Abstract
A chemically synthesized gene encoding human CuZn superoxide dismutase (hSOD) was cloned into the shuttle vector pBAX18R and expressed in Anacystis nidulans 6301 (Synechococcus sp. strain PCC 6301) under the control of a ribulose-1,5-bisphosphate carboxylase/oxygenase gene (rbc) promoter derived from A. nidulans 6301. The sequences immediately upstream from the hSOD coding region and the distances between the ribosomal binding site and ATG initiation codon strongly affected the expression of the hSOD gene in A. nidulans cells. Optimal expression of hSOD was obtained with the expression vector pBAXSOD8-I, which contained a GGAGAG sequence. In defined conditions, irradiation with light increased hSOD enzyme activity in the transformants > 18-fold and the level of the hSOD protein reached a value of about 3% of the total soluble protein. The transformants that expressed hSOD acquired the ability to extenuate photooxidative damage induced by methyl viologen.
Full text
PDF

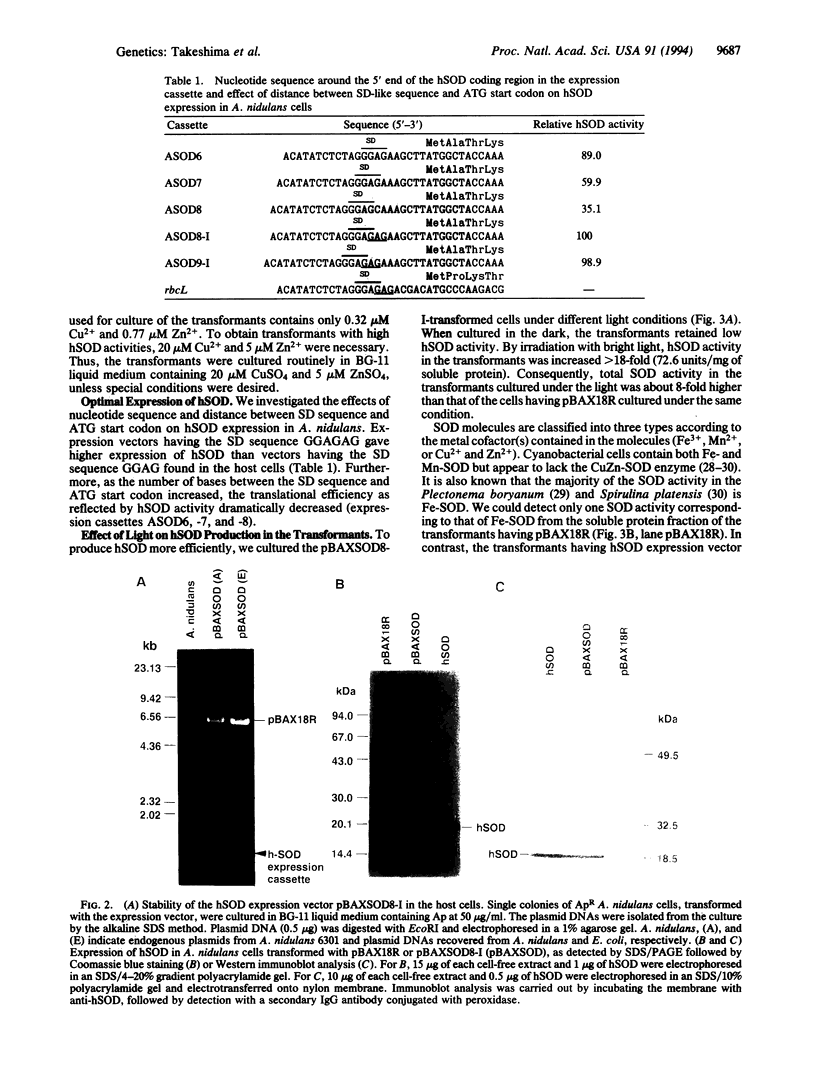
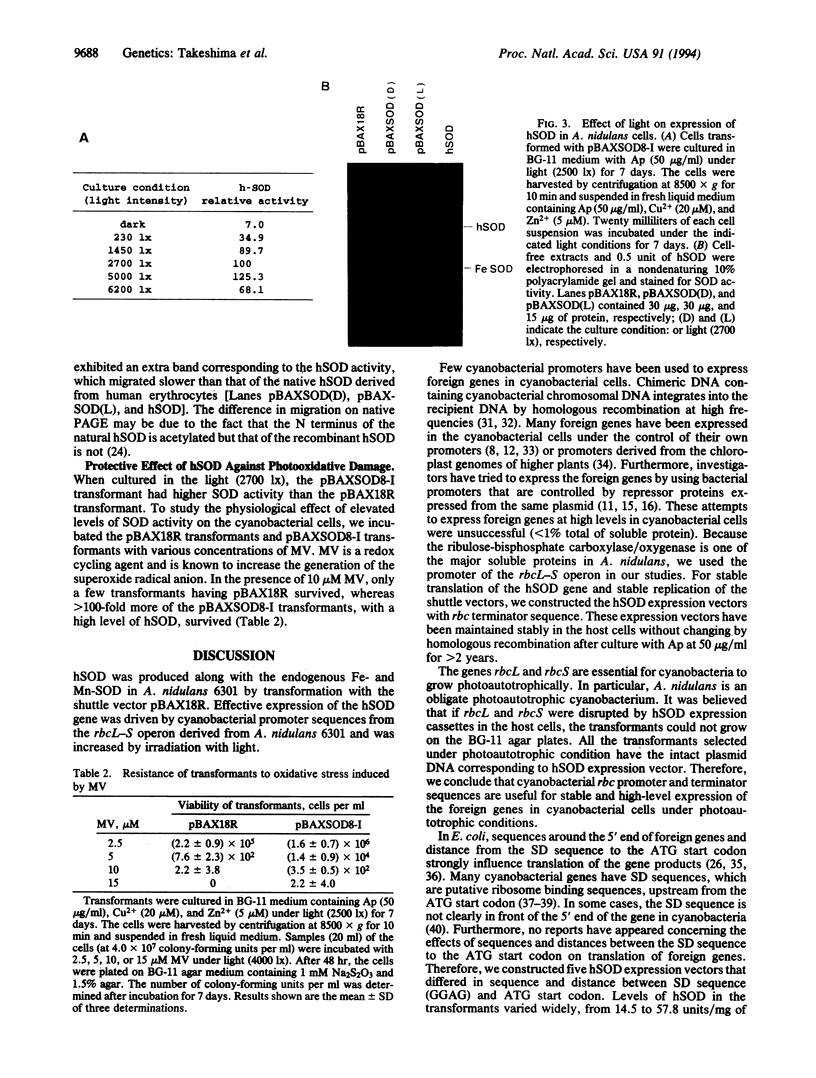

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajlani G., Kirilovsky D., Picaud M., Astier C. Molecular analysis of psbA mutations responsible for various herbicide resistance phenotypes in Synechocystis 6714. Plant Mol Biol. 1989 Nov;13(5):469–479. doi: 10.1007/BF00027307. [DOI] [PubMed] [Google Scholar]
- Angsuthanasombat C., Panyim S. Biosynthesis of 130-kilodalton mosquito larvicide in the cyanobacterium Agmenellum quadruplicatum PR-6. Appl Environ Microbiol. 1989 Sep;55(9):2428–2430. doi: 10.1128/aem.55.9.2428-2430.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Buttarelli F. R., Calogero R. A., Tiboni O., Gualerzi C. O., Pon C. L. Characterization of the str operon genes from Spirulina platensis and their evolutionary relationship to those of other prokaryotes. Mol Gen Genet. 1989 May;217(1):97–104. doi: 10.1007/BF00330947. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983 Dec;47(4):551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. Molecular characterization and evolution of sequences encoding light-harvesting components in the chromatically adapting cyanobacterium Fremyella diplosiphon. J Mol Biol. 1988 Feb 5;199(3):447–465. doi: 10.1016/0022-2836(88)90617-1. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Chisholm R. L. The effect of caffeine, adenosine, and buffer ionic composition on the induction of cell-surface cAMP binding during starvation of Dictyostelium discoideum. Dev Genet. 1988;9(4-5):293–301. doi: 10.1002/dvg.1020090411. [DOI] [PubMed] [Google Scholar]
