Abstract
Background
Chloroquine (CQ) over three days plus primaquine (PQ) for seven days is the treatment of choice of infections by Plasmodium vivax in Bolivia, where 95% of the cases of malaria are attributed to this species. The aim of this study was to evaluate the therapeutic efficacy of CQ in this setting.
Methods
Patients in the Amazon region of northern Bolivia, were included in the study from May to November 2011 and the therapeutic efficacy of CQ was evaluated over a 28-day follow-up period. Patients with P. vivax mono-infection received 25 mg/Kg body weight of CQ over three days. The concentrations of CQ + desethylchloroquine (DCQ) in blood were determined at days 7 and 28 of follow up; at follow-up and on the day of treatment failure was administered PQ.
Results
One hundred patients fulfilled the inclusion criteria, two were lost to follow up and another two were later excluded for protocol violation. Of the 96 patients who completed the follow up 10 showed TF; one presented continued parasitaemia until day 7 of follow up, three on day 21 and six on day 28 of follow up. The geometric mean of CQ + DCQ on day 7 was 321.7 ng/ml (range 197–535 ng/ml). In six patients with TF the CQ + DCQ concentrations in blood on the day of TF were >100 ng/ml. The rate of resistance was 6.5%.
Conclusion
The present study demonstrates the presence of resistance to CQ in the treatment of malaria by P. vivax in the Amazon region of Bolivia. New clinical trials are needed to establish alternative treatments against these parasites in this region of South America.
Keywords: Chloroquine, Plasmodium vivax, Bolivia, Antimalarial drugs
Background
In South America, 60% [1] of the cases of malaria are due to Plasmodium vivax while, in Bolivia, 93% correspond to this parasite and the remaining 7% to Plasmodium falciparum. In Bolivia, the first-line therapeutic schedule for infections by P. vivax includes 25 mg/Kg body weight of chloroquine (CQ) for three days plus 3.5 mg of primaquine (PQ) for 7 days. The first is aimed at reducing the parasitic load in young forms of the parasite and eliminating immature gametocytes while the second drug is active against the gametocytes and hypnozoites of this species [2].
The first description of resistance to CQ in infections by P. vivax was in Papua New Guinea in 1989 [3], with the highest rates of resistance being reported on the same island of New Guinea [4, 5]. In Latin America, the first cases of resistance to CQ in infections by P. vivax were reported in Guyana in 1996, with three patients maintaining parasitaemia in the presence of adequate serum levels of CQ [6]. Since then cases of therapeutic failure (TF) have been described in different areas of the continent. However, confirmed resistance according to the serum levels of CQ and its main metabolite desethylchloroquine (DCQ) has only been reported in Peru with a rate of 1.2% [7] and in Brazil with 5.2% [8].
In 2003, an in vivo evaluation of resistance to CQ was carried out in the municipality of Riberalta in Bolivia, reporting 15% (9/59 cases) of TF over a 28-day period. However, none of the cases showed serum levels of the drug [9]. Another evaluation performed in 2007 in the same municipality showed TF of 6% (5/81), however CQ blood levels on the day of the evaluation were <100 ng/ml [10]. The present study is part of the routine monitoring of the therapeutic efficacy of anti-malarial drugs in Bolivia following the Pan-American Health Organization/World Health Organization (PHO/WHO) evaluation programme.
Methods
Study site
The study was developed in the department of Beni, in the municipality of Riberalta, in the north of the Amazonia of Bolivia where 18% of the diagnoses of malaria and 21% of the cases of malaria by P. vivax are reported in Bolivia [11].
