Abstract
Venous thromboembolism is a common and potentially preventable disease in hospitalized patients. Risk assessment and prophylaxis is also an important quality of care measure. Herein, we discuss the negative impact of VTE on surgical patients, review the risk factors for VTE, the risk assessment tools, available prophylaxis, and then summarize the use of biomarkers for VTE diagnosis.
Keywords: Venous disease, Venous thromboembolism, Thrombosis prophylaxis, Anticoagulation
Venous thromboembolism (VTE) risk assessment and prophylaxis, as currently practiced and recommended, is often under utilized. Venous thrombosis is a major problem worldwide, and is often preventable in the hospitalized setting. It is estimated that approximately 100:100,000 persons have a 1st time VTE event per year. In fact, the incidence of VTE is identical to cardiovascular incidence for patients at moderate risk as shown by the recent Jupiter Trial.1 Thus, at least 200,000 patients per year (as a low estimate) are affected by VTE. About 30% of patients with VTE suffer mortality at 30 days, and 30% have a recurrent VTE at 10 years follow up.2 This overview will focus on epidemiology, VTE risk assessment and quality measures, prophylaxis, and the potential utility of biomarkers.
Risks and VTE Impact
The cumulative risk for VTE is dependent on multiple factors (Table I).3 First, the intrinsic thrombotic risk is mitigated by a small group of inherited risk factors including the deficiencies of natural anticoagulant proteins (e.g. Antithrombin), genetic abnormalities such as factor V Leiden or prothrombin 20210A, and/or the other miscellaneous hypercoagulable states such as anti-phospholipid syndrome. More common are acquired risk factors such as increased age, prior VTE, presence of malignancy, obesity, and use of certain medications such as oral contraceptives or hormone replacement therapy. More variable risk factors are indwelling catheters (including upper extremity catheters), inflammatory bowel disease, and presence of varicose veins. Prothrombotic stress triggers include surgery, pregnancy, trauma, and probably systemic infection. Together, these factors may culminate as a clinical VTE. Prevention of VTE is based on the inventory and knowledge of these risk factors at the patient level.
Table I. VTE Risk Factors.
| Acquired | ||
|---|---|---|
| Occurrence | Thrombotic Potential | |
| Surgery | C | ↑↑ |
| Trauma | C | ↑↑ |
| Infection | C | ↑↑↑ |
| Malignancy | C | ↑↑ |
| h/o VTE | UC | ↑↑↑ |
| FHx VTE | UC | ↑ |
| Age | C | ↑ |
| Inherited | ||
| Anticoagulant III | UC | ↑↑ |
| Protein C | UC | ↑↑ |
| Protein S | UC | ↑↑ |
| FVL | C | ↑ |
| PT20210A | UC | ↑ |
| Miscellaneous | ||
| APL | UC | ↑↑ |
| hyperhomocysteinemia | UC | ↑ |
C = common; UC = uncommon;
We assessed the impact of VTE occurrence on a large group of surgical patients using a hospital administrative discharge database. In brief, approximately 200,000 patients undergoing 6 common cardiovascular procedures were reviewed for a secondary diagnosis of VTE using ICD9-CM codes.4 Overall, the rate of clinical VTE varied from 0.26% to 1.2% depending on the procedure. Carotid endarterectomy had the lowest and abdominal aortic aneurysm had the highest VTE incidence. The occurrence of VTE more than doubled the cost and length of stay and was associated with a 2 fold increased odds of death. In a more detailed analysis using the patient centered Veteran's Affairs National Surgical Quality Improvement (NSQIP) database, 10 common operations were reviewed, encompassing 76,000 patients. Again, carotid endarterectomy had the lowest VTE incidence, while total hip arthroplasty had the highest, at 1.34%. The occurrence of VTE increased the risk of death by 2.4 fold and was markedly associated with postoperative inflammatory states, including those of urinary tract infection, myocardial infarction, and pneumonia; all increasing the VTE risk approximately 2 to nearly 3 fold.5
Malignancy is also a well known risk factor for VTE. When patients with malignancy develop VTE, a significant decrease in survival is observed. Factors which increase VTE risk in cancer patients, based on a large cancer registry, include a solid as compared to hematological tumor, leucopenia, infection, and later stage of cancer.6 Others have shown the site of cancer (gastrointestinal versus non-gastrointestinal), an elevated pre-chemotherapy platelet count, and a low hemoglobin also independently increase the risk of VTE in those with malignancy.7 In cancer patients who require surgery, location of primary tumor, presence of metastases, receipt of chemotherapy, and presence of central venous access are all known to alter VTE risk.8 Thus, stratification of the types of risks is important as not all variables confer the same overall VTE risk.
