Abstract
There are early references of it in ancient text and physicians have discussed its importance and features of its deficiency in the past. Vitamin D has again regained interest with recent dramatic rise in the incidence of deficiency in the developing as well as developing world. In this review article, we discuss the biochemical and role of vitamin D in the skeletal system. We also discuss the recommended dietary requirements and features of skeletal deficiency. Extra-skeletal roles of vitamin D deficiency have been a matter of debate lately and it has also been discussed in detail in this article. In conclusion, it would not be wrong to label vitamin D as one of the most important vitamin involved in the metabolism of the musculoskeletal system and any clinician, especially the orthopaedician, should be well versed with its overall mechanism and roles in the human body.
Keywords: Vitamin D, Rickets, Osteomalacia, Extraskeletal, Metabolism
1. Introduction
Although Vitamin D Deficiency disorder (VDD), namely Rickets was known for centuries, but its causative factor was known only after the discovery of Vitamin D (VD) by E.V. McCollum and his associates in 1913. But it attained a new interest in recent years as VDD has been found to be pandemic worldwide and its association with others diseases.1,2Vitamin D is a group of fat-soluble secosteroids (a steroid with a “broken” ring) plays an important role in bone metabolism and seems to have some anti-inflammatory and immune-modulating properties. Several forms (vitamers) of VD exist namely, VD1 (ergocalciferol with lumisterol), VD2 (ergocalciferol), VD3 (cholecalciferol) VD4 (dihydroergocalciferol), VD5 (sitacalciferol). The most important compounds in this group are VD3 and VD2.3–5 In this review article we discuss vitamin D metabolism and functions in the human body. The common causes of vitamin D deficiency along with daily requirements and prevalence of vitamin D deficiency in the world and India have been discussed along with the extra-skeletal manifestations of vitamin D.
2. Vitamin D metabolism and functions
Ultra Violet (UV)-B irradiation of skin triggers photolysis of 7-dehydrocholesterol (proVD3) to preVD3 in the plasma membrane of human skin keratinocytes.6–8 Once formed in the skin, cell plasma membrane preVD3 is rapidly converted to VD3 by the skin's temperature VD3 from the skin and VD from the diet undergo two sequential hydroxylations, first in the liver to 25(OH)D and then in the kidney to its biologically active form, 1,25-dihydroxyVD (1,25[OH]2D) (Fig. 1).
Fig. 1.
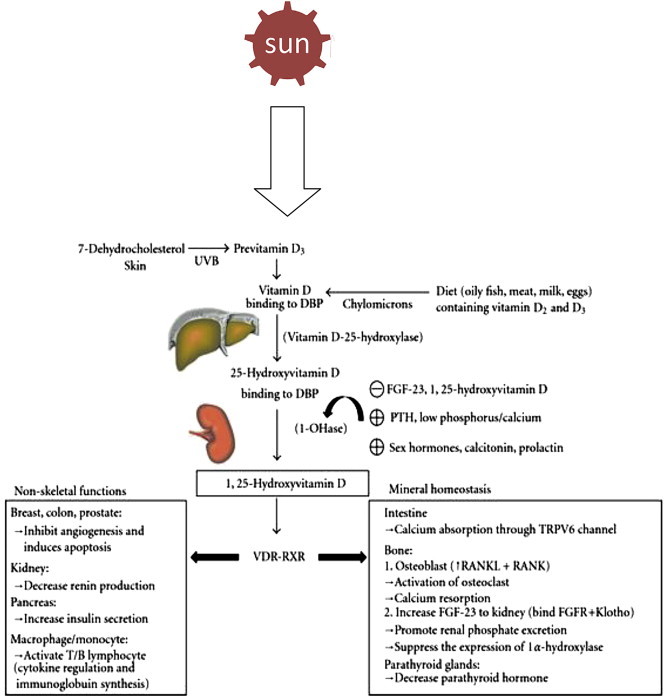
Biosynthesis and metabolism of vitamin D (UVB = Ultraviolet ray B, DBP = VD Binding Protein, FGF = Fibroblast Growth Factor, PTH = Parathormone, VDR = VD Receptor).
3. Mechanism of action
VD acts in 2 ways:
-
I)
Genomic action of VD: VD Receptor (VDR) is a member of the superfamily of nuclear receptors for steroid hormones, which can be categorized as a ligand activated transcription factor.9 VDR is also thought to play an important role in engendering to rapid action of 1, 25 (OH)2D3.10
-
II)
Non genomic action of VD: There exist rapid response non-genomic actions of VD, which are mediated through cell surface receptors, known as 1, 25 D3 MARRS (membrane associated rapid response steroid binding proteins).11
4. Bioactions of VD
On Intestine: 1, 25 (OH)2D3 enhances the efficacy of small intestine to absorb calcium and phosphorus, iron, magnesium, zinc.10
On Skeleton: VD is essential for the development and maintenance of mineralized skeleton.
Growth plate development requires coordinated calcium and 1, 25 (OH)2D3 actions and VDR, where as optimal osteoblastic bone formation and osteoclastic bone resorption demand both 1, 25(OH)2D3 and VDR.1, 25(OH)2D3 regulates osteoclastogenesis in reciprocal regulation of receptor activation of NF-kB (RANK) ligand (RANKL) and osteoprotegerin (OPG).12Interactions between osteoblasts and osteoclasts integrate bone remodelling.
5. Requirement
There is difference in recommendation of daily requirement by different authority and the commonly accepted recommended dietary allowances (RDA) for VD are from Health Canada.13
6. Sources of VD
The main sources of VD2 and D3 include3,14–21: A) Natural sources like Fish (Salmon, Sardines, Mackerel, Tuna etc), Cod Liver oil, mushrooms, egg yolk, sun light exposure etc; B) Fortified foods like butter, milk, yogurts, cheese, margarine, orange juice, breakfast cereals etc; C) Pharmaceutical supplements like VD2, D3 and multivitamin.
