Abstract
The relationship between the antioxidant activities and inhibitory effect of 14 Chinese medicinal herbs against oxidized low-density lipoprotein (LDL) formation was evaluated. Prolongation of the lag phase of LDL oxidation depended on the concentration of the herbs. The concentration of each herb that was able to prolong the lag time by about two-fold was calculated and expressed as doubling-time concentration. The lower the doubling-time concentration, the stronger the inhibitory effect exhibited toward LDL oxidation. Among them, Chrysanthemi Flos (Chrysanthemum morifolium ramat; 甘菊花 gān jú huā), Crataegi Fructus (Crataegus pinnatifida Bge. var. major N.E.Br.; 山楂 shān zhā), and Roselle (Hibiscus sabdariffa Linn.; 洛神 luò shén) showed significant inhibitory effects. Correlation coefficients between doubling-time concentration and radical-scavenging activities were high; the total phenolic content was also high. In conclusion, phenolic compounds contributed not only to antioxidant activities, but also to the inhibitory effect against LDL oxidation. Chrysanthemi Flos, Crataegi Fructus, and H. sabdariffa, with lower doubling-time concentrations, could be potent phytochemical agents to reduce LDL oxidation and prevent the progression of atherosclerosis.
Keywords: Chinese medicinal herbs, low-density lipoprotein, radical-scavenging activity, total phenolics, antioxidant
Graphical abstract
1. Introduction
Cancer, heart disease, diabetes, and brain infarction are the leading causes of death in developed countries, and their impact is steadily growing.1 Chronic diseases, despite being the most serious health problems, are also preventable. A report by the World Health Organization1 stated that avoiding unhealthy diet, practicing sufficient exercise, and stopping tobacco use are important ways of prevention of such diseases.
Several epidemiological findings revealed that high-fat and -sugar diets increase the risk of obesity and adult chronic diseases.2,3 In addition, high-calorie and -fat foods significantly elevate cardiovascular risk factors, including low-density lipoprotein (LDL), total cholesterol, and apolipoprotein-B.4 A recent study indicated that atherogenesis was affected greatly by the formation of oxidized LDL.5 Because atherosclerosis accounts mostly for cardiovascular disease6 and brain infarction, prevention of LDL oxidation should be placed on the front line of the prophylaxis. Intake of dietary antioxidants may be a useful preventive treatment to suppress the formation of oxidized LDL and progression of atherosclerosis. Therefore, red wine, teas, soy foods, coffee, vegetables, and fruits, which contain polyphenols, may reduce the formation of oxidized LDL.7–11
Chinese medicinal herbs such as Roselle (Hibiscus sabdariffa Linn.; 洛神 luò shén) and Salvia miltiorrhiza Bunge (丹參 dān shēn) Bunge received a lot of attention, since they have been proved to have inhibitory effects against LDL oxidation, in vitro12 and in vivo.13 Moreover, a popular formulation of Chinese herbal medicine “Da Chai Hu Tang (大柴胡湯 dà chái hú tāng)” had been used for antihyperlipidemic treatment in ancient times.14
A recent study showed that the 25 types of Chinese medicinal herbs containing phenolic compounds exhibited potent antioxidant activities.15 Steinberg et al16 indicated that phenolic compounds in foods could promote the stability of LDL to oxidation. However, a comparative study of Chinese medicinal herbs on the relationship between their antioxidant activity and LDL oxidation has not yet been attempted. In our previous study, using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2′-deoxyguanosine oxidation methods, we have proved the linear correlations between antioxidant activity and phenolic content.17
Because the traditional Chinese belief in the medicinal values of food is based on the concept that food and medicine share the same origin, this view can be considered a forerunner of nutritional science in the world.18 Many treasured Chinese herbs can be taken as part of a medicinal diet, which is referred to as the homology of medicine and food.19 Screening of the antioxidant activities of these Chinese medicinal herbs has been reported in our previous work.17 The aim of this study is to evaluate the antioxidant effects of Chinese medicinal herbs on LDL oxidation.
2. Materials and methods
2.1. Chemicals and reagents
LDL isolated from human plasma (in 0.1% EDTA, pH 7.4) was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). The Bio-Rad protein assay kit used for determining the concentration of solubilized protein was a product of Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Bovine serum albumin was obtained from Nacalai Tesque Inc. (Kyoto, Japan). Disodium hydrogen phosphate, sodium bromide, sodium chloride, sodium hydroxide, potassium dihydrogen phosphate, and 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) were obtained from Wako Pure Chemical Industries (Osaka, Japan). All the other reagents used were of analytical grade.
