Abstract
Among Yoruba herbalists (Southwest Nigeria), hot water infusion of Mangifera indica L. (芒果 Máng Guǒ) stem bark is reputedly used for the treatment of fever, jaundice and liver disorders. The present study, therefore, investigates the protective effects and mechanism(s) of chemopreventive and curative effects of 125–500 mg/kg/day of Mangifera indica aqueous stem bark extract (MIASE) in acute CCl4-induced liver damage in rats. Rats were treated intragastrically with 125, 250 and 500 mg/kg/day of MIASE for 7 days before and after the administration of CCl4 (3 ml/kg of 20% CCl4, i.p.). The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), total bilirubin (TB), conjugated bilirubin (CB) and fasting blood glucose (FBG) levels were estimated. In addition, hepatic tissue reduced glutathione (GSH) and the malondialdehyde (MDA) concentrations, catalase (CAT), superoxide (SOD) activities in the hepatic homogenate, and histopathological changes in the rat liver sections were determined. Preliminary qualitative phytochemical screening for bioactive compounds in MIASE was also conducted. Results showed that oral treatment with 125–500 mg/kg/day of MIASE significantly attenuated the increase in serum ALT, AST, ALP, FBG, TB, CB and LDL-c levels in acute liver injury induced by CCl4 treatment. Findings also revealed significant elevations in the serum TC, TG, HDL-c, TP and ALB levels. There was marked architectural remodeling in the hepatic lesions of hepatocyte vacuolation and centrilobular necrosis induced by CCl4 treatment, coupled with significant weight loss. MIASE also markedly enhanced SOD and CAT activities while reducing MAD formation; and increased GSH concentration in the hepatic homogenate compared with untreated CCl4-intoxicated group, with more protection offered in the curative than the chemopreventive models of CCl4 hepatotoxicity. Thus, these results indicate that MIASE has a profound protective effect against acute CCl4-induced hepatotoxicity in rats, which may be due to its free radicals scavenging effect, inhibition of lipid peroxidation, and its ability to increase antioxidant activity.
Keywords: Mangifera indica, Stem bark, Carbon tetrachloride, Hepatoprotective, Antioxidant
Graphical abstract
Introduction
Mangifera indica L. (芒果 Máng Guǒ) (family: Anarcardiaceae) tree is among the most economically and culturally important tropical rainforest medicinal plant in Asia and Africa, especially for its edible fruits. It is widely known as Mango. The mango tree is an erect perennial tree with a broad rounded canopy which at full maturity may attain up to 10–30 m and 30–35 m in height and width, respectively.1 Previous studies indicate mango possesses antidiabetic2–5 hypolipidemic, antioxidant, antiviral, cardiotonic, hypotensive, anti-inflammatory and analgesic,6 antibone resorption, antihelminthic, antispasmodic, antipyretic, antidiarrheal, anti-allergic, gastroprotective, hepatoprotective and immunomodulatory properties.1 In African traditional medicine, water infusion of Mangifera indica leaves is highly valued in the local management of feverish and jaundiced conditions for which its antiplasmodial antipyretic effects were reported.7,8
The polyphenolic glucosyl xanthones, mangiferin and vimang, isolated from Mangifera indica have been documented to possess strong antiviral,9,10 antioxidant,11,12 gastroprotective,13 antilipoperoxidation, immunomodulatory and anticancer,14 cardiotonic and hypotensive,15 antihelminthic and anti-allergic,16 wound healing, antidegenerative, antidiabetic and antiatherogenic activities.17 Also reported is the hepatoprotective effect of Vimang isolated from Mangifera indica on primary culture of rat hepatocytes.18
In African folk medicine, water infusions of Mangifera indica stem bark are highly valued in the local treatment of malaria fever, diarrhea, diabetes mellitus and liver diseases.19 In view of its traditional use in the local treatment of hepatic disorders, the present study was designed at evaluating the hepatoprotective effect and mechanism(s) of 125–500 mg/kg/day of MIASE using carbon tetrachloride (CCl4)-induced hepatotoxicity in Wistar rat model.
Materials and methods
Plant collection and identification
Fresh leaves, inflorescence and stem bark of Mangifera indica L. (芒果 Máng Guǒ) plant were collected from the deciduous forest of Odo-Remo in Remo North Local Government Area of Ogun State, Nigeria, in the month of March, 2013. Botanical identification and authentication was done by Mr. T. K. Odewo, Chief Taxonomist in the Department of Botany and Microbiology, University of Lagos, Akoka, Lagos State, Nigeria. Voucher specimen was deposited in the Departmental herbarium with the reference no. LUH 5528, allotted.
Preparation of the plant extract
Fresh peels of Mangifera indica stem bark were harvested and gently washed in tap water to remove dirt. These were then completely air-dried in the laboratory under room temperature (24 ± 2 °C) for 4 weeks protected from direct heat or sunlight. When dried, the stem barks were then pulverized into coarse powder using Laboratory Hammer-mill of the Pharmacognosy Department, Faculty of Pharmacy, University of Lagos, Idi-Araba, Lagos, Nigeria.
40 g of the powdery form was dissolved in 400 ml of distilled water and boiled at 100 °C under continuous stirring with magnetic stirrer for 2 hours. The mixture was then filtered using a piece of clean white cloth. The aqueous filtrate was transferred into an aerated oven already preset at 40 °C to obtain the dried extract of the seed; the yield was 15%.
Qualitative phytochemical analyses of MIASE
Preliminary phytochemical analyses of the Mangifera indica aqueous stem bark extract were done using standard procedure of Sofowora.20 The procedures are summarized below:
Test for tannins
Ferric chloride test: About 0.5 g of the dried powdered samples was boiled in 20 ml of water in a test tube and then filtered. A few drops of 0.1 % ferric chloride was added and observed for brownish green or a blue–black coloration.
Test for phlobatinnins
The plant extract solutions were boiled with 1% aqueous hydrochloric acid on a water bath for 5 minutes then color changes were observed.
Test for saponin
Froth test: 2 g of the powdered sample was boiled in 20 ml of distilled water in a water bath and then filtered. 10 ml of the filtrate was mixed with 5 ml of distilled water and shaken vigorously for a stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken vigorously, then observed for formation of emulsion.
Test for flavonoids
Three methods were used to determine the presence of flavonoids in the plant samples.
-
a.
A portion of the extract solution was in each case heat with 10 ml of ethyl acetate over a steam bath for 3 minutes. The mixture was filtered and 4 ml of filtrate was shaken with 1 ml of dilute ammonia solution and color changes were observed.
-
b.
Lead acetate test: To a solution of 0.5 g extract in water about 1 ml of 10% lead acetate solution was added. Production of yellow precipitate is considered as positive for flavonoids.
-
c.
Few drops of 1% aluminum chloride solution was added to about 2 ml of the plant extract solutions then color changes were observed.
Test for steroidal compound
Two methods were used to determine the presence of terpenoids in the Mangifera indica stem bark aqueous extract sample.
-Lieberman's test: 0.5 g of extract was dissolved in 2 ml of acidic anhydride and cooled well in an ice. Sulfuric acid was then carefully added. A color change from violet to blue or green in some samples indicating the presence of steroidal nucleus (i.e. aglycone portion of the cardiac glycoside).
