Abstract
To determine the influence of smoking on blood and salivary superoxide dismutase enzyme levels among smokers, and to demonstrate the significant alterations in the levels of superoxide dismutase in association with patient age, periodontal disease status, smoking duration, and smoking frequency. This study also aimed to evaluate the use of saliva as a biological fluid for disease diagnosis.
Ninety males aged 25–56 years were selected and included 30 smokers, 30 nonsmokers with chronic periodontitis, and 30 healthy controls. Clinical parameters such as the gingival index, pocket depth, and clinical attachment loss were recorded. Blood and saliva samples were collected and superoxide dismutase enzyme levels were analyzed using spectrophotometric assay.
Superoxide dismutase enzyme levels in the blood and saliva were significantly higher in smokers than in nonsmokers and the controls (p < 0.05). A significant correlation existed between superoxide dismutase levels and clinical parameters. There was also a significant positive correlation between blood and salivary superoxide dismutase levels among the three groups.
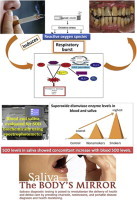
Systemic and local antioxidant status is affected by periodontal disease and by the impact of smoking. The increased blood and salivary superoxide dismutase enzyme levels in smokers may be an adaptive defense mechanism to counteract the increased reactive oxygen species production induced by smoking. This study emphasizes the importance of saliva as an easy noninvasive tool in diagnosing patients who are more prone to precancerous lesions and conditions, and its importance in patient education and motivation programs for smoking cessation.
Keywords: antioxidants, blood, chronic periodontitis, smoking, superoxide dismutase
Graphical abstract
1. Introduction
Most periodontal tissue destruction is caused by an inappropriate host response to microorganisms and their products. To be more specific, a loss of homeostatic balance between proteolytic enzymes (e.g., neutrophil elastase) and their inhibitors (e.g., α1 antitrypsin) and between reactive oxygen species (ROS) and the antioxidant defense systems that protect and repair vital tissue and cellular and molecular components are believed to be responsible.1
Polymorphonuclear leukocytes (PMNLs) are a particularly rich source of ROS, which in the absence of suitable antioxidants can lead to tissue damage. Stimulation by bacterial antigens causes PMNLs to produce superoxide (O2·−) via the metabolic pathway of the “respiratory burst” during phagocytosis. Inflammatory cells such as fibroblasts, vascular endothelial cells, and osteoclasts also produce ROS. They are highly toxic to the ingested microorganisms and to the host cells.2,3
Because cigarette smoke contains a large amount of oxidative species, smoking increases ROS production and is a significant source of oxidative stress. Smokers are nearly four times more likely than nonsmokers to have severe periodontitis.4
A variety of antioxidant defense mechanisms exist to counteract the detrimental effects of ROS in vivo. An antioxidant may be regarded as any substance that, when present in low concentrations (compared to the concentration of an oxidizable substrate), significantly delays or inhibits the oxidation of that substrate.2 The human body has an array of nonenzymatic and enzymatic antioxidant (AO) defense mechanisms to remove harmful ROS and to prevent their deleterious effects. The nonenzymatic antioxidants include vitamins A, C, and E; uric acid; bilirubin; reduced glutathione; albumin; transferrin; lactoferrin; ceruloplasmin; and haptoglobin. The enzymatic antioxidants include superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT).2,5 A delicate balance exists between antioxidant defense repair systems and pro-oxidant mechanisms of tissue destruction; if the balance is shifted in favor of ROS activity, significant tissue damage ensues.6
Within mammalian tissues, the most significant antioxidant is SOD, which catalyses the dismutation of superoxide (O2·−), an oxygen radical that is released in inflammatory pathways and causes connective tissue breakdown. This enzyme is released as a homeostatic mechanism to protect the tissues. It can be detected in extra- and intracellular compartments. Superoxide dismutase has been localized in the human periodontal ligament, and it may represent an important defense within gingival fibroblasts against superoxide release.3
Superoxide dismutase, previously known as erythrocuprein (in humans) or hemocuprein (in bovines), exists as a family of metalloproteins and is widely distributed in mammalian tissues. Erythrocytes only contain the copper/zinc SOD isoenzyme, which is coded by a gene located on chromosome 21. By virtue of their physiological role, erythrocytes are exposed to continuous oxidative stress because oxygen radicals are continuously generated by autoxidation of hemoglobin.7
Saliva, in addition to its lubricant properties, contains many biochemical substances, antibacterial components, and various antioxidants. It therefore could constitute a first line of defense against free radical-mediated oxidative stress.
