Abstract
Cancer is a leading cause of death worldwide and sustained focus is on the discovery and development of newer and better tolerated anticancer drugs especially from plants. The sulforhodamine B (SRB) in vitro cytotoxicity assay, sarcoma-180 (S-180) ascites and solid tumor, and L1210 lymphoid leukemia in vivo models were used to investigate the anticancer activity of root extracts of Aristolochia ringens Vahl. (Aristolochiaceae; 馬兜鈴 mǎ dōu líng). AR-A001 (IC50 values of 20 μg/mL, 22 μg/mL, 3 μg/mL, and 24 μg/mL for A549, HCT-116, PC3, and THP-1 cell lines, respectively), and AR-A004 (IC50 values of 26 μg/mL, 19.5 μg/mL, 12 μg/mL, 28 μg/mL, 30 μg/mL, and 22 μg/mL for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively), were observed to be significantly active in vitro. Potency was highest with AR-A001 and AR-A004 for PC3 with IC50 values of 3 μg/mL and 12 μg/mL, respectively. AR-A001 and AR-A004 produced significant (p < 0.05–0.001) dose-dependent inhibition of tumor growth in the S-180 ascites model with peak effects produced at the highest dose of 120 mg/kg. Inhibition values were 79.51% and 89.98% for AR-A001 and AR-A004, respectively. In the S-180 solid tumor model, the inhibition of tumor growth was 29.45% and 50.50% for AR-A001 (120 mg/kg) and AR-A004 (110 mg/kg), respectively, compared to 50.18% for 5-fluorouracil (5-FU; 20 mg/kg). AR-A001 and AR-A004 were also significantly active in the leukemia model with 211.11% and 155.56% increase in mean survival time (MST) compared to a value of 211.11% for 5-FU. In conclusion, the ethanolic (AR-A001) and dichloromethane:methanol (AR-A004) root extracts of AR possess significant anticancer activities in vitro and in vivo.
Keywords: Anticancer activity, Aristolochia ringens, Cytotoxicity, Lymphoid leukemia, Solid tumor
Graphical abstract
1. Introduction
Cancer is a deadly disease and about one in four people will get it in some form during their lifetime; at the present time, about one in five of all deaths are due to cancer.1 Normal diploid human cells multiply for a finite number of generations and then enter a state of replicative senescence, but cancer cells can proliferate indefinitely.2 About 12.7 million cancer cases and 7.6 million cancer deaths are estimated to have occurred in 2008; of these, 56% of the cases and 64% of the deaths occurred in the economically developing world.3 Surgery is useful in removing visible tumors, but may leave smaller nests of cancer cells in the patient which continue to proliferate, while radiation therapy is relatively imprecise as it can kill both cancer cells and normal cells, and thus has toxic side effects which may themselves be lethal to the patients.2 Chemotherapy with antiproliferative agents, including alkylating agents, antimetabolites, antibiotics, and hormones, apart from being complementary to surgical intervention and radiotherapy, is essential in cases of metastasis.
According to Sikora et al,4 although 92 approved anticancer drugs are available for the treatment of > 200 different tumor entities, effective therapies for most of these tumors are lacking. Furthermore, out of the 92 registered drugs, 17 are considered by oncologists to be more broadly applicable and 12 additional agents are perceived as having certain advantages in some clinical settings.5 Limitations in the application of chemotherapeutic agents include toxicity, manifestation of deleterious side-effects, and a narrow margin of error. These days, renewed and concerted efforts are geared towards the discovery and development of newer and better tolerated anticancer drugs, especially from natural products, mainly plants. New targets for anticancer agent development are rapidly emerging in the post-genome era, and improvements in protein structure determination, combinatorial chemistry, and high-throughput small-molecule screens may accelerate the generation of new agents to be studied in the clinic.6
Aristolochia ringens Vahl. (Aristolochiaceae (AR); 馬兜鈴 mǎ dōu líng) is a glabrous bushy climber native of tropical America, introduced to most West African countries as a garden ornamental, and has become naturalized in roadside bush in Sierra Leone, Ghana, Nigeria,7 and DR Congo.8 The plant is commonly called “Dutchman's pipe” and “Snake work” but local names in Nigeria include “Ako-igun” (Yoruba, Southwest Nigeria) and “Dumandutsee” (Hausa, Northern Nigeria). Preparations of the leaves, roots, and whole plant have been reported to be used traditionally in Nigeria for the treatment of diverse ailments including guinea worm, skin diseases, typhoid, sores, as an antidote to snake poison, an emmenagogue, and an anthelmintic remedy.9 In South America, the plant is used for the treatment of snakebites, fever, ulcers, and colic,10 while the root of the plant is used in Senegal as an antidote for snakebites.11 Sonibare and Gbile12 stated that the root of the plant is used in Southwest Nigeria for the treatment of asthma, while Soladoye et al13 reported its use for the treatment of hemorrhoids. The decoction/infusion of the root of the plant is also used as an antidiabetic.14 Antiinflammatory15 and antitrypanosomal16 activities of the plant have also been reported.
Based on the fact that some species of the genus Aristolochia have been reported to possess anticancer activity,17–19 this study was designed to investigate the anticancer activity of root extracts of AR using in vitro and in vivo methods.
2. Materials and methods
2.1. Plant material
AR roots were obtained from a local herbal market in Mushin, Lagos State, Nigeria. The plant material was identified and authenticated by Mr. T.K. Odewo of the Department of Botany and Microbiology, University of Lagos, Lagos, Nigeria, where a herbarium specimen was deposited with voucher number LUH 4061.