7. Deficiency
VD deficiency is the most common and is the most under-diagnosed medical condition in children and adults. This is largely because patients do not typically present with overt clinical signs and symptoms until the deficiency is severe and prolonged. It is now accepted that the circulating level of 25-hydroxy VD should be used as an indicator of VD status due to its ease of measurement, long half-life in circulation (approximately 2 or 3 weeks), and the correlation of its level with clinical disease states.22–24 Although no consensus on an optimal level of 25-hydroxyVD has been reached, however its level can determine various states of clinical conditions.25–29:
-
A)
Deficiency: <20 ng/ml
-
B)
Insufficiency: 20–29 ng/ml
-
C)
Sufficiency: 30–100 ng/ml
-
D)
Toxicity: >100 ng/ml
8. Deficiency disorder
Children with established VD deficiency present with features of rickets in the form of skeletal abnormalities like knee deformities (Fig. 2), developmental delay, failure to thrive etc., with characteristic biochemical and radiological features. The typical radiological features include widening and cupping of the metaphysis etc, whereas adults present with signs and symptoms of osteomalacia like bone pain and tenderness, proximal muscle weakness presenting as difficulty in rising from a sitting position with biochemical and radiological feature. The typical radiological features include insufficiency fractures or looser zones (Fig. 3), osteoporosis, biconcave discs, ‘rugger jersey’ spine (Fig. 4).
Fig. 2.
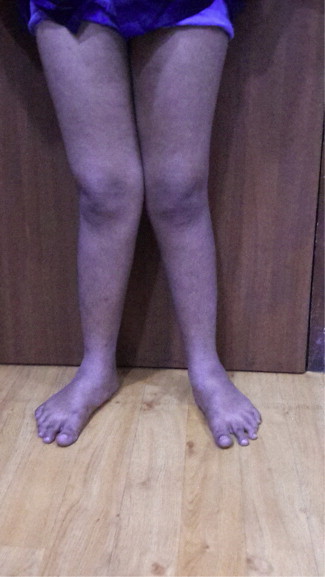
Bilateral genu valgum deformity due to vitamin D deficiency.
Fig. 3.
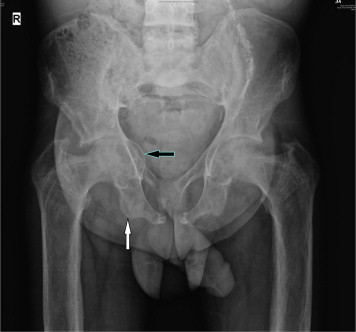
X-ray of the pelvis showing protrusio acetabuli (black arrow) and multiple insufficiency fractures (white arrow).
Fig. 4.
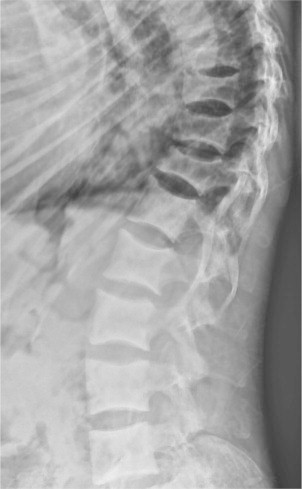
Lateral X-ray of the spine showing rugger jersey spine and multiple biconcave veretbra (cod fish mouth appearance).
9. Causes of VD deficiency
-
A)
UVB-related deficiency
The elderly due to decreased presence of skin 7-dehydrocholesterol, reduced mobility or institutionalization (that discourages sun exposure), reduced renal production of 1,25-dihydroxyVD as well as decreased intake of fortified foods.30,31
-
B)
Dark skin
Melanin competes with 7-dehydrocholesterol for absorption of UVB photons. Dark skin requires 10–50times the exposure to sunlight to produce the same amount of VD as does a white person.32
-
C)
Season, latitude, and the time of day
The thicker the ozone layer is, the fewer amounts of UVB photons can reach the earth, thus few preVD3 can be produced. Zenith angle, defined as the angle of the sunlight reaching the Earth's surface, decides the thickness of ozone layer which sunlight needs to penetrate. Zenith angle is dependent on factors such as time of day, season of the year, and latitude.33,34
-
D)
Sunscreen users
Sunscreens can efficiently absorb UVB radiation. This dramatically prevents the interaction of UVB with 7-dehydrocholesterol, the process of preVD3 generation.8,35
-
E)
Medical/physical condition-related deficiency, fat malabsorption
Crohn's disease, cystic fibrosis (CF), celiac disease, surgical removal of part of the stomach or intestines are associated with fat malabsorption and thus may lead to VD deficiency.36
-
F)
Anticonvulsant use
Long-term use of some antiepileptic drugs, including phenobarbital, phenytoin, and carbamazepine and the antimicrobial agent rifampicin (RIF) can result in osteomalacia.37–41 The induction of the catabolism of 1,25-dihydroxyVD by these drugs is thought to contribute to their deleterious side effects.37–41
-
G)
Chronic kidney disease
Chronic kidney disease, as well as those requiring dialysis, leads to an inability to make sufficient 1,25-dihydroxyVD which has a direct effect in inhibiting parathyroid hormones expression.42,43
-
H)
Liver diseases
Liver is the main site where hydroxylation of VD at position C-25 takes place. Thus, it is not surprising that the degree of liver dysfunction correlates with calcidiol levels44 and that the prevalence of VD insufficiency is particularly high in patients with chronic liver disease.45–48 The levels of 25-hydroxyVD can be low in severe liver diseases and liver diseases directly could lead to impaired absorption of VD.