2.2. Oxidation of LDL
EDTA and salt from the density gradient were removed from the LDL solution using a prepacked column (Econo-Pac 10DG; Bio-Rad, Richmond, CA, USA), as described by Puhl et al.20 The concentration of LDL, free of EDTA, was adjusted to 50 μg/mL of protein with 10mM phosphate-buffered saline (pH 7.4), and it was transferred into a quartz cell for spectrophotometric analysis. An aliquot of the sample (100 μL) was then added to the cell. Oxidation was started at 37°C by the addition of AAPH to a final concentration of 1mM. Trolox, as control, was dissolved in ethanol, dried under nitrogen, and incubated with LDL solution (final Trolox concentrations of 0.625 μg/mL, 1.25 μg/mL, and 2.5 μg/mL). The kinetics of LDL oxidation was determined from the change in conjugated diene formation by monitoring the change in absorbance at 234 nm using a Shimadzu UV-3100 spectrophotometer (UVeVISeNIR scanning; Shimadzu Corp., Kyoto, Japan). Absorbance was recorded every 10 minutes for 4 hours. The changes in absorbance at 234 nm and time were divided into three phases: lag, propagation, and decomposition.
2.3. Preparation of sample
Dried Chinese traditional herbs were purchased from local oriental herbal stores in Kaohsiung, Taiwan. The herbal samples were lyophilized in liquid nitrogen. The lyophilized samples were then ground into a fine powder with a food processor and stored at −80°C until analysis.
2.4. Preparation of herbal sample extracts
The lyophilized herbal samples (0.05–0.2 g) were extracted with 2 mL of methanol and acetic acid mixture (methanol:5% acetic acid = 9:1, v/v). The extraction was centrifuged at 1500 × g for 10 minutes. The extraction step was repeated three times, and the resulting supernatants were combined and dried under nitrogen. Various concentrations of sample were prepared by dissolving the sample residue in 2–5 mL of 10mM phosphate-buffered saline.
2.5. Inhibitory effect of herbal sample extracts on LDL oxidation
The inhibitory effects of selected herb sample extracts on LDL oxidation were measured, according to the method of oxidation of LDL.
3. Results and discussion
3.1. Optimizing the concentration of radical initiator AAPH
The LDL concentration of 50 μg/mL was adopted because this concentration was sufficiently sensitive to evaluate the effects on LDL oxidizability.21,22 To optimize the hydrophilic AAPH concentration, concentrations ranging from 0.5mM to 2.0mM were examined to evaluate their initial lag, propagation, and decomposition phases. As shown in Fig. 1, the lag times for 0.5mM, 1.0mM, and 2.0mM were 100 minutes, 60 minutes, and 40 minutes, respectively. Conjugated-diene formation depended on AAPH concentration, showing that the higher the concentration, the shorter the lag phase. However, an unclear or an unsteady decomposition phase was observed at 0.5mM and 2.0mM, respectively. In addition, the propagation phases for 1.0mM and 2.0mM showed almost the same gradient ratio. Therefore, 1.0mM was considered to be the most optimum concentration of AAPH to oxidize 50 μg/mL LDL.
Fig. 1.
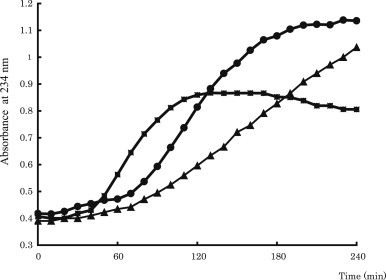
Time course of LDL oxidation at different concentrations of AAPH. Key: ▲, 0.5mM; ●, 1.0mM; and ■, 2.0mM. AAPH = 2,2′-azobis(2-amidinopropane) dihydrochloride; LDL = low-density lipoprotein.
