Abstract
Geriatrics is a medical practice that addresses the complex needs of older patients and emphasizes maintaining functional independence even in the presence of chronic disease. Treatment of geriatric patients requires a different strategy and is very complex. Geriatric medicines aim to promote health by preventing and treating diseases and disabilities in older adults. Development of effective dietary interventions for promoting healthy aging is an active but challenging area of research because aging is associated with an increased risk of chronic disease, disability, and death. Aging populations are a global phenomenon. The most widespread conditions affecting older people are hypertension, congestive heart failure, dementia, osteoporosis, breathing problems, cataract, and diabetes to name a few. Decreased immunity is also partially responsible for the increased morbidity and mortality resulting from infectious agents in the elderly. Nutritional status is one of the chief variables that explains differences in both the incidence and pathology of infection. Elderly people are at increased risk for micronutrient deficiencies due to a variety of factors including social, physical, economic, and emotional obstacles to eating. Thus there is an urgent need to shift priorities to increase our attention on ways to prevent chronic illnesses associated with aging. Individually, people must put increased efforts into establishing healthy lifestyle practices, including consuming a more healthful diet. The present review thus focuses on the phytochemicals of nutraceutical importance for the geriatric population.
Keywords: aging, geriatrics, health, nutraceutical, nutrition, phytochemicals
Graphical abstract
1. Introduction
Aging is a complex and inevitable biological process that is associated with numerous chronically debilitating health effects. It is estimated that there are about 50 million deaths each year worldwide.1 The top 10 leading causes of all deaths worldwide are ischemic heart disease (6.3 million), cerebrovascular accidents (4.4 million), lower respiratory infections (4.3 million), diarrheal disease (2.9 million), perinatal disorders (2.4 million), chronic obstructive pulmonary disease (2.2 million), tuberculosis (2.0 million), measles (1.1 million), and lung cancer (0.9 million). It is evident that in both developing and developed countries of the world, nutrition-modifiable disease is potentially responsible for a substantial portion of global deaths. Important areas of disease and disability in the aging populations in which nutrition may play a role in prevention are: dyslipidemia and heart-related problems; hypertension and stroke; cancer; reduced mobility accompanied by excess body weight with an increased risk of developing type 2 diabetes; Alzheimer’s disease and other cognitive impairments including depression; physical deterioration of bones and joints associated with osteoporosis and arthritis; vision impairment problems including cataracts and macular degeneration; and an increased risk of pulmonary problems and infectious diseases.
A major challenge to health care systems around the world is how to encourage and maintain a healthy lifespan in large and increasing populations of elderly individuals.2
Scientists have identified hundreds of genetic factors called longevity-related genes that modulate lifespan and health-span in model organisms such as yeast, worms, flies, and rodents. A number of the longevity-related genes fall into three conserved nutrient-sensing pathways: target-of-rapamycin, insulin/insulin-like growth factor-1-like signaling, and sirtuin pathways.3–5 These pathways primarily sense cellular amino acid, glucose, and nicotinamide adenine dinucleotide (NAD)+ or NAD+/NADH levels, respectively. There are various hypotheses of aging proposed. The most prominent is the free radical hypothesis, which states that free radicals, such as reactive oxygen species, generated from metabolism inflict oxidative damage to macromolecules, including protein, DNA, and lipid. Accumulation of such oxidative damage with time causes biological aging and eventually results in death.6 However, numerous studies have now shown that this simplified version of the free radical hypothesis of aging is not necessarily sufficient to explain the mechanisms underlying aging processes.