-
I)
Obesity
Obese people have lower 25-hydroxyVD levels. The subcutaneous fat, which is known to store VD, sequestered more of the cutaneous synthesized VD, which results in less release of VD from the skin into the circulation in the obese subject than non-obese subject.49–54
10. Prevalence of VD deficiency in the world and in India
VD deficiency is pandemic, yet it is the most under-diagnosed and under-treated nutritional deficiency in the world.55–57 Several studies showed that 40–100% of U.S. and European elderly men and women still living in the community (not nursing homes) are deficient in VD.25–27 It has already become a largely unrecognized global epidemic. VD inadequacy can be seen in young adults as well as healthy children. For example, 48% of white preadolescent girls in a study in Maine58 and 52% of Hispanic and black adolescents in a study in Boston are VD deficient.59 In Europe, where very few foods are fortified with VD, children and adults would appear to be at especially highrisk.60–62 A study of middle aged British adults showed that 60% are VD insufficient, and the number rose to 90% during winter and spring.63
VD deficiency prevails in epidemic proportions all over the Indian subcontinent, with a prevalence of 70%–100% in the general population. In India, widely consumed food items such as dairy products are rarely fortified with VD. Indian socio religious and cultural practices do not facilitate adequate sun exposure, thereby negating potential benefits of plentiful sunshine. Consequently, subclinical VD deficiency is highly prevalent in both urban and rural settings, and across all socioeconomic and geographic strata. Countrywide studies have reported VD deficiency in as high as 70%–100% of ostensibly healthy individuals. High prevalence of VD deficiency was reported from northern to southern and western to eastern India, in ostensibly healthy children, adolescents, young adults and those ≥50 years old. All over India, VD deficiency was highly prevalent in pregnant women and lactating mothers. VD status of these mothers correlated well with their neonates and their exclusively breastfed infants. Subjects from rural and urban areas presented a similar picture. Relatively, fish are a rich source of VD. The residents of Bengal (eastern India) eat more fish compared to the rest of the Indians. Surprisingly, their VD status appears to be just as poor as in the rest of the country.64 Similarly, even healthy young soldiers with sufficient intake of calcium, adequate sun exposure and regular exercise regimen were found to be VDdeficient,65,66 as were young sportswomen.67 Among resident doctors from Mumbai (western India)68 and also doctors from eastern India,64 most were VD deficient. VD deficiency was also observed in most of 2119 healthcare professionals studied from all over India.69 Evidently, countrywide prevalence of VD deficiency is undeniable.
11. Treatment of disease and supplementation
11.1. VD DOSING, supplementation, and UV irradiation and/or sensible sun exposure
Supplementation with VD has been estimated to prevent VD deficiency in approximately 98% of the general population.70–72 VD supplementation and exposure to sunlight or simulated sunlight have been shown to increase serum 25(OH)D levels in elderly patients.36,73–84 The Institute of Medicine's adequate intake for the United States and Canada is 200 IU/d for all children and adults younger than 51 years, 400 IU/d for people aged 51–70 years, and 600 IU/d for those older than 70 years.72,85 A report by the Scientific Committee for Food, established by the European Commission, indicated that adults 65 years and older should receive 400 IU/d of VD3 and suggested that the requirements of all adults, including those with inadequate sunlight exposure, would be met by this dietary intake.86 This recommendation is consistent with that of the US Food and Drug Administration's daily recommended value of 400 IU/d (10 μg/d) of VD3regardless of age.87 Because it has been suggested that amounts up to 1000 IU/d of VD3 may be needed to maintain a healthy 25(OH)D level of more than 30 ng/mL (75 nmol/L),88–91 an intake of 400 IU/d may represent a minimum. This is especially true in the winter or for children and adults not exposed to sunlight.
11.2. Treatment of severe VD deficiency
Although severe VD deficiency (25[OH] levels <10 ng/mL [25 nmol/L]) is much less common than inadequacy, it does occur, especially in elderly house-bound people. The best method for treating VD deficiency is an oral dose of 50,000 IU/wk of VD2 for 8 weeks, then checking 25(OH)D levels.70,73 In some cases, another once-weekly 8-week course of 50,000 IU of VD2 may be necessary to boost 25(OH)D levels into the desired range of more than 30–50 ng/mL (75–125 nmol/L). For patients prone to developing VD deficiency, after correcting the deficiency, giving patients 50,000 IU every 2 weeks will sustain them in a VD-sufficient state. Alternatively, 1000 IU of VD3 intake should be maintained. Cutaneous exposure to sunlight or artificial UV-B such as a tanning bed is also helpful, especially if the patient is prone to VD deficiency.82–84,92–95 Exposure to direct sunlight typically of no more than 5–10 min on the arms and legs between the hours of 10 AM and 3 PM during the spring, summer, and fall will prevent VD inadequacy.33,70,71
11.3. Hypervitaminosis
VD intoxication is extremely rare. Studies showed that doses of more than 50,000 IU per day, which raises 25-hydroxyVD to more than 150 ng/ml, is associated with hypercalcemia and hyperphosphatemia.96–98 VD toxicity is not caused by sunlight exposure, but can be caused by supplementing with high doses of VD presenting with hypercalcemia as anorexia, nausea, and vomiting, frequently followed by polyuria, polydipsia, weakness, insomnia, nervousness, pruritus, and, ultimately, renal failure. Proteinuria, urinary casts, azotemia, and metastatic calcification (especially in the kidneys) may also develop. Other symptoms of VD toxicity include mental retardation in young children, abnormal bone growth and formation, diarrhea, irritability, weight loss, and severe depression.16,99 Hence, abuse and misuse of VD supplementation by the physician and general public must be stopped and sufficient awareness about it must be created.
12. Extra skeletal effects of VD
The small intestine, kidneys, and bones are the primary organs and tissues responsive to VD that are involved in mineral metabolism that affects skeletal health. However, the effects of VD are not limited to mineral homeostasis and the maintenance of skeletal health. The presence of the VDR in other tissues and organs suggests that VD may also be important in extra skeletal biological processes.100–102 Additionally, the enzyme responsible for conversion of 25(OH)D to the biologically active form of VD (1,25[OH]2D) has been identified in tissues other than kidney,103–106 and evidence is growing that extrarenal synthesis of 1,25(OH)2D may be important for regulating cell growth and cellular differentiation70,99,100,107 via paracrine or autocrine regulatory mechanisms. The VDR is a steroid hormone nuclear receptor that binds 1,25(OH)2D with high affinity and mediates transcriptional gene regulation.70,100,102,107–109 Mounting biochemical and epidemiological evidence suggests that the VDR is also involved in mediating the noncalcemic effects of VD and its analogues and may play a vital role in disease prevention and maintenance of extraskeletalhealth.70,110,111 The VDR has been isolated from many cell types, tissues, and organs, including those not typically associated with calcium homeostasis and bone metabolism. Some of these include the heart, stomach, pancreas, brain, skin, gonads, and various cells of the immune system.70,99,100,107 Genetic variants of the gene encoding the VDR have also been associated with differential risk of developing variouscancers112,113 and immune disorders, including type 1 diabetesmellitus.114,115 In addition, 1,25(OH)2D is involved in non-genomic mediated intracellular signaling pathways.116–119 Both1,25(OH)2D and its synthetic analogues (collectively, VDR ligands) have demonstrated anti proliferative, pro differentiative, and immune modulatory activities (which may be mediated by both the genomic and the nongenomic mechanisms)in several clinical and experimental settings,116 and are being investigated for the potential treatment of many pathologic conditions, including psoriasis, type 1 diabetes mellitus, rheumatoid arthritis, multiple sclerosis, Crohn's disease, hypertension, cardiovascular heart disease, and many common cancers.70,110,120–122 Since the primary function of VD is to modulate calcium homeostasis, the use of analogues for the treatment of conditions other than osteoporosis or osteomalacia could trigger hypercalcemia or other unwanted adverse effects.