3.2. Identification of inhibitory parameter (doubling-time concentration)
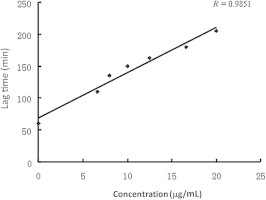
The lag phase in lipid peroxidation processes reflects the antioxidant status of membranes and lipoproteins, and, as a corollary, their resistance to oxidation.23 Fig. 2 shows the relationship between the concentration of Chrysanthemi Flos [(Ju Hua); Chrysanthemum morifolium ramat] and the lag time of LDL oxidation. This result shows that the lag phase of LDL oxidation is concentration dependent at concentrations ranging from 6.7 μg/mL to 20 μg/mL. As shown in Fig. 2, the lag phase was lengthened up to 150 minutes and 205 minutes at concentrations of 10 μg/mL and 20 μg/mL, respectively, indicating that inhibition of LDL oxidation of Chrysanthemi Flos was effective even at low concentrations. Because a linear relationship (R = 0.985) between the lag time and concentration was observed, the inhibitory concentration of Chinese medicinal herbs against LDL oxidation could be calculated from the lag time–concentration standard curve. Therefore, the concentration of herb sample that was able to prolong the lag time by two-fold was calculated and expressed as “doubling-time concentration” for further experimental evaluations.
Fig. 2.
Relationship between the concentration of Chrysanthemi Flos and the lag time of LDL oxidation. LDL oxidation was induced by 1mM AAPH at different concentrations of Chrysanthemi Flos. AAPH = 2,2′-azobis(2-amidinopropane) dihydrochloride; LDL = low-density lipoprotein.
3.3. Inhibitory effect of Chinese medicinal herbs against LDL oxidation
Table 1 shows the inhibitory concentration of Chinese medicinal herbs against LDL oxidation, which are expressed as doubling-time concentrations. This experiment shows that the lower the doubling-time concentration, the stronger the inhibitory effect on LDL oxidation. Among the herbs, Chrysanthemi Flos showed the highest inhibitory effect, followed by Crataegi Fructus (Crataegus pinnatifida Bge. var. major N.E.Br.; 山楂 shān zhā) and Roselle (Hibiscus sabdariffa Linn.; 洛神 luò shén) Besides, Rehmanniae Radix Praeparata (Rehmannia glutinosa Libosch; 熟地黃 shú dì huáng), Cassiae Semen (Cassia obtusifolia L.; 決明子 jué míng zǐ), Glycyrrhizae Radix Et Rhizoma (Glycyrrhiza glabra L.; 甘草 gān cǎo), and Paeoniae Radix Alba (Paeonia lactiflora Pall.; 白芍 bái sháo) showed relatively high inhibitory effects. By contrast, Chuanxiong Rhizoma (Ligusticum chuanxiong Hort.; 川芎 chuān xiōng), Polygoni Multiflori Radix (Polygonum multiflorum Thunb.; 何首烏 hé shǒu wū), and Lycii Fructus (Lycium barbarum L.; 枸杞子 gǒu qǐ zǐ) showed intermediate activity, and Astragali Radix (Astragalus membranaceus (Fisch.); 黄耆 huáng qí), Angelicae Sinensis Radix (Angelica sinensis (Oliv.) Diels.; 當歸 dāng guī), and Ganoderma (Ganoderma lucidum (Leyss. ex Fr.) Karst.; 靈芝 líng zhī) showed lower inhibitory effects. Poria [(Fu Ling); Poria (Poria cocos (Schw.) Wolf; 茯苓 fú líng) showed the lowest inhibitory effect, which was approximately 600-fold lower than that of Chrysanthemi Flos. Although the extract from Chrysanthemi Flos at 10.8 μg/mL only showed ∼ 7% of doubling-time concentration as compared to the control antioxidant chemical Trolox (0.7 μg/mL), the possibility that its total phenolics may have a much higher relative molecular mass than that of the control can be used as an important indicator of the antioxidant capacity of Chrysanthemi Flos.24 Our results were in agreement with previous findings that chrysanthemum has a strong antioxidative ability and inhibitory effects on LDL oxidation.25 Therefore, chrysanthemum, even at low concentrations, may exhibit strong inhibitory effects against LDL oxidation.
Table 1.