2.2. Extraction
Fresh roots of AR were cut into pieces and air-dried until a constant weight was attained. The dried materials were milled and divided into four portions of 100 g each. Three portions were separately macerated in about 1500 mL of alcohol (95% ethanol: AR-A001), hydro-alcohol (ethanol and water, 1:1; AR-A002), and distilled water (AR-A003). Maceration was sustained for 3 hours with mechanical stirring (Heidolph RZR 2051 Control; Heidolph Instruments GmbH & Co. KG, Schwabach, Germany) at 400 × g. Filtration was thereafter carried out using Whatman filter paper (150 mm). Residues were remacerated (× 2) to achieve exhaustive extraction. The combined filtrate of the alcoholic (AR-A001) and hydroalcoholic (AR-A002) extracts were concentrated using a Heidolph Rotavapor (LABORATA 4000) at 120 × g and 40°C. The combined filtrate of the aqueous extract was lyophilized (AR-A003). The fourth portion of 100 g of the powdered plant material was put into 2000 mL separating funnels with the bottom lined with cotton wool and 1 L of dichloromethane:methanol (DCM:MeOH; 70:30) was put into the separating funnel. Twenty-four hours later, the solvent mixture was drained and the extraction liquid was filtered using Whatman filter paper (150 mm). On the 2nd day and 3rd day, 600 mL of the DCM:MeOH solvent mixture was added to the separating funnel, drained 24 hours after, and filtered. The combined DCM:MeOH extract was concentrated with a Rotavapor without vacuum (AR-A004). All dried extracts were weighed and stored in a desiccator. The yields of the extracts were 4.41% (AR-A001), 16.00% (AR-A002), 11.88% (AR-A003), and 5.93% (AR-A004).
2.3. Chemicals
The chemicals used in this study include RPMI-1640, minimum essential medium, fetal calf serum, trypsin, trypan blue, ethanol, penicillin, streptomycin, gentamycin, dimethyl sulfoxide (DMSO), sulforhodamine B (SRB), mitomycin-C, paclitaxel, 5-fluorouracil (5-FU; Sigma Chemical Co., St Louis, MO, USA), phosphate buffer saline (Merck, Darmstadt, Germany); trichloroacetic acid (TCA), distilled water, sodium hydroxide, Tris-EDTA buffer, Tris buffer (Hi-Media, Mumbai, India), acetic acid, sodium bicarbonate, hydrochloric acid (Rankem, New Delhi, India), isopropanol (Sisco, Mumbai, India), and Tris-acetate-EDTA buffer. All other chemicals used in this study were purchased locally and were of analytical grade.
2.4. Cell lines and cell cultures
The human cancer cell lines used in this study, including A549 (lung), HCT-116 (colon), PC3 (prostate), A431 (skin), HeLa (cervix), and THP-1 (leukemia) were obtained from the National Center for Cell Science, Pune, India, or the National Cancer Institute, Frederick, MD, USA. The cells were grown and maintained in appropriate medium, pH 7.4, supplemented with 10% fetal calf serum, glutamine (2mM), penicillin (100 units/mL), and streptomycin (100 μg/mL). The cell cultures were grown in a carbon dioxide incubator (Heraeus, GmbH, Germany) at 37°C with 90% humidity and 5% CO2.20,21
2.5. Animals
The animals used in this study, including inbred BALB/c and DBA/2, outbred Swiss albino, and F1 hybrid CDF1 mice, were obtained from the animal facility of the Indian Institute of Integrative Medicine, Jammu, India and maintained at 22 ± 2°C with 20–25 complete air changes with 100% fresh air. Relative humidity was maintained at 50–60%. Animals were fed with commercially available pelleted feed (M/s Ashirwad Industries, Chandigarh, India) and were provided autoclaved water ad libitum. Animals were housed in transparent polycarbonate filter top cages in animal isolator cabins. The animals selected for the experiments were in the weight range of 18–23 g (about 2 months of age) and were observed to be healthy and free from any disease. All of the animals selected for individual experiments were of the same sex and strain. The protocols adopted in this study were approved by the Institutional Animal Ethics Committee, Indian Institute of Integrative Medicine, Jammu, India.
2.6. In vitro cytotoxicity against human cancer cell lines
The in vitro cytotoxicity of the extracts of AR was determined by a semiautomated assay using SRB.20–22 The human cancer cell lines were grown in tissue culture flasks at 37°C in an atmosphere of 5% CO2 and 90% relative humidity in complete growth medium. Flasks with subconfluent stage of growth were selected and cells were harvested by treatment with trypsin-EDTA. The numbers of cells/mL of suspension were counted using hemocytometer. The cell density was adjusted to 10,000 cells/100 μL, or as appropriate for each cell line, in the cell suspension. A volume of 100 μL of cell suspension was added to each well of 96 well plates with the help of handy-step. The plates were incubated at 37°C in an atmosphere of 5% CO2 and 90% relative humidity for 24 hours. Thereafter, 100 μL of a working solution of each test material was added to the wells of the 96 well plates. The stock solutions of the extracts (20 mg/mL) were prepared in DMSO and serially diluted with complete growth medium such that 100 μL of working solutions of each extract gave concentrations of 10 μg/mL, 30 μg/mL, and 100 μg/mL [final DMSO concentration was from 0.5% (highest) to 0.001% (lowest)] added to the 96-well cell culture plates. The 96-well cell culture plates contained appropriately seeded cells (e.g., 8000 cells/100 μL for HCT-116 and A431; 10,000 cells/100 μL for HeLa), and all vehicle controls contained the same concentration of DMSO. The plates were incubated for 48 hours at 37°C in an atmosphere of 5% CO2 and 90% relative humidity. Thereafter, 50 μL of chilled 50% TCA was gently added to each well of the plate, making a final concentration of 10%. The plates were incubated at 4°C for 1 hour to fix the cells attached to the bottom of the wells. The plates were then washed five or six times with distilled water and thereafter air-dried. To each well, 100 μL of SRB dye (0.4% w/v in 1% acetic acid) was added and left at room temperature for 30 minutes. Thereafter, the plates were washed with 1% acetic acid. The plates were again air-dried and 100 μL of Tris buffer (10mM; pH 10.5) was added to each well. The plates were shaken gently for 10–15 minutes on a mechanical shaker. The optical density of the plate wells was recorded with a microplate reader at 540 nm and data were maintained. Growth inhibition was calculated as the percent survival of treated cells over control cells × 100 (T/C%).
