) and local recurrence rate (34.5% vs 34.5%), with a positive resection margin rate (21.1% vs 17.0%; P = .542). During the mean follow-up period of 35.5 months, the overall survival of those whose outcomes were expressed in months was 67.5 and 61.0 months for the LA and OA groups, respectively, and for those whose outcomes were expressed as a percentage, survival was 66.8% and 63.5%, respectively. The remaining 19 publications were mainly case series of LA (minor studies), and the overall sample amounted to 151 patients. Most of the articles reported series of LA for adrenal masses, of which ACC formed a small subgroup. These reports are summarized in Table 2. In this group, the median size of the lesions was 6.4 cm (3.1–9 cm). The overall recurrence rate in patients who underwent LA was 26%, with 11.8% having peritoneal carcinomatosis and 7.8% having local recurrence, with a positive resection margin of 6.2%. The overall survival during a mean follow-up period of 22 months was 41 months and, for those whose data were expressed as a percentage, it was 84.3%. As stated above, the paired t test was used to determine the significance of the difference between the study parameters in the LA and OA groups. Except for the tumor size (LA mean = 7.1, OA mean = 11.2; P = .0003), no significant difference was found between LA and OA observations, for any of the parameters under study. The error bars showing the mean ± SD of the 2 groups for the parameters are shown in Figure 1.
Table 1.
Major Studies Comparing LA with OA for ACC
| Author | Year | No. ACC | No. of Patients LA/OA | Median Tumor Size (cm, LA/OA) | Overall Recurrence (% LA/OA) | P Car (% LA/OA) | Median TTR (Months LA/OA) | PRM (%, LA/OA) | LRR (%, LA/OA) | Overall Survival (LA/OA) | Median Follow-Up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donatini et al6 | 2014 | 34 | 13/21 | 5.5/6.8 | 31/24 | NR | 4/5 | NR | 7/9 | 85/81a | 60 |
| Mir et al4 | 2013 | 44 | 18/26 | 7/13 | 55/46 | 5/0 | 9.7/13.8 | 39/38 | NR | 54/58a | 22 |
| Cooper et al16 | 2013 | 92b | 46/46 | 8/12.3 | 76/58 | 54/20 | 10.9/19.6 | 28.3/8.7 | 76/58 | 54/110c | 29/38 |
| Fossa et al11 | 2013 | 32 | 17/15 | 8/13 | 70/100 | 2/0 | 15/8 | 5/3 | 3/5b | 103/36c | 29/8 |
| Lombardi et al13 | 2012 | 156 | 30/126 | 7.3/9 | 26/38 | NR | 29/27 | 0/0 | 21/19 | 108/60c | 42 |
| Brix et al7 | 2012 | 152 | 35/117 | 6.2/8 | 77/69 | 3/3 | 24.2/21.5 | 8.6/15.4 | 77/69 | ND | 39.3 |
| Miller et al10 | 2010 | 88 | 17/71 | 7/12.3 | 63/65 | 18/11 | 9.6/19.2 | 50/18 | 25/20 | NR | 36.5 |
| Porpiglia et al8 | 2010 | 43 | 18/25 | 9/10.5 | 50/65 | 0/0 | 23/18 | NR | 33/24 | 95/72a | 35 |
| Leboulleux et al14 | 2010 | 64 | 6/58 | 7/14 | 67/27 | 67/27 | 20 | 17/36 | 34/72 | 5/38c | 35 |
| Gonzalez et al15 | 2005 | 139 | 6/133 | 6/13 | 100/86 | 83/13 | NR/13 | NR | 50/38 | 33 /43a | 28 |
| 844 | 206/638 | 7.1/11.2 | 61.5/57.9 | 29/9.7 | 16.11/16.5 | 21.1/17 | 36.2/34.8 | 67.5/61c 66.8/63.5a |
35.6 |
Abbreviations: P Car=peritoneal carcinomatosis; TTR=time to recurrence; LRR=local recurrence rate; PRM=positive resection margin; NR=not reported; ND=no difference between the 2 groups, with an HR for death of 0.79 (95% CI, 0.36–1.72).
Percentage.
Includes only cases performed in the authors' hospital and not the ones referred to them after initial operation in another hospital.
Months.
Table 2.