Another suggestion is the hormesis hypothesis of aging, which has been frequently used to interpret the prolongevity effects induced by nutraceuticals. Hormesis theory states that mild stress-induced stimulation of the defense response at the organism level results in biologically beneficial effects and extends lifespan and health span. The defense response involves many protective mechanisms and influences on gene expression and metabolism.6,7 Therefore, significant efforts in developing dietary interventions for promoting healthy aging have been devoted identifying effective ways to modulate metabolism and stress.4
It is believed that half of the world’s oldest populations are found in five countries: China, India, USA, Japan, and Russia. Currently, out of the 600 million older persons in the world, 370 million live in developing countries. A survey indicates that by 2020, 70% of the world's one billion elderly persons will be living in developing countries. While in 1950 only 8% of the world’s population was over 60 years old; that proportion will be up to 21% by the year 2050. The elderly population will make up a substantial proportion of many areas of the globe.1
The financial burden of caring for an aging population is substantial. According to the Center for Disease Control and Prevention, the costs for health care, long-term care and hospice for people with Alzheimer’s disease and other dementias alone is expected to increase from $183 billion in 2011 to $1.1 trillion in 2050, in current dollars. Healthy aging can be achieved by adopting healthy lifestyle practices and consuming a healthful diet. There are three main contributions to healthy aging: genetics and family history; lifestyle practices and exercise; and diet and nutrition. The first of these three factors is immutable, but the remaining two can be modified to improve health.8
Additionally, as people grow older, they need fewer calories but more nutrients to maintain proper health. Generally speaking, people burn fewer calories during physical activity when they age, but even the most active aging body gradually loses lean muscle tissue, and less muscle translates to a lower calorie requirement. At the same time, however, their appetites decrease while, as previously noted, their needs for several nutrients goes up or at least remains the same in order to enable the body to run at peak efficiency as the years pass. To fill these nutrient gaps, fortified food or beverage products will continue to grow in popularity and will become a mainstay with any consumer who embraces the concept of healthy aging.8
2. Primary disease concerns in older populations
With the advent of the developed medical facilities and technologies, the average life span of people has increased but unfortunately longevity is often accompanied by significant disability, despite new medications and surgical techniques. The most widespread conditions affecting older people are shown in Table 1.
Table 1.
Diseases common in geriatrics.
| Disease/condition | Disease/condition |
|---|---|
| Hypertension | Osteoporosis |
| Vascular disease | Diabetes |
| Congestive heart failure | Breathing problems |
| Coronary heart disease | Frequent falls/bone fractures |
| Dementia (Alzheimer’s disease) | Parkinson’s disease |
| Depression | Cancer |
| Incontinence | Cataracts |
| Arthritis | Glaucoma |
| Macular degeneration | Impaired immunity |
According to the Centers for Disease Control and Prevention, 80% of older adults have at least one of these conditions and 50% have at least two.
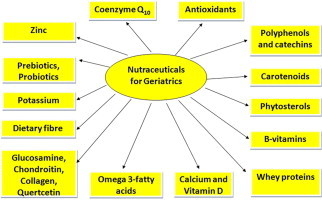
3. Nutraceuticals
The term nutraceutical encompasses a broad spectrum of commercially available products in which a part or a part of food (nutrient) is intended to provide medical or health benefits, including the prevention and treatment of disease (pharmaceutical). Nutraceuticals have no formal regulatory definition but they can be broadly defined to include functional foods, dietary supplements, and medical foods. Like nutraceuticals, functional foods have no legal definition but are distinguished from other types of nutraceuticals because they are recognizable as conventional food products. In contrast, dietary supplements are legally defined, which expressly states, among other requirements, that a product labeled as a dietary supplement may not be represented as a conventional foodstuff. A final category, medical foods, is distinguishable from functional foods and dietary supplements by the requirement that medical foods meet distinctive nutritional requirements of a disease or condition. Medical foods must be a food for oral or tube feeding, be labeled for dietary management of a specific medical disorder, disease, or condition for which there are established nutritional requirements and be intended for use under medical supervision.
Chemically, nutraceuticals may be classified as isoprenoid derivatives (terpenoids, carotenoids, saponins, tocotrienols, tocopherols, terpenes), phenolic compounds (couramines, tannins, lignins, anthocyanins, isoflavones, flavanones, flavanoids), carbohydrate derivatives (ascorbic acid, oligosaccharides, nonstarch polysaccharides), fatty acid and structural lipids (n-3 polyunsaturated fatty acids, conjugated linoleic acid, monounsaturated fatty acids, sphingolipids, lecithins), amino acid derivatives (amino acids, allyl-S compounds, capsaicinoids, isothiocyanates, indoles, folate, choline), microbes (probiotics, prebiotics), and minerals (Ca, Zn, Cu, K, Se).9,10 They play a crucial role in maintaining optimal immune response, such that deficient or excessive intakes can have negative impact on health.
Nutraceuticals, including dietary supplements and functional foods, are a $152 billion world market. The percentage of those aged 65 years and older using nutraceutical products is higher than for any other age group and has doubled in recent years. Aging is associated with decreased immunity, increased morbidity and mortality resulting from infectious agents, and poor nutritional status. Deficiencies in vitamin E, vitamin B6, folate, zinc, and selenium are particularly common, and deficits in these micronutrients have been reported to influence immunity negatively. Thus, if nutraceutical products can improve micronutrient status, the regular use of nutraceuticals by the elderly population may provide an opportunity to enhance immunity in this at-risk population.9
Numerous studies have demonstrated the effects of nutraceuticals from fruit or plant extracts in reducing oxidative damage and promoting healthy aging in invertebrate models. The active ingredients in nutraceuticals that are generally produced by plants as secondary compounds appear to help plants overcome stressful conditions. The beneficial properties of nutraceuticals can be attributed to the varieties of phytochemicals, such as flavonoids, anthocyanin glycosides, triterpenoids, and proanthocyanidin oligomers.9,11,12 In this review, the phytochemicals with nutraceutical potential for geriatric individuals will be discussed briefly.