2.7. Evaluation of in vivo anticancer activity
2.7.1. Sarcoma-180 ascites model
This was carried out according to the method described by Chashoo et al.23 Sarcoma-180 (S-180) cells were harvested from the peritoneal cavity of Swiss albino mice, used for propagation, harboring 8–10 days old ascitic tumors. On Day 0, 1 × 107 cells/animal were injected intraperitoneally (i.p.) into the peritoneal cavity of BALB/c mice of same sex. The tumor inoculated animals were then randomized and divided into different groups based on the treatment schedule, including one control (normal saline) group and one standard drug (5-FU) group. From Day 1 to Day 9, the different treatment groups were administered i.p. AR-A001, AR-A002, AR-A003 (100 mg/kg), and AR-A004 (80 mg/kg), 5-FU (20 mg/kg), and normal saline (0.2 mL/mouse). On Day 12, all of the animals were sacrificed under diethyl ether anesthesia and the ascitic fluid was collected from the peritoneal cavity of each mouse for the evaluation of tumor weight, volume, and cell number. The percent inhibition of tumor was calculated as under:
Based on the highest value of tumor growth inhibition and least mortality, AR-A001 and AR-A004 were subjected to a graded dose (80 mg/kg i.p., 100 mg/kg i.p., and 120 mg/kg i.p.) evaluation in this model.
2.7.2. S-180 solid tumor model
This evaluation followed the same procedure outlined in the ascites model except that tumor cells (1 × 107 cells/animal) were injected intramuscularly (i.m.) into the right thigh of BALB/c mice of the same sex on Day 0. Based on the effectiveness in the ascites model, AR-A001 and AR-A004 were evaluated in this model at the dose of 120 mg/kg and 110 mg/kg, respectively. The extracts were administered i.p. Normal saline (0.2 mL/mouse) and 5-FU (20 mg/kg) given i.p. served as control and standard drug, respectively. On Day 13, the longest and shortest diameters of tumors were measured with Vernier calipers and tumor volume was determined according to the formula:24,25
Percent inhibition of tumor:
2.7.3. L1210 lymphoid leukemia model
L1210 lymphocytic leukemia cells were harvested from the peritoneal cavity of DBA/2 mice, used for propagation, harboring 7-day-old tumors. On Day 0, 2.5 × 106 cells were injected i.p. into CDF1 mice of the same sex. From Day 1 to Day 9, the different treatment groups were administered i.p. AR-A001 and AR-A004 (100 mg/kg), 5-FU (20 mg/kg), and normal saline (0.2 mL/mouse). The animals were observed for mortality and the mean survival time (MST, days) was calculated as given below:
where S5 = number of survivors on Day 5, ƩS = sum of daily survivors from Day 6 to Day 18, and NT = number of no takes (survivors beyond Day 18).
where T = mean survival time (MST, days) of the drug treated mice, C = mean survival time (MST, days) of untreated control animals, T/C% < 125% = toxic/inactive, and T/C% > 125% = significant antileukemic effect.26
2.8. Statistical analysis
Results are expressed as mean ± standard error of the mean. Data were analyzed using one-way analysis of variance followed by Dunnett's multiple comparison test using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Values were considered significant at p < 0.05.
3. Results
3.1. In vitro cytotoxic activity
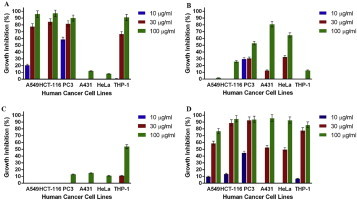
Figs. 1–4 show the effects of AR-A001, AR-A002, AR-A003, and AR-A004, respectively, against the human cancer cell lines used in this study. AR-A001 produced significant cytotoxic activity against A549, HCT-116, PC3, and THP-1 human cancer cell lines with IC50 values of 20 μg/mL, 22 μg/mL, 3 μg/mL, and 24 μg/mL, respectively. AR-A002 and AR-A003 did not elicit significant activity against the human cancer cell lines used in this study, with IC50 values generally > 100 μg/mL. AR-A004 showed significant and consistent cytotoxic activity against all the human cancer cell lines in this study, with IC50 values of 26 μg/mL, 19.5 μg/mL, 12 μg/mL, 28 μg/mL, 30 μg/mL, and 22 μg/mL for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively.
Fig. 1.
