Abstract
Andrographolide is a major bioactive secondary plant metabolite isolated Andrographis paniculata (Burm. F.) Wall. Ex. Nees. (穿心蓮 chuān xīn lián), a well-known traditionally used medicinal herb. The aim of the study was to pharmacologically evaluate the beneficial effect of andrographolide on stress-induced thermoregulatory and other physiological responses in mice. A stress-induced hyperthermia test was conducted in mice. The test agents were orally administered once daily for 11 consecutive days, and treatment effects on body weight changes, basal rectal temperature, and foot-shock-triggered hyperthermic responses were quantified on Day 1, Day 5, Day 7, and Day 10 of the experiments. Pentobarbital-induced hypnosis was quantified on the 11th day of treatment. Observations made during a pilot dose finding experiment revealed that, like A. paniculata extracts, pure andrographolide also possess adaptogenic properties. Observed dose-dependent efficacies of 3 mg/kg/d, 10 mg/kg/d, and 30 mg/kg/d andrographolide in the pilot experiment were reconfirmed by conducting two further analogous experiments using separate groups of either male or female mice. In these confirmatory experiments, efficacies of andrographolide were compared with that of 5 mg/kg/d oral doses of the standard anxiolytic diazepam. Significantly reduced body weights and elevated core temperatures of the three vehicle-treated control groups observed on the 5th day and subsequent observational days were completely absent even in the groups treated with the lowest andrographolide dose (3 mg/kg/d) or diazepam (5 mg/kg/d). Benzodiazepine-like potentiation of pentobarbital hypnosis was observed in andrographolide-treated animals. These observations reveal that andrographolide is functionally a diazepam-like desensitizer of biological mechanisms, and processes involved in stress trigger thermoregulatory and other physiological responses.
Keywords: andrographolide, foot-shock stress, hyperthermia, pentobarbital hypnosis, adaptogen
Graphical abstract

1. Introduction
Andrographis paniculata (Burm. F.) Wall. Ex. Nees. (穿心蓮 chuān xīn lián) is a herbaceous plant of the Acanthaceae family, often cultivated in India, China, and other countries for medicinal purposes. Due to its extremely bitter taste, it is often referred to as the “the king of bitters,” and is used as a bitter tonic in Ayurvedic and other traditionally known health care systems of India and many other Asian countries. In traditional Chinese medicine, Andrographis paniculata (Burm. F.) Wall. Ex. Nees. (A. paniculata) is indicated for conditions of “heat,” particularly in the lungs, throat, and urinary tract, as well as for manifestations of “fire poison” on the skin, such as sores and carbuncles.1 During more recent decades, the plant has attracted considerable attention of modern drug discoverers and herbal researchers, and several reports revealing diverse therapeutic potentials of different types of A. paniculata extracts in properly controlled clinical trials have also appeared.2–5 Andrographolide, structurally a labdane diterpenoid (Fig. 1), is quantitatively the major bitter-tasting secondary metabolite of the plant, and it is now often considered to be the major bioactive constituent of the plant involved in its observed therapeutically interesting bioactivities.6–8 Although a very broad spectrum of therapeutically interesting pharmacological properties of A. paniculata extracts and pure andrographolide have now been revealed, many questions concerning their pharmacological targets and sites of actions still remain unanswered.9,10 Available information on their medicinal chemistry and pharmacology suggest, though, that like various other bioactive secondary plant metabolites, andrographolide is also a pharmacologically pleiotropic or polyvalent agent, and that most probably several biological targets and mechanisms are involved in its modes of actions.
Fig. 1.

HPLC fingerprint and chemical structure of andrographolide. HPLC = high-performance liquid chromatography.
Apart from andrographolide, > 20 other structurally analogous diterpenoids as well as > 10 flavonoids have been isolated and pharmacologically characterized from diverse types of A. paniculata extracts currently commercialized for medicinal purposes.2 It has been suggested that clinically observed beneficial effects of such extracts against upper respiratory tract infections could also be due to their adaptogenic-like properties,11 and several modern scholars, researchers, and practitioners of Ayurvedic and other traditionally known systems of medicine consider A. paniculata to be another adaptogenic herb.12–15 However, as yet little efforts have been made to experimentally verify the possibility that andrographolide can also be quantitatively the major adaptogenic secondary metabolite of the plant. Pharmacologically, adaptogens are characterized by their broad spectra of bioactivities in animal models commonly used for identifying antistress, nootropic, immune function modulating, and antioxidant properties of test agents, and numerous better scrutinized adaptogenic herbs still continue to be recommended by modern herbalists for prevention and cure of mental health problems.16 Consequently, at present, diverse combinations of behavioral and other models usually used for discovering and developing psychoactive drugs are now commonly used for predicting or estimating their therapeutic potentials as adaptogenic herbs and their bioactive constituents.17,18
During the course of our psychopharmacological studies with traditionally known herbal remedies,19 a modified version of the conventionally known stress-induced hyperthermia test for anxiolytics was identified as a convenient test for assessing pharmacologically interesting dose ranges and treatment regimen of adaptogenic herbal extracts.20 This foot-shock stress-induced hyperthermia test is now regularly used in our laboratories for primary pharmacological screening of commercialized extracts from adaptogenic herbs, or for identifying their bioactive properties and comparing their adaptogenic potentials with those of the parent extracts. Observations made during our efforts to define pharmacologically interesting doses and treatment regimen of a medicinally used A. paniculata extract have revealed that its efficacy in this bioassay appears gradually after its repeated daily dose only.21 This extract is highly rich in andrographolide (> 30% w/w), and diverse other types of A. paniculata extracts rich in andrographolide are used in India, China, and other Asian countries for treatment of upper respiratory tract infections.11,22 Therefore, it was of interest to test whether andrographolide could also be its quantitatively major adaptogenic constituent involved in its clinically observed symptomatic relief in patients suffering from the common cold. Results of these very first sets of experiments conducted to experimentally verify this possibility are described and discussed in this article. Possible implications of these findings for discovering drug leads from andrographolide and other structurally or functionally analogous secondary plant metabolites are also pointed out.