Doubling-time concentration, DPPH, peroxyl radical-scavenging activity, and total phenolics of Chinese medicinal herbs.
| Chinese medicinal herbs | Doubling time concentration (μg/mL) a | Radical-scavenging activity (μmol Trolox eq./100 g dry weight) |
Total phenolics (μmol gallic acid eq./100 g dry weight) | |
|---|---|---|---|---|
| DPPH | Peroxyl | |||
| Chrysanthemi Flos (甘菊花 gān jú huā) | 10.8 ± 1.6 | 38,460 | 17,760 | 13,500 |
| Crataegi Fructus (山楂 shān zhā) | 12.0 ± 1.4 | 23,620 | 13,600 | 11,430 |
| Hibiscus sabdariffa (洛神花 luò shén huā) | 15.5 ± 3.5 | 17,650 | 11,560 | 9340 |
| Rehmanniae Radix Praeparata (熟地黃 shú dì huáng | 53.0 ± 7.5 | 6080 | 7260 | 5588 |
| Cassiae Semen (決明子 jué míng zǐ) | 65.7 ± 4.9 | 13,200 | 8440 | 7135 |
| Glycyrrhizae Radix Et Rhizoma (甘草 gān cǎo) | 69.3 ± 3.6 | 9200 | 13,320 | 10,000 |
| Paeoniae Radix Alba (白芍 bái sháo) | 70.7 ± 3.8 | 9850 | 3493 | 4602 |
| Chuanxiong Rhizoma (川芎 chuān xiōng) | 87.5 ± 17.7 | 5150 | 6109 | 4920 |
| Polygoni Multiflori Radix (何首烏 hé shǒu wū) | 93.8 ± 9.0 | 9520 | 6602 | 4580 |
| Lycii Fructus (枸杞子 gǒu qǐ zǐ) | 117.3 ± 6.4 | 5610 | 7420 | 6172 |
| Astragali Radix (黄耆 huáng qí) | 330.0 ± 28.3 | 810 | 3350 | 1280 |
| Angelicae Sinensis Radix (當歸 dāng guī) | 240.7 ± 25.3 | 1620 | 3497 | 3013 |
| Ganoderma (靈芝 líng zhī) | 350.0 ± 55.7 | 1260 | 1210 | 1156 |
| Poria (茯苓 fú líng) | 5850.0 ± 919.0 | 37 | 170 | 100 |
| Trolox | 0.7 ± 0.2 | |||
DPPH = 1,1-diphenyl-2-picrylhydrazyl.
Data are mean values ± SD of three determinations.
Protocatechuic acid and esculetin contained in H. sabdariffa reportedly possess stronger antioxidant activities than vitamin E in oxidative LDL induced by copper ion or nitric oxide donor.12 In addition, the inhibitory effects of H. sabdariffa and Crataegi Fructus extracts on LDL oxidation are concentration dependent.26,27 Despite different radical initiators being used in various studies, similar trends were observed, suggesting that some of the Chinese medicinal herbs investigated in the study had perceived inhibitory effects against LDL oxidation. A previous study demonstrated that Angelicae Sinensis Radix has therapeutic effects on atherosclerosis, due to its functional properties such as effects of antilipid peroxidation, uptake of oxygen radicals, inhibition of platelet aggregation, prevention of thrombogenesis, and antiproliferation of vascular smooth muscle cells.28 Therefore, Angelicae Sinensis Radix, which showed a relatively low inhibitory effect in this study, has also been proved to have protective effects against LDL oxidation.28 Furthermore, the inhibitory effects of Chrysanthemi Flos, Crataegi Fructus, and H. sabdariffa were roughly 20 times more powerful than that of Angelicae Sinensis Radix. This indicated that the tested Chinese medicinal herbs with a lower doubling-time concentration might be potent natural antioxidants to attenuate atherosclerosis.
3.4. Relationship between antioxidant activities, total phenolics, and doubling-time concentration
Table 1 also shows the relationship between DPPH radical-scavenging activity, peroxyl radical-scavenging activity, total phenolics, and doubling-time concentration. The data on DPPH and peroxyl radical-scavenging activity were obtained from our previous work.17 On average, an inverse relationship among the parameters could be observed, indicating that the higher the antioxidant activities or total phenolics, the lower the doubling-time concentration (i.e., the stronger the inhibitory effect against LDL oxidation). Therefore, Chrysanthemi Flos, Crataegi Fructus, and H. sabdariffa, which have high antioxidant activities and total phenolics, exhibited the strongest inhibitory effects on LDL oxidation. On the contrary, Poria, which demonstrated the lowest antioxidant activity and total phenolics, exhibited the weakest inhibitory effect.