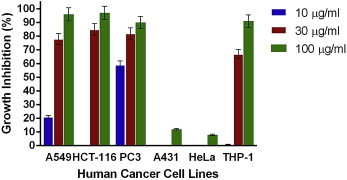
In vitro cytotoxic activity of AR-A001 against various human cancer cells lines in the sulforhodamine B assay. Estimated IC50 values are 20 μg/ml, 22 μg/ml, 3 μg/ml, >100 μg/ml, >100 μg/ml, and 24 μg/ml for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively. Samples were assayed in quadruplicate. Values are mean ± standard deviation.
Fig. 2.
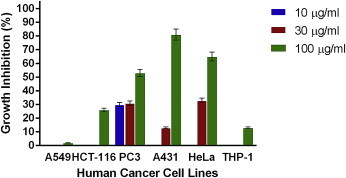
In vitro cytotoxic activity of AR-A002 against various human cancer cells lines in the sulforhodamine B assay. Estimated IC50 values are >100 μg/ml, >100 μg/ml, >100 μg/ml, 67 μg/ml, 66 μg/ml, and >100 μg/ml for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively. Samples were assayed in quadruplicate. Values are mean ± standard deviation.
Fig. 3.
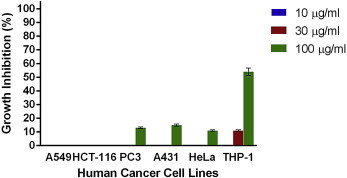
In vitro cytotoxic activity of AR-A003 against various human cancer cells lines in the sulforhodamine B assay. Estimated IC50 values are NA, NA, >100 μg/ml, >100 μg/ml, >100 μg/ml, and 93 μg/ml for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively. Samples were assayed in quadruplicate. Values are mean ± standard deviation. NA = not active.
Fig. 4.
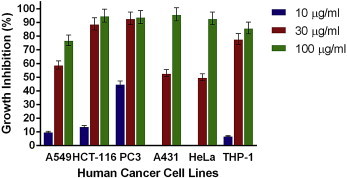
In vitro cytotoxic activity of AR-A004 against various human cancer cells lines in the sulforhodamine B assay. Estimated IC50 values are 26 μg/ml, 19.5 μg/ml, 12 μg/ml, 28 μg/ml, 30 μg/ml and 22 μg/ml for A549, HCT-116, PC3, A431, HeLa, and THP-1, respectively. Samples were assayed in quadruplicate. Values are mean ± standard deviation.
3.2. In vivo anticancer activity of AR extracts against S-180 ascites in BALB/c mice
Initial screening results for the effects of AR extracts on the S-180 ascites model in mice are shown in Table 1. AR-A001 (100 mg/kg) and AR-A004 (80 mg/kg) elicited 73.56% and 72.16% inhibition in tumor growth with no mortality recorded. These effects were highly significant (p < 0.001) in respect of the number of tumor cells present in the peritoneal cavity of treated mice as compared to control mice, but significantly lower (p < 0.05, 0.01) as compared to 5-FU (97.84% inhibition). AR-A002 and AR-A003 (100 mg/kg) elicited 80.66% and 77.24% tumor growth inhibition with mortalities of 57.14% and 71.43%, respectively. As shown in Table 2, the effects of AR-A001 and AR-A004 were dose-dependent with peak tumor cell growth inhibitory effects elicited at a dose of 120 mg/kg (79.51% and 89.98% inhibition, respectively). However, mortality was not recorded at this dose for AR-A001, while it was 60% in respect of AR-A004.
Table 1.
Effects of Aristolochia ringens (AR) extracts against sarcoma-180 (ascites) in BALB/c mice.
| Treatments | Dose (mg/kg) | Body weight (g) |
Day 12 |
Tumor growth inhibition (%) | Mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 9 | Body weight (g) | Weight of tumor (g) | Volume of ascitic fluid (mL) | Number of tumor cells (× 107) | ||||
| Normal saline | (0.2 mL/mouse) | 22.91 ± 0.74 | 24.82 ± 0.86 | 28.64 ± 1.19 | 29.36 ± 1.44 | 9.73 ± 1.00 | 9.88 ± 0.96 | 277.86 ± 37.20 | 0 | 0 |
| 5-FU | 20 | 21.14 ± 0.55* | 20.86 ± 0.63*** | 17.71 ± 0.84*** | 16.60 ± 0.75*** | 0.50 ± 0.21*** | 0.52 ± 0.22*** | 6.00 ± 2.45*** | 97.84 | 0 |
| AR-A001 | 100 | 20.43 ± 0.48** | 20.86 ± 0.55*** | 21.43 ± 0.95***,## | 21.43 ± 1.00***,## | 5.86 ± 1.37*,## | 6.01 ± 1.35*,## | 73.47 ± 15.71***,## | 73.56 | 0 |
| AR-A002 | 100 | 20.57 ± 0.37** | 18.43 ± 0.20***,# | 16.83 ± 0.48*** | 16.67 ± 1.33*** | 1.85 ± 0.98*** | 2.00 ± 1.04*** | 53.75 ± 27.17*** | 80.66 | 57.14 |
| AR-A003 | 100 | 20.71 ± 0.42* | 17.71 ± 0.42***,### | 16.50 ± 0.22*** | 15.00 ± 3.00* | 2.71 ± 2.71 | 2.75 ± 2.75 | 63.25 ± 63.25 | 77.24 | 71.43 |
| AR-A004 | 80 | 21.00 ± 0.62* | 20.14 ± 0.55c | 18.57 ± 0.65*** | 17.29 ± 1.32*** | 2.56 ± 0.71***,# | 2.73 ± 0.74***,# | 77.34 ± 26.44***,# | 72.16 | 0 |
Data are presented as mean ± standard error of the mean (n = 7, n = 11 for control).