Acute oral toxicity test of MIASE using preliminary dose test of up and down procedure
The Acute oral toxicity study was conducted using the limit dose test of up and down procedure according to OECD/OCDE Test Guidelines on Acute Oral Toxicity under a computer-guided Statistical Programme-AOT425statPgm, version 1.0,21 at a limit dose of 5000 mg/kg body weight/oral route and default of Sigma at 0.5. A total of three female young adult Wistar rats were systematically selected out of a population of 10 Wistar rats (6–8 weeks old) by systematic randomization techniques. The population sample was selected such that the weight differences do not exceed ± 10% of the mean initial weight of the sample population. The rats were fasted of rat chow overnight prior to dosing on each occasion. A rat was picked at a time, weighed and dosed with equivalent 5000 mg/kg body weight of the crude extract dissolved in 10 ml/kg of 0.9% normal saline used as the vehicle. Feeding was done using metallic gastric feeding tube. After the extract administration, each rat was observed for the first 5 minutes after oral administration for signs of possible regurgitation and then kept in a cage for observation. The treated rat was watched for every 15 min in the first 4 hour after dosing, then every 30 minutes for the successive 6 hour and then daily for the successive 38 hour for the short-term outcome and the remaining 12 days for the long-term possible lethal outcome which in this case was “death”. Behavioral manifestations of acute oral toxicity were also observed. All observations were systematically recorded with individual records being maintained for each rat.
Experimental animals
After institutional ethical clearance for the study was obtained, a total of eighty-four young adult Male Wistar rats aged 8–10 weeks and with weight range of 110 g–150 g were purchased (in two separate batches of forty-two rats in each batch) from the Laboratory Animal Centre of the College of Medicine, University of Lagos, Idi-Araba, Lagos State and were kept in the Rat Colony of the Animal House of Lagos State University College of Medicine, Ikeja, Lagos State, where the laboratory work was carried out. On each occasion, the rats were allowed one week of acclimatization before being used for experimentation. The animals were housed in standard metal cages, maintained under standard laboratory conditions and fed standard rat pellets and potable water ad libitum.
Experimental design
Drug-induced hepatic and renal toxicity models used in the conduct of this study was done using 30% carbon tetrachloride dissolved in olive oil according to the modified method of Lu et al. 22 The study was conducted in two phases (chemopreventive and curative) with each phase consisting of 36 male Wistar rats. The rats in each model were grouped into six groups of six rats each - three control and treatment groups, respectively.
Induction of CCl4-induced hepatotoxicity and oral drug treatment in the chemopreventive model
In this model of chemically-induced hepatotoxicity, rats were randomly divided into 6 groups of 6 rats each such that the weight differences within and between groups did not exceed ± 20%. Group I rats consist of rats pretreated with 10 ml/kg of 0.9% normal saline while rats in Group II–VI were orally pretreated on daily basis with 10 ml/kg of 0.9% normal saline, 10 mg/kg of ascorbic acid, 125 mg/kg of MIASE, 250 mg/kg of MIASE, 500 mg/kg of MIASE, respectively, all dissolved in 0.9% normal saline and pretreated for 7 days. Twenty-four hours after the last oral pretreatment with ascorbic acid and graded dose of MIASE, rats were treated with single intraperitoneal injection of 3 ml/kg of 30% CCl4 dissolved in olive oil. Ascorbic acid, being a known potent antioxidant, hepatoprotectant was used as a standard reference drug. Treated rats were then sacrificed humanely forty-eight hours post-CCl4 treatment.
Induction of CCl4-induced hepatotoxicity and oral drug treatment in the curative model
In this model of chemically-induced hepatotoxicity, rats were also randomly divided into 6 groups of 6 rats each such that the weight differences within and between groups did not exceed ± 20%. Group I rats consist of rats pretreated with 1 ml/kg of 0.9% normal saline given intraperitoneally while Groups II–VI rats were intraperitoneally treated on daily basis with 3 ml/kg of 30% CCl4 1 hour before oral treatment with 10 mg/kg of ascorbic acid, 125 mg/kg of MIASE, 250 mg/kg of MIASE, 500 mg/kg of MIASE, respectively, all dissolved in 0.9% normal saline. All treatment lasted 7 days. Twenty-four hours after the last treatment, the rats were sacrificed humanely under diethyl ether anesthesia. Again, ascorbic acid, being a known potent antioxidant, hepatoprotectant was used as a standard reference drug.
Collection of blood samples
Prior to the termination of the studies, rat feed was withdrawn in order to fast the rats and their beddings changed. However, drinking water was provided ad libitum. On the termination day, rats were anesthetized using inhalational diethyl ether after which blood samples for hepatic function tests were obtained directly from the heart chamber using a 21G needle mounted on a 5 ml syringe plunger and collected into plain sample bottle.
Collection of livers for hepatic tissue oxidative stress markers
After blood collection through cardiac puncture, a deep longitudinal incision was made into the ventral surface of the rat abdomen. The livers were identified and carefully dissected out en bloc from each rat. The right lobe of the liver was rinsed in ice cold 1.15% KCl solution in order to preserve the oxidative enzyme activities of the liver before being stored in a clean sample bottle which itself was in an ice-pack filled cooler. This is to prevent the breakdown of the hepatic antioxidant biomarkers.
Determination of liver and renal tissue superoxide dismutase activity
Superoxide dismutase activity was determined by its ability to inhibit the auto-oxidation of epinephrine by the increase in absorbance at 480 nm as described by Sun and Zigman.23 The reaction mixture (3 ml) contained 2.95 ml 0.05 M sodium carbonate buffer pH 10.2, 0.02 ml of liver homogenates, and 0.03 ml of epinephrine in 0.005 N HCl was used to initiate the reaction. The reference curette contained 2.95 ml buffer, 0.03 ml of substrate (epinephrine), and 0.02 ml of water. Enzyme activity was calculated by measuring the change in absorbance at 480 nm for 5 minutes.
Determination of liver tissue catalase activity
Hepatic tissue catalase activity was determined according to Kakkar et al.24 by measuring the decrease in absorbance at 240 nm due to the decomposition of H2O2 in UV recording spectrophotometer. The reaction mixture (3 ml) contained 0.1 ml of serum in phosphate buffer (50 mM, pH 7.0) and 2.9 ml of 30 mM of H2O2 in the phosphate buffer pH 7.0. An extinction coefficient for H2O2 at 240 nm of 40.0 M−1 cm−1 according to Aebi25 was used for calculation. The specific activity of catalase was expressed as moles of H2O2 reduced per minutes per mg protein.
Determination of liver tissue reduced glutathione activity
The reduced glutathione (GSH) content in the liver tissue was estimated according to the method described by Sedlak and Lindsay.26 To the homogenate 10% TCA was added and centrifuged. One milliliter of the supernatant was treated with 0.5 ml of Ellman’s reagent (19.8 mg of 5,5-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm.
Determination of liver tissue malondialdehyde activity
Malondialdehyde (MDA) an index of lipid peroxidation was determined using the method of Buege and Aust.27 One milliliter of supernatant was added to 2 ml of (1:1:1 ratio) TCA-TBA-HCl reagent (thiobarbituric acid 0.37%, 0.24 N HCl and 15% TCA) tricarboxylic acid, thiobarbituric acid, reagent boiled at 100 °C for 15 minutes, and allowed to cool. Flocculent material was removed by centrifuging at 3000 rpm for ten minutes. The supernatant was removed and the absorbance was read at 532 nm against a blank. MDA was calculated using the molar extinction for MDATBA-complex of 1.56 × 105 m−1 cm−1.
Determination of serum hepatic function parameters
Serum aspartate transaminase and alanine transaminase determination
The method of Reitman and Frankel28 was used. Into a test tube, 0.1 ml substrate (D, L-aspartate, 0.2 mol/L and α-ketoglutaric acid, 1.8 mmol/L in phosphate buffer, pH 7.5) solution was pipetted and placed in a 37 °C water bath to warm 0.2 ml plasma was added and shaken gently to mix. Exactly one hour after adding plasma, 1.0 ml color reagent.