12.1. VD and cancer
VD is one of the most potent hormones for regulating cell growth; 1,25(OH)2D inhibits proliferation and induces differentiation into normally functioning cells.70,99,102,107,123 Some evidence suggests that 1,25(OH)2D helps to regulate cell growth and prevent cancer progression70,99,100,124 by reducingangiogenesis,125 increasing cell differentiation and apoptosis of cancer cells, and reducing cell proliferation126–128 and metastases.70,99,100,106,107,120,126,129
12.2. VD and cardiovascular disease
Adding to the evidence of the effect of VD on extraskeletal tissues are data that suggest that inadequate VD and calcium and living at higher latitudes may being dependent contributing factors in the pathogenesis and progression of hypertension and cardiovascular disease.70,110,130,131
12.3. VD and multiple sclerosis
As with previous epidemiological data reporting a latitudinal risk gradient for cancer and cardiovascular disease110,132–137 a similar risk gradient exists for developing multiple sclerosis.138–141 One double-blinded RCT involving patients with multiples clerosis who were randomized to receive either VD supplementation or placebo showed that patients who received supplementation had increased serum transforming growth factor β1 levels vs those who did not receive supplementation.142 Elevated transforming growth factor β1 levels have been associated with the stable phase of multiple sclerosis, whereas reduced levels have been associated with relapsing-remitting multiple sclerosis.143,144
12.4. VD and type I diabetes mellitus
1,25(OH)2D acts as an immune modulator, reducing cytokine production and lymphocyte proliferation, which have been implicated in the destruction of insulin-secreting β cells in the pancreas and the development of type 1diabetes mellitus.87 In addition, β-islet cells express the VDR and respond to 1,25(OH)2D by increasing insulinproduction.70,87,145,146
12.5. VD and psoriasis
One of the great successes of VD therapy for treating an extraskeletal disorder is in the treatment of psoriasis.122,147 Smith et al.148 showed that 1,25(OH)2D3 inhibited the proliferation of human keratinocytes that express the VDR in vitro and accelerated their differentiation. This suggested that hyper proliferative skin disorders such as psoriasis might be responsive to treatment with 1,25(OH)2D3.149 Initial treatments with topical 1,25(OH)2D3 showed great improvements in reducing the severity and area of psoriatic lesions, with little or no adverse effects.122,150–152 Today, three VD analogues including calcipotriene, 1,24(OH)2D3, and 22-oxo-1,25(OH)2D3, are among the first-line treatments used for psoriasis.122,147,151,152
12.6. VD in OA and degenerative disc disease
There is a small but statistically significant clinical benefit to VD treatment in patients with Knee OA.153 VD deficiency is greatly associated with early stage of OA, suggesting VD supplementation before cartilage damage occur.154 Several clinical trial have ensued and are ongoing. These studies are in the initial stage, and to date no strong evidence has been available establish the role of vitamin D in OA. There is also some evidence of association with degenerative disc disease with VD deficiency.155,156
12.7. VD in other diseases
A possible role of VD has also been implicated in several other diseases, including rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, osteoarthritis, and periodontal disease.
13. Conclusion
VD inadequacy is a global problem and widespread prevalence of its deficiency in India is undeniable. Although recommendations of daily VD intake have been provided, higher levels are required in order to have real preventive or treatment effects as numerous studies have proved. UVB radiation plays an alternative in improving VD content other than oral supplementation. Its advantage is that it wills not cause VD intoxication since excessive VD will be broken down by UVB. However, a number of factors of the UVB such as wavelength, duration of exposure are needed to be carefully controlled so as to avoid erythema. Factually, sun exposure is an untenable solution, for most individuals in India, towards attaining VD sufficiency. Low calcium intake in conjunction with VD deficiency makes matters worse. The need for improvement in vitamin status of the Indian population is both important and urgent. The Indian government needs to take substantive measures in this direction. Revision of recommended daily allowance of calcium and VD is required. Despite the close link of VD with human health, VD inadequacy is not widely recognized as a problem by physicians and patients. Not only VD deficiency produces skeletal changes but may also be responsible for causation and or progression of many other non musculoskeletal diseases. Hence, greater awareness of this problem is required among researchers, clinician, and patients about VD inadequacy and its consequences. On the contrary, the misuse and abuse of VD supplementation must also be highlighted as it can cause life threatening hypercalcemia is some patients. The value of extra skeletal use of VD, at present, is doubtful in various chronic diseases as its use in these conditions had been based on observational data and mixed quality evidence from predominately small trials. Appropriate interpretation of the data is further muddied by seemingly endless media reports suggesting vitamin D as a panacea for chronic disease.157
Conflicts of interest
All authors have none to declare.
References
- 1.McCollum E.V., Simmonds N., Becker J.E., Shiply P.G. Studies on experimental rickets. An experimental demonstration the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 2.Wolf G. The discovery of vitamin D: the contribution of Adolf Windaus. J Nutr. 2004;134:1299–1302. doi: 10.1093/jn/134.6.1299. [DOI] [PubMed] [Google Scholar]
- 3.Holick M.F. High prevalence of VD inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–375. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Holick M.F. The use and interpretation of assays for VD and its metabolites. J Nutr. 1990;120(suppl 11):1464–1469. doi: 10.1093/jn/120.suppl_11.1464. [DOI] [PubMed] [Google Scholar]
- 5.Vieth R. Why ‘vitamin D’ is not a hormone, and not a synonym for 1,25dihydroxy-vitamin D, its analogs or deltanoids. J Steroid Biochem Mol Biol. 2004;89–90:571–573. doi: 10.1016/j.jsbmb.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Holick M.F. McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 7.MacLaughlin J.A., Anderson R.R., Holick M.F. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 8.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K. VD supplementation for prevention of mortality in adults. Cochrane Database Syst Rrev. 2014;1:CD007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke N.E., Haddad J.G. VD binding protein (Gc-globulin) Endocr Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 10.Brown A.J., Dusso A., Slatopolsky E., Vitamin D. Am J Physiol Renal Physiol. 1999;277:157–175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 11.Boyan B.D., Dean D.D., Sylvia V.L., Schwartz Z. Nongenomic regulation of extracellular matrix events by VD metabolites. J Cell Biochem. 1994;56:331–339. doi: 10.1002/jcb.240560309. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 13.Dietary reference intakes tables [Health Canada 2005].