Free radical scavenging and the antioxidant defense system have an important role in maintaining normal cellular physiology, facing diseases, and promoting immunity.8 There are sparse previous studies that considered the effects of smoking on the antioxidant status of blood and saliva with regard to periodontal conditions, and have yielded conflicting results.3,5,9 Hence, the present study was performed to evaluate the influence of smoking on periodontal health by estimating the SOD enzyme level in the blood and saliva of smokers and nonsmokers with chronic periodontitis. The enzyme levels in blood and saliva among healthy, smokers and nonsmokers were also compared and correlated with clinical findings.
2. Materials and methods
Ninety male participants in the age range of 25–56 years were selected by random sampling from the outpatient Department of Periodontics, P.M. Nadagouda Memorial Dental College and Hospital (Bagalkot, Karnataka, India). They were divided into three categories, based on the clinical periodontal parameters, smoking status, and inclusion criteria: Group I comprised 30 healthy individuals with no clinical and radiographic manifestations of periodontal disease; Group II comprised 30 nonsmokers with chronic periodontitis and at least 20 natural teeth and a minimum of six periodontal pockets ≥5 mm or the loss of attachment of ≥3 mm10; and Group III comprised 30 smokers (based on the self-reported smoking status) with chronic periodontitis. Current smokers who smoked ≥10 cigarettes per day and who fulfilled the criteria of chronic periodontitis were enrolled in the study.3,11 All included participants were systemically healthy; had no history of antibiotic, anti-inflammatory, or antioxidant drug treatment within the previous 6 months; and had not undergone periodontal treatment for at least 6 months prior to sampling and recording.
Females; individuals with systemic diseases such as diabetes mellitus, hepatitis, rheumatoid arthritis, cardiovascular disease, and human immunodeficiency virus infection; and regular users of vitamin supplements were excluded from the study. The need and design of the study were explained to all potential participants. Only those who gave written informed consent were included in the study. Ethical clearance for the study was obtained from the ethical committee of P.M. Nadagouda Memorial Dental College and Hospital in Bagalkot.
The gingival index (Loe and Silness),12 probing pocket depth (PD),13,14 and clinical attachment loss (CAL)13,14 were assessed. Blood and saliva samples were collected from all participants 48 hours after the clinical measurements in the morning after an overnight fast. The study participants were asked not to eat or drink (except water) prior to sample collection.
2.1. Collection of saliva samples
Unstimulated whole saliva samples were used in this study. After rinsing the mouth with 15 mL of plain water to remove exfoliated cells and debris, the participants were asked to allow saliva to pool in the bottom of the mouth and spit it on to ice chilled sterile polypropylene tubes. Approximately 1 mL of whole saliva was collected and centrifuged immediately at 3000 × g at 4 °C for 5 minutes. The resultant supernatant was aspirated and assayed biochemically for the estimation of the SOD enzyme level.15
2.2. Collection of blood samples
Two milliliters of venous blood was drawn from the antecubital vein of all participants by using a disposable syringe. It was transferred to sterile vial containing the anticoagulant EDTA.
2.3. Preparation of erythrocyte lysate
The blood was centrifuged at 2000 × g for 20 minutes at 25°C. The plasma and the upper layer of the red blood cell pellet, which contains the buffy coat, were removed aseptically. The red blood cell (RBC) pellet was washed three times with sterile saline (0.85 gm/100 mL) to ensure complete removal of the plasma, leukocytes, and platelets. The washed RBCs were hemolyzed by adding sterile distilled water (1:5 by volume). The lysate was then centrifuged at 800 × g for 15 minutes at 4°C to make the lysate ghost free. The supernatant was used as the source for the SOD enzyme estimation.16 All samples were immediately prepared and assayed on the same day.