2. Materials and methods
2.1. Animals
Male and female Swiss albino mice (20 ± 5 g) were acquired from the Central Animal House of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India (Registration Number: 542/AB/CPCSEA). Prior approval was granted by the Central Animal Ethical Committee of the Banaras Hindu University, Varanasi, India for the study protocol (Dean/11-12/CAEC/325, dated November 30, 2011), and the “Principles of laboratory care” (NIH publication number 85-23, revised during 1985) guidelines were always followed. The animals were housed in groups of six in polypropylene cages, and maintained at an ambient temperature of 25 ± 1°C and 45–55% relative humidity, with a 12:12 hours of light/dark cycle. They were provided with commercial food pellets and tap water ad libitum, and acclimatized to laboratory conditions for at least 1 week prior to using them in the experiment. All test parameters were assessed between 9.00 hours and 14.00 hours.
2.2. Drugs and chemicals
Analytically pure andrographolide [99.0% by high-performance liquid chromatography (HPLC); Fig. 1] isolated from A. paniculata was generously supplied by R&D Center, Natural Remedies Pvt. Ltd., Bangalore, India. Details of the extraction procedure and analytical method used for standardization of the A. paniculata extract used in our earlier studies have been reported elsewhere.23 Briefly, extraction of coarsely ground A. paniculata leaves was performed with methanol for 3 hours, in a stainless–steel-jacketed extractor fitted with a reflux condenser. The liquid extract was removed, and the remaining raw material was re-extracted two more times with methanol in a similar manner. The resulting extracts were combined, concentrated, and dried under vacuum (at < 55°C). The yield of the dried extract was 6% (w/w). Analytically, the extract was standardized to contained andrographolide (> 30.0%, w/w), isoandrographolide (> 0.3%, w/w), neoandrographolide (> 1.0%, w/w), andrograpanin (> 0.3%, w/w), and 14-deoxy-11,12 didehydroandrographolide (< 5.0%, w/w). The extract was subjected to liquid–liquid partitioning between ethyl acetate and water. The ethyl acetate layer was repeatedly chromatographed over silica gel using a combination of hexane:ethyl acetate and chloroform:methanol. Purity of the isolated compounds was determined by HPLC. Diazepam (Cipla, Ahmadabad, India), Tween 80 (Sisco Research Lab., Mumbai, India), Agar (Central Drug House, Delhi, India), and other chemicals and reagents used were obtained from commercial sources.
2.3. Animal grouping and drug administration
In the pilot experiment, equal numbers of male and female animals were allotted to each treatment group, whereupon the vehicle-treated control group consisted of 12, and the drug-treated ones consisted of six animals each. The six groups used in this experiment were as follows: Group I (negative control)—treated with vehicle; Group II—treated with andrographolide (3 mg/kg, p.o.); Group III—treated with andrographolide (10 mg/kg, p.o.); Group IV—treated with andrographolide (30 mg/kg, p.o.); Group V—treated with andrographolide (100 mg/kg, p.o.), and Group VI—treated with andrographolide (300 mg/kg, p.o.). Based on the observation made in the pilot experiment, two further confirmatory experiments using either male or female mice (6 animals per group) were conducted. In each of these two experiments, the animals were allotted to the following experimental groups: Group I (negative control)—treated with vehicle; Group II—treated with andrographolide (3 mg/kg, p.o.), Group III—treated with andrographolide (10 mg/kg, p.o.), Group IV—treated with andrographolide (30 mg/kg, p.o.), and Group V (positive control)—treated with diazepam (5 mg/kg, p.o.). For oral administrations, the test agents were macerated with 2% Tween 80 and then suspended in 0.2% aqueous agar, and were administered once daily for 11 consecutive days. The control groups were treated with this vehicle combination only. All procedures used have been well standardized and regularly used in our laboratory for evaluating adaptogenic potentials of herbs and other test agents. Selection of the andrographolide doses for the pilot dose-finding experiment and the test procedure used were based on our earlier observation with a therapeutically used A. paniculata extract rich in andrographolide (> 30% w/w).21,24
2.4. Stress-induced hyperthermia and pentobarbital-induced hypnosis
Treatments on Day 1, Day 5, Day 7, and Day 10 were given after measuring their basal core temperatures with a calibrated rectal thermometer (Digital Thermometer; Dr. Gene, New Delhi, India). Tests were always conducted 60 minutes after the day’s treatments, and observations were made in a blinded manner. All animals were subjected to a foot-shock stress-triggered hyperthermia test on Day 1, Day 5, Day 7, and Day 10 of the experiment. For such purposes, they were individually placed in a black box (24 × 29 × 40 cm3) with a grid floor for 1 minute. During this period, the animals received five consecutive foot-shocks (2 mA, 50 Hz of 2-millisecond duration each) at 10-second intervals from the grid floor, after which they were immediately placed back to their home cages. Rectal temperatures of the animals were then measured again after 10 minutes of the foot-shock exposure by a blinded observer.25 Calculated difference between the first and the last temperature measurements of an animal on a test day was used to quantify stress-induced hyperthermia.