- 14.Calvo M.S., Whiting S.J., Barton C.N. VD intake: a global perspective of current status. J Nutr. 2005;135:310–316. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Norman A.W. From VD to hormone D: fundamental of the VD endocrine system essential for good health. Am J Clin Nutr. 2008;88:491s–499. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 16.Holick M.F. VDD. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Schoenmakers I., Goldberg G.R., Prentice A. Abundant sunshine and VDD. Br J Nutr. 2008;99:1171–1173. doi: 10.1017/S0007114508898662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DRI, Dietary Reference Intakes: For Calcium, Phosphorus, Magnesium, Vitamin D, and fluoride. National Academy Press; Washington, D.C: 1997. p. 250. [PubMed] [Google Scholar]
- 19.Wang T., Bengtsson G., Kärnefelt I. Provitamins and vitamins D2 and D3 in Cladina spp. over a latitudinal gradient: possible correlation with UV levels. J Photochem Photobiol B, Biol. September 2001;62:118–122. doi: 10.1016/s1011-1344(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 20.Holick M.F. The VD epidemic and its health consequences. J Nutr. 2005;135 doi: 10.1093/jn/135.11.2739S. 2739S–48S. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi A., Okano T., Sayamoto M. Tissue distribution of 7-dehydrocholesterol, vitamin D3 and 25-hydroxyvitamin D3 in several species of fishes. J Nutr Sci Vitaminol. 1986;32:13–22. doi: 10.3177/jnsv.32.13. [DOI] [PubMed] [Google Scholar]
- 22.Wolpowitz D., Gilchrest B.A. The VD questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301–317. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- 23.Adams J.S., Clemens T.L., Parrish J.A., Holick M.F. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D deficient subjects. N Engl J Med. 1982;306:722–725. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 24.Reichel H., Koeffler H.P., Norman A.W. The role of the VD endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 25.Hollis B.W., Wagner C.L. Assessment of dietary VD requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 26.Holick M.F., Siris E.S., Binkley N. Prevalence of VD inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 27.Lips P., Hosking D., Lippuner K. The prevalence of VD inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas K.K., Lloyd-Jones D.M., Thadhani R.I. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 29.Dawson-Hughes B., Heaney R.P., Holick M.F., Lips P., Meunier P.J., Vieth R. Estimates of optimal VD status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 30.Bell N.H. VD metabolism, aging, and bone loss. J Clin Endocrinol Metab. 1995;80:1051. doi: 10.1210/jcem.80.4.7714064. [DOI] [PubMed] [Google Scholar]
- 31.Need A.G., Morris H.A., Horowitz M., Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 32.Clemens T.L., Henderson S.L., Adams J.S., Holick M.F. Increased skin pigment reduces the capacity of skin to synthesis vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 33.Webb A.R., Kline L., Holick M.F. Influence of season and latitude on the cutaneous synthesis of vitaminD3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 34.Lu Z., Chen T.C., Kline L. Photosynthesis of previtamin D3 in cities around the world. In: Holick M.F., Kligman A., editors. Biologic Effects of Light. Walter De Gruyter; Berlin: 1992. pp. 48–51. (Symposium proceedings, October 13-15:1991). [Google Scholar]
- 35.Matsuoka L.Y., Ide L., Wortsman J., MacLaughlin J., Holick M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 36.Lo C.W., Paris P.W., Clemens T.L., Nolan J., Holick M.F. VD absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42:644–649. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 37.Pack A.M., Morrell M.J. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24–S29. doi: 10.1016/j.yebeh.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Andress D.L., Ozuna J., Tirschwell D. Antiepileptic drug induced bone loss in young male patients who have seizures. Arch Neurol. 2002;59:781–786. doi: 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 39.Burt R., Freston J.W., Tolman K.G. The influence of phenobarbital on biotransformation of 25-hydroxycholecalciferol. J Clin Pharmacol. 1976;16:393–398. doi: 10.1002/j.1552-4604.1976.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 40.Shah S.C., Sharma R.K., Chitle A.R. Rifampicin induced osteomalacia. Tubercle. 1981;62:207–209. doi: 10.1016/0041-3879(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 41.Karaaslan Y., Haznedaroglu S., Ozturk M. Osteomalacia associated with carbamazepine/valproate. Ann Pharmacother. 2000;34:264–265. doi: 10.1345/aph.19099. [DOI] [PubMed] [Google Scholar]
- 42.Dusso A.S., Sato T., Arcidiacono M.V. Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int Suppl. 2006;102:S8–S11. doi: 10.1038/sj.ki.5001595. [DOI] [PubMed] [Google Scholar]
- 43.Correa P., Segersten U., Hellman P., Akerstrom G., Westin G. Increased 25-hydroxyvitamin D3 1α-hydroxylase and reduced 25-hydroxyvitamin D3 24-hydroxylase expression in parathyroid tumors – new prospects for treatment of hyperparathyroidism with vitamin D. J Clin Endocrinol Metab. 2002;87:5826–5829. doi: 10.1210/jc.2002-021356. [DOI] [PubMed] [Google Scholar]
- 44.Putz-Bankuti C., Pilz S., Stojakovic T. Association of 25-hydroxyVDlevels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845–851. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 45.Arteh J., Narra S., Nair S. Prevalence of VDD in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 46.Malham M., Jorgensen S.P., Ott P. VDD in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–925. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miroliaee A., Nasiri-Toosi M., Khalilzadeh O., Esteghamati A., Abdollahi A., Mazloumi M. Disturbances of parathyroid hormone-VDaxis in non-cholestatic chronic liver disease: a cross-sectional study. Hepatol Int. 2010;4:634–640. doi: 10.1007/s12072-010-9194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher L., Fisher A. VDand parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513–520. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Liel Y., Ulmer E., Shary J., Hollis B.W., Bell N.H. Low circulating VD in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 50.Compston J.E., Vedi S., Ledger J.E., Webb A., Gazet J.C., Pilkington T.R.E. VD status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 51.Hey H., Stockholm K.H., Lund B.J., Sorensen O.H. VDD in obese patients and changes in circulating VD metabolites following jejunoileal bypass. Int J Obes. 1982;6:473–479. [PubMed] [Google Scholar]