2.4. Estimation of SOD enzyme
The superoxide dismutase level was estimated in saliva and erythrocytic lysate by using the method of Misra and Fridovich.17
2.5. The principle
The ability of SOD to inhibit the autoxidation of adrenaline to adrenochrome at pH 10.2 was the basis for this assay. The superoxide (O2·−) anion, which is the substrate for the SOD enzyme, is generated indirectly by the oxidation of epinephrine by oxygen in an alkaline pH. The SOD enzyme reacts with the O2·− formed during the epinephrine oxidation, and therefore slows the rate and the amount of adrenochrome formation.
2.6. Reagents
The reagents used were the following: (1) sodium carbonate buffer 0.1M (pH 10.2; supplied by HiMedia Laboratories Pvt. Ltd. A-516,Swastik Disha Business Park,Via Vadhani Ind. Est., LBS Marg, Mumbai-400086, India) and (2) epinephrine (1 mM; supplied by Sigma-Aldrich, E 1635-5G, shanghai, China).
2.7. Procedure
The supernatant sample (1 mL) was added into test tubes containing 0.5 mL of carbonate buffer (0.1M, pH 10.2). Water was added to this mixture to a final volume of 2.5 mL. This mixture was pipetted into a cuvette and 0.5 mL of epinephrine was added to it to initiate the reaction. The reaction was monitored at 12-second intervals for 1 minute at 480 nm and 25°.
C using a UV-visible spectrophotometer (Beijing, China (Mainland)). A suitable control that lacked the enzyme was run simultaneously. The change in the absorbance due to the inhibition of the conversion of epinephrine to adrenochrome was measured. The enzyme unit was calculated as the amount of the enzyme required to inhibit the auto-oxidation of epinephrine by 50%.17
2.8. Calculations
The percentage inhibition versus the concentration of SOD standards was plotted. Logarithmic transformation values of SOD standards were used. The SOD levels of the unknown samples were determined from the standard curve by using the percentage inhibition.
The percentage inhibition was calculated by the following formula:
| [1] |
One unit of SOD was defined as the amount of enzyme required to inhibit the auto-oxidation of epinephrine by 50%. The SOD levels in erythrocytes were expressed as units/mL in the RBC suspension and as units/mL in saliva.
2.9. Statistical evaluation
The descriptive data are expressed as the mean and standard deviation. The statistical software [SPSS 15.0 (SPSS Inc., Chicago, IL, USA), Stata 8.0, Medical 9.0.1 and Systat 11.0 (Chicago, USA)] were used for the analysis. Statistical significance was set at p < 0.05. Comparison of clinical parameters of the three groups was analyzed using the Kruskal–Wallis analysis of variance (ANOVA) test and by one-way ANOVA test. Comparison of the blood and saliva SOD enzyme levels among the three groups was analyzed by one-way ANOVA test. Pair-wise comparison was performed by Tukey's honest significant difference (HSD) post hoc procedures. Karl Pearson's correlation coefficient was used to correlate between blood and salivary SOD levels with clinical parameters among the three groups. Blood and salivary SOD levels were also correlated with each other in all three groups.
3. Results
The mean age of the study participants was 39.77 ± 9.33 years in Group I; 39.10 ± 9.72 years in Group II; and 38.90 ± 9.65 years in Group III. There was no statistically significant difference in the mean age among the three study groups (p > 0.05).
The mean gingival index score among the smokers (1.19 ± 0.55) was significantly less than that of nonsmokers (2.13 ± 0.52); whereas the mean pocket probing depth and CAL were higher in the smokers than in the nonsmokers. All values were statistically significant (p < 0.05) (Table 1).
Table 1.
Comparison of the three groups with respect to clinical parameters.
| Clinical parameters | Group I (control) mean ± SD | Group II (nonsmokers) mean ± SD | Group III (smokers) mean ± SD | Significance | p |
|---|---|---|---|---|---|
| Gingival index | 0.08 ± 0.08 | 2.13 ± 0.52 | 1.19 ± 0.55 | H value, 70.7233 | 0.0000∗ |
| Pocket depth | 2.05 ± 0.25 | 5.97 ± 0.61 | 6.82 ± 0.95 | F value, 435.5330 | 0.0000∗ |
| Clinical attachment loss | 0.09 ± 0.11 | 5.57 ± 1.15 | 6.50 ± 1.52 | F value, 294.9099 | 0.0000∗ |
*Indicates 5% level of significance (p < 0.05).