*p < 0.05, **p < 0.01, ***p < 0.001 versus normal saline.
#p < 0.05, ##p < 0.01, ###p < 0.001 versus 5-FU (one-way analysis of variance followed by Dunnett's multiple comparison test).
5-FU = 5-fluorouracil.
Table 2.
Effects of AR-A001 (80 mg/kg, 120 mg/kg) and AR-A004 extracts (100 mg/kg, 120 mg/kg) against sarcoma-180 (ascites) in BALB/c mice.
| Treatments | Dose (mg/kg) | Body weight (g) |
Day 12 |
Tumor growth inhibition (%) | Mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | Weight of tumor (g) | Volume of ascitic fluid (mL) | Number of tumor cells (× 107) | |||||||
| Day 1 | Day 5 | Day 9 | ||||||||
| Normal saline | (0.2 mL/mouse) | 21.40 ± 0.75 | 23.80 ± 0.86 | 27.20 ± 1.24 | 27.60 ± 1.12 | 9.81 ± 1.01 | 9.96 ± 0.77 | 474.25 ± 45.26 | 0 | 0 |
| 5-FU | 20 | 21.20 ± 0.58 | 22.60 ± 0.75 | 21.40 ± 1.08** | 20.60 ± 1.44** | 0.50 ± 0.22*** | 0.48 ± 0.22*** | 10.00 ± 4.18*** | 97.89 | 0 |
| AR-A001 | 80 | 20.80 ± 0.66 | 21.80 ± 1.07 | 22.60 ± 1.69* | 23.00 ± 1.70* | 4.91 ± 1.06**,## | 5.10 ± 1.07**,## | 138.55 ± 30.42***,## | 70.79 | 0 |
| 120 | 20.80 ± 0.66 | 21.20 ± 0.80* | 21.20 ± 1.07** | 20.80 ± 1.93* | 3.71 ± 1.08**,# | 3.70 ± 1.12**,# | 97.18 ± 31.07***,# | 79.51 | 0 | |
| AR-A004 | 100 | 20.80 ± 0.66 | 20.20 ± 1.07* | 18.60 ± 1.03***,# | 18.60 ± 1.12*** | 2.35 ± 0.92*** | 2.52 ± 0.99*** | 55.14 ± 22.88*** | 88.37 | 0 |
| 120 | 20.80 ± 0.66 | 20.80 ± 0.97* | 19.60 ± 1.50** | 22.50 ± 2.50 | 2.36 ± 2.36 | 2.50 ± 2.50 | 47.50 ± 47.50** | 89.98 | 60 | |
Data are presented as mean ± standard error of the mean (n = 5).
*p < 0.05, **p < 0.01, ***p < 0.001 versus normal saline.
#p < 0.05, ##p < 0.01 versus 5-FU (one-way analysis of variance followed by Dunnett’s multiple comparison test).
5-FU = 5-fluorouracil.
3.3. In vivo anticancer activity of AR-A001 and AR-A004 extracts against S-180 solid tumor in BALB/c mice
The effects of active extracts against S-180 solid tumor are shown in Table 3. AR-A001 (120 mg/kg) and AR-A004 (110 mg/kg) produced a significant reduction (p < 0.05) in the body weight of mice and significant inhibition in tumor growth on Day 9 and Day 13 as compared to control animals. The inhibition in solid tumor growth produced by AR-A001 was reduced from 31.81% on Day 9 to 29.45% on Day 13. The effect of AR-A001 on tumor volume on Day 9 was significantly lower (p < 0.05) than that produced by 5-FU (63.39%) while the effect of the extract on Day 13 was comparable and not significantly different (p > 0.05) from that elicited by 5-FU (50.18%). Tumor growth inhibition produced by AR-A004 reduced from 55.74% on Day 9 to 50.50% on Day 13, with the later effect being comparable and not significantly different (P > 0.05) relative to 5-FU.
Table 3.
Effects of AR-A001 and AR-A004 extract against sarcoma-180 (solid) tumor in BALB/c mice.
| Treatments | Dose (mg/kg) | Body weight (g) |
Day 9 |
Day 13 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 9 | Day 13 | Tumor volume (mm3) | Inhibition (%) | Tumor volume (mm3) | Inhibition (%) | ||
| Normal saline | (0.2 mL/mouse) | 21.80 ± 0.58 | 23.00 ± 0.55 | 23.80 ± 0.58 | 24.00 ± 0.32 | 1848.50 ± 108.31 | 0.00 | 2283.50 ± 136.58 | 0.00 |
| 5-FU | 20 | 21.80 ± 0.58 | 20.60 ± 1.17 | 18.80 ± 2.08* | 19.80 ± 2.15 | 676.80 ± 188.15*** | 63.39 | 1137.60 ± 247.45** | 50.18 |
| AR-A001 | 120 | 20.80 ± 0.86 | 21.20 ± 1.02 | 20.60 ± 1.21* | 20.80 ± 1.02* | 1260.40 ± 237.90*,# | 31.81 | 1611.00 ± 290.72* | 29.45 |
| AR-A004 | 110 | 20.80 ± 0.86 | 20.20 ± 1.50 | 19.80 ± 1.39* | 19.60 ± 1.21** | 818.10 ± 124.32*** | 55.74 | 1130.40 ± 137.88*** | 50.50 |
Values are mean ± standard error of the mean (n = 5).
*p < 0.05, **p < 0.01, ***p < 0.001 versus normal saline.
#p < 0.05 versus 5-FU (one-way analysis of variance followed by Dunnett’s multiple comparison test).