On the 11th day, treatments were administered without prior rectal temperature measurements and 60 minutes prior to pentobarbital (40 mg/kg, i.p.) challenge. Rectal temperatures were measured on this day immediately prior to the pentobarbital challenge. The time necessary for pentobarbital-induced sleep induction (loss of righting reflex) and duration of sleep were recorded by a blinded observer.26
2.5. Statistical analysis
Mean ± standard error of mean was calculated from the values observed in each experimental group. Unless stated otherwise, statistical analysis was performed by one-way analysis of variance followed by Student–Newman–Keuls multiple comparison test. GraphPad Prism-5 software (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis and calculation of ED50 value. A p value of < 0.05 for differences was considered to be statistically significant.
3. Results
Body weights and rectal temperatures of all age-matched animals used in the three described experiments on the initial experimental days were within physiological ranges. During the course of the experiments, the mean body weights of all the three vehicle-treated control groups decreased consistently, and their mean basal core temperatures recorded on Day 5, Day 7, Day 10, and Day 11 of the experiments were significantly (p < 0.05) higher than those observed on the initial experimental days (Fig. 2A and B). Such elevations of basal core temperature of the control groups in the two experiments, conducted only with male or female animals, occurred gradually during the course of the experiments, and remained constant on the 10th experimental day and 11th experimental day (Fig. 2B and C). This was not the case for the control group in the pilot experiment, where equal numbers of male and female animals were used in all experimental groups. In this experiment, the mean basal rectal temperatures of the control group recorded on the 5th day were almost equal in magnitude to those recorded on subsequent observational days of the experiment (Fig. 2B). The magnitudes of this elevation in basal core temperatures due to daily handling and transient foot-shocks observed on the 10th day and 11th day in the control groups of all the three experiments were almost equal, and such was also the case for the magnitude of foot-shock stress-triggered transient hyperthermia in all the three vehicle-treated control groups (Fig. 2C). The mean time of onset of sleep and durations of sleep observed after pentobarbital challenge on the 11th day of the experiments observed in all the three control groups were also almost equal in magnitudes (Fig. 2D).
Fig. 2.
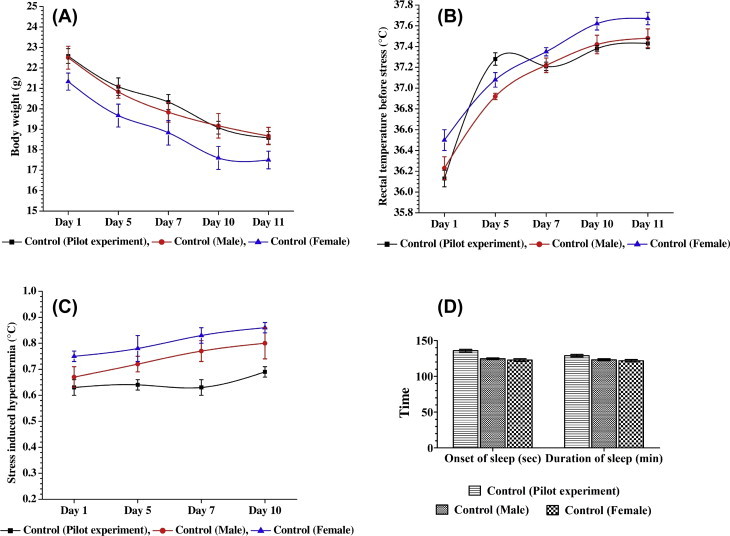
Mean (± SEM) of (A) body weights, (B) basal rectal temperatures, (C) foot-shock stress-induced hyperthermic responses, and (D) sleep onset periods and durations of sleep induced by pentobarbital in the vehicle-treated control groups used in the three reported experiments. The number of control animals used in the pilot experiment was 12, and that in each of the two confirmatory experiments was 6. No statistically significant differences (2-tailed t test) were detected between the values observed in separate groups of male and female animals used in the confirmatory experiments. SEM = standard error of mean.
Observed effects of daily oral doses of andrographolide and the number of treatment days on the four parameters quantified in the pilot experiment are summarized in Fig. 3. These results revealed that even a very high single oral dose of andrographolide (300 mg/kg) did not alter the basal rectal temperature of the animals and had no statistically significant effects on their acute responses to foot-shock-triggered hyperthermia. However, significant (p < 0.05) inhibitory effects of even the lowest tested andrographolide doses (3 mg/kg/d) on both these quantified parameters were apparent on the 5th day and on subsequent days of the experiment. Body weight losses of the animals of the 3 mg/kg/d andrographolide-treated group on the 5th day and on subsequent experimental days were also less severe than those observed in the vehicle-treated control group on these days. The mean body weights of the animals of all other andrographolide-treated groups on 5th day were significantly (p < 0.05) higher than those recorded for the groups on Day 1 of the experiment and remained almost constant during the subsequent experimental days (Fig. 3A). The mean basal core temperatures in the groups treated with 3 mg/kg/d, 10 mg/kg/d, and 30 mg/kg/d andrographolide on the 1st experimental day and 5th experimental day were almost identical to each other. Numerically, the mean basal core temperatures in the 100 mg/kg/d and 300 mg/kg/d andrographolide-treated groups on Day 5 were somewhat higher than (but not statistically significantly different from) those recorded for the groups on Day 1. The mean basal core temperatures in all andrographolide-treated groups on the 7th day, 10th day, and 11th days remained almost constant, and were always significantly (p < 0.05) lower than those of the control group on these days (Fig. 3B).