VTE as a Quality Indicator
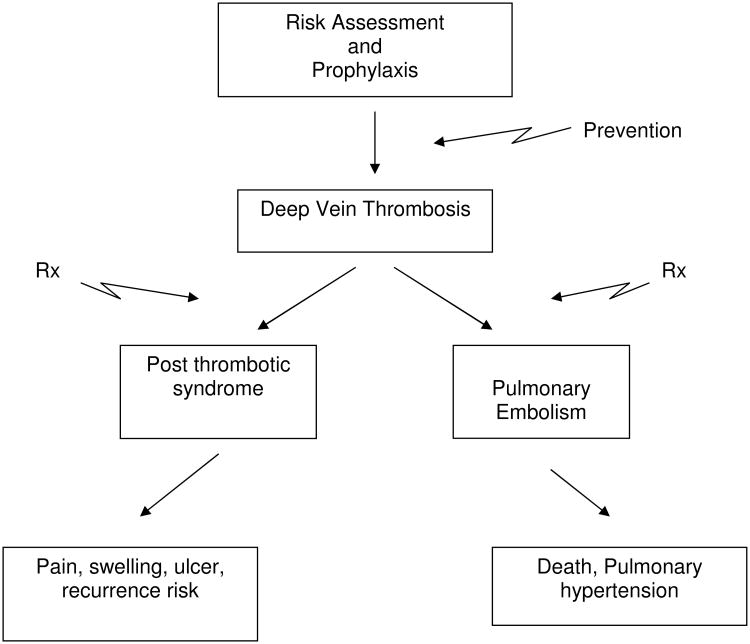
Venous thrombosis and the sequelae are now a high profile disease for which prevention is key (Figure 1). The Federal Government has become focused on many processes related to quality of care. For example, the Agency for Healthcare Research and Quality (AHRQ) ranked 90 variables for patient safety and quality of care, of which the foremost is appropriate use of VTE prophylaxis in patients at risk (www.psnet.ahrq.gov). The Center for Medicare and Medicaid services considered listing VTE as a ‘never event’, for total knee and total hip arthroplasty in 2009 (www.cms.hhs.gov/). Furthermore, the Surgical Care Improvement Project (www.jointcommission.org/preformancemeasures) also includes assessment of DVT, specifically measuring the prophylaxis and risk assessments rates. Confirming the importance of VTE nationally is the recent call to action by the United States Surgeon General.9
Figure 1.
Simplified overview of DVT – VTE areas of prevention and treatment.
International survey data on at risk patients suggests at least 50% of patients may not receive adequate prophylaxis, including both medical and surgical groups.10 In some patients, contraindications to VTE prophylaxis may be present. However, the leading cause of inadequate VTE prophylaxis is likely medical professionals not performing risk assessment, and furthermore the risk level of the patient may not match the prescribed prophylaxis. Various VTE assessment models have been proposed. An ideal model would both identify an individual patient's VTE risk and recommend appropriate prophylaxis. This risk model would identify patients at highest and lowest VTE risk, be evidence based, and be validated in a diverse patient population.
The American College of Chest Physicians (ACCP) guidelines suggests group VTE prophylaxis is most appropriate, with low, moderate, and high risk patients categories as the best strategy.11 Concerns with this approach include under or over estimating the VTE risk, as well as not distinguishing the variables that contribute to the category classification. Additionally, the broad classification scheme makes formal validation difficult. In contrast to the ACCP group prophylaxis model, the Caprini Risk Assessment Model12 creates an individualized risk assessment score based on presence or absence of over 35 risk factors. The Caprini model's ability to predict VTE risk has been validated for general, urologic, and vascular surgery patients,13 as well as those patients having post-bariatric body contouring surgery.14
We have adopted the Caprini Risk Assessment Model12 and have integrated this tool into our electronic medical record. The utility of this individual risk assessment approach is supported by a retrospective study of 8,216 patients between July 2001 and January of 2008 at the University of Michigan.13 Using the Caprini risk factor scoring system, we found that those patients who had a score of 0 – 1 had 0% VTE incidence, while those with a score >8 had approximately 6.5% incidence of VTE at 30 days. The independent factors associated with increased VTE for the whole group included recent pregnancy, sepsis, malignancy, a history of VTE, as well as central venous access. From these data, we concluded that the Caprini score accurately reflects the anticipated 30 day clinical VTE incidence. This score, if very high, might further identify patients who are likely to benefit from extended out of hospital prophylaxis, and there might be a ‘super high risk’ category such that prophylactic full strength anticoagulation may be indicated.15
VTE risk assessment represents an ongoing research endeavor. What remains unknown is the level of prophylaxis to provide for a specific risk score or categorization, in addition to the appropriate weighting of various risk factors when the risk score or categorization is generated. Further validation studies of existing risk assessment models are needed, including head-to-head comparisons between group risk categorization and individual risk assessment. Finally, studies that examine efficacy, safety, and cost of prophylaxis regimens recommended by existing risk assessment models are required. These studies must be updated as new chemoprophylaxis agents are developed.