5-FU = 5-fluorouracil.
3.4. Effects of AR-A001 and AR-A004 extracts against L1210 lymphoid leukemia in CDF1 mice
The effects of AR-A001 and AR-A004 (100 mg/kg) on L1210 lymphoid leukemia model are shown in Table 4. AR-A001 increased the MST from 9 days in control to 19 days, corresponding to a 211.11% increase in MST. This value was the same as for 5-FU. In respect of AR-A004, MST was 14 days compared to 9 days for control, corresponding to 155.56% increase in MST.
Table 4.
Effects of AR-A001 and AR-A004 extracts against L1210 lymphoid leukemia in CDF1 mice.
| Treatments | Dose (mg/kg) | Mean survival time (days) | % Increase in mean survival time | Inference |
|---|---|---|---|---|
| Normal saline | (0.2 mL/mouse) | 9 | – | – |
| 5-FU | 20 | 19 | 211.11 | Significant activity |
| AR-A001 | 100 | 19 | 211.11 | Significant activity |
| AR-A004 | 100 | 14 | 155.56 | Significant activity |
5-FU = 5-fluorouracil.
T/C% < 125% = toxic/inactive; T/C% > 125% = significant antileukemic effect.
4. Discussion
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries.27 The burden of cancer is increasing in economically developing countries as a result of population aging and growth, and adoption of cancer-associated lifestyle choices including smoking, physical inactivity and “westernized” diets.3 Schwartsmann et al6 stated that the total number of new cases of cancer will rise from 10 million in year 2000 by approximately 25% in each decade, reaching 24 million new cases/year in the year 2050. The total number of deaths will rise from 6 million in the year 2000 to 10 million in 2020, and to > 16 million in the year 2050.28,29 Furthermore, it was reported that in the year 2050, there will be 17 million new cases of cancer in less developed countries, while only 7 million new cases of cancer will occur in the more developed countries, with the assertion that public health authorities should expect cancer to become a major challenge not only in developed countries but especially in developing countries as well.29–31
The major challenges associated with currently available anticancer agents include selectivity, toxicity, resistance, and development of a secondary malignancy. These drawbacks have motivated the search for newer, more efficacious, and better tolerated antitumor drugs, with natural products, especially plants, offering an inexhaustible reservoir for new drug discovery and development.
In this study, the DCM:MeOH extract of the root of AR (AR-A004) was significantly active against PC3, HCT-116, THP-1, A549, A431, and HeLa human cancer cell lines with an IC50 range of 12–30 μg/mL in the SRB cytotoxicity assay. Based on the recommendation of the National Cancer Institute (NCI, USA), 30 μg/mL is the upper IC50 limit considered promising for purification of a crude extract.32 The ethanol extract (AR-A001) was also found to be significantly active against PC3, A549, HCT-116, and THP-1 cell lines, with an IC50 range of 3–24 μg/mL. AR-A004 but not AR-A001 was found to be active in the S-180 solid tumor model (50.50% tumor growth inhibition at a dose of 110 mg/kg relative to a value of 50.18% for 5-FU at 20 mg/kg). The initial activity against S-180 ascites was statistically significant (72.16%, 88.37%, and 89.98% tumor growth inhibition at doses of 80 mg/kg, 100 mg/kg, and 120 mg/kg as compared to 97.89% for 5-FU at 20 mg/kg). The dose of AR-A004 used in the solid tumor model was 110 mg/kg, because although the highest tumor growth inhibitory activity was observed at a dose of 120 mg/kg, mortality was 60% at this dose. AR-A001 also produced dose-dependent antitumor activity in the S-180 ascites model, with tumor growth inhibition values of 73.56%, 70.79%, and 79.51% at doses of 80 mg/kg, 100 mg/kg, and 120 mg/kg, respectively. However, the tumor growth inhibition value was just 29.45% in the S-180 solid tumor model for AR-A001. Both AR-A001 and AR-A004 (100 mg/kg) were also significantly active in the L1210 lymphoid leukemia model (T/C 211.11 and 155.56%, respectively, relative to 211.11% for 5-FU), with AR-A001 showing greater activity, comparable to 5-FU, in this model. This is in view of the interpretation by Todorova et al26 that T/C% value < 125% indicates toxicity/inactivity while a value > 125% depicts a significant antileukemic effect. The results obtained in this study established for the first time the in vitro and in vivo anticancer activity of AR with the DCM:MeOH and ethanolic root extracts showing significant activity in the solid tumor and leukemia models used in this study.
In this study, AR-A002 and AR-A003 did not elicit significant activity against the human cancer cell lines used, with IC50 values generally > 100 μg/mL. However, these extracts elicited significant tumor growth inhibition in the S-180 ascites model, although mortality was significant. It should be noted that inactivity in vitro does not automatically translate to inactivity in vivo, because in some cases, inactive phytochemical principles may need metabolic activation which is not possible under the in vitro environment.