Study design
The screening methods for surveillance of anti-malarial drug efficacy (MSADE) of the WHO [12] were followed. The sample size was defined as established in MSADE determined by the proportion of TF in South America and corresponding to no more than 5%, with a confidence interval of 95% and a precision of 5%. Thus, 73 patients were to be included with a maximum period of seven months. The patients were recruited and followed by multidisciplinary teams in two health care centers: La Unidad health care centre (LU) and the Cesar Moscoso health care centre (CM) in the urban area of the Riberalta, being equal distance from each other (3,000 m) The inclusion criteria were: patients greater than five years of age diagnosed with mono-infection with P. vivax, a parasite density of asexual forms of 250–100,000 parasites/μl of blood, a temperature >37.5°C or in its absence a history of a rise in temperature within the last 48 h. Microscopic diagnosis was performed according to the practical guidelines of diagnosis of the National Malaria Control Programme [13], (thick blood film, Giemsa staining) and parasite density was calculated per microlitre of blood by dividing the number of parasites counted multiplied by 6,000, divided by leucocytes counted. The results were obtained from the average of two experienced microscopists. In the case of a difference in parasitic density greater than 50%, the final result was considered by averaging the result of a third microscopist. The presence of gametocytes was also determined in this way.
The exclusion criteria were: pregnant women confirmed by rapid pregnancy diagnostic tests (detection of human chorionic gonadotropin in urine), patients with signs and symptoms of severe malaria, a history of allergy to anti-malarial drugs, concomitant presence of severe or chronic diseases such as tuberculosis or HIV/AIDS and a previous history of having received anti-malarial drugs.
The treatment consisted of the use of chloroquine phosphate (Lote FCV 002A, Macleods Pharmaceuticals Ltd, India) 25 mg/Kg body weight administered orally over 3 days: on days 0 and 1 of the study 10 mg/Kg body weight were given and on day 2, 5 mg/Kg body weight were administered under strict supervision of the investigative team. Patients vomiting within half an hour after the administration of the drug were given another treatment cycle with the same dose and patients with more than two episodes of vomiting were excluded from the study.
The patients who accepted to participate in the study were followed on days 2, 3, 7, 14, 21 and 28 after the treatment. The day of diagnosis was denominated day 0. After the 28 days of follow-up all the patients were treated with PQ 0.5 mg/Kg body weight/day administered orally during seven days. Patients with TF or those in whom mixed malaria was detected were excluded from the study. These patients were then administered artemisinin-based combination therapy (ACT) used in Bolivia for the treatment of P. falciparum, plus PQ 0.5 mg/Kg over 7 days.
Two milliliters of blood were obtained by venopuncture from all the patients in the study, with or without parasitaemia, on days 7 and 28 of the follow up. The concentrations of CQ and DCQ in blood were also determined by high performance liquid chromatography (HPLC) according to the standardized technique of the Center for Disease Control in Atlanta, USA in patients with TF. The samples obtained were kept at 4°C until analysis.
The HPLC was performed using the Shimadzu LC-10ATVP with RF-10 AXL fluorescence detector under the following chromatographic conditions: chromatographic column: Agilent Zorbax SIL, silane packing (corresponding to L3 according to USP). Detector: fluorescence, emission 380 nm, excitation 320 nm, mobile phase A: methanol and diethylamine (100:0.3); mobile phase B: n-hexane, t-butyl methyl ether and diethylamine (1:1:0.003), flow gradient: 1.0 ml/min; injection volume 7 µl and column temperature: 30°C. A concentration of CQ + DCQ of 70–99 ng/ml was considered effective to eliminate all the asexual and sexual forms of P. vivax in blood [14] and a CQ + DCQ concentration >100 ng/ml in the presence of parasitaemia was considered to demonstrate resistance to CQ [15–17].
Statistical analysis
The Tableau® 7 (Professional edition) software was used to analyse the data of frequency and to measure the data of CQ + DCQ. The statistical package SPSS® v 20 (Chicago, IL, USA) 14 was used to compare the means and medians of the results of CQ + DCQ and associated variables, with parametric and non-parametric models of analysis of variance. Kaplan-Meier analysis of survival and confidence intervals was analysed by the WHO programme for study the in vivo therapeutic efficacy [12]. All the contrasts of hypothesis were evaluated with an alpha risk of 5% and the estimations were made with a confidence interval of 95%.