Studies of the Outcome of LA for ACC Not Included in Table 1
| Author | Year | ACC Patients (n) | Median Tumor Size (cm) | Overall recurrence (%) | P Car (%) | Median TTR (Months) | PRM (%) | LRR (%) | Survival (% or Months) | Median Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Greco et al50 | 2011 | 34 | NR | 2.9a | 2.9a | NR | 0 | 0 | NR | NR |
| Gaujoux et al58 | 2011 | 8b | >6 | 12.5 | 0 | NR | NR | 12.5 | 32c | 32 |
| Lupascu et al57 | 2011 | 4 | 7.0–7.4 | NR | 75 | 0 | 19 | NR | 25d | 28.9 |
| Conzo et al56 | 2009 | 8 | <4 to >6 | NR | NR | NR | NR | NR | NR | NR |
| Mellon et al59 | 2008 | 3 | 4.9 ± 2.1 | 0 | O | – | 0 | 0 | 100d | 27.4 |
| Castillo et al48 | 2008 | 38 | 7.3 ± 3.5 | NR | NR | NR | NR | NR | NR | NR |
| Soon et al64 | 2008 | 3e | 3.1 | 0 | 0 | – | NR | 0 | 100d | 18 |
| Parnaby et al55 | 2008 | 3 | 6 | 0 | 0 | – | NR | 0 | 100d | 48 |
| Hemal et al53 | 2008 | 5 | 7.8 | 60 | 0 | NR | NR | 0 | 13–60 | |
| Nocca et al30 | 2007 | 4 | 8.5 | 25 | 0 | 75d | 34 | |||
| Lodin et al54 | 2007 | 5 | 3.5–8 | 20 | NR | – | NR | NR | – | 58 |
| Liao et al45 | 2006 | 4 | 5–8.5 | 75 | 75 | NR | 0 | 25 | 6–38 | |
| Lombardi et al52 | 2006 | 4e | 5.9 | 25 | 25 | NR | NR | O | 100d | 23 |
| Palazzo et al33 | 2006 | 3 | 6.9 | 33 | 0 | NR | NR | 0 | 75d | 13–32 |
| Moinzadeh et al49 | 2005 | 6 | 3.6–9 | 60 | 0 | NR | 25 | 33 | 33c | 26 |
| Castilho et al48 | 2003 | 4 | <5 | 0 | 0 | 0 | 0 | 0 | 17c | 17 |
| Kebebow et al26 | 2002 | 5 | 6.6 | 60 | 0 | NR | 0 | 40 | 40c | 39 |
| Henry et al43 | 2002 | 6f | 7.7 | 16.6 | 0 | 20 | NR | 0 | 83c | 8–83 |
| Hobart et al47 | 2000 | 4 | 8 | 0 | 0 | – | NR | 0 | 100d | 9.9 |
| Total | 151 | 6.4 (3.1–9) | 26 (0–75) | 11.8 (0–75) | – | 6.2 (0–25) | 7.8 (0–40) | 41 (months) 84.3% | 23 |
Abbreviations: NR=not reported; ACC=adrenocortical cancer; P Car=peritoneal carcinomatosis; rec=recurrence; PRM=positive resection margin; LRR=local recurrence rate.
Port site metastasis.
Includes 4 conversions.
Months.
Percentage.
Includes1 conversion.
Ncludes of 2 conversions.
Figure 1.
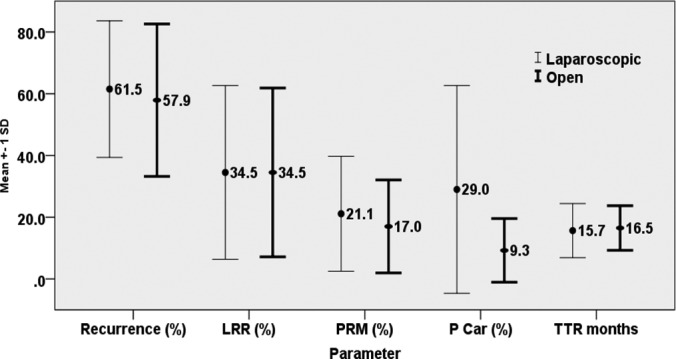
Comparisons of several LA and OA outcome parameters. LRR, local recurrence rate; PRM, positive resection margin; P Car, peritoneal carcinomatosis; TTR, time to recurrence.
DISCUSSION
The emphasis of this review was on studies that compare the results between OA and LA. Unfortunately, studies addressing the comparison of outcomes between LA and OA were limited to 10. In addition, some of these studies were deficient in outlining the follow-up, surveillance, and statistical analysis. Moreover, most of them were retrospective, with the potential risk of having a heterogenous sample of patients, thus raising the question of selection bias.
Outcomes: Disease-Free Survival, Local Recurrence, Peritoneal Metastasis, and Mortality
The data were extracted from 10 studies that compared the oncologic outcome of 2 approaches (LA and OA) after adrenalectomy (Table 1). They were broadly grouped into those that were favorable and those that were clearly against the laparoscopic approach. Five of the most recent studies showed comparable outcomes between the 2 groups,6,8,11,13,25 whereas the remainder did not.10,14–16
In a recent study, Donatini et al6 reported a comparable outcome between LA and OA for patients with ACC stages I or II and with tumors <10 cm in size. Although the patients undergoing LA had a shorter length of hospital stay and no difference in morbidity, the approach did not compromise the long-term oncological outcome in these patients. After a similar follow-up (66 ± 52 months for LA and 51 ± 43 months for OA), the disease-specific and disease-free survivals were identical.
Fossa et al 11 echoed similar observations of favorable outcomes in their recent study, involving 32 patients with ACC stages I–III, of whom 17 underwent LA and 15, OA. They noted that LA offered both short-term advantages and similar long-term outcomes, when compared with OA. The 2 groups (LA and OA) were similar in local, peritoneal, and distant metastases; the median progression-free survival was 15.2 months versus 8.1 months; and the overall survival was 103.6 months versus 36.5 months.
In one of the largest series (152 patients) with tumors <10 cm, Brix et al7 observed that, in patients with ACC of limited size, survival after LA and OA was comparable when the procedures were performed by an experienced surgeon. They noted that, in patients undergoing adrenalectomy (LA: median size, 6.2 cm; OA: median size, 8 cm; P < .001), 35 underwent LA, with 12 conversions to open, and 117 underwent OA). Tumor recurrence and peritoneal carcinomatosis of 77% and 3% were seen in patients after LA versus 69% and 3% of patients after OA, respectively; the difference, however, was not statistically significant. In a matched-pair analysis, disease-specific and disease-free survival did not differ between the LA and OA groups (hazard ratio [HR] for death: 0.79 [95% confidence interval [CI], 0.36–1.72; P = .55; HR for recurrence: 1.07 [95% CI, 0.61–1.87]; P = .82).7 Moreover, the 2 groups did not differ with regard to peritoneal metastasis or port site recurrence. Porpiglia et al8 were also in favor of LA for ACC in stages I and II, as the results were comparable with those of OA from an oncologic perspective. In their study of 43 patients, stage I or II, of whom 18 underwent LA, the median recurrence-free survival (LA 23 months vs OA 18 months; P = .8) and the percentage of patients still alive after a 3-year follow-up (OA 84% vs LA 100%; P = .3) was comparable in both groups. No difference was noted in patterns of recurrence, including port site recurrence.8
Lombardi et al13 also showed comparable results in the LA and OA groups. Analysis of their results among the 126 patients who underwent OA and 30 who underwent LA showed that the local recurrence was 19% for OA and 21% for LA (P = .497), and distant metastasis was 31% versus 17%. The mean recurrence time of 27 ± 27 and 29 ± 33 months, respectively (P = .839), and the 5-year disease-free survival (38% vs 58.2%) and 5-year survival rates (48% vs 67%; P = .200) were also comparable.