By contrast, the number and position of hydroxyl groups, related glycosylation, and other substitutions might largely affect the radical-scavenging activities of phenolic compounds.29 Moreover, Crataegi Fructus, Glycyrrhizae Radix Et Rhizoma, H. sabdariffa, Cassiae Semen, and Lycii Fructus were found to have higher contents of phenolic compounds.17 Based on this reason, a paradox might have occurred in the case of Glycyrrhizae Radix Et Rhizoma and Lycii Fructus; in other words, the total amount of phenolic compounds might not reflect the doubling-time concentration.
Overall, the doubling-time concentration correlated well with DPPH and peroxyl radical-scavenging activities, as well as total phenolics (Fig. 3). Because the variations in doubling concentration (∼600-fold) and DPPH radical-scavenging activity (∼1000-fold) were significantly larger than the other two parameters (∼130-fold), the data were thus converted to logarithms. High correlation coefficients of 0.967, 0.928, and 0.945 were obtained for DPPH radical-scavenging activity versus concentration, peroxyl radical-scavenging activity versus concentration, and total phenolics versus concentration, respectively. Consequently, phenolic compounds contributed mostly not only to antioxidant activities, but also to inhibitory effects against LDL oxidation.
Fig. 3.
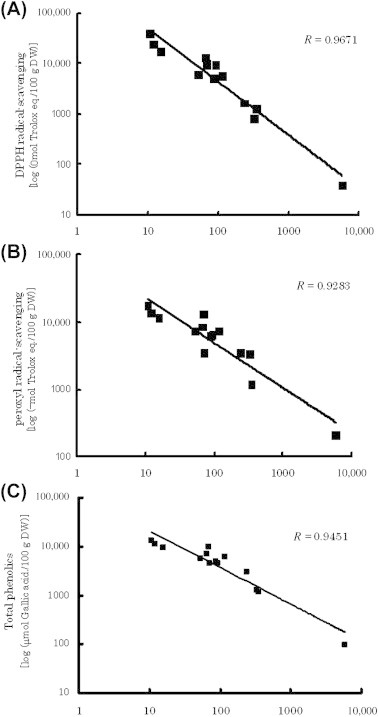
Relationship between doubling-time concentration and (A) DPPH radical-scavenging activity, (B) peroxyl radical-scavenging activity, and (C) total phenolics. DPPH = 1,1-diphenyl-2-picrylhydrazyl; DW = dry weight.
The traditional Chinese medicinal herb tea “Ju Hua Shan Zha Cha”, which included Chrysanthemi Flos and Crataegi Fructus, has widely been used as antihyperlipidemic and anti-atherosclerotic agents.30 Therefore, the above results present strong evidence to prove that Chrysanthemi Flos and Crataegi Fructus may be potent phytochemical agents that inhibit formation of oxidized LDL and thus prevent the progression of atherosclerosis.
4. Conclusion
In conclusion, phenolic compounds contribute not only to antioxidant activities, but also to the inhibitory effects against LDL oxidation. Therefore, Chinese medicinal herbs identified with higher inhibitory effects can be used as potent phytochemical agents in therapeutic treatment of atherosclerosis and other high-risk diseases.
Conflicts of interest
All authors have none to declare.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Guilbert J.J. Education for Health; Abingdon: 2003. The World Health Report 2002: REDUCING Risks, Promoting Healthy Life. [DOI] [PubMed] [Google Scholar]
- 2.Astrup A. The role of dietary fat in the prevention and treatment of obesity. Efficacy and safety of low-fat diets. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S46–S50. doi: 10.1038/sj.ijo.0801698. [DOI] [PubMed] [Google Scholar]
- 3.Stroehla B.C., Malcoe L.H., Velie E.M. Dietary sources of nutrients among rural Native American and white children. J Am Diet Assoc. 2005;105:1908–1916. doi: 10.1016/j.jada.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Requejo A., Sanchez-Bayle M., Baeza J. Relations between nutrient intake and serum lipid and apolipoprotein levels. J Pediatr. 1995;127:53–57. doi: 10.1016/s0022-3476(95)70256-3. [DOI] [PubMed] [Google Scholar]
- 5.George J., Blank M., Hojnik M. Oxidized low density lipoprotein (Ox-LDL) but not LDL aggravates the manifestations of experimental antiphospholipid syndrome (APS) Clin Exp Immunol. 1997;108:227–233. doi: 10.1046/j.1365-2249.1997.d01-1019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000;33(Suppl):S85–S97. [PubMed] [Google Scholar]
- 7.Cordova A.C., Jackson L.S.M., Berke-Schlessel D.W., Sumpio B.E. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto R., Yaita M., Tanaka K., Hara Y., Kojo S. Inhibition of radical reaction of apolipoprotein B-100 and alpha-tocopherol in human plasma by green tea catechins. J Agric Food Chem. 2000;48:6380–6383. doi: 10.1021/jf000973i. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi R., Ohmori R., Kiyose C., Momiyama Y., Ohsuzu F., Kondo K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J Agric Food Chem. 2005;53:4578–4582. doi: 10.1021/jf048062m. [DOI] [PubMed] [Google Scholar]