Ethical aspects
The study was performed following the recommendations of the National Committee of Bioethics of Bolivia and the Ethical Committee of PAHO (PAHOERC). All patients provided informed consent to participate in the study, and in those under legal age informed consent was obtained from their legal guardians.
Results
From May to November 2011, 656 cases of malaria by P. vivax were reported out of a total of 5,290 cases evaluated in the urban area of Riberalta. One hundred of these patients (51 in CM and 49 in LU) were included in the study. Of the total number of cases evaluated 64% were males and 36% females, with a median age of 20 years (range 5–69 years), and 25% of whom were under the age of 15 years. Thirty-one percent (n = 31) had an axillary temperature >37°C, the geometric mean of PD on day 0 was 3,837.43 parasites/µl (range 252.35–29,987.15 parasites/µl), The geometric mean of CQ administered over the three days of treatment was 1,332.19 mg (range 375–2,250 mg). The mean haemoglobin values on day 0 in the females was 10 g/dl (range 7–14 g/dl) and 12 g/dl (range 8–16 g/dl) in males, and 75% (n = 75) had anaemia before initiating the treatment (Table 1).
Table 1.
Characteristics of the patients included in the study
| Characteristics | |
|---|---|
| Total number of patients recruited | 100 |
| Age | |
| Median age (year, range) | 20 (5–69) |
| Number of patients <15 years of age | 25% (n = 25) |
| Sex | |
| Female | 36% (n = 36) |
| Male | 64% (n = 64) |
| History of fever | 97% (n = 97) |
| Axillary temperature >37°C (day 0) | 31% (n = 31) |
| Geometric mean of parasites/μl (day 0) (CI 95%) | 3,837.43 (252.35–29,987.15) |
| Geometric mean CQ concentration in mg at day 3 (CI 95%) | 1,332.19 (375-2,250) |
| Mean haemoglobin value (day 0) | |
| Female, g/dl; range | 10 (7–14) |
| Male, g/dl; range | 12 (8–16) |
| No. (%) with anaemiaa | 75 (75%) |
aHaemoglobin level <12 g/dl for females and <13 g/dl for males (according to WHO criteria).
Response to treatment
Of the 100 patients included in the study two were lost to follow up and another two were later excluded; one for presenting co-infection by P. falciparum and the other for violating the protocol. Therefore, a total of 96 patients were followed until day 28 after treatment. Of these 96 patients 59% (n = 57) eliminated the parasitaemia on day 2; 91% (n = 87) on day 3 and 99% (n = 95) on day 7 of follow up. Parasitaemia or clinical deterioration were not observed until the end of follow up in 89.6% (n = 86). Treatment failure was observed on days 7, 21 and 28 follow-up, with 1.04% (n = 1) on day 7, 3.1% (n = 3) on day 21 and 6.3% (n = 6) on day 28. The median age of the patients cured was 21 years (range 5–61 years) and that of patients with TF was 14 years (range 5–32 years), with the differences being statistically significant (P = <0.05). With the Kaplan-Meier analysis the cumulative incidence of treatment failure was 0.104 (95% CI 0.057–0.185).
Blood concentrations of CQ + DCQ
The geometric mean CQ + DCQ value in blood of all the patients followed with and without TF (n = 96) on day 7 was 319.46 ng/ml (range 50–743 ng/ml), being 109.24 ng/ml (range 34–602 ng/ml) on day 28 of follow up (n = 92). In the patient with continued presence of parasites in blood until day 7 inclusive the CQ + DCQ values on day 7 were 535 µg/ml and the patient was excluded from the study. In the 10 patients presenting TF the geometric mean of CQ + DCQ was 321.7 ng/ml (range 197–535 ng/ml) on day 7, being 113.46 ng/ml (range 75–223 ng/ml) on the day of TF. In six patients the CQ + DCQ level on the day of TF was above the minimum elimination concentration (MEC) (100 ng/ml) (Table 2; Figure 1). Using Kaplan-Meier survival analysis, the cumulative incidence of therapeutic failure due to resistance was 0.65 (95% CI 0.029–0.139) (Table 3).