The H value is based on Kruskal–Wallis analysis of variance (ANOVA); the F value is based on one-way ANOVA.
Blood SOD enzyme levels in the smoker and nonsmoker periodontitis groups were higher than in the control group (Fig. 1). The mean blood SOD value of 19.09 ± 9.89 units/mL RBC suspensions was significantly the highest in the smokers (Table 2). Salivary SOD enzyme levels were significantly higher in smokers, followed in descending order by nonsmokers and the controls with the mean values of 23.11 ± 8.11 U/mL saliva, 13.37 ± 6.47 U/mL saliva, and 4.73 ± 3.36 U/mL saliva, respectively (Table 3). With respect to blood and saliva SOD levels, a pair-wise comparison between the groups also showed significant results.
Fig. 1.
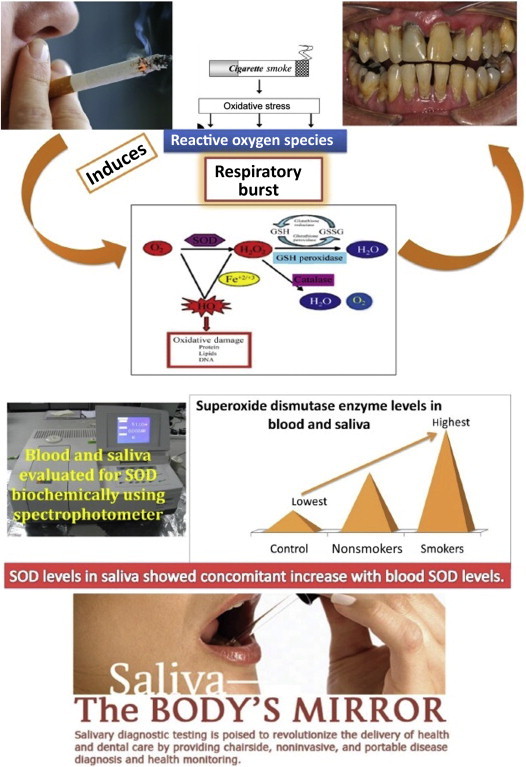
Schematic representation of mechanism of “Respiratory burst”, leading to periodontal breakdown. Also showing elevated levels of superoxide dismutase enzyme levels among smokers. GSH = reduced glutathione; GSSG = oxidized glutathione; SOD = superoxide dismutase.
Table 2.
Comparison of the three groups with respect to blood superoxide dismutase enzyme levels.
| Group | Mean | SD | |
|---|---|---|---|
| I | 4.96 | 2.91 | F = 35.2838 |
| II | 10.92 | 4.69 | p = 0.0000* |
| III | 19.09 | 9.89 | |
| Pair-wise comparison is by Tukey's HSD post hoc procedures | |||
| Control vs. nonsmokers | 0.0020* | ||
| Control vs. smokers | 0.0001* | ||
| Nonsmokers vs. smokers | 0.0001* | ||
*Indicates 5% level of significance (p < 0.05).
HSD = honest significant difference.
Table 3.
Comparison of the three groups with respect to salivary superoxide dismutase enzyme levels.
| Group | Mean | SD | |
|---|---|---|---|
| I | 4.73 | 3.36 | F = 64.0218 |
| II | 13.37 | 6.47 | p = 0.0000* |
| III | 23.11 | 8.11 | |
| Pair-wise comparison by Tukey's HSD post hoc procedures | |||
| Control vs. nonsmokers | 0.0001* | ||
| Control vs. smokers | 0.0001* | ||
| Nonsmokers vs. smokers | 0.0001* | ||
*Indicates 5% level of significance (p < 0.05).
HSD = honest significant difference.
The correlation between superoxide dismutase enzyme levels with age and clinical parameters among the groups showed an insignificant correlation between SOD levels and clinical parameters in Group I; a statistically significant positive correlation between the CAL and the SOD levels of blood and saliva in Group II; and a significant negative correlation between the mean gingival index score and blood SOD levels in Group III. There was a positive correlation between blood and salivary SOD with mean probing PD and CAL.