- 10.Vinson J.A., Su X., Zubik L., Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 11.Yukawa G.S., Mune M., Otani H. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Moscow) 2004;69:70–74. doi: 10.1023/b:biry.0000016354.05438.0f. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.J., Chou F.P., Tseng T.H., Hsieh M.H., Lin M.C., Wang C.J. Hibiscus protocatechuic acid or esculetin can inhibit oxidative LDL induced by either copper ion or nitric oxide donor. J Agric Food Chem. 2002;50:2130–2136. doi: 10.1021/jf011296a. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y.J., Hong C.Y., Lin S.J., Wu P., Shiao M.S. Increase of vitamin E content in LDL and reduction of atherosclerosis in cholesterol-fed rabbits by a water-soluble antioxidant-rich fraction of Salvia miltiorrhiza. Arterioscler ThrombVasc Biol. 1998;18:481–486. doi: 10.1161/01.atv.18.3.481. [DOI] [PubMed] [Google Scholar]
- 14.Qian X., Zhang M. Clinical research on asymptomatic hyperlipemia treated by Da Chai Hu Tang. Forum Tradit Chin Med. 2001;16:11–12. [Google Scholar]
- 15.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y.C., Chuah A.M., Yamaguchi T., Takamura H., Matoba T. Antioxidant activity of traditional Chinese medicinal herbal materials. Food Sci Technol Res. 2008;14:205–210. [Google Scholar]
- 18.Lin P.H. The concept of Chinese medicines and foods having the same origin and diets and therapies to treat pain: taking Tangkuei (Angelicae Sinensis Radix) as an example. J Chin Diet Cult. 2008;4:53–80. [Google Scholar]
- 19.Zhang Q.H. Homology of medicine and food and Chinese herb taken as food. J Liaoning Univ Tradit Chin Med. 2009;11:54–55. [Google Scholar]
- 20.Puhl H., Waeg G., Esterbauer H. Methods to determine oxidation of low-density lipoproteins. Methods Enzymol. 1994;233:425–441. doi: 10.1016/s0076-6879(94)33049-2. [DOI] [PubMed] [Google Scholar]
- 21.Kleinveld H.A., Hak-Lemmers H.L., Stalenhoef A.F., Demacker P.N. Improved measurement of low-density-lipoprotein susceptibility to copper-induced oxidation: application of a short procedure for isolating low-density lipoprotein. Clin Chem. 1992;38:2066–2072. [PubMed] [Google Scholar]
- 22.Hatch F.T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- 23.Cadenas E., Sies H. The lag phase. Free Radic Res. 1998;28:601–609. doi: 10.3109/10715769809065816. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Qiu N., Ding H., Yao R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Int. 2008;41:363–370. [Google Scholar]
- 25.Wu T.-Y., Khor T.O., Saw C.L.L., Loh S.C., Chen A.I., Lim S.S. Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13:1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirunpanich V., Utaipat A., Morales N.P. Antioxidant effects of aqueous extracts from dried calyx of Hibiscus sabdariffa Linn. (Roselle) in vitro using rat low-density lipoprotein (LDL) Biol Pharm Bull. 2005;28:481–484. doi: 10.1248/bpb.28.481. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Chang Q., Zhu M., Huang Y., Ho W.K.K., Chen Z.Y. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–152. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 28.Xiaohong Y., Jing-Ping O.-Y., Shuzheng T. Angelica protects the human vascular endothelial cell from the effects of oxidized low-density lipoprotein. in vitro. Clin Hemorheol Microcirc. 2000;22:317–323. [PubMed] [Google Scholar]
- 29.Son S., Lewis B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure–activity relationship. J Agric Food Chem. 2002;50:468–472. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 30.Qu Y.X. Study on processing technology of natural compound health drink made from hawthorn, jujube, Chinese wolfberry, and chrysanthemum. Food Sci. 2008;29:710–713. [Google Scholar]