However, authors of several other studies have voiced concern regarding the laparoscopic approach for resection in ACC.10,14–16 Mir et al4 in a study of 44 cases of ACC, with 18 undergoing LA and 26 undergoing OA, although finding no statistically significant increase in recurrence and death between the 2 groups, recommended OA in patients with ACC.
Cooper et al16 noted that, despite the use of the LA approach for smaller tumors, these patients developed peritoneal carcinomatosis more frequently than those who underwent OA. Hence, the authors concluded that the oncological benefits of an open approach far outweigh the short-term benefits of minimally invasive surgery.
Miller et al10 who reviewed 88 patients with ACC of whom 17 had undergone LA in other institutions, reported that a positive resection margin and tumor capsule breach were more frequent in the LA group (LA 50%, OA 18%; P < .01), despite the smaller mean tumor size compared with that in patients who underwent OA (7.0 cm vs 12.3 cm). Tumor size influenced the mean time to recurrence, as it was significantly lower in LA group (LA, 9.6 months vs OA 19.2 months; P < .005). However, the overall recurrence rates were comparable (LA 63% vs OA 65%; P = .22).10 Similar discouraging results were reported by Leboulleux et al,14 who noted a significantly higher rate of peritoneal carcinomatosis in patients who underwent LA compared with those who underwent OA (HR, 3.8; [95% CI, 1.2–12.3]; P = .016). Gonzalez et al15 echoed the same concern of higher risk of local recurrence and peritoneal carcinomatosis in patients undergoing LA. In a large study of 160 patients of whom 6 underwent LA, the local recurrence and peritoneal carcinomatosis was 100% and 83% in the LA group compared with 35% and 8%, respectively, in the OA group.
Unfortunately, the dichotomy of results in oncological outcome in these studies is related to several factors, including the retrospective nature of the studies, and hence the inherent risk of bias is present. The variability in the experience of the surgeons, referral patterns, and volume of cases can significantly influence the outcome, particularly when LA is advocated for lesions >6 cm. The procedure being performed in nonspecialized centers could increase the risk of nonradical resection and capsule rupture, with imminent risk of local recurrence or peritoneal carcinomatosis. The significant difference between the results could also be caused by the variance in the baseline characteristics of the patients and tumors (smaller size of tumor, younger patients, lower stage tumor, and the use of adjuvant mitotane, which may influence the outcome favorably in some series). Moreover, some consider tumor biology to play a significant role in the outcome, as they note that low-grade tumors are less aggressive and tend to recur later, with metastasis to distant site at longer intervals than high-grade tumors.10
The following factors influence the feasibility and outcome of LA.
Tumor Size, Local Infiltration, and Recurrence
LA for ACC has been performed for lesions >6 and ≤10 cm with comparable oncological outcome.6,8,11,13,25 However, with progressive increase in size, the risk of capsule rupture increases, mainly during handling and dissection of a large tumor.10,40,63 Although some have reported comparable results with larger lesions with regard to tumor recurrence and positive margin,6,8,11,13,25 others have found that this risk increases proportionally with the size of the lesion.10,14,15 In one of the reports, the recurrence rate (local or peritoneal) was 38% and 20%, with a positive margin of 50% and 20% for patients undergoing resection of lesion <6 cm by LA or OA, respectively. However, when LA and OA were performed for lesions >10 cm the recurrence rate was 50 and 42%, with a positive margin of 50 and 7%, respectively.10 Preoperative imaging by magnetic resonance imaging (MRI), computed tomography (CT), and even positron emission tomography (PET) have dramatically improved the preoperative visualization and diagnosis of ACC and hence have aided in the decision of whether a laparoscopic approach is feasible or appropriate.65,66 This decision is of particular importance, as two-thirds of the lesions >6 cm are benign, and these patients should not be deprived of the advantage of laparoscopic resection because of inadequate diagnosis. In ACC lesions, the feature that must be clearly defined is a distinct fat plane between the adrenal gland, kidney, and inferior vena cava, depicting an absence of invasion of surrounding structures.19,65 In the presence of infiltration of adjoining structures, seen in about one-third of cases,19 and in the presence of intravenous thrombus, LA is contraindicated.9,16 Although there are reports of radical resection being performed laparoscopically on the left side for locally invasive ACC, with concomitant resection of adjoining structures including spleen, tail of the pancreas, and diaphragm,19,67,68 these are exceptions rather than recommendations to be used in routine practice.1,9 Such resection would invariably require an open approach.15,68
The increased risk of peritoneal carcinomatosis after LA compared to OA is a concern raised by some.10,14–16 However the present literature is inconclusive.6,8,10,13,14,16,25 Some find LA for ACC inappropriate,10,14–16 but other recent reports take a contrary view.6,8,11,13,25 The possibility of tumor cells being close to the capsule and the diminished tactile sensation in the laparoscopic compared to the open approach may increase the risk of shedding tumor cells on laparoscopic instruments without the surgeon's being aware of it.10 In this regard, the nature of recurrence (localized or generalized) is important. Some are of the opinion that LA increases the risk of a multifocal pattern of peritoneal recurrence, making these patients less likely to be candidates for salvage surgery.15,16 Patients who are amenable to complete surgical resection have been shown to have a significantly higher survival rate than those whose recurrence cannot be resected.69 As the only definitive treatment of ACC is complete margin-free (R0) surgical resection and avoiding violation of capsule integrity, all surgeons should endeavor to achieve this goal. LA performed in specialized centers may ensure this to a large extent. A high risk of recurrence is related to an initial tumor size of >8 cm, microscopic invasion of blood vessels and tumor capsule or a Ki-67 index of >10%.10,19,35,68