- 52.Hyldstrup L., Andersen T., McNair P., Breum L., Transbol I. Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol. 1993;129:393–398. doi: 10.1530/acta.0.1290393. [DOI] [PubMed] [Google Scholar]
- 53.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of VD in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 55.Van Schoor N.M., Lips P. Worldwide VD status. Best Pract Res Clin Endocrinol.Metab. 2011;25:671–680. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Mithal A., Wahl D.A., Bonjour J.P. Global VD status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 57.Van der Meer I.M., Middelkoop B.J., Boeke A.J., Lips P. Prevalence of VDD among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: an overview. Osteoporos Int. 2011;22:1009–1021. doi: 10.1007/s00198-010-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan S.S., Rosen C.J., Halteman W.A., Chen T.C., Holick M.F. Adolescent girls in Maine at risk for VD insufficiency. J Am Diet Assoc. 2005;105:971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Gordon C.M., DePeter K.C., Feldman H.A., Grace E., Emans S.J. Prevalence of VDD among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 60.Lips P. VDD and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 61.Bakhtiyarova S., Lesnyak O., Kyznesova N., Blankenstein M.A., Lips P. VD status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int. 2006;17:441–446. doi: 10.1007/s00198-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 62.McKenna M.J. Differences in VD status between countries in young adults and the elderly. Am J Med. 1992;93:69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 63.Hypponen, Power Hypovitaminosis D in British adults at age 45y: nationwide cohort study on dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 64.Baidya A., Chowdhury S., Mukhopadhyay S., Ghosh S. Profile of VD in a cohort of physicians and diabetologists in Kolkata. Indian J Endocrinol.Metab. 2012;16:S416–S417. doi: 10.4103/2230-8210.104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goswami R., Gupta N., Goswami D., Marwaha R.K., Tandon N., Kochupillai N. Prevalence and significance of low 25-hydroxy VD concentrations in healthy subjects in Delhi. Am J.Clin. Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 66.Tandon N., Marwaha R.K., Kalra S., Gupta N., Dudha A., Kochupillai N. Bone mineral parameters in healthy young Indian adults with optimal VD availability. Natl Med J.India. 2003;16:298–302. [PubMed] [Google Scholar]
- 67.Marwaha R.K., Puri S., Tandon N. Effects of sports training & nutrition on bone mineral density in young Indian healthy females. Indian J Med Res. 2011;134:307–313. [PMC free article] [PubMed] [Google Scholar]
- 68.Multani S.K., Sarathi V., Shivane V., Bandgar T.R., Menon P.S., Shah N.S. Study of bone mineral density in resident doctors working at a teaching hospital. J Postgrad Med. 2010;56:65–70. doi: 10.4103/0022-3859.65272. [DOI] [PubMed] [Google Scholar]
- 69.Beloyartseva M., Mithal A., Kaur P. Widespread VDD among Indian health care professionals. Arch Osteoporos. 2012;7:187–192. doi: 10.1007/s11657-012-0096-x. [DOI] [PubMed] [Google Scholar]
- 70.Holick M.F. Sunlight and VD for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6,suppl l):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 71.Holick M., Jenkins M. iBooks; NewYork, NY: 2003. The UV Advantage. [Google Scholar]
- 72.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine . DietaryReference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1997. Vitamin D; pp. 250–287. [Google Scholar]
- 73.Malabanan A., Veronikis I.E., Holick M.F. Redefining VD insufficiency [letter] Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 74.Eriksen E.F., Glerup H. VDD and aging: implications for general health and osteoporosis. Biogerontology. 2002;3:73–77. doi: 10.1023/a:1015263514765. [DOI] [PubMed] [Google Scholar]
- 75.Glerup H., Mikkelsen K., Poulsen L. Commonly recommended daily intake of VD is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 76.Feskanich D., Willett W.C., Colditz G.A. Calcium, vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77:504–511. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 77.Dawson-Hughes B., Harris S.S., Krall E.A., Dallal G.E. Effect of calcium and VD supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 78.Lips P., Wiersinga A., van Ginkel F.C. The effect of VD supplementation on VD status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–650. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 79.Grant A.M., Avenell A., Campbell M.K. RECORD Trial Group. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised evaluation of calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 80.Chapuy M.C., Chapuy P., Thomas J.L., Hazard M.C., Meunier P.J. Biochemical effects of calcium and VD supplementation in elderly, institutionalized, vitamin D-deficient patients. Rev Rhum Engl Ed. 1996;63:135–140. [PubMed] [Google Scholar]
- 81.Chel V.G.M., Ooms M.E., Popp-Snijders C. Ultraviolet irradiation corrects VDD and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13:1238–1242. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 82.Tangpricha V., Turner A., Spina C., Decastro S., Chen T.C., Holick M.F. Tanning is associated with optimal VD status (serum 25-hydroxy VD concentration) and higher bone mineral density. Am J Clin Nutr. 2004;80:1645–1649. doi: 10.1093/ajcn/80.6.1645. [DOI] [PubMed] [Google Scholar]
- 83.Chuck A., Todd J., Diffey B. Subliminal ultraviolet-B irradiation for the prevention of VDD in the elderly: a feasibility study. Photodermatol Photoimmunol Photomed. 2001;17:168–171. doi: 10.1034/j.1600-0781.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 84.Shao Q., Chen T.C., Holick M.F. Sun-tanning bed radiation increases VD synthesis in human skin in vivo. In: Holick M.F., Kligman A., editors. Biological Effects of Light. Walter De Gruyter; Berlin, Germany: 1992. pp. 62–66. [Google Scholar]
- 85.Holick M.F. VD requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int. 1998;8(suppl 2):S24–S29. doi: 10.1007/pl00022729. [DOI] [PubMed] [Google Scholar]
- 86.Scientific Committee on Food. Opinion on the Tolerable Upper Intake Level of Vitamin D. December 4, 2002. www.imace.org/nutrition/pdf/poster.pdf Available at: Accessibility verified January 31, 2006. [Google Scholar]
- 87.United States Food and Drug Administration Web site. Reference Daily Intakes, Recommended Dietary Allowances. Available at: www.fda.gov/fdac. Accessibility verified February 13, 2006.