Fig. 3.
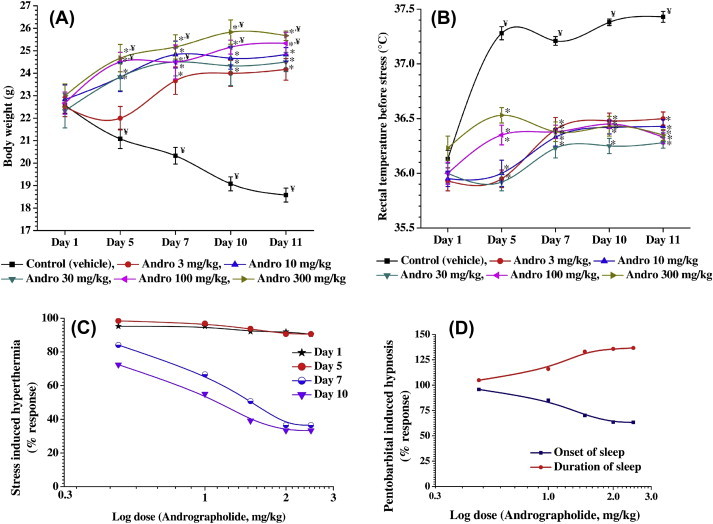
Effects of once-daily oral doses of andrographolide on the parameters quantified in the pilot experiment for 10 consecutive days observed. (A) Mean body weights (B) and basal core temperature were quantified on different days of the experiment. Andrographolide dose–response curves (C) in the foot-shock stress-induced hyperthermia test conducted on Day 1, Day 5, Day 7, and 10 of the experiment and (D) in the pentobarbital sleep test conducted on Day 11 of the test are shown in this figure. Observed statistically significant (p < 0.05) mean values of andrographolide-treated groups against the mean values of the control group on the same day are marked with *, and such differences (p < 0.05) between the mean values of a group on Day 1 and on subsequent days of the experiment are marked with the symbol ¥. Percent response values used in the dose–response curves were calculated by considering the responses of the control group on the day of the experiment as 100%.
Statistically significant (p < 0.05) dose and duration of treatment-dependent antagonistic effects of andrographolide on foot-shock-triggered hyperthermia were also observed on the 5th day, 7th day, and 10th day of the pilot experiment (Fig. 3C). The efficacy of andrographolide observed on the 5th day was much lower than those observed on the 7th day and 10th day. Hereupon, efficacies of the highest two tested doses were almost identical. However, even after administering the highest andrographolide dose tested (300 mg/kg/d), the foot-shock stress-triggered hyperthermic responses were only partially antagonized on both of these days. The estimated ED50 values of andrographolide in this test on the 7th day and 10th day of the experiment were 10.88 mg/kg/d and 10.35 mg/kg/d, respectively. Dose-dependent potentiating effects of 10 daily andrographolide treatments on pentobarbital-induced sedation and hypnosis were observed on the 11th day of the experiment. Log dose–response curves for these effects of andrographolide are shown in Fig. 3D. The estimated ED50 vales of andrographolide for prolongation of onset time and duration of sleep induced by pentobarbital were 13.76 mg/kg/d and 12.36 mg/kg/d, respectively.
The subsequent two experiments were conducted not only to verify the observations made in the pilot experiment, but also to test whether the observed efficacy of andrographolide could be different in male and female animals. Results of these two experiments are summarized in Figs. 4–6. Although somewhat lower mean body weights, and higher basal rectal temperatures and calculated values of foot-shock-triggered hyperthermia were observed in the vehicle-treated female control group on the 1st day and on subsequent observational days of the experiments, these mean values were not statistically significantly different from the corresponding values of the vehicle-treated male control group (Fig. 2), and no statistically significant differences between the effects of daily handling and transient foot-shocks on male and female mice were observed during the entire experimental period. Such were also the cases for all dose-dependent effects of andrographolide or those of the tested dose (5 mg/kg/d) of the anxiolytic diazepam used as a reference standard in these experiments.
Fig. 4.

Effects of intermittent foot-shocks and daily handling on (A and B) body weights and (C and D) basal rectal temperatures of male and female mice treated with vehicle, or andrographolide, or diazepam. * p < 0.05 versus control values on the same day. ¥p < 0.05 versus Day 1 values of the same group.
Fig. 5.