With regard to the correlation between smoking status and clinical parameters, the number of cigarettes smoked per day and the number of pack years showed a significant positive correlation with the PD and clinical attachment loss (CAL; p < 0.05). The duration of smoking showed a positive correlation with clinical parameters, but the value was not statistically significant. A highly significant positive correlation existed between CAL and the number of cigarettes smoked per day and the number of pack years, as analyzed by Karl Pearson's correlation coefficient method.
When blood SOD was correlated with saliva SOD, each group showed a statistically significant positive correlation, as evaluated by Karl Pearson's correlation coefficient method (Table 4).
Table 4.
The correlation between blood superoxide dismutase and salivary superoxide dismutase in all three groups.
| Group | r | p |
|---|---|---|
| I | 0.6340 | 0.0002∗ |
| II | 0.4959 | 0.0053∗ |
| III | 0.3053 | 0.0041∗ |
*Indicates 5% level of significance (p < 0.05).
r = correlation coefficient.
4. Discussion
Smoking has long been recognized as a risk factor for periodontal disease. A great deal of research into the detrimental effects of tobacco smoking has concluded that it has widespread systemic effects, many of which may provide mechanisms that increase an individual's susceptibility to periodontal disease and affect their response to treatment by stimulating destructive/inflammatory responses and impairing protective/reparative responses.18 Tobacco constituents can exacerbate aspects of the respiratory burst and enhance ROS production.19 Superoxide dismutase is an antioxidant enzyme that acts against the superoxide oxygen radical released in inflammatory pathways and causes connective tissue breakdown.20 This study attempted to estimate and compare the level of SOD enzyme levels in the blood and saliva of smokers and nonsmokers with chronic periodontitis and correlate these levels with the severity of periodontal diseases. To the best of our knowledge, this is the first study simultaneously evaluating the SOD enzyme levels in blood erythrocytes and saliva of smokers and nonsmokers with periodontitis.
In the present study, smokers were enrolled based on their self-reported smoking status. Only current smokers, who smoked ≥10 cigarettes per day (i.e., heavy smokers) were included in the study.3,11 The prevalence of female smokers in India is low (≤4%)3; for this reason and to avoid the sex-influenced changes on the SOD enzyme levels, female patients were not enrolled in the present study.
Smokers had significantly increased PD (6.82 ± 0.95 mm) and CAL (6.50 ± 1.52 mm), compared to nonsmokers. However, the gingival index was significantly higher in nonsmokers with periodontitis (2.13 ± 0.52) than in smokers (1.19 ± 0.55). This supports the concept that smokers generally present with reduced gingival inflammation and bleeding on probing, compared to nonsmokers, because smoking has a strong, chronic, dose-dependent suppressive effect on gingival inflammation and bleeding on probing, as documented in the third National Health and Nutritional Examination Survey (NHANES III).11
Results showed significantly increased SOD enzyme levels in the blood (i.e., in erythrocytes) and saliva of smokers with periodontitis, compared to nonsmokers with periodontitis and healthy controls. Various medical literature reports have shown that erythrocyte SOD activities are increased in cigarette smokers, irrespective of periodontal disease.21–24 It is primarily attributed to the fact that erythrocyte antioxidants have protective effects against oxidative damage induced by smoking. The result of our study explains the hypothesis that an exaggerated response by erythrocytes occurs in response to increased oxidative stress in smokers. In 2011, Russo et al25 also stated that acute exposure to high levels of free radicals may downregulate the gene expression of antioxidant enzymes, whereas chronic continuous exposure may increase the gene expression of these enzymes. Tonguc et al5 demonstrated higher blood and gingival tissue SOD levels among the smokers with periodontitis, compared to nonsmokers and formers smokers.