[2, 4-dinitrophenylhydrazine (DNPH) approximately 20 mg/100 ml, in 10% HCl solution] was added and mixed gently and left at room temperature (18–26 °C). Twenty minutes after adding color reagent, 10 ml 0.40 N sodium hydroxide solution was added and mixed by inversion. Five minutes after, absorbance was read at 340 nm using water as reference. AST activity in Sigma-Frankel units/ml was determined from calibration curve. The same procedure was carried out for ALT except that procedures were started 30 minutes after starting AST. Substrate for ALT was L-alanine (0.2 mol/L) and α-ketoglutaric acid (1.8 mmol/L) in phosphate buffer, pH 7.5.28
Serum alkaline phosphatase determination
Alkaline phosphate was determined using the colorimetric end-point method of Tietz et al. 29 and adapted by Teco Diagnostics Kits. The principle is based on the fact that alkaline phosphatase acts upon the AMP-buffered sodium thymolphthalein monophosphate. The addition of an alkaline reagent stops enzyme activity and simultaneously develops a blue chorogem, which is measured photometrically. For each sample 0.5 ml of alkaline phosphatase substrate was dispensed into labeled test tubes and equilibrated to 37 °C for 3 minutes. At timed intervals 0.5 ml of standard, control and sample were added to their respective test-tube and mixed gently deionized water was used as blank. The samples were incubated for exactly ten minutes at 37 °C following the same sequences, 2.5 ml of alkaline phosphatase color developer at timed intervals were added. The wavelength of the spectrometer was set at 590 nm.
Determination of serum lipids, albumin, proteins and fasting blood glucose
Serum total protein was estimated by Biuret method30 while that of albumin was determined by bromocresol green.31 The total bilirubin and the conjugated bilirubin were determined by Jendrassik–Grof method.32 The serum total cholesterol and its fractions were determined by the Lierberman–Burchard quantitative test.33 Fasting blood glucose was determined by glucose oxidase method of Trinder,34 using One Touch Basic Blood Glucose Monitoring System (LifeScan Inc., Milpitas, California, U.S.A.).
Histopathology of the treated rat livers
The remaining part of the liver harvested is gently but briskly rinsed in 0.9% normal saline and fixed in 10% formo-saline before being completely dehydrated in absolute (100%) ethanol. The liver was then embedded in routine paraffin blocks. From the embedded paraffin blocks, 4–5 μm thick sections of each tissue was prepared and stained with hematoxylin-eosin. These were examined under a photomicroscope (Model N-400ME, CELTECH Diagnostics, Hamburg, Germany) connected with a host computer. Sections were illuminated with white light from a 12V halogen lamp (100 W) after filtering with a 520 nm monochromatic filter. The slides were examined for associated histopathological lesions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (Graph Pad Software – Version 5.0. Graph Pad Software Inc., La Jolla, California, U.S.A.). Data were expressed as mean ± S.D. for body weights and relative kidney weights and mean ± S.E.M. for biochemical and hematological assays. The data were analyzed using the one-way ANOVA for comparison between the control and treated groups and post hoc test conducted using Newman-Keuls'-test. Level of statistical significance was considered at p < 0.05, p < 0.001 and p < 0.0001.
Results
Extraction and phytochemical screening of MIASE
Aqueous extraction of pulverized stem bark of Mangifera indica L. (芒果 Máng Guǒ) and its complete drying off yielded a chocolate-color, sweet-smelling solid residue with a yield of 15%. Preliminary qualitative phytochemical screening of MIASE for its secondary metabolites showed that the extract contains alkaloids, tannins, cardiac glycosides, flavonoids, phlobatinnins, reducing sugars and saponins with anthocyanosides and anthraquinones being absent.
Acute oral toxicity test of MIASE
Single oral treatment of sequentially treated young adult, nulliparous, non-pregnant female Wistar rats with 5000 mg/kg of MIASE resulted in no mortality and fatality as the short-term and delayed-term outcome of the oral toxicity test (Table 1). However, behavioral toxicities manifested by the treated rats include body scratching, feed refusal, reduced locomotor activity, and watery stooling.
Table 1.
Sequence and results of the limit dose test of MIASE in young female Wistar rats.
| Test sequence | Dose (mg/kg) | Short-term result (48 h) | Long-term result (12 days) |
|---|---|---|---|
| 01 | 5000 | Survival | Survival |
| 02 | 5000 | Survival | Survival |
| 03 | 5000 | Survival | Survival |
Effect of 125–500 mg/kg of MIASE on FBG levels and %ΔFBG in the chemopreventive and chemocurative models of CCl4-treated rats
Intraperitoneal injection of CCl4 caused significant (p < 0.001) increases in the FBG levels when compared to untreated normal rats (Table 2). With oral pre-treatment with 125–500 mg/kg/day of MIASE, there were significant (p < 0.05, p < 0.001 and p < 0.0001) dose-dependent decreases in the FBG when compared to the untreated CCl4-treated (Group II) values (Table 2). In the chemocurative model, intraperitoneal injection of CCl4 caused significant (p < 0.05) decreases in the FBG levels when compared to untreated normal rats (Table 3). However, with post-CCl4 oral treatment with 125–500 mg/kg/day of MIASE, there non-significant decreases in the FBG value when compared to the untreated CCl4-treated (Group II) values (Table 3).
Table 2.
Effects of oral pretreatments of 125–500 mg/kg of MIASE on the fasting blood glucose concentration (FBG) (mg/dl) and %Δ FBG of CCl4-treated rats.
| Groups | FBG (mg/dl) on |
||
|---|---|---|---|
| Day 1 | Day 8 | %Δ FBG | |
| I | 57.67 ± 3.03 | 55.83 ± 1.76 | 5.33 ± 2.87 |
| II | 59.83 ± 3.04 | 65.33 ± 1.45b | 8.33 ± 4.61 |
| III | 56.00 ± 3.52 | 45.67 ± 3.29f | -29.47 ± 12.71f |
| IV | 59.50 ± 3.35 | 53.00 ± 1.73f | -11.73 ± 4.43d |
| V | 53.17 ± 3.15 | 45.33 ± 1.76f | -18.61 ± 7.14e |
| VI | 54.00 ± 3.79 | 41.50 ± 1.80f | -33.76 ± 8.37f |
b represents a significant increase at p < 0.001 when compared to Group I values while d, e and f represent significant decreases at p < 0.05, p < 0.001 and p < 0.0001 when compared to Group II values, respectively.
Table 3.
Effects of oral pretreatments of 125–500 mg/kg of MIASE on the fasting blood glucose concentration (FBG) (mg/dl) and %Δ FBG of CCl4-chemocurative rats.
| Groups | FBG (mg/dl) on |
||
|---|---|---|---|
| Day 1 | Day 8 | %Δ FBG | |
| I | 55.50 ± 0.96 | 55.50 ± 0.85 | 0.06 ± 1.90 |
| II | 59.33 ± 1.45 | 42.00 ± 1.77a− | -29.13 ± 2.95b− |
| III | 58.17 ± 0.75 | 48.83 ± 2.52 | -15.79 ± 5.14 |
| IV | 58.50 ± 0.76 | 52.50 ± 4.06 | -10.60 ± 5.88 |
| V | 59.83 ± 1.49 | 48.83 ± 3.54 | -18.29 ± 6.05a− |
| VI | 58.67 ± 1.17 | 41.67 ± 2.40a− | -28.89 ± 4.26b− |
a− and b− represent significant decreases at p < 0.05 and p < 0.001 when compared to Group I values, respectively.
Effect of 125–500 mg/kg of MIASE on the liver function parameters in the CCl4-chemopreventive and chemocurative rats
Oral pre-treatment with 125, 250 and 500 mg/kg/day of MIASE before induction of hepatotoxicity with 3 ml/kg of 30% CCl4 as measured by the effects of MIASE on liver function parameters (ALT, AST, ALP, TB, CB) as depicted in Tables 4a and b. Oral pretreatments with 125–500 mg/kg of MIASE significantly (p < 0.001) attenuated increases in the serum ALT, AST, ALP, TB and CB levels when compared to untreated CCl4-treated rats (Table 4a). Similarly, 125–500 mg/kg of MIASE significantly (p < 0.05, p < 0.001, p < 0.0001) attenuated decreases in and improved the serum TP, ALB, TG, TC and HDL-c levels when compared to that of untreated CCl4-treated rats (Group II) values (Table 4b). Although intraperitoneal CCl4 treatment caused no significant (p > 0.05) alterations in the serum LDL-c levels in the treated rats when compared to untreated control (Group I), however, oral treatment with 125–500 mg/kg of MIASE caused dose-related significant (p < 0.001) decreases in the serum LDL-c levels when compared to untreated CCl4-treated rats (Table 4b).