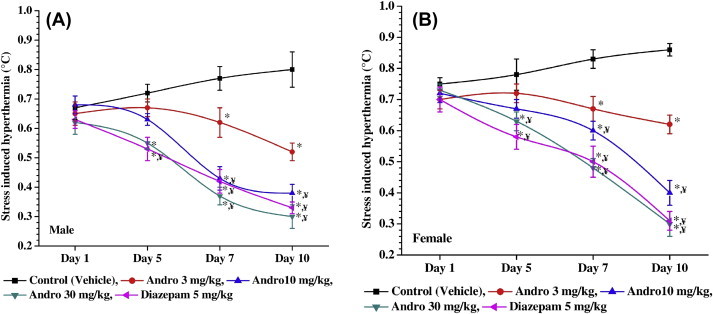
Effects of daily handling on foot-shock stress-triggered hyperthermia on Day 1, Day 5, Day 7, and Day 10 in (A) male and (B) female mice treated with vehicle, or andrographolide, or diazepam. * p < 0.05 versus control values on the same day. ¥p < 0.05 versus Day 1 values of the same group.
Fig. 6.
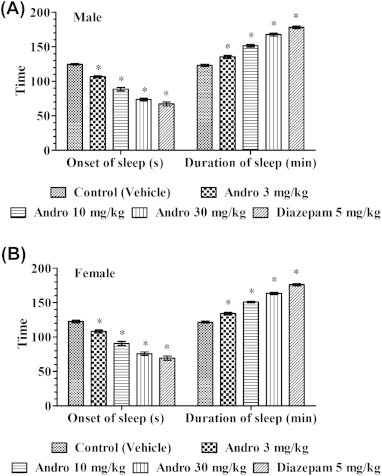
Effects of andrographolide or diazepam treatments on pentobarbital-induced sleep parameters quantified on Day 11 of treatments in (A) male and (B) female mice. * p < 0.05 versus control.
4. Discussion
Observations reported in this communication were made during our efforts to pharmacologically validate the described bioassay for identifying bioactive properties of adaptogenic herbs (Fig. 7). They revealed that not only physiological effects of daily handling and intermittent foot-shocks on body weights and thermoregulatory mechanisms of male and female adult mice are indistinguishable under the experimental conditions used in our laboratories, but also the observed effects of andrographolide on the quantified parameters are qualitatively analogous to those observed for the medicinally used A. paniculata extract in our laboratories earlier.21 All quantified effects of andrographolide were qualitatively analogous to those observed after daily oral administration of the 5 mg/kg dose of diazepam.
Fig. 7.

Graphical summary.
Diazepam is an anxiolytic agent often used as a reference drug for pharmacological validation of animal models (including diverse versions of stress-induced hyperthermia tests) for the anxiety state of animals or for detecting acute dose anxiolytic-like activities of test agents.20 However, in both the confirmatory experiments reported in this communication, no acute dose effects of the anxiolytic drug were observed. These observations could indicate that physiological processes and mechanisms involved in the foot-shock stress-triggered hyperthermia are not identical to those involved in transient hyperthermia triggered by milder physiological stimuli commonly used in other versions of stress-induced hyperthermia tests for anxiolytics, or for identifying gamma-aminobutyric acid (GABA) receptor function modulating agents. A literature survey revealed, though, that most studies on the effects of diazepam in diverse versions of stress-induced hyperthermia test were conducted after intraperitoneal administration of its acute doses only. However, it has also been reported that oral doses of diazepam < 6 mg/kg are ineffective in one of the most commonly used versions of the stress-induced hyperthermia test using mice as experimental animals,27 and that, at lower doses, it also affords protection against diverse stress-triggered pathologies. Because we were interested in comparing the effects of repeated daily doses of andrographolide on thermoregulatory processes, and from our earlier observations we had also known that 5 mg/kg daily oral doses of diazepam is effective in desensitizing mild stress-triggered slight elevation of rectal temperature, this dose of the anxiolytic was considered appropriate for our purpose.
It is well known that, apart from its anxiolytic activity, diazepam also possesses diverse other stress response modulating effects28,29 and that, depending on its dose administered for protracted treatments, tolerance liability of diazepam varies considerably.30 Our observations clearly revealed that all physiological responses quantified in the bioassay used are antagonized by diazepam after its 5 mg/kg repeated daily oral doses only, and that its activity profile observed after this dose of the anxiolytic administered for > 5 days is almost identical to that observed after 10 mg/kg/d oral treatment regimen of pure andrographolide. Therefore, it seems reasonable to assume that the biological processes and mechanisms involved in the observed effects of the two agents must have some similarities. However, since even after the administration of very high single oral doses of andrographolide, it did not have any effects on stress-induced hyperthermia, its mode and site of action cannot be identical to those of benzodiazepines.
In any case, it remains certain that qualitatively the activity profile of an A. paniculata extract observed earlier in our laboratories in the bioassay used is identical to that of pure andrographolide observed in the experiments described in this communication.21 However, the ED50 values of pure andrographolide estimated in this study were always significantly lower than those calculated from the dose–response curves of andrographolide present in the tested extract administered and using the same bioassay procedure.21 Therefore, it appears that some other bioactive constituents present in the tested extract modulate the stress response desensitizing effects of andrographolide observed in the bioassay.