In our study, unstimulated whole saliva was used for analysis. The salivary SOD level was significantly higher in smokers (23.11 ± 8.11 U/mL) than in the nonsmokers (13.37 ± 6.47 U/mL) and the control groups (4.73 ± 3.36 U/mL). This is in accordance with a study by Kanehira et al26 in which a comparison of salivary antioxidant enzyme levels in elderly smokers and nonsmokers showed a significant increase in the SOD level among the smokers. Studies by Nagler27 and Baharavand et al28 showed similar results. Saliva, in addition to its cleansing and lubricating properties, constitutes a first line of defense against free radical-mediated oxidative stress. Most research reports of biomarkers and periodontitis have used Gingival Crevicular Fluid (GCF) as a sample fluid. However, sampling GCF is time-consuming and only reflects the gingival inflammation at each specific site sampled. In contrast to GCF, saliva is abundant and sampling it is much easier, less costly, and better tolerated by a patient. In addition, because whole saliva represents a pooled sample with contributions from all periodontal sites, the analysis of biomarkers in saliva may provide an overall assessment of the disease status, as opposed to the site-specific GCF analysis.15 For analyzing the antioxidant status, whole saliva is the most relevant because it contains gingival crevicular fluid, immune cells, and tissue metabolites, and reflects most closely the predominant intraoral condition. By contrast, stimulation may increase the flow of gingival crevicular fluid and this may result in false increases in the concentration of antioxidants in saliva.4
Studies evaluating the effects of smoking on SOD activity, regardless of periodontal status, have suggested that smoking increases the SOD activity in blood and saliva.21,23,26,29,30 It has also been reported that after smoking cessation30 and periodontal therapy,31,32 the increased SOD activity in smokers had decreased to the level in nonsmokers.
In contrast to our results, some studies have shown significantly lower activity of SOD among smokers with chronic periodontitis in blood,16 in saliva,3 in periodontal tissues,16 and GCF3; whereas other studies have reported no changes in blood33,34 and salivary4 antioxidant capacity among smokers and nonsmokers. These studies suggest that increased oxidative stress induced by smoking would have resulted in the depletion and inactivation of SOD caused by increased production of hydrogen peroxide. However, the increased level of SOD in our study confirms several findings in medical literature on oxidant–antioxidant imbalance. The SOD activity increases directly after oxidative stress. This elevation of the SOD level in blood and saliva occurs as a protective defense mechanism to scavenge the excessive superoxide radical produced by smoking-induced oxidative stress.20
There was a positive correlation between the SOD values in blood and in saliva and the gingival index, PD, and CAL in nonsmoker patients with periodontitis. However, only CAL showed a significant value in blood SOD and salivary SOD (p < 0.05). This is in agreement with the findings of other studies32,35,36 that show that SOD activity increases with the progression of inflammation in chronic periodontitis; human periodontal ligament possesses the enzyme SOD, which may afford biological protection against ROS (particularly superoxide) during the inflammatory response.37 The increase in production of superoxide radical may have resulted in oxidative stress, and then caused an increased need for SOD production to establish a ROS–AO balance to protect the tissue. Kim et al31 showed that when periodontitis is in the active state, the immune defense mechanism is activated and PMNLs increase their release of ROS, which results in the release of endogenous antioxidants in the body with a concomitant increase in the antioxidant level in saliva.
Smoking induces a selective increase of antioxidant enzyme activity in tissues as a self defense mechanism; this increase is insufficient to protect tissues from the direct harmful effects of smoking.6,30,38 Excessive SOD produced as a defense mechanism may have resulted in the overproduction of hydrogen peroxide (H2O2) because of dismutation of the superoxide radical. The H2O2 must then be detoxified by the enzyme glutathione peroxidase (GSH-Px). It is possible that the GSH-Px level or activity may have been adversely affected by increased SOD level or activity after a person is exposed to cigarette smoke.21,26 This accumulation of undetoxified H2O2, which belongs to the ROS group, may act as a constant source of oxidative stress and increase periodontal destruction in smokers.
When blood SOD was correlated with salivary SOD, a significant positive correlation was seen among all three groups. This indicates that the body attempts to counteract ROS by locally and systemically increasing endogenous antioxidant enzyme activity to protect the tissues as the oxidative stress increases.
This signifies that the biochemical changes occurring in blood during the disease process occurs simultaneously in saliva. Hence, as a future perspective, saliva can be considered as a non invasive diagnostic fluid over blood analysis in disease diagnosis.