- 88.Tangpricha V., Koutkia P., Rieke S.M., Chen T.C., Perez A.A., Holick M.F. Fortification of orange juice with vitamin D: a novel approach to enhance VD nutritional health. Am J Clin Nutr. 2003;77:1478–1483. doi: 10.1093/ajcn/77.6.1478. [DOI] [PubMed] [Google Scholar]
- 89.Heaney R.P., Dowell M.S., Hale C.A., Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 90.Vieth R. Why the optimal requirement for vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89–90:575–579. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 91.Hollis B.W. Circulating 25-hydroxy VD levels indicative of VD sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 92.Koutkia P., Lu Z., Chen T.C., Holick M.F. Treatment of VDD due to Crohn's disease with tanning bed ultraviolet B radiation. Gastroenterology. 2001;121:1485–1488. doi: 10.1053/gast.2001.29686. [DOI] [PubMed] [Google Scholar]
- 93.Freedman D.M., Dosemeci M., McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59:257–262. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Barger-Lux M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [published correction appears in. [DOI] [PubMed] [Google Scholar]
- 95.Matuulla-Nolte B., Krause B., Schmidt-Gayk H. Serial UVB irradiation can influence secondary hyperparathyroidism in VDD. In: Holick M.F., Jung E.G., editors. Biologic Effects of Light: Proceedings of a Symposium, Basel, Switzerland, November 1-3, 1998. Kluwer Academic Publishers; Boston, Mass: 1999. pp. 121–123. [Google Scholar]
- 96.Holick M.F., Garabedian M. Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. In: Favus M.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th ed. American Society for Bone and Mineral Research; Washington, DC: 2006. pp. 129–137. [Google Scholar]
- 97.Bouillon R. Vitamin D: from photosynthesis, metabolism, and action to clinical applications. In: DeGroot L.J., Jameson J.L., editors. Endocrinology. W.B. Saunders; Philadelphia: 2001. pp. 1009–1028. [Google Scholar]
- 98.Holick M.F. Resurrection of VDD and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Insel P.M., Turner E.R., Ross D. 2nd ed. Jones and Bartlett Publishers; Boston: 2006. Discovering Nutrition. [Google Scholar]
- 100.Holick M.F. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 101.DeLuca H. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6, suppl l):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 102.Stumpf W.E., Sar M., Reid F.A., Tanaka Y., DeLuca H.F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 103.Mawer E.B., Hayes M.E., Heys S.E. Constitutive synthesis of 1,25-dihydroxyvitamin D3 by a human small cell lung cancer cell line. J Clin Endocrinol Metab. 1994;79:554–560. doi: 10.1210/jcem.79.2.8045976. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz G.G., Whitlatch L.W., Chen T.C., Lokeshwar B.L., Holick M.F. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25- hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–395. [PubMed] [Google Scholar]
- 105.Cross H.S., Bareis P., Hofer H. 25-Hydroxyvitamin D3-1α-hydroxlyase and VD receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–292. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 106.Tangpricha V., Flanagan J.N., Whitlatch L.W. 25-Hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 107.Holick M.F. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [published correction appears in Am J Clin Nutr. 2004;79:890] [DOI] [PubMed] [Google Scholar]
- 108.Chen T.C., Holick M.F. VD and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14:423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 109.Rachez C., Freedman L.P. Mechanisms of gene regulation by vitamin D3 receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 110.Grant W.B., Holick M.F. Benefits and requirements of VD for optimal health: a review. Altern Med Rev. 2005;10:94–111. [PubMed] [Google Scholar]
- 111.Holick M.F. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes. 2002;9:87–98. [Google Scholar]
- 112.Uitterlinden A.G., Fang Y., van Meurs J.B., van Leeuwen H., Pols H.A. VD receptor gene polymorphisms in relation to VD related disease states. J Steroid Biochem Mol Biol. 2004;89–90:187–193. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 113.Slattery M.L., Neuhausen S.L., Hoffman M. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111:750–756. doi: 10.1002/ijc.20330. [published correction appears in Int J Cancer. 2004;111:983] [DOI] [PubMed] [Google Scholar]
- 114.Pani M.A., Knapp M., Donner H. VD receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes. 2000;49:504–507. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 115.McDermott M.F., Ramachandran A., Ogunkolade B.W. Allelic variation in the VD receptor influences susceptibility to IDDM in Indian Asians. Diabetologia. 1997;40:971–975. doi: 10.1007/s001250050776. [DOI] [PubMed] [Google Scholar]
- 116.Nagpal S., Na S., Rathnachalam R. Noncalcemic actions of VD receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 117.Omdahl J.L., Morris H.A., May B.K. Hydroxylase enzymes of the VD pathway: expression, function, and regulation. Annu Rev Nutr. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 118.Falkenstein E., Tillmann H.C., Christ M., Feuring M., Wehling M. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 119.Norman A.W., Bishop J.E., Bula C.M. Molecular tools for study of genomic and rapid signal transduction responses initiated by 1α,25(OH)2- vitamin D3. Steroids. 2002;67:457–466. doi: 10.1016/s0039-128x(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 120.Chen T.C., Holick M.F., Lokeshwar B.L., Burnstein K.L., Schwartz G.G. Evaluation of VD analogs as therapeutic agents for prostate cancer. Recent Results Cancer Res. 2003;164:273–288. doi: 10.1007/978-3-642-55580-0_20. [DOI] [PubMed] [Google Scholar]
- 121.Harris S.S. VDand type 1 diabetes [letter] Am J Clin Nutr. 2004;79:889–890. doi: 10.1093/ajcn/79.5.889. [DOI] [PubMed] [Google Scholar]
- 122.Holick M.F. Clinical efficacy of 1,25-dihydroxyvitamin D3 and its analogues in the treatment of psoriasis. Retinoids. 1998;14:12–17. [Google Scholar]