Table 4a.
Effect of oral pretreatments with 125–500 mg/kg/day of MIASE on serum ALT, AST, ALP, TB and CB in the CCl4-treated rats.
| Groups | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | TB (mg/dl) | CB (mg/dl) |
|---|---|---|---|---|---|
| I | 29.83 ± 1.72 | 20.67 ± 2.82 | 77.41 ± 6.63 | 8.68 ± 0.45 | 2.43 ± 0.37 |
| II | 87.00 ± 2.41c | 65.33 ± 3.71c | 162.30 ± 10.29c | 20.02 ± 0.36c | 12.75 ± 0.36c |
| III | 36.33 ± 3.73e | 21.17 ± 2.20e | 98.72 ± 14.03d | 14.10 ± 1.78d | 3.78 ± 0.34e |
| IV | 79.17 ± 4.95 | 47.83 ± 2.41d | 92.27 ± 1.14d | 9.90 ± 0.78e | 11.62 ± 0.55c+ |
| V | 36.50 ± 4.95e | 18.00 ± 2.34f | 61.50 ± 5.28e | 9.43 ± 0.34e | 2.68 ± 0.27f |
| VI | 23.80 ± 1.28f | 14.83 ± 1.17f | 69.85 ± 0.51f | 10.39 ± 0.37e | 2.88 ± 0.28f |
c represents a significant increase at p < 0.0001 when compared to Group I values while d, e and f represent significant decreases at p < 0.05, p < 0.001 and p < 0.0001, respectively, when compared to Group II values.
Table 4b.
Effect of oral pretreatments with 125–500 mg/kg/day of MIASE on serum ALT, TP, ALB, TG, TC, HDL-c and LDL-c in the CCl4-treated rats.
| Groups | TP (g/dl) | ALB (g/dl) | TG (mmol/l) | TC (mmol/l) | HDL-c (mmol/l) | LDL-c (mmol/l) |
|---|---|---|---|---|---|---|
| I | 68.47 ± 1.74 | 46.55 ± 0.95 | 1.28 ± 0.07 | 3.09 ± 0.20 | 1.43 ± 0.11 | 1.27 ± 0.17 |
| II | 39.33 ± 1.58c− | 21.04 ± 1.06c− | 0.50 ± 0.06c− | 2.02 ± 0.11c− | 0.50 ± 0.0c− | 1.03 ± 0.05 |
| III | 67.10 ± 1.91c+ | 14.10 ± 1.78a+ | 0.97 ± 0.05 | 2.94 ± 0.23b+ | 1.21 ± 0.08c+ | 1.30 ± 0.05 |
| IV | 58.44 ± 3.46a+ | 42.75 ± 2.27c+ | 0.76 ± 0.15 | 2.65 ± 0.11a+ | 1.72 ± 0.10c+ | 0.55 ± 0.17f |
| V | 62.85 ± 3.02a+ | 43.64 ± 1.79 | 0.93 ± 0.14 | 2.67 ± 0.06c+ | 1.80 ± 0.06c+ | 0.53 ± 0.09f |
| VI | 69.85 ± 0.51c+ | 43.81 ± 0.55c+ | 1.23 ± 0.11c+ | 3.01 ± 0.17b+ | 1.59 ± 0.11c+ | 0.46 ± 0.18f |
c− represents a significant decrease at p < 0.0001 when compared to Group I values while a+, b+ and c+ represent significant increases at p < 0.05, p < 0.001 and p < 0.0001, respectively, when compared to Group II values; f represents significant a decreases at p < 0.0001 decreases when compared to Group II values.
Oral treatment with 125, 250 and 500 mg/kg/day of MIASE 1 hour post-CCl4 treatment with 3 ml/kg of 30% CCl4 as measured by their effects on liver function parameters (ALT, AST, ALP, TB, CB) significantly (p < 0.001 and p < 0.0001) attenuated increases in the levels of these liver function parameters (Table 5a). Similarly, 125–500 mg/kg of MIASE significantly (p < 0.05, p < 0.0001) attenuated decreases in and improved the serum TP, ALB, TG, TC, HDL-c, and LDL-c levels when compared to that of untreated CCl4-treated rats (Group II) values (Table 5b).
Table 5a.
Effect of post-CCl4 oral treatments with 125–500 mg/kg/day of MIASE on serum ALT, AST, ALP, TB and CB in the CCl4-treated rats.
| Groups | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | TB (mg/dl) | CB (mg/dl) |
|---|---|---|---|---|---|
| I | 15.50 ± 0.76 | 36.00 ± 2.24 | 27.70 ± 1.81 | 1.29 ± 0.07 | 0.24 ± 0.00 |
| II | 54.83 ± 2.65c | 97.83 ± 2.69c | 69.13 ± 3.81c | 1.98 ± 0.04c | 1.39 ± 0.06c |
| III | 15.00 ± 1.37f | 21.17 ± 2.20e | 41.73 ± 1.86f | 1.18 ± 0.11f | 0.30 ± 0.01f |
| IV | 23.17 ± 1.28e | 64.33 ± 1.61e | 51.37 ± 4.54e | 1.58 ± 0.10e | 0.30 ± 0.01f |
| V | 15.50 ± 0.76f | 50.67 ± 4.49f | 41.70 ± 3.17f | 1.16 ± 0.12f | 0.28 ± 0.02f |
| VI | 12.67 ± 0.71f | 42.67 ± 2.62f | 32.72 ± 2.10f | 1.21 ± 0.07f | 0.29 ± 0.02f |
c represents a significant increase at p < 0.0001 when compared to Group I values while e and f represent significant decreases at p < 0.001 and p < 0.0001, respectively, when compared to Group II values.
Table 5b.
Effect of post-CCl4 oral treatments with 125–500 mg/kg/day of MIASE on serum ALT, TP, ALB, TG, TC, HDL-c and LDL-c in the CCl4-treated rats.
| Groups | TP (g/dl) | ALB (g/dl) | TG (mmol/l) | TC (mmol/l) | HDL-c (mmol/l) | LDL-c (mmol/l) |
|---|---|---|---|---|---|---|
| I | 78.54 ± 2.35 | 46.03 ± 0.63 | 0.77 ± 0.05 | 2.56 ± 0.07 | 1.67 ± 0.06 | 0.59 ± 0.09 |
| II | 40.42 ± 0.31c− | 25.37 ± 1.03c− | 0.44 ± 0.03c− | 1.46 ± 0.14c− | 1.16 ± 0.07c− | 1.16 ± 0.07c |
| III | 77.54 ± 1.51c+ | 44.80 ± 0.76a+ | 0.68 ± 0.04a+ | 1.87 ± 0.06a+ | 1.15 ± 0.03 | 0.51 ± 0.05f |
| IV | 59.25 ± 2.85b+ | 35.27 ± 0.49 | 0.86 ± 0.05a+ | 1.65 ± 0.09 | 0.93 ± 0.12 | 0.35 ± 0.06f |
| V | 78.24 ± 1.10c+ | 46.22 ± 0.70c+ | 1.12 ± 0.15c+ | 1.49 ± 0.12 | 0.95 ± 0.07 | 0.30 ± 0.03f |
| VI | 81.62 ± 2.43c+ | 56.75 ± 0.66c+ | 1.69 ± 0.02c+ | 1.85 ± 0.02 | 1.61 ± 0.02a+ | 0.05 ± 0.01f |
c− and c represent significant decreases and increases at p < 0.0001, respectively when compared to Group I values while a+, b+ and c+ represent significant increases at p < 0.05, p < 0.001 and p < 0.0001, respectively, when compared to Group II values. f represents significant decreases at p < 0.001 when compared to Group II values.