A Weiss score ≥3 is used as a pathologic criterion for the diagnosis of ACC.69,70 The greater the Weiss score, the higher the risk of recurrence.71 Some of these patients present with heterogeneous behavior of the tumor and remain healthy, even after 10 years,70 perhaps because the Weiss system has a gray zone for a tumor grade of 3, which may contain both adrenocortical adenoma and carcinoma.6,72 Some with adenoma may have better long-term outcomes than those with carcinoma; the latter are more likely to recur.69,70
Other Approaches
Robot-assisted LA for ACC has been reported.73,74 Although, once mastered, robotic surgery can be performed with comfort and ease, no distinct advantage has been attributed to this approach, particularly when considering the cost and learning curve of performing LA for a rare malignancy.73,74 Reports of retroperitoneoscopic adrenalectomy for ACC are limited.7,50,75 The procedure has been performed in occasional cases (5.7%) by some,7 whereas others have performed adrenalectomy by transabdominal and retroperitoneal approaches in equal numbers (50% of 34 cases).50 The potential advantages reported are avoiding breach of the peritoneum (and hence the entry of the intraperitoneal cavity), limiting the risk of recurrence locally (retroperitoneally) if it were to occur, and a limited hospital stay.50,75 In a recent report, miniretroperitoneoscopic adrenalectomy was performed with 3-mm instruments for lesions <6 cm with excellent outcome; however, conversion to conventional laparoscopy was necessary in 8% of the cases.76
Lymphadenectomy
The role of lymph node dissection (LND) in patients with ACC and its influence on the outcome is controversial.77–80 Periadrenal lymph nodes and those along the renal vessels are routinely excised by some in patients with stage I or II ACC,77,78 whereas others would limit it to cases that present in stage III with local invasion.79,80 The German ACC study group published the results after a median follow-up of 59 and 39 months, respectively, of 283 patients undergoing adrenalectomy with LND (47 cases) and without (236 cases).81 Multivariate analysis in this group revealed a significant reduction in the risk of recurrence and disease-related deaths and suggested an improvement in oncological outcome in patients who undergo wide local resection of ACC.81 However, there are others who report no improvement in oncological outcome, despite routine lymph node excision.82 Reports suggest that lymph node metastasis is not as frequent in patients with ACC as believed; in one of the reports, it was noted in only 10% of the cases (2/20 of LND).4,81 Despite the lack of evidence, the general trend is toward a more limited resection in stages I and II and extended resection in stage III.9
Conversion From Laparoscopic to Open Surgery
Laparoscopic conversion may be necessary in the event of technical difficulty, unanticipated tumor characteristics (such as local invasion), dense adhesions, intraoperative complications such as significant bleeding, and inability to adhere to oncological principles while performing the resection.7–9,58,83 Some suggest that even patients with large and potentially malignant nonfunctional tumors with no preoperative radiologic evidence of local invasion or metastasis should undergo a trial laparoscopic dissection.64 Conversion would be necessary in the presence of laparoscopic signs of invasion, regional lymphadenopathy, or aberrant vasculature.9,64,83 Some would beg to differ with this view and would expect the characteristics of the tumor and invasion to be delineated with the present imaging modalities well before surgery.16 The impact of conversion on the risk of recurrence and peritoneal carcinomatosis is not well established,3 even though there are such reports for other cancers.84 In one of the reports where conversion was necessary in one-third of the patients, no difference in outcome was noted between the 2 groups.7 However, an early conversion, before manipulation of the tumor or breach of the tissue plane or capsule disruption, logically should not affect the outcome negatively.
Experience of the Surgeon and Volume of Cases
The relation of surgical outcome to the surgeon's experience and the volume of cases managed in a center applies to all types of surgery, and LA is no exception. This association has been noted in some of the reports for patients undergoing LA for ACC.3,4,85–87 However, the number of cases a surgeon should perform per year to qualify, or his overall experience in LA, is arbitrary. The present view of some is that a surgeon should have performed at least 40 LAs4,87 for benign conditions or the center should treat a minimum of 10 to 20 such patients per year,3 before performing LA for ACC. This is a demanding surgery technically, particularly for large tumors, and hence should be performed in a specialized referral center.3,85,86 These centers, while providing the required expertise to perform this surgery, are likely to have the benefit of a multidisciplinary service to manage this rare malignancy.3
Uncertain Preoperative Diagnosis
Diagnostic uncertainty is likely to arise in patients with incidentalomas or small lesions without enough characteristics to suggest malignancy.56,78,87 The sensitivity and specificity of the CT scan in cases of atypical adenoma are 71% and 98%, respectively.78 Unfortunately, in 30% of cases, CT cannot distinguish benign from malignant lesions. MRI, too, in 10–30% of cases cannot distinguish the character of adrenal tumors.78 Although some surgeons have established the diagnosis with fine-needle aspiration cytology in a nonfunctioning adrenal tumor, others have treated them with adrenalectomy in the presence of features of high risk of malignancy (based on the size of the lesion and its radiologic characteristics).69,87 The choice of approach between open and laparoscopic is not certain, based on the available literature. However, irrespective of the approach, the outcome is likely to be favorable if the principles of oncological resection are followed.