- Federspiel N. A., Grossman A. R. Characterization of the light-regulated operon encoding the phycoerythrin-associated linker proteins from the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1990 Jul;172(7):4072–4081. doi: 10.1128/jb.172.7.4072-4081.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Seijffers J. Controlled gene expression utilising lambda phage regulatory signals in a cyanobacterium host. Mol Gen Genet. 1986 Jun;203(3):505–510. doi: 10.1007/BF00422077. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gruber M. Y., Glick B. R., Thompson J. E. Cloned manganese superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2608–2612. doi: 10.1073/pnas.87.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S. K., Samson G., Fork D. C., Laudenbach D. E. Characterization of damage to photosystems I and II in a cyanobacterium lacking detectable iron superoxide dismutase activity. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8716–8720. doi: 10.1073/pnas.89.18.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., de Marsac N. T. Cyanobacterial genetic tools: current status. Methods Enzymol. 1988;167:808–847. doi: 10.1016/0076-6879(88)67092-3. [DOI] [PubMed] [Google Scholar]
- Kanematsu S., Takeshima Y., Hagiwara H. Chemically-synthesized gene encoding modified human superoxide dismutase: its construction, expression and properties of the product. Free Radic Res Commun. 1991;12-13 Pt 2:829–836. doi: 10.3109/10715769109145864. [DOI] [PubMed] [Google Scholar]
- Kolowsky K. S., Williams J. G., Szalay A. A. Length of foreign DNA in chimeric plasmids determines the efficiency of its integration into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1984 Mar;27(3):289–299. doi: 10.1016/0378-1119(84)90073-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Cammack R., Hall D. O. Purification and physicochemical properties of superoxide dismutase from two photosynthetic microorganisms. Biochim Biophys Acta. 1976 Jul 8;438(2):380–392. doi: 10.1016/0005-2744(76)90255-2. [DOI] [PubMed] [Google Scholar]