The limitation of our present study is that the smoking status was recorded by the self-report of the study participants. However, the estimation by serum cotinine assay would be more reliable for the evaluation of the smoking status of an individual. Therefore, further studies should be considered that use serum cotinine assay and that include individuals by severity of periodontitis.
Conflicts of interest
All authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Chapple I.L.C., Mathews J.B. The role of reactive oxygen species and antioxidant species in the periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 2.Chapple I.L.C. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 3.Agnihotri R., Pandurang P., Kamath S.U. Association of cigarette smoking with superoxide dismutase enzyme levels in subjects with chronic periodontitis. J Periodontol. 2009;80:657–662. doi: 10.1902/jop.2009.080545. [DOI] [PubMed] [Google Scholar]
- 4.Buduneli N., Kardesler L., Isik H. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–164. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 5.Tonguc M.O., Ozturk O., Sutcu R. The impact of smoking status on antioxidant enzyme activity and malondialdehyde levels in chronic periodontitis. J Periodontol. 2011;82:1320–1328. doi: 10.1902/jop.2011.100618. [DOI] [PubMed] [Google Scholar]
- 6.Chapple I.L.C., Brock G., Eftimiadi C., Matthews J.B. Glutathione in gingival crevicular fluid and its relationship to local antioxidant capacity in periodontal health and disease. Mol Pathol. 2002;55:367–373. doi: 10.1136/mp.55.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casado A., De la Torre R., Lopez-Fernandez M.E. Copper/zinc superoxide dismutase activity in newborns and young people in Spain. Indian J Med Res. 2007;125:655–660. [PubMed] [Google Scholar]
- 8.Mates J.M., Perez-Gomez C., Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 9.Battino M., Ferreiro M.S., Gallardo I., Newman H.N., Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29:189–194. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 10.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Novak M.J., Novak K.F. Smoking and periodontal disease. In: Newman M.G., Takei H.H., Klokkevold P.R., Carranza F.A., editors. Carranza's Clinical Periodontology. 9th ed. Saunders; Philadelphia, PA: 2003. pp. 245–252. [Google Scholar]
- 12.Loe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 13.Newman M.G., Takei H.H. Diagnosis, prognosis, and treatment plan. In: Newman M.G., Takei H.H., Klokkevold P.R., Carranza F.A., editors. Carranza's Clinical Periodontology. 9th ed. Saunders; Philadelphia, PA: 2003. pp. 441–447. [Google Scholar]
- 14.Salvi G.E., Lindhe J., Lang N.P. Examination of patients with periodontal diseases. In: Lindhe J., Lang N.P., Karring T., editors. Clinical Periodontology and Implant Dentistry. 5th ed. Blackwell Publishing Ltd; Victoria, Australia: 2008. pp. 577–580. [Google Scholar]
- 15.Khalili J., Biloklytska H.F. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008;14:754–760. doi: 10.1111/j.1601-0825.2008.01464.x. [DOI] [PubMed] [Google Scholar]
- 16.Garg N., Singh R., Dixit J., Jain A., Tewari V. Levels of lipid peroxides and antioxidants in smokers and nonsmokers. J Periodont Res. 2006;41:405–410. doi: 10.1111/j.1600-0765.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 17.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 18.Ryder M.I. The influence of smoking on host responses in periodontal infections. Periodontol 2000. 2007;43:267–277. doi: 10.1111/j.1600-0757.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 19.Iho S., Tanaka Y., Takauji R. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-κB. J Leukoc Biol. 2003;74:942–951. doi: 10.1189/jlb.1202626. [DOI] [PubMed] [Google Scholar]
- 20.Akalin F.A., Toklu E., Renda N. Analysis of superoxide dismutase activity levels in the gingiva and gingival crevicular fluid in patients with chronic periodontitis and periodontally healthy control. J Clin Periodontol. 2005;32:238–243. doi: 10.1111/j.1600-051X.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 21.Ozguner F., Koyu A., Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005;21:21–26. doi: 10.1191/0748233705th211oa. [DOI] [PubMed] [Google Scholar]