The recent better outcomes of LA, in some of the reports, are attributable to certain factors:
LA has been restricted to cases with tumors <10 cm. Lesions larger than those are likely to increase the risk of tumor spillage and local recurrence, even when it is technically feasible to excise them.3,4,7–9,13–16
LA is contraindicated if tumor extension to adjacent structures is noted before or during the operation. A tumor extension noted during LA warrants a conversion to the open approach. Hence, only in stages I and II ACC are tumors excised laparoscopically.3,7,13,15,16,68
LA should be performed by an experienced surgeon who should have performed at least 40 LAs for benign lesions. Such experience is likely to reduce the risk of excessive manipulation by an inexperienced surgeon, leading to a capsule rupture.3,4,12,85
An anterior approach is generally preferred to a posterior approach. The posterior approach is more suitable for small bilateral lesions, which are likely to be metastatic malignant lesions.3,7,9,12,16,72,73,88
Some of these factors form the basis of change in the recent guidelines issued by the European Society of Endocrine Surgeons, where LA is suggested in stages I and II of ACC.60
The major concern in adrenalectomy for ACC is the risk of recurrence and peritoneal carcinomatosis. Risks of recurrence have been reported in several studies and are attributable to the following:
Advanced stage of ACC (stages III and IV). Adequate preoperative staging is a must before opting for LA for ACC. The risk of recurrence and peritoneal carcinomatosis is higher in patients with stage III or IV.3,4,9,11,12,15,18
Tumor spillage during LA or a positive margin of resected tumor.3,4,8,9,12
However, this review reveals no difference in outcome between the LA and OA approaches, when performed across the various disease stages. The only significant difference was the size of the tumor in each of the approaches.
CONCLUSION
No randomized controlled studies have been conducted that compared the outcomes of OA versus LA in patients with ACC, particularly when the tumor is >6 cm in size. Although there are some recent reports that suggest that the risk of local recurrence and peritoneal carcinomatosis are comparable in the 2 approaches, most of the other results are equivocal, inconclusive, or inferior in outcome in patients with LA. Although an open approach is recommended in patients with tumor invasion, vascular thrombi, and lesions larger than 10 cm, there is concern regarding the ability to achieve consistent oncological resection of lesions between 6 and 10 cm by the laparoscopic approach. This review reveals that outcomes of adrenalectomy performed across the different disease stages show no statistically significant difference between the 2 approaches with regard to peritoneal carcinomatosis, positive resection margin, and time to recurrence. Well-conducted randomized controlled studies would go a long way toward further clarification. The question of whether it is safe to stretch the envelope in these cases is not entirely answered, as there have been no stage-based studies that compared the 2 approaches. In view of the rarity of this cancer, the only way forward is a multicenter trial to attain better statistical power.
Contributor Information
Norman Oneil Machado, Department of Surgery, Sultan Qaboos University Hospital, Muscat, Oman..
Hani al Qadhi, Department of Surgery, Sultan Qaboos University Hospital, Muscat, Oman..
Khalifa al Wahaibi, Department of Surgery, Sultan Qaboos University Hospital, Muscat, Oman..
Syed G. Rizvi, Department of Family Medicine and Public Health, Muscat, Oman..
References:
- 1. Rodgers SE, Evans DB, Lee JE, et al. Adrenocortical carcinoma. Surg Oncol Clin N Am. 2006;15:535–553. [DOI] [PubMed] [Google Scholar]
- 2. Lafemina J, Brennan MF. Adrenocortical carcinoma: past, present, and future. J Surg Oncol. 2012;106:586–594. [DOI] [PubMed] [Google Scholar]
- 3. Jurowich C, Fassnacht M, Kroiss M, Deutschbein T, Germer CT, Reibetanz J. Is there a role for laparoscopic adrenalectomy in patients with suspected adrenocortical carcinoma? A critical appraisal of the literature. Horm Metab Res. 2013;45:130–136. [DOI] [PubMed] [Google Scholar]
- 4. Mir MC, Klink JC, Guillotreau J, et al. Comparative outcome of laparoscopic and open adrenalectomy for adrenocortical carcinoma: single, high volume center experience. Ann Surg Oncol. 2013;20:1456–1461. [DOI] [PubMed] [Google Scholar]
- 5. Toniato A. Minimally invasive surgery for malignant adrenal tumours. The Surgeon. 2013;11:253–257. [DOI] [PubMed] [Google Scholar]
- 6. Donatini G, Caiazzo R, Do Cao CD, et al. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol. 2014;21:284–291. [DOI] [PubMed] [Google Scholar]
- 7. Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol. 2010;58:609–615. [DOI] [PubMed] [Google Scholar]