Table 2.
Results on the day of therapeutic failure (TF)
| Center | Day TF | Age years | Weight (Kg) | CQ administered mg | CQ + DCQ D7a | CQ + DCQ TFb |
|---|---|---|---|---|---|---|
| LU 10 | 7 | 32 | 62 | 1.500 | 535 | |
| CM 02 | 21 | 17 | 72 | 1.875 | 351 | 102 |
| CM 35 | 21 | 9 | 29 | 750 | 247 | 80 |
| LU 07 | 21 | 7 | 21 | 525 | 449 | 83 |
| CM 42 | 28 | 5 | 16 | 450 | 263 | 117 |
| CM 23 | 28 | 14 | 56 | 1.500 | 315 | 183 |
| CM 43 | 28 | 19 | 61 | 1.575 | 197 | 148 |
| LU 12 | 28 | 14 | 57 | 1.500 | 431 | 223 |
| LU 28 | 28 | 14 | 42 | 1.050 | 408 | 86 |
| LU 38 | 28 | 9 | 27 | 675 | 199 | 75 |
Concentrations of CQ + DCQ on day 7 and on the day of TF.
aCQ + DCQ D7 chloroquine + desethylchloroquine on day 7 of follow up.
bCQ + DCQ TF chloroquine + desethylchloroquine on the day of therapeutic failure.
Figure 1.
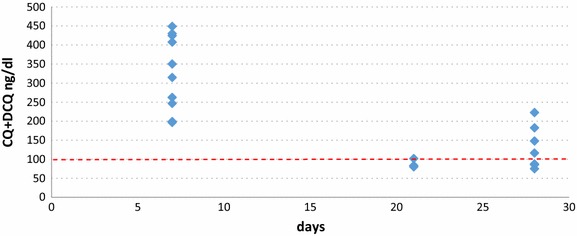
Chloroquine + desethylchloroquine (CQ + DCQ) concentrations in blood on day 7 (D7). Follow and on the day of therapeutic failure (DTF) in the 10 patients with therapeutic failure.
Table 3.
Estimation of cumulative incidence (risk) of current parasitaemia after chloroquine therapy and malaria by P. vivax adjusted for the concentration of chloroquine + desethylchloroquine (CQ + DCQ) in blood on the day of therapeutic failure (TF)
| Day | Number of patients | TF/CQ + DCQ >100 ng/dl | Censored | Failure cumulative incidence |
|---|---|---|---|---|
| 0 | 96 | 0 | 0 | 0 |
| 2 | 96 | 0 | 3 | 0 |
| 3 | 93 | 0 | 0 | 0 |
| 7 | 93 | 1 | 1 | 0.01 |
| 14 | 91 | 0 | 0 | 0.01 |
| 21 | 91 | 1 | 0 | 0.022 |
| 28 | 90 | 4 | 0 | 0.065 |
| Total | 86 | 6 | 4 | (95% CI 0.029–0.139) |
Discussion
According to the MSADE [12], the presence of parasites from day 7 to 28 of follow up at CQ + DCQ blood concentrations >100 ng/ml indicates resistance to CQ. Thus, in the Amazon region of Bolivia there is evidence of resistance to CQ in P. vivax infections.
The concentration of CQ-DCQ in blood on day 7 in all the patients with TF was above the MEC of the drug. In addition, the geometric mean was 321.7 ng/ml (range 197–535 ng/ml) demonstrating the CQ presented adequate absorption through the intestinal tract [18]. In 6 of the 10 patients with TF it was observed that the CQ + DCQ levels on the day of TF were >100 ng/ml using Kaplan-Meier survival analysis, the cumulative incidence of therapeutic failure due to resistance was 0.65 (95% CI 0.029–0.139).