VTE Prophylaxis Options and Ways to Improve Compliance
The currently recommended measures for VTE prophylaxis include pharmacological and mechanical. These measures aim to either decrease the thrombosis propensity by affecting the clotting system or improve venous hemodynamics by increasing flow. Pharmacologic choices include low dose unfractionated heparin 5000 units/TID, low molecular weight heparin daily or BID, fondaparinux (anti Xa inhibitor) daily, or a low dose vitamin K antagonist (for the highest risk patients) (Table II).11 The risk of major bleeding on pharmacological prophylaxis is less than 3%.16 A recent meta-analysis of bleeding complications in general surgery patients examined 33 studies in which chemoprophylaxis was provided. Hematoma requiring a second operation occurred in only 1% of patients.17 Intermittent pneumatic compression placed on the lower limbs are only effective if actually on the patient and the device turned on.18 Too many times, the IPCs are actually treating the bedpost.
Table II. Prophylaxis Measures.
| Type | Mechanism | Complications |
|---|---|---|
| LMWH | Anti FXa, IIa | Bleeding, occasional HIT |
| uFH | Anti FXa, IIa | Bleeding, HIT |
| Fondaparinux | Anti FXa | Bleeding, no Antidote |
| VKA | Anti Factor II, VII, IX, IX | Bleeding, drug interactions |
| Mechanical | Increase venous blood flow, possibly activate endogenous, fibrinolytic system. | Local irritation, discomfort |
LMWH = low molecular weight heparin; uFH = unfractionate heparin; HIT = heparin induced thrombocytopenia; VKA = vitamin K antagonist
Chemoprophylaxis should be continued while the patient is non-ambulatory and in-active. With increasing usage of early discharge and broad use of home health care, consideration of post-discharge prophylaxis is important. Literature supports that postoperative orthopedic, intra-abdominal, and pelvic malignancy patients should receive prophylaxis for 30 days after their surgery.11, 19 Recent results from the UK's Million Women Study demonstrate that VTE risk may remain substantially elevated for at least 90 days after surgery.20 It is now recognized that a VTE is associated with an incident surgical or stress event if it occurs within 90 days of the procedure.21, 22
Prophylaxis adherence can be improved via evidence based guidelines that are actively implemented, as opposed to passively disseminated. The most effective processes are active reminders for the clinician to assess VTE risk, coupled with recommendations for appropriate prophylaxis. An example would be to distill the current ACCP guidelines as interactive reminders and ready information for clinicians. This includes an active audit and feedback system to the clinicians.23 Computer alert systems seem to be most promising, and coalesce with the advent of the electronic medical health record at many centers. A recent randomized controlled trial to issue alerts to physicians facilitated the detection of high risk patients and increased the use of VTE prophylaxis from 14.5% to 33.5%. These interventions reduced VTE incidence by 41%.21
Biomarkers and VTE
At our institution, about 85 – 90% of all venous duplex scans for limb symptoms or DVT suspicion are negative studies. Biomarkers for VTE should improve the pretest probability to better ‘rule in’ or ‘rule out’ a VTE diagnosis (Table III). While currently used in the outpatient setting, these need to be tested in the post operative or hospital setting to detect those patients at high risk, potentially obviating an ultrasound scan. The most commonly used biomarker is D-dimer, which has an excellent sensitivity, but low specificity. Thus, D-dimer has a high certainty to rule out VTE.24 D-dimer is also useful in determining if active thrombus metabolism is occurring and allow modification of VKA duration. Thrombin generation has been assayed in at risk patients and allows prediction of recurrence risk but has not been studied for incident VTE diagnosis.25 Another potentially newer biomarker to ‘rule in’ the diagnosis of VTE is P-selectin. P-selectin is a cell adhesion molecule, present in platelets, endothelium, as well as leukocytes. Strong experimental data suggests its role as a pathophysiologic factor in DVT, and correlative studies are present in humans.26, 27 Forthcoming data suggests that serum P-selectin levels are sensitive and specific for VTE, in contrast to D-dimer. Whether P-selectin levels are associated with VTE in surgical patients is as of yet unclear, but studies are ongoing.