- 8. Porpiglia F, Fiori C, Daffara F, et al. Retrospective evaluation of the outcome of open versus laparoscopic adrenalectomy for stage I and II adrenocortical cancer. Eur Urol. 2010;57:873–878. [DOI] [PubMed] [Google Scholar]
- 9. Carnaille B. Adrenocortical carcinoma: which surgical approach. Langenbecks Arch Surg. 2012;397:195–199. [DOI] [PubMed] [Google Scholar]
- 10. Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–1385. [DOI] [PubMed] [Google Scholar]
- 11. Fossa A, Rosok BI, Kazaryan AM, et al. Laparoscopic versus open surgery in stage I-III adrenocortical carcinoma: a retrospective comparison of 32 patients. Acta Oncol. 2013;52:1771–1777. [DOI] [PubMed] [Google Scholar]
- 12. Stroka G, Slijper N, Shteinberg D, Mady H, Galili O, Matter I. Laparoscopic adrenalectomy for malignant lesions : surgical principles to improve oncologic outcomes. Surg Endoc. 2013;27:2321–2326. [DOI] [PubMed] [Google Scholar]
- 13. Lombardi CP, Raffaelli M, De Crea C, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstituitional Italian survey. Surgery. 2012;152:1158–1164. [DOI] [PubMed] [Google Scholar]
- 14. Leboulleux S, Deandreis D, Al Ghuzlan A, et al. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol. 2010;162:1147–1153. [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 2005;138:1078–1085. [DOI] [PubMed] [Google Scholar]
- 16. Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013;27:4026–4032. [DOI] [PubMed] [Google Scholar]
- 17. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA. Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 18. Crucitti F, Bellantone R, Ferrante A, Boscherini M, Crucitti P, The ACC Italian Registry Study Group. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstituitional series of 129 patients. Surgery. 1996;119:161–170. [DOI] [PubMed] [Google Scholar]
- 19. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. [DOI] [PubMed] [Google Scholar]
- 20. Porpiglia F, Miller BS, Manfredi M, Fiori C, Doherty GM. A debate on laparoscopic versus open adrenalectomy for adrenocortical carcinoma. Horm Cancer. 2011;2:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stefanidis D, Goldfarb M, Kercher K, Hope W, Richardson W, Fanelli R. Guidelines for the Minimally Invasive Treatment of Adrenal Pathology. Society of American Gastrointestinal and Endoscopic Surgeons. Surg Endosc. 2013;27(11):3960–3980. [DOI] [PubMed] [Google Scholar]
- 22. Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–1385. [DOI] [PubMed] [Google Scholar]
- 23. Villar JM, Moreno P, Ortega J, et al. Results of adrenal surgery: data of a Spanish National Survey. Langenbecks Arch Surg. 2010;395:837–843. [DOI] [PubMed] [Google Scholar]
- 24. Kazaryan AM, Marangos IP, Rosseland AR, et al. Laparoscopic adrenalectomy: Norwegian single-center experience of 242 procedures. J Laparoendosc Adv Surg Tech A. 2009;19:181–189. [DOI] [PubMed] [Google Scholar]
- 25. Foxius A, Ramboux A, Lefebvre Y, Broze B, Hamels J, Squifflet J. Hazards of laparoscopic adrenalectomy for Conn's adenoma when enthusiasm turns to tragedy. Surg Endosc. 1999;13:715–717. [DOI] [PubMed] [Google Scholar]
- 26. Kebebew E, Siperstein AE, Clark OH, et al. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasm. Arch Surg. 2002;137:948–953. [DOI] [PubMed] [Google Scholar]
- 27. Moizandeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005;173:519–525. [DOI] [PubMed] [Google Scholar]
- 28. Zeh HJ, Udelsman R. One hundred laparoscopic adrenalectomies: a single surgeon's experience. Ann Surg Oncol. 2003;10:1012–1017. [DOI] [PubMed] [Google Scholar]
- 29. Prager G, Heinz-Peer G, Passler C, Kaczirek K, Scheuba C, Niederle B. Applicability of laparoscopic adrenalectomy in a prospective study in 150 consecutive patients. Arch Surg. 2004;139:46–49. [DOI] [PubMed] [Google Scholar]
- 30. Nocca D, Aggarwal R, Mathieu A, et al. Laparoscopic surgery and corticoadrenalomas. Surg Endosc. 2007;21:1373–1376. [DOI] [PubMed] [Google Scholar]
- 31. Eto M, Hamaguchi M, Harano M, et al. Laparoscopic adrenalectomy for malignant tumors. Int J Urol. 2008;15:295–298. [DOI] [PubMed] [Google Scholar]
- 32. Corcione F, Miranda L, Marzano E, et al. Laparoscopic adrenalectomy for malignant neoplasm. Surg Endosc. 2005;19:841–844. [DOI] [PubMed] [Google Scholar]
- 33. Palazzo FF, Sebag F, Sierra M, et al. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumor. World J Surg. 2006;30:893–898. [DOI] [PubMed] [Google Scholar]
- 34. Zafar SS, Abaza R. Robot-assisted laparoscopic adrenalectomy for adrenocortical carcinoma: initial report and review of the literature. J Endourol. 2008;22:985–989. [DOI] [PubMed] [Google Scholar]
- 35. Schlamp A, Hallfeldt K, Mueller-Lisse U, et al. Recurrent adrenocortical carcinoma after laparoscopic resection. Nat Clin Pract Endocrinol Metab. 2007;3:191–195. [DOI] [PubMed] [Google Scholar]
- 36. Ramacciato G, Mercantini P, La Torre M, et al. Is laparoscopic adrenalectomy safe and effective for adrenal masses larger than 7 cm? Surg Endosc. 2008;22:516–521. [DOI] [PubMed] [Google Scholar]