Ruebsh et al. confirmed resistance to CQ in infections by P. vivax with the levels of the drug in blood, describing resistance in two out of 177 patients in the Amazon region of Peru [7]. Santana Filho reported parasitaemia above the MEC of the drug in 11 out of 109 patients in the Amazon region of Brazil, although DCQ concentrations were not determined [19]. In addition, Marquez et al. described resistance in seven out of 135 patients receiving CQ + PQ in the same Amazon region of Brazil [8].
According to the recommendations of the WHO, a change in the treatment schedule should be considered in cases of proven resistance >10% [2]. The rate of resistance to CQ in Latin America is rising [20] and treatment alternatives should be investigated to achieve better control against malaria in this region. At present combined treatments with ACT are recommended for infections by P. falciparum because of their demonstrated rapid effectiveness in eliminating the parasitic load and achieving complete cure of the disease [2]. This is the alternative treatment which should be implemented in the treatment of malaria by P. vivax. Several clinical trials have demonstrated the efficacy of the ACT in the treatment of malaria P. vivax, with dihydroartemisinin-piperaquine being the ACT most frequently studied [21]. Taking into account the low prevalence of glucose 6 phosphate dehydrogenase deficiency in the Amazon region of South America [22], the chosen ACT could be combined with PQ [23, 24], with the aim of preventing a recurrence of parasitaemia due to circulating hypnozoites, however further studies are still required to use these drugs simultaneous.
Conclusions
In conclusion, 6.5% of resistance to CQ was observed in infections by P. vivax in the Amazon region of Bolivia. Considering the percentage of resistance found and the rate of TF with MEC of the drug in blood on the day of TF this resistance could be greater. New treatment schedules should be evaluated to guarantee the control of malaria in this region.
Authors’ contributions
AA designed and coordinated the study, MM, AL and CG determined CQ concentrations in blood; MC and SG performed the patient follow up, CA did the statistical analysis, AA wrote the article. All authors read and approved the final manuscript.
Acknowledgements
This study received financial support from the Iniciativa Amazónica contra la Malaria/Red Amazónica de la Vigilancia de las Drogas Antimaláricas AMI/RAVREDA, supported by Ministry of Health of Bolivia, the board of the network of Riberalta and the Organización Panamericana de la Salud. We would like to thank all the patients who donated blood for the study as well as Mrs. Maritza Morales Technician. Angelo Roca, Fátima Tuno, Zulma Queteguari, Mrs. Sulfia, Paola Languide and Francisco Ramos, Jorge Castillo, and Dr. Jorge Cuba for their help in patient adherence to the study.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- CQ
chloroquine
- DCQ
desethylchloroquine
- PQ
primaquine
- TF
treatment failure
- ACT
artemisinin-based combination therapy
- MEC
minimum effective concentration
- LU
La Unidad health care centre
- CM
Cesar Moscoso health care centre
- PD
parasitic density
- MSADE
screening methods for surveillance of anti-malarial drug efficacy
Contributor Information
Arletta Añez, Email: arlettarocio@gmail.com.
Manuel Moscoso, Email: molomos@yahoo.es.
Ángel Laguna, Email: aplaguna@hotmail.com.
Cecilia Garnica, Email: cgarnicalopez@yahoo.es.
Viviana Melgar, Email: irvime@yahoo.es.
Mauren Cuba, Email: maurencc@hotmail.com.
Sonia Gutierrez, Email: higueras_74@hotmail.com.
Carlos Ascaso, Email: carlosascaso@ub.edu.