Table III. Biomarkers.
| Type | Use |
|---|---|
| D – dimer | To improve pretest probability; May allow timing of AC cessation |
| Thrombin - Antithrombin | Prediction of VTE recurrence |
| P-Selectin | To increase pretest probability; May allow ‘rule in’ to rule of DVT |
AC = anticoagulant
Conclusion
Hospitals and physicians currently possess all the tools required to appropriately risk-stratify patients for VTE, provide adequate prophylaxis, and systematically decrease VTE occurrence in hospitalized patients. New biomarkers may better assist with VTE risk assessment and early detection (despite prophylaxis). Ongoing refinements of existing risk-stratification tools, including risk factor identification and weighting, will further determine appropriate levels and duration of prophylaxis. Finally, VTE risk is dynamic during a patient's hospital course, and prophylaxis requirements must be regularly re-evaluated and modified as clinical circumstances change.
References
- 1.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N Engl J Med. 2008 doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Circulation. 2009. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 3.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henke P, Froehlich J, Upchurch G, Jr, Wakefield T. The significant negative impact of in-hospital venous thromboembolism after cardiovascular procedures. Ann Vasc Surg. 2007;21(5):545–550. doi: 10.1016/j.avsg.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, Henke PK. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335–341. doi: 10.1016/j.jvs.2006.10.034. discussion 341-332. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Wakefield TW, Henke PK. Risk factors associated with venous thromboembolic events in patients with malignancy. Blood Coagul Fibrinolysis. 2006;17(4):265–270. doi: 10.1097/01.mbc.0000224845.27378.c3. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborne NH, Wakefield TW, Henke PK. Venous Thromboembolism in Cancer Patients Undergoing Major Surgery. Ann Surg Oncol. 2008 doi: 10.1245/s10434-008-0151-4. [DOI] [PubMed] [Google Scholar]
- 9.Wakefield TW, McLafferty RB, Lohr JM, Caprini JA, Gillespie DL, Passman MA. Call to action to prevent venous thromboembolism. J Vasc Surg. 2009;49(6):1620–1623. doi: 10.1016/j.jvs.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, Huang W, Zayaruzny M, Emery L, Anderson FA., Jr Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 11.Geerts W. Antithrombotic and Thrombolytic Therapy. Chest. 2008;133(8th Ed: ACCP Guidelines):381s–451s. [Google Scholar]
- 12.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Annals of surgery. 251(2):344–350. doi: 10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 14.Hatef DA, Kenkel JM, Nguyen MQ, Farkas JP, Abtahi F, Rohrich RJ, Brown SA. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122(1):269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 15.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 199(1 Suppl):S3–10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 17.Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141(8):790–797. doi: 10.1001/archsurg.141.8.790. discussion 797-799. [DOI] [PubMed] [Google Scholar]
- 18.Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost. 2007;5(9):1854–1861. doi: 10.1111/j.1538-7836.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346(13):975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 20.Sweetland S, Green J, Liu B, Berrington de Gonzalez A, Canonico M, Reeves G, Beral V. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, Goldhaber SZ. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 22.Henke PK, Ferguson E, Varma M, Deatrick KB, Wakefield GT, Woodrum DT. Proximate versus nonproximate risk factor associated primary deep venous thrombosis: clinical spectrum and outcomes. J Vasc Surg. 2007;45(5):998–1003. doi: 10.1016/j.jvs.2007.01.042. discussion 1003-1004; quiz 1005-1007. [DOI] [PubMed] [Google Scholar]
- 23.Tooher R, Middleton P, Pham C, Fitridge R, Rowe S, Babidge W, Maddern G. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Annals of surgery. 2005;241(3):397–415. doi: 10.1097/01.sla.0000154120.96169.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, Biel RK, Bharadia V, Kalra NK. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140(8):589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. Jama. 2006;296(4):397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 26.Smith A, Quarmby JW, Collins M, Lockhart SM, Burnand KG. Changes in the levels of soluble adhesion molecules and coagulation factors in patients with deep vein thrombosis. Thromb Haemost. 1999;82(6):1593–1599. [PubMed] [Google Scholar]
- 27.Rectenwald JE, Myers DD, Jr, Hawley AE, Longo C, Henke PK, Guire KE, Schmaier AH, Wakefield TW. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94(6):1312–1317. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]