- 37. Ushiyama T, Suzuki K, Kageyama S, Fujita K, Oki Y, Yoshimi TA. Case of Cushing's syndrome due to adrenocortical carcinoma with recurrence 19 months after laparoscopic adrenalectomy. J Urol. 1997;157:2239. [PubMed] [Google Scholar]
- 38. Hamoir E, Meurisse M, Defechereux T. Is laparoscopic resection of a malignant corticoadrenaloma feasible? Case report of early, diffuse and massive peritoneal recurrence after attempted laparoscopic resection. Ann Chir. 1998;52:364–368. [PubMed] [Google Scholar]
- 39. Höfle G, Gasser RW, Lhotta K, Janetschek G, Kreczy A, Finkenstedt G. Adrenocortical carcinoma evolving after diagnosis of preclinical Cushing's syndrome in an adrenal incidentaloma: a case report. Horm Res. 1998;50:237–242. [DOI] [PubMed] [Google Scholar]
- 40. Deckers S, Derdelinckx L, Col V, Hamels J, Maiter D. Peritoneal carcinomatosis following laparoscopic resection of an adrenocortical tumor causing primary hyperaldosteronism. Horm Res. 1999;52:97–100. [DOI] [PubMed] [Google Scholar]
- 41. MacGillivray DC, Whalen GF, Malchoff CD, Oppenheim DS, Shichman SJ. Laparoscopic resection of large adrenal tumors. Ann Surg Oncol. 2002;9:480–485. [DOI] [PubMed] [Google Scholar]
- 42. Valeri A, Borrelli A, Presenti L, et al. The influence of new technologies on laparoscopic adrenalectomy: our personal experience with 91 patients. Surg Endosc. 2002:16:1274–1279. [DOI] [PubMed] [Google Scholar]
- 43. Henry JF, Sebag F, Iacobone M, et al. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg. 2002;26:1043–1047. [DOI] [PubMed] [Google Scholar]
- 44. Heniford BT, Arca MJ, Walsh RM, Gill IS. Laparoscopic adrenalectomy for cancer. Semin Surg Oncol. 1999;16:293–306. [DOI] [PubMed] [Google Scholar]
- 45. Liao CH, Chueh SC, Lai MK, Hsiao PJ, Chen J. Laparoscopic adrenalectomy for potentially malignant adrenal tumors greater than 5 centimeters. J Clin Endocrinol Metab. 2006;91:3080–3083. [DOI] [PubMed] [Google Scholar]
- 46. Kirshtein B, Yelle JD, Moloo H, et al. Laparoscopic adrenalectomy for adrenal malignancy: a preliminary report comparing the short-term outcomes with open adrenalectomy. J Laparoendosc Adv Surg Tech A. 2008;18:42–46. [DOI] [PubMed] [Google Scholar]
- 47. Hobart MG, Gill IS, Schweizer D, Sung GT, Bravo EL. Laparoscopic adrenalectomy for large-volume (≥5 cm) adrenal masses. J Endourol. 2000;14:149–154. [DOI] [PubMed] [Google Scholar]
- 48. Castilho LN, Mitre AI, Arap S. Laparoscopic adrenal surgery in a Brazilian center. J Endourol. 2003;17:11–18. [DOI] [PubMed] [Google Scholar]
- 49. Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005;173:519–525. [DOI] [PubMed] [Google Scholar]
- 50. Greco F, Hoda MR, Rassweiler J, et al. Laparoscopic adrenalectomy in urological centers: the experience of the German Laparoscopic Working Group. BJUI. 2011;108:1646–1651. [DOI] [PubMed] [Google Scholar]
- 51. Palazzo FF, Sebag F, Sierra M, Ippolito G, Souteyrand P, Henry JF. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006:30:893–898. [DOI] [PubMed] [Google Scholar]
- 52. Lombardi CP, Raffaelli M, De Crea C, Bellantone R. Role of laparoscopy in the management of adrenal malignancies. J Surg Oncol. 2006;94:128–131. [DOI] [PubMed] [Google Scholar]
- 53. Hemal AK, Singh A, Gupta NP. Whether adrenal mass more than 5 cm can pose problem in laparoscopic adrenalectomy? An evaluation of 22 patients. World J Urol. 2008;26:505–508. [DOI] [PubMed] [Google Scholar]
- 54. Lodin M, Privitera A, Giannone G. Laparoscopic adrenalectomy (LA): keys to success—correct surgical indications, adequate preoperative preparation, surgical team experience. Surg Laparosc Endosc Percutan Tech. 2007;17:392–395. [DOI] [PubMed] [Google Scholar]
- 55. Parnaby CN, Chong PS, Chisholm L, Farrow J, Connell JM, O'Dwyer PJ. The role of laparoscopic adrenalectomy for adrenal tumors of 6 cm or greater. Surg Endosc. 2008;22:617–621. [DOI] [PubMed] [Google Scholar]
- 56. Conzo G, Tricarico A, Belli G, et al. Adrenal incidentalomas in the laparoscopic era and the role of correct surgical indications: observations from 255 consecutive adrenalectomies in an Italian series. Can J Surg. 2009;52:E281–E285. [PMC free article] [PubMed] [Google Scholar]
- 57. Lupascu C, Tarcoveanu E, Bradea C, Andronic D, Ursulescu C, Niculescu D. Laparoscopic adrenalectomy for large solid cortical tumors: is it appropriate? Chirurgia (Bucur) 2011;106:315–320. [PubMed] [Google Scholar]
- 58. Gaujoux S, Bonnet S, Leconte M, et al. Risk factors for conversion and complications after unilateral laparoscopic adrenalectomy. Br J Surg. 2011;98:1392–1399. [DOI] [PubMed] [Google Scholar]
- 59. Mellon MJ, Sundaram CP. Laparoscopic adrenalectomy for pheochromocytoma versus other surgical indications. JSLS. 2008;12:380–384. [PMC free article] [PubMed] [Google Scholar]
- 60. Henry JF, Peix JL, Kraimps JL. Positional statement of the European Society of Endocrine Surgeons (ESES) on malignant adrenal tumors. Langenbecks Arch Surg. 2012;397:145–146. [DOI] [PubMed] [Google Scholar]