Effect of 125–500 mg/kg of MIASE on the tissue antioxidant status in the CCl4-chemopreventive and chemocurative rats
Table 6 shows the effects of oral pre-treatments with 125–500 mg/kg/day of MIASE and subsequent intraperitoneal CCl4 treatment on the antioxidant markers (SOD, CAT, GSH and MAD) in the treated rats. CCl4 treatment caused significant (p < 0.05 and p < 0.001) decreases in the hepatic tissue SOD, CAT and GSH while causing significant (p < 0.001 and p < 0.0001) increases in the hepatic and renal tissue MAD (Table 6). Oral pretreatment with 250 and 500 mg/kg of MIASE significantly (p < 0.05 and p < 0.0001) attenuated decreases in the hepatic tissue levels of SOD, CAT and GSH levels while significantly attenuated increases in the hepatic tissue MAD levels (Table 6). These effects were comparable to that recorded for the 10 mg/kg of the standard antioxidant (ascorbic acid) used. However, 125 mg/kg/day of MIASE did cause significant (p > 0.05) alterations in the hepatic tissue SOD, CAT, GSH and MAD levels when compared to that of the untreated CCl4-treated (Group II) rats (Table 6). Similarly, Table 7 shows the effects of 125–500 mg/kg of MIASE on hepatic tissue antioxidant markers (SOD, CAT, GSH and MAD) in the post-CCl4 treated rats. CCl4 treatment caused significant (p < 0.05 and p < 0.0001) decreases on the hepatic and renal SOD, CAT and GSH while causing significant (p < 0.001 and p < 0.0001) increases in the hepatic and renal MAD values (Table 7). However, post-CCl4 oral treatments with 125–500 mg/kg/day of MIASE significantly (p < 0.05, p < 0.001 and p < 0.0001) reversed and improved the values of these markers when compared to values for the untreated CCl4-treated rats and returning them to near normal values (Table 7).
Table 6.
Effect of pre-CCl4 oral treatments with 125–500 mg/kg/day of MIASE on hepatic tissue SOD, CAT, GSH and MDA in the CCl4-treated rats.
| Group | SOD (U/mg protein) | CAT (U/mg protein) | GSH (U/mg protein) | MDA (U/mg protein) |
|---|---|---|---|---|
| I | 4.04 ± 0.52 | 23.48 ± 2.77 | 2.08 ± 0.22 | 0.16 ± 0.03 |
| II | 2.29 ± 0.29a− | 13.42 ± 0.92b− | 1.25 ± 0.09 | 0.38 ± 0.10b |
| III | 3.14 ± 0.36 | 16.53 ± 0.32 | 1.78 ± 0.16 | 0.08 ± 0.02f |
| IV | 3.47 ± 0.30 | 17.66 ± 1.08 | 1.77 ± 0.32 | 0.13 ± 0.01e |
| V | 4.83 ± 0.22c+ | 27.79 ± 2.01c+ | 3.12 ± 0.30c+ | 0.11 ± 0.02e |
| VI | 5.69 ± 0.45c+ | 35.18 ± 1.69c+ | 4.51 ± 0.23c+ | 0.04 ± 0.01f |
a− and b− represent significant decreases at p < 0.05 and p < 0.001, respectively, when compared to Group I values while b+ and c+ represent significant increases at p < 0.001 and p < 0.0001, respectively, when compared to Group II values. b represents a significant increase at p < 0.001 when compared to Group II values while e and f represent significant decreases at p < 0.001 and p < 0.0001, respectively, when compared to Group II values.
Table 7.
Effect of oral treatments with 125–500 mg/kg/day of MIASE on hepatic tissue SOD, CAT, GSH and MDA in post-CCl4-treated rats.
| Group | SOD (U/mg protein) | CAT (U/mg protein) | GSH (U/mg protein) | MDA (U/mg protein) |
|---|---|---|---|---|
| I | 2.74 ± 0.19 | 24.84 ± 1.66 | 0.92 ± 0.07 | 0.10 ± 0.00 |
| II | 1.59 ± 0.17c− | 11.29 ± 0.63c− | 0.48 ± 0.03a− | 0.15 ± 0.03b |
| III | 3.68 ± 0.26c+ | 27.50 ± 1.87c+ | 1.72 ± 0.06c+ | 0.02 ± 0.01f |
| IV | 3.68 ± 0.26c+ | 18.24 ± 1.21b+ | 0.99 ± 0.20a+ | 0.09 ± 0.01e |
| V | 3.92 ± 0.22c+ | 20.71 ± 1.15c+ | 1.25 ± 0.19c+ | 0.11 ± 0.01 |
| VI | 4.07 ± 0.12c+ | 26.45 ± 1.50c+ | 1.72 ± 0.05c+ | 0.08 ± 0.01e |
a− and c− represent significant decreases at p < 0.05 and p < 0.0001, respectively, when compared to Group I values while a+,b+ and c+ represent significant increases at p < 0.05, p < 0.001 and p < 0.0001, respectively, when compared to Group II values. b represents a significant increase at p < 0.001 when compared to Group II values while e and f represent significant decreases at p < 0.001 and p < 0.0001, respectively, when compared to Group II values.
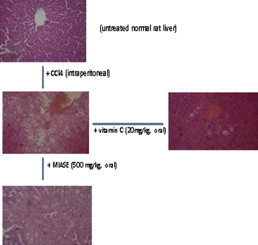
Histopathological evaluation
Figs. 1–6 are the photomicrographs of the lesions induced by CCl4-treatment and pre-CCl4 oral treatments with 125–500 mg/kg/day of MIASE on the treated rat livers. Intraperitoneal CCl4 injection was marked by hepatic centrilobular vacuolation and vascular congestion indicative of early hepatic necrosis (Fig. 2) when compared to normal hepatic architecture (Fig. 1). However, oral pretreatments with 10 mg/kg/day of vitamin C and 125–500 mg/kg/day of MIASE for 7 days caused amelioration of the CCl4-induced histological lesions of hepatic necrosis (Figs. 3–6). Similarly, Figs. 7–11 show the histopathological findings of post-CCl4 oral pretreatments with 125–500 mg/kg/day of MIASE on the liver tissue of CCl4-treated rats. Single intraperitoneal treatment with CCl4 caused marked vascular congestion with ballooning and vacuolation of periportal hepatocytes and hepatocytes around the central hepatic vein (Fig. 7) when compared to normal hepatic architecture (Fig. 1). With repeated daily oral pretreatments with 10 mg/kg of vitamin C and 125–500 mg/kg/day of MIASE, these histological changes were ameliorated in a dose-related fashion (Figs. 9–11).
Fig. 1.
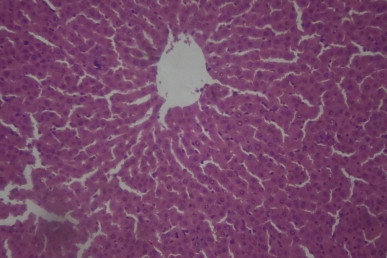
A sectional representative of normal rat liver showing normal hepatic architecture (Hematoxylin & Eosin, x100 magnification).
Fig. 2.
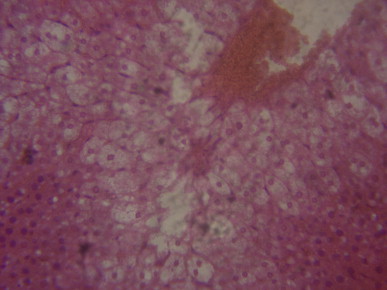
A sectional representative of untreated CCl4-treated rat liver showing hepatocyte ballooning and hepatic centrilobular necrosis and vascular congestion indicating early hepatic necrosis (Hematoxylin & Eosin stain, x400 magnification).