- Matteucci M. D., Heyneker H. L. Targeted random mutagenesis: the use of ambiguously synthesized oligonucleotides to mutagenize sequences immediately 5' of an ATG initiation codon. Nucleic Acids Res. 1983 May 25;11(10):3113–3121. doi: 10.1093/nar/11.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mohamed A., Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989 Dec;13(6):693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- Pierce J., Carlson T. J., Williams J. G. A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5753–5757. doi: 10.1073/pnas.86.15.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO(2)-Requiring Phenotype : Evidence for a Central Role for Carboxysomes in the CO(2) Concentrating Mechanism. Plant Physiol. 1989 Oct;91(2):505–513. doi: 10.1104/pp.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Tomioka N., Yamada C., Sugiura M. Cloning and characterization of a plasmid DNA from anacystis nidulans 6301. Gene. 1982 Sep;19(2):221–224. doi: 10.1016/0378-1119(82)90009-9. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Yamada C., Takahata N., Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart L. B., McIntosh L. Expression of photosynthesis genes in the cyanobacterium Synechocystis sp. PCC 6803: psaA-psaB and psbA transcripts accumulate in dark-grown cells. Plant Mol Biol. 1991 Nov;17(5):959–971. doi: 10.1007/BF00037136. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., de la Torre F., Szulmajster J. Expression of the larvicidal gene of Bacillus sphaericus 1593M in the cyanobacterium Anacystis nidulans R2. Mol Gen Genet. 1987 Sep;209(2):396–398. doi: 10.1007/BF00329671. [DOI] [PubMed] [Google Scholar]
- Van der Plas J., Bovy A., Kruyt F., de Vrieze G., Dassen E., Klein B., Weisbeek P. The gene for the precursor of plastocyanin from the cyanobacterium Anabaena sp. PCC 7937: isolation, sequence and regulation. Mol Microbiol. 1989 Mar;3(3):275–284. doi: 10.1111/j.1365-2958.1989.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Guglielmi G., Bryant D. A., Stevens S. E., Jr Functional expression of plastid allophycocyanin genes in a cyanobacterium. J Bacteriol. 1987 May;169(5):1830–1835. doi: 10.1128/jb.169.5.1830-1835.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]