- 61. Brunt LM, Doherty GM, Norton JA, Soper NJ, Quasebarth MA, Moley JF. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg.1996;183:1–10. [PubMed] [Google Scholar]
- 62. Dudley NE, Harrison BJ. Comparison of open posterior versus transperitoneal laparoscopic adrenalectomy. Br J Surg. 1999;86:656–660. [DOI] [PubMed] [Google Scholar]
- 63. Suzuki K, Ushiyama T, Ihara H, Kageyama S, Mugiya S, Fujita K. Complications of laparoscopic adrenalectomy in 75 patients treated by the same surgeon. Eur Urol. 1999;36:40–47. [DOI] [PubMed] [Google Scholar]
- 64. Soon PS, Yeh MW, Delbridge LW, et al. Laparoscopic surgery is safe for large adrenal lesions. Eur J Surg Oncol. 2008;34:67–70. [DOI] [PubMed] [Google Scholar]
- 65. Bharwani N, Rockall AG, Sahdev A, et al. Adrenocortical carcinoma: the range of appearances on CT and MRI. AJR Am J Roentgenol. 2011;196:W706–W714. [DOI] [PubMed] [Google Scholar]
- 66. Groussin L, Bonardel G, Silvera S, et al. 18F-fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94:1713–1722. [DOI] [PubMed] [Google Scholar]
- 67. Corcione F, Miranda L, Marzano E, et al. Laparoscopic adrenalectomy for malignant neoplasm. Surg Endosc. 2005;19:841–844. [DOI] [PubMed] [Google Scholar]
- 68. Causeret S, Monneuse O, Mabrut JY, Berger N, Peix JL. Adrenocortical carcinoma: prognostic factors for local recurrence and indications for reoperation—a report on a series of 22 patients. Ann Chir. 2002;127:370–377. [DOI] [PubMed] [Google Scholar]
- 69. Wang C, Sun Y, Wu H, Zhao D, Chen J. Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers: distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology. 2014;64:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tissier F. Classification of adrenal cortical tumors: what limits for the pathological approach? Best Pract Res Clin Endocrinol Metab. 2010;24:877–885. [DOI] [PubMed] [Google Scholar]
- 71. Hermsen IG, Kerkhofs TM, Butter G, et al. Surgery in adrenocortical carcinoma: importance of national cooperation and centralized surgery. Surgery. 2012;152:50–56. [DOI] [PubMed] [Google Scholar]
- 72. Mondal SK, Dasgupta S, Jain P, Mandal PK, Sinha SK. Histopathological study of adrenocortical carcinoma with special reference to the Weiss system and TNM staging and the role of immunohistochemistry to differentiate it from renal cell carcinoma. J Cancer Res Ther. 2013;9:436–441. [DOI] [PubMed] [Google Scholar]
- 73. Zafar SS, Abaza R. Robot-assisted laparoscopic adrenalectomy for adrenocortical carcinoma: initial report and review of the literature. J Endourol. 2008;22:985–989. [DOI] [PubMed] [Google Scholar]
- 74. Brunaud L, Bresler L, Ayav A, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg. 2008;195:433–438. [DOI] [PubMed] [Google Scholar]
- 75. Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy: results of 560 procedures in 520 patients. Surgery. 2006;140:943–948. [DOI] [PubMed] [Google Scholar]
- 76. Porpiglia F, Fiori C, Bertolo R, et al. Mini-retroperitoneoscopic adrenalectomy: our experience after 50 procedures. Urology. 2014;84:596–601. [DOI] [PubMed] [Google Scholar]
- 77. Aspinall SR, Imisairi AH, Bliss RD, Scott-Coombes D, Harrison BJ, Lennard TW. How is adrenocortical cancer being managed in the UK? Ann R Coll Surg Engl. 2009;91:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zeiger MA, Thompson GB, Duh QY, et al. , and the American Association of Clinical Endocrinologists; American Association of Endocrine Surgeons. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1):1–20. [DOI] [PubMed] [Google Scholar]
- 79. National Cancer Institute. Adrenocortical carcinoma (PDQ1) treatment. June 3, 2003. Available at http://www.nci.nih.gov/cancerinfo/pdq/treatment/adrenocortical/healthprofessional Accessed May 16, 2008.
- 80. Gill IS. The case for laparoscopic adrenalectomy. J Urol. 2001;166:429–436. [PubMed] [Google Scholar]
- 81. Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg. 2012;255:363–369. [DOI] [PubMed] [Google Scholar]
- 82. Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–289. [DOI] [PubMed] [Google Scholar]
- 83. Saunders BD, Doherty GM. Laparoscopic adrenalectomy for malignant disease. Lancet Oncol. 2004;5:718–726. [DOI] [PubMed] [Google Scholar]
- 84. Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. [DOI] [PubMed] [Google Scholar]
- 85. Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbecks Arch Surg. 2012;397:201–207. [DOI] [PubMed] [Google Scholar]
- 86. Guerrieri M, De Sanctis A, Crosta F, et al. Adrenal incidentaloma: surgical update. J Endocrinol Invest. 2007;30:200–204. [DOI] [PubMed] [Google Scholar]
- 87. Birsen O, Akyuz M, Dural C, et al. A new risk stratification algorithm for the management of patients with adrenal incidentalomas. Surgery. 2014;156:959–965. [DOI] [PubMed] [Google Scholar]
- 88. Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. [DOI] [PubMed] [Google Scholar]