Fig. 3.
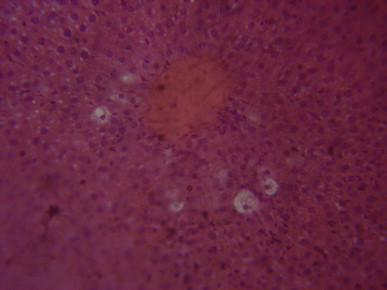
A sectional representative of rat liver pretreated with 10 mg/kg/day of vitamin C before intraperitoneal CCl4 treatment showing focal hepatocyte vacuolation and central venous congestion (Hematoxylin & Eosin stain, x400 magnification).
Fig. 4.
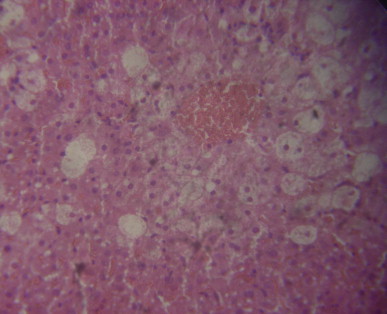
A sectional representative of pretreated with 125 mg/kg/day of MIASE before CCl4 intraperitoneal treatment showing global moderate-to-severe hepatocyte vacuolation and severe central venous congestion (Hematoxylin & Eosin stain, x400 magnification).
Fig. 5.
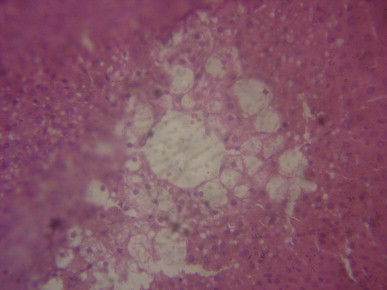
A sectional representative of rat liver pretreated with 250 mg/kg/day of MIASE before CCl4 intraperitoneal treatment showing moderate focal centrilobular hepatocyte vacuolation and mild central venous congestion (Hematoxylin & Eosin stain, x400 magnification).
Fig. 6.
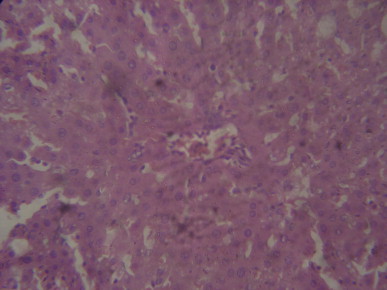
A representative section of rat liver pretreated with 500 mg/kg/day of MIASE before CCl4 treatment showing hepatic sinusoid congestion and mild central venous congestion with normal hepatocytes (Hematoxylin & Eosin stain, x400 magnification).
Fig. 7.
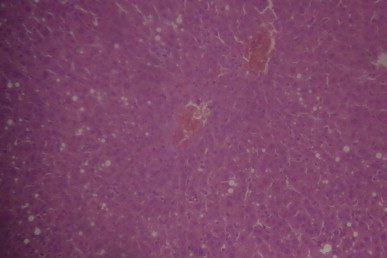
A sectional representative of untreated CCl4-treated rat liver showing marked vascular congestion with ballooning and vacuolation of periportal hepatocytes and hepatocytes around the central hepatic vein (Hematoxylin & Eosin, x100 magnification).
Fig. 8.
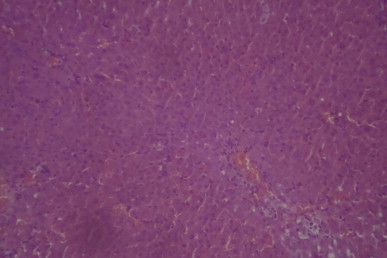
A sectional representative of post-CCl4 treated rat liver treated with 10 mg/kg/day of vitamin C showing moderate hepatic vascular congestion (Hematoxylin & Eosin, x100 magnification).
Fig. 9.
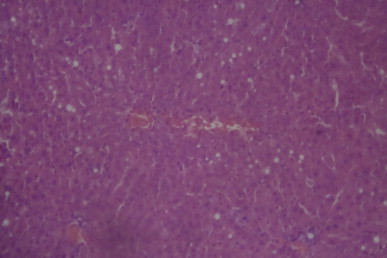
A sectional representative of post-CCl4 treated rat liver orally treated with 125 mg/kg/day of MIASE showing marked global vacuolation and patchy necrosis of hepatocytes around congested hepatic vascular channels (Hematoxylin & Eosin, x100 magnification).
Fig. 10.
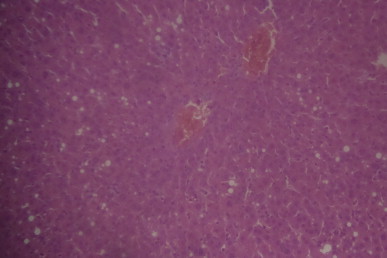
A sectional representative of CCl4-treated rat liver pretreated with 250 mg/kg/day of MIASE showing mild congested hepatic vascular channels and patchy hepatic vacuolation (Hematoxylin & Eosin, x100 magnification).
Fig. 11.
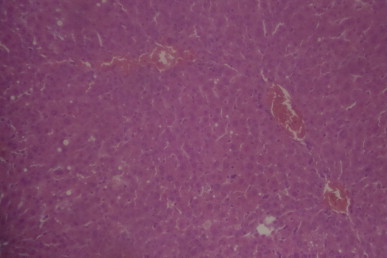
A sectional representative of post-CCl4 treated rat liver treated with 500 mg/kg/day of MIASE showing mild congested hepatic vascular channels (Hematoxylin & Eosin, x100 magnification).
Discussion
The liver, as the key organ of drug metabolism and excretion, is naturally endowed with the major task of drug detoxification. Hepatotoxicants including viruses, fungal products, bacterial metabolites, minerals, environmental pollutants and chemotherapeutic agents can induce various and varying degrees of injury to the liver.35 CCl4-induced hepatic injury is a classical and reliable model of xenobiotic-induced hepatotoxicity that has been extensively used for the screening of drugs for their possible antihepatotoxic/hepatoprotective activity.36
In the present study, the preventive and curative hepatoprotective effects of MIASE against CCl4-induced hepatotoxicity in rats were evaluated. Intraperitoneal injection of CCl4 elicited significant increases in the serum ALT, AST, ALP, TB and CB levels with concomitant significant decreases in the TP, ALB, TC, HDL-c and LDL-c levels. Significant serum elevations in these hepatic biochemical markers have been attributed to the compromised structural and functional integrity of the hepatocytes.37 Both preventive and curative oral treatments with 125–500 mg/kg/day of MIASE for 7 days significantly reversed the altered serum liver function parameters in the CCl4-treated rats with more significant hepatoprotective effect offered in the curative treatment. Results of this present study on the hepatoprotective activity of MIASE is in line with that earlier reported for the lupeol and mango pulp extract in carcinogen-induced hepatotoxicity in Swiss Albino mice despite differences in the doses, plant parts, and animal models studied.38 Again, the fact that MIASE significantly reversed the deleterious effect of CCl4 serum lipids is a strong indication of the anti-atherogenic potential of MIASE which lends support to the earlier report on the antihyperglycemic and anti-atherogenicity of mangiferin in streptozotocin diabetic rats.39 In the same vein, intraperitoneal treatment with CCl4 elicited hyperglycemia which was significantly (p < 0.05, p < 0.001 and p < 0.0001) reduced dose-dependently by oral treatments with 125–500 mg/kg/day of MIASE. Although, earlier studies have reported CCl4-induced hyperglycemia in rats treated with CCl4,37,40,41 the marked reduction in FBG values, suggests the antihyperglycemic potential of MIASE in CCl4-induced hyperglycemia in rats. Although several studies have independently reported the antihyperglycemic effect of MIASE in different diabetic rat models,3,4,42 this study reports for the first time the hypoglycemic effect of MIASE in the CCl4-induced hepatotoxic rats.
