Abstract
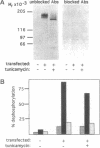
cDNA encoding a receptor-like protein-tyrosine-phosphatase (PTP) termed DEP-1 was isolated from a HeLa cell library. The cDNA predicts an enzyme consisting of an extracellular segment containing eight fibronectin type III repeats, a single transmembrane segment, and a single intracellular PTP domain. Following expression of DEP-1 cDNA in COS cells a glycoprotein of 180 kDa was detected and PTP activity was demonstrated in immunocomplexes with a C-terminal peptide antiserum. Endogenous DEP-1 was detected in WI-38 human embryonic lung fibroblasts by immunoblotting and immunocomplex PTP assays. Immunoblot analysis of DEP-1 expression in WI-38 cells revealed dramatically increased levels and activity of the PTP in dense cultures relative to sparse cultures. Also, DEP-1 activity, detected in PTP assays of immunocomplexes, was increased in dense cell cultures. In contrast, the expression levels of PTP-1B did not change with cell density. This enhancement of DEP-1 expression with increasing cell density was also observed in another fibroblast cell line, AG1518. The increase in DEP-1 occurs gradually with increasing cell contact and is initiated before saturation cell density is reached. These observations suggest that DEP-1 may contribute to the mechanism of contact inhibition of cell growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki J., Umeda M., Takio K., Titani K., Utsumi H., Sasaki M., Inoue K. Neural cell adhesion molecule mediates contact-dependent inhibition of growth of near-diploid mouse fibroblast cell line m5S/1M. J Cell Biol. 1991 Dec;115(6):1751–1761. doi: 10.1083/jcb.115.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Doolittle R. F. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8990–8994. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S. M., Flint A. J., Tonks N. K. Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993 Aug;122(4):961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Shimer S., Johnson K. A., Hill D. E., Bruskin A. M. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992 Jan 15;52(2):478–482. [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Erikson R. L. Extracellular signals and reversible protein phosphorylation: what to Mek of it all. Cell. 1993 Jul 30;74(2):215–217. doi: 10.1016/0092-8674(93)90411-i. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Fantl W. J., Johnson D. E., Williams L. T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Flint A. J., Gebbink M. F., Franza B. R., Jr, Hill D. E., Tonks N. K. Multi-site phosphorylation of the protein tyrosine phosphatase, PTP1B: identification of cell cycle regulated and phorbol ester stimulated sites of phosphorylation. EMBO J. 1993 May;12(5):1937–1946. doi: 10.1002/j.1460-2075.1993.tb05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink M. F., Zondag G. C., Wubbolts R. W., Beijersbergen R. L., van Etten I., Moolenaar W. H. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993 Aug 5;268(22):16101–16104. [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Chuang P. T., Rubin G. M. Cloning and characterization of a receptor-class phosphotyrosine phosphatase gene expressed on central nervous system axons in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11266–11270. doi: 10.1073/pnas.88.24.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. P., Wang H., D'Eustachio P., Musacchio J. M., Schlessinger J., Sap J. Cloning and characterization of R-PTP-kappa, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993 May;13(5):2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Main A. L., Harvey T. S., Baron M., Boyd J., Campbell I. D. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992 Nov 13;71(4):671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Suzuki T., Uchida T., Inazawa J., Ariyama T., Matsuda K., Horita K., Noguchi H., Mizuno H., Sakamoto C. Molecular cloning of a human transmembrane-type protein tyrosine phosphatase and its expression in gastrointestinal cancers. J Biol Chem. 1994 Jan 21;269(3):2075–2081. [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Mooney R. A., Freund G. G., Way B. A., Bordwell K. L. Expression of a transmembrane phosphotyrosine phosphatase inhibits cellular response to platelet-derived growth factor and insulin-like growth factor-1. J Biol Chem. 1992 Nov 25;267(33):23443–23446. [PubMed] [Google Scholar]
- Oon S. H., Hong A., Yang X., Chia W. Alternative splicing in a novel tyrosine phosphatase gene (DPTP4E) of Drosophila melanogaster generates two large receptor-like proteins which differ in their carboxyl termini. J Biol Chem. 1993 Nov 15;268(32):23964–23971. [PubMed] [Google Scholar]
- Pallen C. J., Tong P. H. Elevation of membrane tyrosine phosphatase activity in density-dependent growth-arrested fibroblasts. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6996–7000. doi: 10.1073/pnas.88.16.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijksen G., Völler M. C., Van Zoelen E. J. Orthovanadate both mimics and antagonizes the transforming growth factor beta action on normal rat kidney cells. J Cell Physiol. 1993 Feb;154(2):393–401. doi: 10.1002/jcp.1041540223. [DOI] [PubMed] [Google Scholar]
- Sap J., Jiang Y. P., Friedlander D., Grumet M., Schlessinger J. Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding. Mol Cell Biol. 1994 Jan;14(1):1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991 Oct;3(5):786–791. doi: 10.1016/0955-0674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Tonks N. K. Introduction: protein tyrosine phosphatases. Semin Cell Biol. 1993 Dec;4(6):373–377. doi: 10.1006/scel.1993.1044. [DOI] [PubMed] [Google Scholar]
- Walton K. M., Dixon J. E. Protein tyrosine phosphatases. Annu Rev Biochem. 1993;62:101–120. doi: 10.1146/annurev.bi.62.070193.000533. [DOI] [PubMed] [Google Scholar]
- Wary K. K., Lou Z., Buchberg A. M., Siracusa L. D., Druck T., LaForgia S., Huebner K. A homozygous deletion within the carbonic anhydrase-like domain of the Ptprg gene in murine L-cells. Cancer Res. 1993 Apr 1;53(7):1498–1502. [PubMed] [Google Scholar]
- Wieser R. J., Schütz S., Tschank G., Thomas H., Dienes H. P., Oesch F. Isolation and characterization of a 60-70-kD plasma membrane glycoprotein involved in the contact-dependent inhibition of growth. J Cell Biol. 1990 Dec;111(6 Pt 1):2681–2692. doi: 10.1083/jcb.111.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford-Thomas T. A., Rhodes J. D., Dixon J. E. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992 Apr;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Tonks N. K. Isolation of a cDNA clone encoding a human protein-tyrosine phosphatase with homology to the cytoskeletal-associated proteins band 4.1, ezrin, and talin. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5949–5953. doi: 10.1073/pnas.88.14.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]