References
- 1.WHO (2012) World Malaria report 2012. World Health Organization, Geneva
- 2.PAHO (2011) Directrices para el tratamiento de la malaria. 2da ed. Organización Panamericana de la Salud, Washington DC
- 3.Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg. 1992;86:121–122. doi: 10.1016/0035-9203(92)90531-G. [DOI] [PubMed] [Google Scholar]
- 4.Sumawinata IW, Bernadeta, Leksana B, Sutamihardja A, Purnomo, Subianto B et al (2003) Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am J Trop Med Hyg 68:416–420 [PubMed]
- 5.Baird JK, Basri H, Subianto B, Fryauff DJ, Mcelroy PD, Leksana B, et al. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J Infect Dis. 1995;171:1678–1682. doi: 10.1093/infdis/171.6.1678. [DOI] [PubMed] [Google Scholar]
- 6.Phillips EJ, Keystone JS, Kain KC. Failure of combined chloroquine and high dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis. 1996;23:1171–1173. doi: 10.1093/clinids/23.5.1171. [DOI] [PubMed] [Google Scholar]
- 7.Ruebush TK, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- 8.Marques MM, Costa MR, Santana Filho FS, Vieira JL, Nascimento MT, Brasil LW, et al. Plasmodium vivax chloroquine resistance and anemia in the western Brazilian Amazon. Antimicrob Agents Chemother. 2013;58:342–347. doi: 10.1128/AAC.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez E, Yucra WO, Castro V, Figueroa RV, De la Cruz L, Téllez C, et al. Evaluación de la eficacia de la cloroquina para el tratamiento de la malaria por Plasmodium vivax en Yacuiba, Tarija, Bolivia. Cuad Hosp Clín. 2009;54:27–33. [Google Scholar]
- 10.Añez A, Navarro-Costa D, Yucra O, Garnica C, Melgar V, Moscoso M, et al. Respuesta terapéutica de Plasmodium vivax a la cloroquina, en Riberalta, Guayaramerín y Yacuiba, Bolivia. Biomédica. 2012;32:527–535. doi: 10.7705/biomedica.v32i4.750. [DOI] [PubMed] [Google Scholar]
- 11.MSyD (2011) Situación Actual de la malaria en Bolivia. Parte Epidemiológico 2011, Ministerio de Salud y Deportes, La Paz
- 12.WHO (2009) Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva
- 13.MSyD (2010) Guía practica de diagnóstico de la malaria. Serie Documentos Técnicos Normativos 154, Ministerio de Salud y Deportes, La Paz
- 14.Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565–1577. doi: 10.1056/NEJMra043207. [DOI] [PubMed] [Google Scholar]
- 15.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi et al (1997) Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg 56:621–626 [DOI] [PubMed]
- 16.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;11:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy GS, Basri H, Purnomo, Andersen EM, Bangs MJ, Mount DL et al (1993) Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96–100 [DOI] [PubMed]
- 18.WHO (2011) Methods and techniques for assessing exposure to antimalarial drugs in clinical field studies, World Health Organization, Geneva
- 19.De Santana Filho FS, Lima-Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, et al. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–1129. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonçalves LA, Cravo P, Ferreira MU. Emerging Plasmodium vivax resistance to chloroquine in South America. An overview. Mem Inst Oswaldo Cruz. 2014;109:534–539. doi: 10.1590/0074-0276130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair D, Gogtay N, Brand F, Olliaro P. Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database Syst Rev. 2011;6:CD008492. doi: 10.1002/14651858.CD008492.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Mezzacappa MA, Facchini FP, Pinto AC, Cassone AE, Souza DS, Bezerra MA, et al. Clinical and genetic risk factors for moderate hyperbilirubinemia in Brazilian newborn infants. J Perinatology. 2010;30:819–826. doi: 10.1038/jp.2010.48. [DOI] [PubMed] [Google Scholar]
- 23.Wilairatana P, Silachamroon U, Krudsood S, Singhasivanon P, Treeprasertsuk S, Bussaratid V, et al. Efficacy of primaquine regimens for primaquine-resistant Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg. 1999;61:973–977. doi: 10.4269/ajtmh.1999.61.973. [DOI] [PubMed] [Google Scholar]
- 24.Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA, et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother. 2013;57:1128–1135. doi: 10.1128/AAC.01879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