CCl4, as a potent chemical hepatotoxin, is widely used for the induction of experimental hepatotoxicity in animal models. CCl4 after its administration undergoes reductive metabolism by the hepatic CYP2E1 into a highly reactive trichloromethyl radical (CCl3•-) that initiates lipid peroxidation, disrupts membrane integrity and causes hepatocyte necrosis and death.43 In all of these, the role of oxidative stress in the etiopathogenesis of hepatic disorders is well documented.44 The free radicals generated as a result of reductive halogenation of CCl4, in the presence of oxygen, lead to auto-oxidation of free fatty acids and cause functional and morphological alterations in the hepatocyte cell membrane after binding covalently to membrane lipids and proteins, or abstracting a hydrogen atom from and unsaturated lipid and initiating lipid peroxidation.43,45 Thus, prevention and/or inhibition of free radical generation and promotion of antioxidant activity constitute important defense system against CCl4-induced hepatic injury.46,47 In measuring free radicals-induced oxidative stress, enzyme markers such superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and for lipid peroxidation, catabolite such as malondialdehyde (MAD), are widely accepted and used.48
In order to evaluate the effect of MIASE on CCl4-induced oxidative stress, hepatic tissue levels of GSH and activities of antioxidant enzymes CAT, SOD, and MAD were measured. Administration of CCl4 by intraperitoneal route profoundly elevated the tissue activities of these antioxidant enzymes while significantly decreasing the hepatic tissue GSH concentration. Accompanying these biochemical changes are histological changes of hepatocyte vacuolation/ballooning and hepatic centrilobular necrosis. These biochemical and histopathological results are in strong consonance with the earlier findings of Brattin et al.,49 Fukao et al.50 and Padhy et al.51 However, daily oral treatment with 125–500 mg/kg/day of MIASE over 7 days afforded hepatoprotection dose-dependently with better protection offered by MIASE administered curatively than that offered prophylactically. In addition, MIASE administered post-CCl4 treatment produced marked dose-related reductions in the hepatic MAD level, MAD being a reliable index of lipid peroxidation. Thus, this results strongly further suggests anti-lipoperoxidation activity of MIASE. The antioxidant and anti-lipoperoxidative properties of MIASE could be attributed to its constituent flavonoids and other polyphenolics as these phytocomponents have been widely reported to possess antioxidant and anti-lipoperoxidative activities.52,53 Preliminary qualitative phytochemical screening of MIASE for its secondary metabolites showed that the extract contains alkaloids, tannins, cardiac glycosides, flavonoids, phlobatinnins, reducing sugars and saponins with anthocyanosides and anthraquinones being absent. This is in complete agreement with other earlier reports.35,36
Conclusion
The present study suggests that MIASE has a potent hepatoprotective activity in CCl4-induced hepatic injury in rats. MIASE may possess both antioxidant and anti-lipoperoxidative activities by inhibiting and scavenging free radicals generated by CCl4. These findings provide biochemical and histological data supporting folkloric use of MIASE in the local treatment of some hepatic disorders.
Acknowledgment
The authors would like express their profound gratitude to Mr. Sunday Adenekan of the Department of Biochemistry, Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, Idi-Araba, Surulere, Lagos State, Nigeria, for his technical assistance in the areas of the biochemical assays.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Abbreviations
- ALB
albumin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AOT
acute oral toxicity
- AST
aspartate aminotransferase
- CAT
catalase
- CB
conjugated bilirubin
- CCl4
carbon tetrachloride
- DTNB
dithiobisnitro benzoic acid
- FBG
fasting blood glucose
- GSH
reduced glutathione
- HCl
hydrochloric acid
- HDL-c
high density lipoprotein cholesterol
- H2O2
hydrogen peroxide
- i.p.
intraperitoneal route
- KCl
potassium chloride
- LDL-c
low density lipoprotein cholesterol
- MDA
malondialdehyde
- MIASE
Mangifera indica L. (芒果 Máng Guǒ) aqueous stem bark extract
- OECD
Organization for Economic Community Development
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
- TC
total cholesterol
- TCA
tricarboxylic acid
- TG
triglyceride
- TP
total protein
- UV
ultraviolet light
References
- 1.Shah K.A., Patel M.B., Patel R.J., Parmar P.K. Mangifera indica (Mango) Phcog Rev. 2010;4(7):42–48. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aderibigbe A.O., Emudianughe T.S., Lawal B.S. Antihyperglycemic effect of Mangifera indica in rats. Phytother Res. 1999;13:504–507. doi: 10.1002/(sici)1099-1573(199909)13:6<504::aid-ptr533>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Aderibigbe A.O., Emudianughe T.S., Lawal B.A. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother Res. 2001;15:456–458. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 4.Perpétuo G.F., Salgado J.M. Effect of mango (Mangifera indica, L.) ingestion on blood glucose levels of normal and diabetic rats. J Plant Foods Hum Nutr. 2003;58:1–12. [Google Scholar]
- 5.Amrita B., Liakot A., Masfida A., Begum R. Studies on the antidiabetic effects of Mangifera indica stem-barks and leaves on non-diabetic, type 1 and type 2 diabetic model rats. Bangladesh J Pharmacol. 2009;4:110–114. [Google Scholar]
- 6.Ojewole J.A. Anti-inflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anarcardiaceae) stem-bark aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:547–554. doi: 10.1358/mf.2005.27.8.928308. [DOI] [PubMed] [Google Scholar]
- 7.Awe S.O., Olajide O.A., Oladiran O.O., Makinde J.M. Antiplasmodial and antipyretic screening of Mangifera indica extract. Phytother Res. 1998;12:427–438. [Google Scholar]
- 8.Bidla G., Titanji V.P., Jako B., Bolad A., Berzins K. Antiplasmodial activity of seven plants used in African folk medicine. Indian J Pharmacol. 2004;36:245–246. [Google Scholar]
- 9.Zheng M.S., Lu Z.Y. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin Med J. 1990;103:160–165. [PubMed] [Google Scholar]
- 10.Zhu X.M., Song J.X., Huang Z.Z., Whu Y.M., Yu M.J. Antiviral activity of mangiferin against herpes simplex virus type 2 in vitro. Zhongguo Yao Li Xue Bao. 1993;14:452–454. [PubMed] [Google Scholar]
- 11.Martinez G., Delgado R., Perez G., Garrido G., Nunez Selles A.J., Leon O.S. Evaluation of the in-vitro antioxidant activity of Mangifera indica L. extract (Vimang) Phytother Res. 2000;14:424–427. doi: 10.1002/1099-1573(200009)14:6<424::aid-ptr643>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Muruganandan S., Gupta S., Kataria M., Lal J., Gupta P.K. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176:165–173. doi: 10.1016/s0300-483x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 13.Pardo-Andreu G.L., Sanchez-Baldoquín C., Avila-González R., Yamamoto E.T., Revilla A., Uyemura S.A., Naal Z., Delgado R., Curti C. Interaction of Vimang (Mangifera indica L. extract) with Fe(III) improves its antioxidant and cytoprotective activity. Pharmacol Res. 2006;54:389–395. doi: 10.1016/j.phrs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Guha S., Ghosal S., Chattopadyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin: a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- 15.Beltrán A.E., Alvarez Y., Xavier F.E., Hernanz R., Rodriguez J., Núñez A.J., Alonso M.J., Salaices M. Vascular effect of Mangifera indica L. extract (Vimang) Eur J Pharmacol. 2004;499:297–305. doi: 10.1016/j.ejphar.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Garcia D., Escalante M., Delgado R., Ubeira F.M., Leiro J. Antihelminthic and anti-allergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother Res. 2003;17:1203–1208. doi: 10.1002/ptr.1343. [DOI] [PubMed] [Google Scholar]