The possibility that bioactive agents could have adaptogenic properties evolved from pharmacological observations made initially in Russia with a structurally well-defined molecule during the 1940s.31 Since then, the concept that adaptogenic properties of herbal remedies are involved in their medicinal benefits has been well accepted by almost all scholars, researchers, and practitioners of integrative medicine who are also well trained in modern medical sciences.16 By definition, adaptogens are nontoxic, or well-tolerated, bioactive substances capable of counteracting or preventing homeostatic disturbances triggered by metabolic, environmental, and mental stresses. The facts that andrographolide is a nontoxic substance is evident from a recently reported toxicological study,32 and is reconfirmed by the observations made in the pilot experiment described in this communication. This experiment was conducted to estimate the pharmacologically interesting dose and treatment regimen of andrographolide using its daily oral doses of up to 300 mg/kg/d. Even after administering its highest tested dose, no apparent behavioral and other apparent toxicities of andrographolide were observed in this experiment during the 11 observational days. By contrast, even the lowest andrographolide dose tested (3 mg/kg/d) completely prevented the daily handling- and intermittent foot-shock-triggered body weight losses, and the slight elevation of core body temperatures within physiological ranges observed in control animals. Moreover, dose- and treatment regimen-dependent partial protection against transient foot-shock-triggered hyperthermia, with ED50 values around 10 mg/kg/d, was observed in the pilot as well as in both the confirmatory experiments. Because the dose and duration of treatment dependence of these observed effects of andrographolide were not identical, it seems reasonable to assume that different physiological thermoregulatory mechanisms and biological processes are involved in its diazepam-like beneficial effects observed only after their fairly low daily oral doses.
Numerous behavioral alterations induced by daily handling of experimental animals have long since been known to experimental pharmacologists,33 and it is also well recognized that almost all handling procedures necessary for in vivo bioassays drastically alter their metabolic status, basal core temperature, and numerous other physiological parameters.34 However, until now, daily handling stress-induced homeostatic and behavioral alterations have seldom been used for identifying adaptogenic or antistress potentials of test agents. Stress-induced hyperthermia paradigm is now regularly used by numerous drug discoverers for identifying acute dose effects of structurally and functionally novel psychoactive drugs potentially useful for treatment of exaggerated anticipatory anxiety,35,36 or for overall estimation of stress responses in experimental and other animals.37 Because clear dose-dependent diazepam-like effects of andrographolide against foot-shock stress-triggered hyperthermia were apparent after its repeated daily doses, it seems reasonable to assume that neurotransmitter mechanisms modulated by such treatments are analogous to those of conventionally known benzodiazepine-like anxiolytics. The observations that daily treatments with andrographolide also potentiate pentobarbital-induced sedation and hypnosis, and that its dose–response curves for such effects are almost identical to those observed on the 7th observational day and 10th observational day in the stress-induced hyperthermia test are in agreement with this possibility. As both benzodiazepines and barbiturates are well-known modulators of central GABAA receptors, modulation of functions of the GABAergic system may also be involved in the observed effects of andrographolide after its repeated daily doses.
Among diverse therapeutic potentials of andrographolide identified to date, those dealing with its anti-inflammatory and anticancer activities have attracted the most attention of modern drug discovers.2–8,22 Since available information on its pharmacokinetics and metabolism has revealed its low oral bioavailability,38 efforts are now being made in several laboratories to identify structurally novel and orally better bioavailable andrographolide derivatives and their analogs as potential drug leads.39 All such drug discovery approaches are based on the conviction that blood levels of andrographolide dictate its pharmacological efficacy. This may not necessarily be the case as clearly indicated not only by the observation reported in this communication, but also by our earlier report on nervous system function modulating effects of an A. paniculata extract rich in andrographolide (>30% w/w).21
Medicinal values of andrographolide-enriched A. paniculata extracts for treatment of gastrointestinal and hepatic disorders have long since been well recognized by Ayurvedic and other traditionally known medical practitioners. Efficacy of one such extract for obtaining symptomatic relief in patients with inflammatory bowel diseases has also been demonstrated in a recently reported clinical trial.40 Moreover, therapeutic benefits of such extracts against other diverse chronic inflammatory disorders, now commonly grouped under the heading “central sensitivity syndrome,” have repeatedly been demonstrated by several other properly controlled clinical trials.10,41 It is also now well recognized that the gut microbiota ecology and the gut brain axis play important roles in altering central sensitivity of almost all inflammatory diseases,42,43 and that the gut microbiota ecology of laboratory mice is altered by daily handling of them for experimental purposes.44 Since antimicrobial, antifungal, and antiparasitic activities of andrographolide and other A. paniculata extract components are known,45 it could also be that the reported therapeutic benefits of the extracts and the observed effects of andrographolide against stress-induced hyperthermia reported in this communication are possibly due to their modulating effects on the gut microbiota ecology. Efforts to experimentally verify this possibility are now being made in our laboratories.
Maximal suppressive effects of andrographolide against hyperthermia induced by daily handling was observed after its five daily doses of 3 mg/kg, and its daily dose of 3 mg/kg for a period of 30 days has been reported to be its maximally effective dose in three different gastric ulcer models.46 However, antihyperglycemic and antidiabetic activities of pure andrographolide have also been observed after its oral doses as low as 1.5 mg/kg.47,48 Our observations, together with others reported to date on pharmacological activity profile of andrographolide after its repeated oral doses, strongly suggest that it is an orally active antihyperglycemic agent with gastric ulcer protective and antistress activity, and that its structural modulations are not essential prerequisites for obtaining such therapeutic benefits from this naturally abundant phytochemical. Therefore, efforts to experimentally verify the possibility that andrographolide itself can also be useful for the prevention and cure of metabolic disorders triggered by or associated with environmental and metabolic stress seem to be an urgent therapeutic necessity. During such efforts, due attention has to be paid to the adaptogenic effects of andrographolide induced after its repeated daily doses.