Other studies have reported the anticancer activity of the DCM and MeOH,17 chloroform,33,34 and aqueous19 extracts of other species of the Aristolochia family. The anticancer activity of various species of Aristolochia has been reported but not of AR. Voloudakis-Baltatzis et al35 evaluated the anticancer activity of the Greek herb substances IBV-BK (Aristolochia, Iska) on human primary cultures derived from malignant and nonmalignant breast tissues in vitro and in vivo in rats with Walker carcinoma. Based on findings in the study, the authors concluded that IBK-BK, with Aristolochia as one of the constituents, possesses potent anticancer activity in vitro and in vivo. Mongelli et al17 investigated the cytotoxic and DNA interaction of extracts of Argentinean medicinal plants including Aristolochia triangularis. Aristolochia triangularis, along with other plants, was established to contain cytotoxic compounds against KB cells based on inhibition of growth of crown gall tumors. The assay employed showed good correlation with the 3PS (in vivo murine leukemia) antitumor assay.36 Masud Rana and Khanam33 investigated the antitumor effect of the whole plant extract of Aristolochia indica against Ehrlich ascites carcinoma in mice. The crude chloroform extract resulted in significant tumor cell growth inhibition and increased the MST. The root extract of Aristolochia indica has been reported to inhibit the growth of human CA-755 and HeLa cells and to be effective against adenocarcinoma.37 Chaouki et al34 investigated the antiproliferative effect of four different polarity extracts of Aristolochia baetica on the human breast cancer cell line MCF-7 and reported the chloroform extract to be the most active. Benarba et al19 investigated the cytotoxic and apoptogenic activities of the aqueous extract of Aristolochia longa in Burkitt's lymphoma BL41 cells by flow cytometry. Based on the significant efficacy demonstrated in the study, the aqueous extract of Aristolochia longa was reported to be a promising source of novel treatment of Burkitt's and other lymphomas. The establishment of anticancer activity of AR in this study is in agreement with the report of antiproliferative effects for other species of the genus.
5. Conclusion
In conclusion, the findings in this study suggest that the ethanolic (AR-A001) and DCM:MeOH (AR-A004) root extracts of AR possess significant anticancer activities in vitro and in vivo. AR-A001 was significantly active in the S-180 ascites and L1210 lymphoid leukemia in vivo models. AR-A004 was additionally significantly active in the S-180 solid tumor model. Further extensive studies are required to afford the identification, isolation, and characterization of specific phytomolecules responsible for bioactivities observed in this study and their inherent mechanism(s) of action.
Conflicts of interest
We declare that there are no conflicts of interest, financial or otherwise, pertaining to this study.
Acknowledgments
The authors are profoundly grateful to the Federation of Indian Chambers of Commerce and Industry (FICCI) and the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India, for the CV Raman Postdoctoral Fellowship award (June 5, 2010–December 1, 2012) given to Dr. Abidemi James Akindele. Dr. Ram A. Vishwakarma, Director IIIM (Indian Institute of Integrative Medicine, Jammu, India), is also very much appreciated for accepting to host the first author for the postdoctoral research. The immense support and guidance of Dr. A.K. Saxena, Chief Scientist and Head, Cancer Pharmacology Division, IIIM, is also appreciated.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Abidemi James Akindele, Email: ajakindele@cmul.edu.ng.
Dilip Manikrao Mondhe, Email: dmmondhe@iiim.ac.in.
References
- 1.Chhajed M., Sowle A., Baraskar R., Parihar N. Buserelin as a wonder drug for prostate cancer: a comprehensive study. Int J Pharm Res Sci. 2012;1:150–172. [Google Scholar]
- 2.Rao M.R.P., Adagale U.R., Shetty A., Namjoshi P., Gaitonde P., Jain P. 2007. Cancer Immunotherapy.http://www.pharmainfo.net/reviews/cancer-immunotherapy Accessed on 20th July, 2013, [Google Scholar]
- 3.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Sikora K., Advani S., Koroltchouk V. Essential drugs for cancer therapy: a World Health Organization consultation. Ann Oncol. 1999;10:385–390. doi: 10.1023/a:1008367822016. [DOI] [PubMed] [Google Scholar]
- 5.Aherne W., Garret M., McDonald T., Workman P. Mechanism-based high-throughput screening for novel anticancer drug discovery. In: Baguley B.C., Kerr D.J., editors. Anticancer Drug Development. Academic Press; London, UK; San Diego, CA: 2002. pp. 249–267. [Google Scholar]
- 6.Schwartsmann G., Ratain M.J., Cragg G.M., Wong J.E., Saijo N., Parkinson D.R. Anticancer drug discovery and development throughout the World. J Clin Oncol. 2002;20:47s–59s. [PubMed] [Google Scholar]
- 7.Burkill H.M. Vol 1. Royal Botanic Gardens; Kew: 1985. (The Useful Plants of West Tropical Africa). [PubMed] [Google Scholar]
- 8.De Groot H., Wanke S., Neinhuis C. Revision of the genus Aristolochia (Aristolochiaceae) in Africa, Madagascar and adjacent islands. Bot J Linn Soc. 2006;151:219–238. [Google Scholar]
- 9.2013. Anonymous. Nigerian medicinal plants database. www.medicinalplantsinnigeria.com/Plantsdatabase.pdf. Accessed on 3rd January. [Google Scholar]
- 10.Van Wyk B., Wink M. Timber Press; Portland, OR, USA: 2004. Medicinal Plants of the World: An Illustrated Scientific Guide to the Important Medicinal Plants and Their Uses. [Google Scholar]