- 17.Parmar H.S., Kar A. Possible amelioration of atherogenic diet induced dyslipidemia, hypothyroidism and hyperglycemia by the peel extracts of Mangifera indica, Cucumis melo and Citrullus vulgaris fruits in rats. Biofactors. 2008;33(1):13–24. doi: 10.1002/biof.5520330102. [DOI] [PubMed] [Google Scholar]
- 18.Rodeiro I., Donato M.T., Jimnenez N., Garrido G., Delgado R., Gomez-Lechon M.J. Effects of Mangifera indica L. aqueous extract (Vimang) on primary culture of rat hepatocytes. Food Chem Toxicol. 2007;45:2506–2512. doi: 10.1016/j.fct.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Gill L.S. Ethnomedical Uses of Plants in Nigeria. Uniben Press; Nigeria, Benin-City: 1992. Mangifera indica L; p. 155. [Google Scholar]
- 20.Sofowora A. 2nd ed. Spectrum Books Ltd; Nigeria, Ibadan: 1993. Medicinal Plants and Traditional Medicine in Africa; p. 150. [Google Scholar]
- 21.Acute Oral Toxicity (OECD Test Guideline 425) 2001. Statistical Programme (AOT425StatPgm)http://www.oecd.org/oecd/pages/home/displaygeneral/0, 3380, EN-document-524-nodirectorate-no-24-6775-8,FF.html Version 1.0. [Google Scholar]
- 22.Lu K.L., Tsai C.C., Ho L.K., Lin C.C., Chang Y.S. Preventive effect of the Taiwan folk medicine Ixeris laevigata var. oldhami on α-naphthyl-isothiocyanate and carbon tetrachloride-induced acute liver injury in rats. Phytother Res. 2002;16:S45–50. doi: 10.1002/ptr.801. [DOI] [PubMed] [Google Scholar]
- 23.Sun M., Zigman S. An improved spectrophotometric assay of superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;90:81–89. doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 24.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.Sedlak J., Lindsay R.H. Estimation of total, protein-bound and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:1192–1205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 27.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 28.Reitman S., Frankel S. Determination of serum glutamate-oxaloacetic and glutamic pyruvic acid transaminase. Am J Clin Pathol. 1957;28:56–66. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Trietz N.W., Rinker A.D., Shaw L.M. International Federation of Clinical Chemistry. IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1.3.1). IFCC document stage 2, draft 1, 1983-03 with a view to an IFCC recommendation. Clin Chim Acta. 1983;135:339–367. doi: 10.1016/0009-8981(83)90294-2. [DOI] [PubMed] [Google Scholar]
- 30.Treitz N.W. W.B. Sanders; U.S.A., Philadelphia: 1970. Fundamentals of Clinical Chemistry with Clinical Correlation; pp. 280–284. [Google Scholar]
- 31.Waterborg H.H. Springer; Berlin, Heidelberg: 2002. The Lowry Method for Protein Quantitation. The Protein Protocols Handbook; pp. 7–9. [Google Scholar]
- 32.Zelenka J., Lenícek M., Muchová L., Jirsa M., Kudla M., Balaz P., Zadinová M., Ostrow J.D., Wong R.J., Vítek L. High sensitive method for quantitative determination of bilirubin in biological fluids and tissues. J Chromatogr B Analyt Techno Biomed Life Sci. 2008;867:37–42. doi: 10.1016/j.jchromb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Cox R.A., García-Palmieri M.R. Cholesterol, Triglycerides, and Associated Lipoproteins. In: Walker H.K., Hall W.D., Hurst J.W., editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; Massachusetts, Boston: 1990. http://www.ncbi.nlm.nih.gov/books/NBK351/ Chapter 31. Available from: [PubMed] [Google Scholar]
- 34.Trinder P. Determination of blood glucose using 4-aminophenzone as oxygen acceptor. J Clin Path. 1969;22:246–248. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Mahmoud M.A. Antibacterial efficacy of stem bark extracts of Mangifera indica against some bacteria associated with respiratory tract infections. Sci Res Essays. 2009;4:1031–1037. [Google Scholar]
- 36.Oyewo O.O., Onyije F.M., Akintunde O.W., Ashamu E.A., Oyinbo C.A. Effects of crude aqueous stem bark extract of Mangifera indica on the histology of the kidney of Wistar rats. J Vet Adv. 2012;2:60–64. [Google Scholar]
- 37.Jatwa R., Kar A. Protective effect of L-ornithine-L-aspartate and silymarin on chemically induced kidney toxicity and thyroid dysfunction in mice. EXCLI J. 2008;7:139–150. [Google Scholar]
- 38.Prasad S., Kalra N., Shukla Y. Hepatoprotective effects of lupeol and mango pulp extract of carcinogen-induced alteration in Swiss albino mice. Mol Nutr Food Res. 2007;51:352–359. doi: 10.1002/mnfr.200600113. [DOI] [PubMed] [Google Scholar]
- 39.Muruganadan K., Srinivasan S., Gupta P.K., Gupta J.L. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005;93:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Pingale S.S. Hepatosuppression by Adhatoda vasica against CCl4-induced liver toxicity in rat. Pharmacologyonline. 2009;3:633–639. [Google Scholar]
- 41.Khan R.A., Khan M.R., Ahmed M., Shah N.A. Carbon tetrachloride-induced lipid peroxidation and hyperglycemia in rat: A novel study. Toxicol Ind Health. 2013 doi: 10.1177/0748233713475503. [DOI] [PubMed] [Google Scholar]
- 42.Das J., Ghosh J., Roy A., Sil P.C. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFκB pathways. Toxicol Appl Pharmacol. 2012;260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–127. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 44.Cesaratto L., Vascotto C., Calligaris S., Tell G. The importance of redox state in liver damage. Ann Hepatol. 2004;3:86–92. [PubMed] [Google Scholar]
- 45.Pramyothin P., Janthasoot W., Pongnimitprasert N., Phrukudo S., Ruangrungsi N. Hepatotoxic effect of (+) usnic acid from Usnea siammensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. J Ethnopharmacol. 2004;90:381–387. doi: 10.1016/j.jep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Wei W., Wang N.P., Gui S.Y., Wu L., Sun W.Y., Xu S.Y. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77:1902–1915. doi: 10.1016/j.lfs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Murugesan P., Muthusamy T., Balasubramania K., Arunakaran J. Effects of vitamins C and E on steroidogenic enzymes mRNA expression in polychlorinated biphenyl (Aroclor 1254) exposed adult rats Leydig cells. Toxicology. 2007;232:170–182. doi: 10.1016/j.tox.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Wang T., Sun N.-L., Zhang W.-D., Li H.-L., Lu G.-C., Yuan B.-J., Jiang H., She J.-H., Zhang C. Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J Ethnopharmacol. 2008;117:300–308. doi: 10.1016/j.jep.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Brattin W.J., Glende E.A.J., Jr., Recknagel R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 50.Fukao T., Hosono T., Misawa S., Seki T., Ariga T. The effect of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol. 2004;42:743–749. doi: 10.1016/j.fct.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Padhy B.M., Srivastava A., Kumar V.L. Calotropis procera latex affords protection against carbon tetrachloride induced hepatotoxicity in rats. J Ethnopharmacol. 2007;113:498–502. doi: 10.1016/j.jep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Roy S., Sehgal R., Padhy B.M., Kumar V.L. Antioxidant and protective effects of latex of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol. 2005;102:470–473. doi: 10.1016/j.jep.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 53.Yang J., Li Y., Wang F., Wu C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem. 2010;58(10):6525–6531. doi: 10.1021/jf903070a. [DOI] [PubMed] [Google Scholar]