- 123.Holick M.F. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 124.Van den Bemd G.J., Chang G.T. VD and VD analogs in cancer treatment. Curr Drug Targets. 2002;3:85–94. doi: 10.2174/1389450023348064. [DOI] [PubMed] [Google Scholar]
- 125.Abe J., Nakamura K., Takita Y., Nakano T., Irie H., Nishii Y. Prevention of immunological disorders in MRL/1 mice by a new synthetic analogue of vitamin D3: 22-oxa-1α,25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 126.Dalhoff K., Dancey J., Astrup L. A phase II study of the VD analogue seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer. 2003;89:252–257. doi: 10.1038/sj.bjc.6601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mantell D.J., Owens P.E., Bundred N.J., Mawer E.B., Canfield A.E. 1α,25-Dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 128.Ylikomi T., Laaksi I., Lou Y.R. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 129.Sambrook P.N., Chen J.S., March L.M. Serum parathyroid hormone predicts time to fall independent of VD status in a frail elderly population. J Clin Endocrinol Metab. 2004;89:1572–1576. doi: 10.1210/jc.2003-031782. [DOI] [PubMed] [Google Scholar]
- 130.Krause R., Buhring M., Hopfenmuller W., Holick M.F., Sharma A.M. Ultraviolet B and blood pressure [letter] Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 131.Rostand S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2,pt 1):150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 132.Zhao X.Y. Feldman D.The role of VD in prostate cancer. Steroids. 2001;66:293–300. doi: 10.1016/s0039-128x(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 133.Apperly F.L. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191–195. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 134.Grant W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 135.John E.M., Schwartz G.G., Dreon D.M., Koo J. VDand breast cancer risk: the NHANES I epidemiologic follow-up study, 1971–1975 to 1992: National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 136.Grant W.B. An ecologic study of the role of solar UV-B radiation in reducing the risk of cancer using cancer mortality data, dietary supply data and latitude for European countries. In: Holick M.F., editor. Biologic Effects of Light 2001: Proceedings of a Symposium, Boston, Massachusetts. Mass:Kluwer Academic Publishing; Boston: 2002. pp. 267–276. [Google Scholar]
- 137.Li Y.C. VDregulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 138.Hernan M.A., Olek M.J., Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53:1711–1718. doi: 10.1212/wnl.53.8.1711. [DOI] [PubMed] [Google Scholar]
- 139.Leibowitz U., Sharon D., Alter M. Geographical considerations in multiple sclerosis. Brain. 1967;90:871–886. doi: 10.1093/brain/90.4.871. [DOI] [PubMed] [Google Scholar]
- 140.Ponsonby A.L., McMichael A., van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181–182:71–78. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 141.Embry A.F., Snowdon L.R., Vieth R. VD and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis [letter] Ann Neurol. 2000;48:271–272. [PubMed] [Google Scholar]
- 142.Mahon B.D., Gordon S.A., Cruz J., Cosman F., Cantorna M.T. Cytokine profile in patients with multiple sclerosis following VD supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 143.Carrieri P.B., Provitera V., De Rosa T., Tartaglia G., Gorga F., Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20:373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- 144.Losy J., Michalowska-Wender G. In vivo effect of interferon-beta 1a on interleukin-12 and TGF-beta(1) cytokines in patients with relapsing-remitting multiple sclerosis. Acta Neurol Scand. 2002;106:44–46. doi: 10.1034/j.1600-0404.2002.01209.x. [DOI] [PubMed] [Google Scholar]
- 145.Lee S., Clark S.A., Gill R.K., Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: VDreceptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 146.Mathieu C., Badenhoop K. VD and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Guenther L., Cambazard F., Van De Kerkhof P.C.M. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147:316–323. doi: 10.1046/j.1365-2133.2002.04967.x. [DOI] [PubMed] [Google Scholar]
- 148.Smith E.L., Walworth N.C., Holick M.F. Effect of 1α,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86:709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- 149.MacLaughlin J.A., Gange W., Taylor D., Smith E., Holick M.F. Cultured psoriatic fibroblasts from involved and uninvolved sites have a partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1985;82:5409–5412. doi: 10.1073/pnas.82.16.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bikle D.D. Vitamin D: role in skin and hair. In: Feldman D., editor. 2nd ed. vol 1. Elsevier Academic Press; San Diego, Calif: 2005. pp. 609–630. (Vitamin D). [Google Scholar]
- 151.Smith E.L., Pincus S.H., Donovan L., Holick M.F. A novel approach for the evaluation and treatment of psoriasis: oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516–528. doi: 10.1016/s0190-9622(88)70207-8. [DOI] [PubMed] [Google Scholar]
- 152.Perez A., Chen T.C., Turner A. Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134:238–246. [PubMed] [Google Scholar]
- 153.Sanghi D., Mishra A., Sharma A.C. Does vitamin D improve osteoarthritis of knee: a randomized controlled pilot tril. Clin Orthop Relat Res. 2013;471:3556–3562. doi: 10.1007/s11999-013-3201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Castillo E.C., Hernendez-Cueto M.A., Vega-lopez C. Effect of vitamin D supplementation during the induction and progression of osteoarthritis in rat model. Evid Based Complement Alternat Med. 2012:156563. doi: 10.1155/2012/156563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh A., Agarwal A.C., Kalia R.B. Vitamin D deficiency and degenerative disc disease in childhood. Orthop J MP. 2014;20:58–60. [Google Scholar]
- 156.Colombini A., Cauci S., Lombardi G. Relationship between vitamin D receptor gene(VDR) polymorphism, vitamin d status, osteoarthritis and intervertebral disc degeneration. J Steroid Biochem Mol Biol. 2013;138:24–40. doi: 10.1016/j.jsbmb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Welsh P., Sattar N. Vitamin D and chronic disease prevention. BMJ. 2014;348:g2280. doi: 10.1136/bmj.g2280. [DOI] [PubMed] [Google Scholar]