5. Conclusion
Andrographolide is quantitatively the major adaptogenic secondary metabolite of A. paniculata. Downregulation of the functions of the benzodiazepine site of GABA-A receptors involved in stress-triggered physiological responses by repeated daily intake of andrographolide is most probably involved in its adaptogenic activity.
Conflicts of interest
The authors declare that they have no conflicts of interest to disclose.
Acknowledgments
A.K.T. gratefully acknowledges the Department of Science and Technology, Government of India, New Delhi, India, for awarding him the INSPIRE Fellowship (IF110595). Thanks are also due to Natural Remedies Pvt. Ltd., Bangalore, India, for generously supplying the gift sample of pure andrographolide isolated from A paniculata along with its HPLC fingerprint.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bensky D., Gamble A. Eastland Press; Washington: 1986. Andrographis paniculata: Chinese Herbal Medicine Materia Medica. [Google Scholar]
- 2.Chao W.W., Lin B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian) Chin Med. 2010;5:17.1–17.15. doi: 10.1186/1749-8546-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Dora J., Singh A., Tripathi R. A review on king of bitter (Kalmegh) Int J Res Pharm Chem. 2012;2:116–124. [Google Scholar]
- 4.Mishra S.K., Sangwan N.S., Sangwan R.S. Andrographis paniculata (Kalmegh): a review. Pharmacogn Rev. 2007;1:283–298. [Google Scholar]
- 5.Valdiani A., Kadir M.A., Tan S.G., Talei D., Abdullah M.P., Nikzad S. Nain-e Havandi Andrographis paniculata present yesterday, absent today: a plenary review on underutilized herb of Iran's pharmaceutical plants. Mol Biol Rep. 2012;39:5409–5424. doi: 10.1007/s11033-011-1341-x. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo M.A., Hanke J.L., Bertoglio J.C., Burgos R.A. Andrographolide a new potential drug for long term treatment of rheumatoid arthritis disease. In: Matsuno H., editor. Innovative Rheumatology. InTech; Croatia: 2013. pp. 247–270. [Google Scholar]
- 7.Jayakumar T., Hsieh C.Y., Lee J.J., Sheu J.R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med. 2013;846740 doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim J.C., Chan T.K., Ng D.S., Sagineedu S.R., Stanslas J., Wong W.S. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 9.Levita J., Nawawi A., Mutalib A., Ibrahim S. A review of its anti-inflammatory activity via inhibition of NF-kappaB activation from computational chemistry aspects. Int J Pharmacol. 2010;6:569–576. [Google Scholar]
- 10.Subramanian R., Zaini Asmawi M., Sadikun A. A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem Rev. 2012;11:39–75. [Google Scholar]
- 11.Panossian A., Wikman G. Efficacy of Andrographis paniculata in upper respiratory tract infectious diseases and the mechanism of action. In: Wagner H., Ulrich-Merzenich G., editors. Evidence and Rational Based Research on Chinese Drugs. Springer; Vienna: 2013. pp. 137–179. [Google Scholar]
- 12.Govindarajan R., Vijayakumar M., Pushpangadan P. Antioxidant approach to disease management and the role of 'Rasayana' herbs of Ayurveda. J Ethnopharmacol. 2005;99:165–178. doi: 10.1016/j.jep.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Thakur A.K., Chatterjee S.S., Kumar V. General neuropharmacological screening of standardized extract of Andrographis paniculata in rodents. Ann Neurosci. 2012;19:S36–S37. [Google Scholar]
- 14.Thakur M., Weng A., Fuchs H. Rasayana properties of Ayurvedic herbs: are polysaccharides a major contributor. Carbohydr Polymers. 2012;87:3–15. doi: 10.1016/j.carbpol.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Williamson E.M. Churchill Livingstone; China: 2002. Major Herbs of Ayurveda. [Google Scholar]
- 16.Winston D., Maimes S. Healing Art Press; Vermont: 2007. Adaptogens: Herbs for Strength, Stamina, and Stress Relief. [Google Scholar]
- 17.Pawar V.S., Shivakumar H. Screening methods for evaluation of adaptogenic agents: a review. J Pharm Res. 2011;4:763–765. [Google Scholar]
- 18.Pawar V.S., Shivakumar H. A current status of adaptogens: natural remedy to stress. Asian Pac J Trop Dis. 2012;2:S480–S490. [Google Scholar]
- 19.Chatterjee S.S., Kumar V. Holistic psychopharmacology and promiscuous plants and principles of Ayurveda. Am J Plant Sci. 2012;3:1015–1021. [Google Scholar]
- 20.Vinkers C.H., van Bogaert M.J., Klanker M. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur J Pharmacol. 2008;585:407–425. doi: 10.1016/j.ejphar.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 21.Thakur A.K., Chatterjee S.S., Kumar V. Neuropsychopharmacology of a therapeutically used Andrographis paniculata extract: a preclinical study. Orient Pharm Exp Med. 2014;14:181–191. [Google Scholar]