- 22.Srivatsan R., Das S., Gadde R. Antioxidants and lipid peroxidation status in diabetic patients with and without complications. Arch Iran Med. 2009;12:121–127. [PubMed] [Google Scholar]
- 23.Kocyigit A., Erel O., Gur S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin Biochem. 2001;34:629–633. doi: 10.1016/s0009-9120(01)00271-5. [DOI] [PubMed] [Google Scholar]
- 24.Hulea S.A., Olinescu R., Nita S., Crocnan D., Kummerow F.A. Cigarette smoking causes biochemical changes in blood that are suggestive of oxidative stress: a case control study. J Environ Pathol Toxicol Oncol. 1995;14(3–4):173–180. [PubMed] [Google Scholar]
- 25.Russo M., Cocco S., Secondo A. Cigarette smoking condensate causes a decrease of the gene expression of Cu-Zn superoxide dismutase, Mn superoxide dismutase, glutathione peroxidase, catalase, and free radical induced cell injury in SH-SY5Y human neuroblastoma cells. Neurotox Res. 2011;19:49–54. doi: 10.1007/s12640-009-9138-6. [DOI] [PubMed] [Google Scholar]
- 26.Kanehira T., Shibata K., Kashiwazaki H., Inoue N., Morita M. Comparison of antioxidant enzymes in saliva of elderly smokers and nonsmokers. Gerodontology. 2006;23:38–42. doi: 10.1111/j.1741-2358.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 27.Nagler R.M. Altered salivary profile in heavy smokers and its possible connection to oral cancer. Int J Biol Markers. 2007;22:274–280. doi: 10.1177/172460080702200406. [DOI] [PubMed] [Google Scholar]
- 28.Baharvand M., Maghami A.G., Azimi S., Bastani H., Ahmadieh A., Taghibakhsh M. Comparison of superoxide dismutase activity in saliva of smokers and nonsmokers. South Med J. 2010;103:425–427. doi: 10.1097/SMJ.0b013e3181d7e0d8. [DOI] [PubMed] [Google Scholar]
- 29.Giuca M.R., Giuggioli E., Metelli M.R. Effects of cigarette smoke on salivary superoxide dismutase and glutathione peroxidase activity. J Biol Regul Homeost Agents. 2010;24:359–366. [PubMed] [Google Scholar]
- 30.McCusker K., Hoidal J. Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis. 1990;141:678–682. doi: 10.1164/ajrccm/141.3.678. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.C., Kim O.S., Kim O.J., Kim Y.J., Chung H.J. Antioxidant profile of whole saliva after scaling and root planning in periodontal disease. J Periodontal Implant Sci. 2010;40:164–171. doi: 10.5051/jpis.2010.40.4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei D., Zhang X.L., Wang Y.Z., Yang C.X., Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis before and after periodontal therapy. Aust Dent J. 2010;55:70–78. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 33.Bolzan A.D., Bianchi M.S., Bianchi N.O. Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex, age and cigarette smoking. Clin Biochem. 1997;30:449–454. doi: 10.1016/s0009-9120(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 34.Leonard M.B., Lawton K., Watson I.D., MacFarlane I. Free radical activity in young adult cigarette smokers. J Clin Pathol. 1995;48:385–387. doi: 10.1136/jcp.48.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panjamurthy K., Manoharan S., Ramachandran C.R. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett. 2005;10:255–264. [PubMed] [Google Scholar]
- 36.Tulunoglu O., Alacam A., Bastug M., Yavuzer S. Superoxide dismutase activity in healthy and inflamed pulp tissues of permanent teeth in children. J Clin Pediatr Dent. 1998;22:341–345. [PubMed] [Google Scholar]
- 37.Jacoby B.H., Davis W.L. The electron microscopic immunolocalization of copper-zinc superoxide dismutase in association with collagen fibres of periodontal soft tissues. J Periodontol. 1991;62:413–420. doi: 10.1902/jop.1991.62.7.413. [DOI] [PubMed] [Google Scholar]
- 38.Sohn H.O., Lim H.B., Lee Y.G., Lee D.W., Kim Y.T. Effect of subchronic administration of antioxidants against cigarette smoke exposure in rats. Arch Toxicol. 1993;67:667–673. doi: 10.1007/BF01973689. [DOI] [PubMed] [Google Scholar]