- 11.Neuwinger H.D. Medpharm Scientific Publishers; Stuttgart, Germany: 2000. African Traditional Medicine. A Dictionary of Plant Use and Application. [Google Scholar]
- 12.Sonibare M.A., Gbile Z.O. Ethnobotanical survey of anti-asthmatic plants in South Western Nigeria. Afr J Tradit Complement Altern Med. 2008;5:340–345. doi: 10.4314/ajtcam.v5i4.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soladoye M.O., Adetayo M.O., Chukwuma E.C., Adetunji A.N. Ethnobotanical survey of plants used in the treatment of haemorrhoids in South-Western Nigeria. J Adv Dev Res. 2011;2:100–111. [Google Scholar]
- 14.Olabanji S.O., Omobuwajo O.R., Ceccato D., Adebajo A.C., Buoso M.C., Moschini G. Accelerator-based analytical technique in the study of some anti-diabetic medicinal plants of Nigeria. Nucl Instrum Methods Phys Res B. 2008;266:2387–2390. [Google Scholar]
- 15.Aigbe F.R., Adeyemi O.O. The aqueous root extract of Aristolochia ringens (Vahl.) prevents chemically induced inflammation. Planta Med. 2011;77:PF28. [Google Scholar]
- 16.Osho I.B., Lajide L. Prescreening evaluation of some plant extracts used in ethno-veterinary practices as antitrypanosomal agents. J Med Plants Res. 2012;6:2056–2060. [Google Scholar]
- 17.Mongelli E., Pampuro S., Coussio J., Salomon H., Ciccia G. Cytotoxic and DNA interaction activities of extracts from medicinal plants used in Argentina. J Ethnopharmacol. 2000;71:145–151. doi: 10.1016/s0378-8741(99)00195-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruffa M.J., Ferraro G., Wagner M.L., Calcagno M.L., Campos R.H., Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J Ethnopharmacol. 2002;79:335–339. doi: 10.1016/s0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- 19.Benarba B., Ambroise G., Aoues A., Meddah B., Vazquez A. Aristolochia longa aqueous extract triggers the mitochondrial pathway of apoptosis in BL41 Burkitt's lymphoma cells. Int J Green Pharm. 2012;6:45–49. [Google Scholar]
- 20.Samanta S., Pain A., Dutta S., Saxena A.K., Shanmugavel M. Antitumor activity of nitronaphthal-NU, a novel mixed function agent. J Exp Ther Oncol. 2005;5:15–22. [PubMed] [Google Scholar]
- 21.Sharma P.R., Mondhe D.M., Muthiah S., Pal H.C., Shahi A.K., Saxena A.K. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem Biol Interact. 2009;179:160–168. doi: 10.1016/j.cbi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Storeng P.S., Scudiero R.D., Monks A., Mc Mahon J., Vistica D. New colorimetric cytotoxicity assay for anti-cancer drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Chashoo G., Singh S.K., Sharma P.R., Mondhe D.M., Hamid A., Saxena A. A propionyloxy derivative of 11-keto-boswellic acid induces apoptosis in HL-60 cells mediated through topoisomerase I & II inhibition. Chem Biol Interact. 2011;189:60–71. doi: 10.1016/j.cbi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Sourbier C., Scroggins B.T., Ratnayake R., Prince T.L., Lee S., Lee M.J. Englerin A stimulates PKCθ to inhibit insulin signaling and to simultaneously activate HSF1: pharmacologically induced synthetic lethality. Cancer Cell. 2013;23:228–237. doi: 10.1016/j.ccr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X.R., Wei J., Lei G., Wang J., Lu J., Xia W. Polyclonal HER2-specific antibodies induced by vaccination mediate receptor internalization and degradation in tumor cells. Breast Cancer Res. 2012;14:R89. doi: 10.1186/bcr3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todorova N., Ilarionova M., Todorov K., Todorov D. Antileukemic activity of epirubicin conjugated with biopolymer dextran against lymphoid leukemia l1210 as tumor model. Biotechnol & Biotechnol Eq. 2004;18:128–130. [Google Scholar]
- 27.World Health Organization . World Health Organization; Geneva: 2008. The Global Burden of Disease: 2004 Update. [Google Scholar]
- 28.Murray C.J., Lopez A. Harvard University Press; Cambridge, MA, USA: 1996. The Global Burden of Disease. [Google Scholar]
- 29.United Nations Development Programme (UNDP) Oxford University Press; Oxford, United Kingdom: 2000. Human Development Report 2000. [Google Scholar]
- 30.Ferlay J., Bray F., Pisani P., Parkin D.M. IARC Press; Lyon, France: 2001. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0 (IARC, CancerBase No 5) [Google Scholar]
- 31.Schwartsmann G. Breast cancer in South America: challenges to improve early detection and medical management of a public health problem. J Clin Oncol. 2011;19:118s–124s. [PubMed] [Google Scholar]
- 32.Mothana R.A., Lindequist U., Gruenert R., Bednarski P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern Med. 2009;9:7. doi: 10.1186/1472-6882-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masud Rana A.Y.K.M., Khanam J.A. Aristolochia indica whole plant extract as an antineoplastic agent. J Med Sci. 2002;2:202–205. [Google Scholar]
- 34.Chaouki W., Leger D.Y., Eljastimi J., Beneytout J.-L., Hmamouchi M. Antiproliferative effect of extracts from Aristolochia baetica and Origanum compactum on human breast cancer cell line MCF-7. Pharm Biol. 2010;48:269–274. doi: 10.3109/13880200903096588. [DOI] [PubMed] [Google Scholar]
- 35.Voloudakis-Baltatzis I.E., Karydas I., Pateras C. Experimental applications of herbs of Greek flora with anticancer properties in breast cancer in vitro and in rats in vivo. Anticancer Res. 1992;12:1883. [Google Scholar]
- 36.Bryant F.O., Cutler H.G., Parker S.R. Effect of fungal natural products in an Agrobacterium tumefaciens potato disc assay. J Nat Prod. 1994;57:640–643. [Google Scholar]
- 37.Klein S. Substances which inhibit the growth of tumors. J Am Chem Soc. 1962;57:2350. [Google Scholar]