- 22.Saxena R.C., Singh R., Kumar P. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmColdTM) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178–185. doi: 10.1016/j.phymed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekaran C.V., Thiyagarajan P., Sundarajan K. Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmCold™) Food Chem Toxicol. 2009;47:1892–1902. doi: 10.1016/j.fct.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Thakur A.K., Chatterjee S.S., Kumar V. Therapeutic potential of traditionally used medicinal plant Andrographis paniculata (Burm. F.) against diabesity: an experimental study in rats. TANG Int J Genuine Tradit Med. 2014;4:1–8. [Google Scholar]
- 25.Zethof T.J., Van der Heyden J.A., Tolboom J.T., Olivier B. Stress-induced hyperthermia in mice: a methodological study. Physiol Behav. 1994;55:109–115. doi: 10.1016/0031-9384(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho-Freitas M.I., Costa M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol Pharm Bull. 2002;25:1629–1633. doi: 10.1248/bpb.25.1629. [DOI] [PubMed] [Google Scholar]
- 27.Groenink L., van der Gugten J., Zethof T.J., van der Heyden J.A., Olivier B. Neuroendocrine effects of diazepam and flesinoxan in the stress-induced hyperthermia test in mice. Pharmacol Biochem Behav. 1996;54:249–254. doi: 10.1016/0091-3057(95)02177-9. [DOI] [PubMed] [Google Scholar]
- 28.Beracochea D., Tronche C., Coutan M., Dorey R., Chauveau F., Pierard C. Interaction between diazepam and hippocampal corticosterone after acute stress: impact on memory in middle-aged mice. Front Behav Neurosci. 2011;5:14.1–14.9. doi: 10.3389/fnbeh.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez-Cuesta L.A., Marquez-Valadez B., Perez-De La Cruz V. Diazepam blocks striatal lipid peroxidation and improves stereotyped activity in a rat model of acute stress. Basic Clin Pharmacol Toxicol. 2011;109:350–356. doi: 10.1111/j.1742-7843.2011.00738.x. [DOI] [PubMed] [Google Scholar]
- 30.Divljakovic J., Milic M., Timic T., Savic M.M. Tolerance liability of diazepam is dependent on the dose used for protracted treatment. Pharmacol Rep. 2012;64:1116–1125. doi: 10.1016/s1734-1140(12)70908-8. [DOI] [PubMed] [Google Scholar]
- 31.Brekhman, Dardymov New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–430. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 32.Bothiraja C., Pawar A., Shende V., Joshi P. Acute and subacute toxicity study of andrographolide bioactive in rodents: evidence for the medicinal use as an alternative medicine. Comp Clin Pathol. 2013;22:1123–1128. [Google Scholar]
- 33.Claassen V. Neglected factors in pharmacology and neuroscience research. In: Huston J.P., editor. Techniques in the Behavioral and Neural Science. Elsevier; New York: 1994. pp. 422–459. [Google Scholar]
- 34.Balcombe J.P., Barnard N.D., Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 35.Adriaan Bouwknecht J., Olivier B., Paylor R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Van der Heyden J.A., Zethof T.J., Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- 37.Careau V., Reale D., Garant D., Speakman J.R., Humphries M.M. Stress-induced rise in body temperature is repeatable in free-ranging Eastern chipmunks (Tamias striatus) J Comp Physiol B. 2012;182:403–414. doi: 10.1007/s00360-011-0628-5. [DOI] [PubMed] [Google Scholar]
- 38.Yang T., Shi H.X., Wang Z.T., Wang C.H. Hypolipidemic effects of andrographolide and neoandrographolide in mice and rats. Phytother Res. 2013;27:618–623. doi: 10.1002/ptr.4771. [DOI] [PubMed] [Google Scholar]
- 39.Zhou B., Zhang D., Wu X. Biological activities and corresponding SARs of andrographolide and its derivatives. Mini Rev Med Chem. 2013;13:298–309. [PubMed] [Google Scholar]
- 40.Sandborn W.J., Targan S.R., Byers V.S. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am J Gastroenterol. 2013;108:90–98. doi: 10.1038/ajg.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yunus M.B. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 43.Thakur A.K., Shakya A., Husain G.M., Emerald M., Kumar V. Gut-microbiota and mental health: current and future perspectives. J Pharmacol Clin Toxicol. 2014;2:1–15. [Google Scholar]
- 44.Ma B.W., Bokulich N.A., Castillo P.A. Routine habitat change: a source of unrecognized transient alteration of intestinal microbiota in laboratory mice. PloS One. 2012;7:e47416. doi: 10.1371/journal.pone.0047416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niranjan A., Tewari S.K., Lehri A. Biological activities of Kalmegh (Andrographis paniculata Nees) Indian J Nat Prod Resour. 2012;1:125–135. [Google Scholar]
- 46.Saranya P., Geetha A., Selvamathy S.M. A biochemical study on the gastroprotective effect of andrographolide in rats induced with gastric ulcer. Indian J Pharm Sci. 2011;73:550–557. doi: 10.4103/0250-474X.99012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nugroho A.E., Andrie M., Warditiani N.K., Siswanto E., Pramono S., Lukitaningsih E. Antidiabetic and antihiperlipidemic effect of Andrographis paniculata (Burm. f.) Nees and andrographolide in high-fructose-fat-fed rats. Indian J Pharmacol. 2012;44:377–381. doi: 10.4103/0253-7613.96343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu B.C., Hung C.R., Chen W.C., Cheng J.T. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003;69:1075–1079. doi: 10.1055/s-2003-45185. [DOI] [PubMed] [Google Scholar]