4. Phytochemicals for age-related disease and disability
Table 2 lists some important phytochemicals that can be potentially helpful in the prevention of certain age-related chronic diseases and associated disability.
Table 2.
Phytochemicals of nutraceutical importance for geriatrics.
| Phytochemical/nutraceutical | Target disease/condition |
|---|---|
| Calcium and vitamin D | Osteoporosis, cancer, diabetes |
| Antioxidants (vitamin E, vitamin C, polyphenols) | Cancer, heart disease, neurodegenerative disease |
| B vitamins (folate, vitamin B6, vitamin B12) | Heart disease, cognition |
| Omega-3 fatty acids (fish oil, DHA, EPA) | Inflammation, heart disease, stroke |
| Plant stanols/sterols | Elevated blood cholesterol, heart disease |
| Glucosamine, chondroitin, and collagen | Osteoarthritis |
| Lutein, zeaxanthin, and lycopene | Macular degeneration |
| EGCG | Cancer |
| Fiber (soluble and insoluble) | Diabetes, constipation |
| Prebiotics and probiotics | Diarrhea |
| Potassium | Hypertension |
| Whey protein | Sarcopenia |
| Zinc | Immunity, macular degeneration |
| Coenzyme Q10 | Inflammation, endothelial dysfunction |
DHA = docosahexaenoic acid; EGCG = epigallocatechin-3-gallate; EPA = eicosapentaenoic acid.
5. Nutraceuticals for geriatrics
5.1. Antioxidants
A prominent theory of aging and chronic disease has been the free radical theory, in which a lifelong accumulation of cellular damage due to free radicals leads to an increased risk of disease and disability. In living cells, two antioxidant defense system are present against free radical damage. The first line of defense includes antioxidant enzymes (such as superoxide dismutase, catalase, and glutathione peroxidase) and the second line includes low molecular weight nonenzymatic antioxidants (thioredoxin, glutathione, vitamins A, C, E, lycopene, lutein, polyphenols, quercetin, etc.). It is thought, therefore, that diets rich in antioxidants, such as vitamin E and vitamin C and many bioactive polyphenol compounds found in fruits and vegetables will help to combat free radical damage and improve health. This theory is consistent with strong association with better health outcomes, and may have positive effects on cancer, heart disease, and neurodegenerative diseases.13,14
These antioxidants inhibit the formation of free radicals by breaking the chain reaction or can reduce the concentration of free radicals by donating hydrogen and electron. They also act as peroxide decomposers (vitamin E), enzyme inhibitors, singlet oxygen quenchers (vitamin E), synergists, and metal-chelating agents (transferritin). To provide maximum intracellular protection, antioxidants are strategically compartmentalized throughout the cell. So that free radical produced in intracellular and extracellular during metabolism, both enzymatic and nonenzymatic antioxidants are able to detoxify free radical.13
Certain antioxidant enzymes (superoxide dismutase, catalase, and glutathione) are produced within the body. Other antioxidant agents are found in foods such as green leafy vegetables and it is believed that diets rich in antioxidants (such as β-carotene and vitamins A, C, and E) are beneficial to human health.15 Therefore, antioxidants naturally present in body or supplied in the form of diet (phytonutrients) play an important role to control various diseases resulting from oxidative stress. Raw fresh fruits and vegetables are of more importance than cooked, because of their high concentration and maximum absorption of antioxidants. Researchers are trying to determine the relationship between antioxidants and prevention of some diseases, such as cardiovascular disease and cancer.13
5.2. Plant polyphenols and catechins: turmeric (薑黃 jiāng huáng), green tea (綠茶 lǜ chá), grape seed
Polyphenols are naturally occurring compounds found largely in fruits, vegetables, legumes, cereals, and beverages. Legumes and chocolate also contribute to the polyphenolic intake. These molecules are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens. Basic research and epidemiological studies have shown an inverse association between risk of degenerative diseases and intake of diet rich in polyphenols. Epidemiological studies provide convincing evidences that diet rich in antioxidants is associated with a lower incidence of degenerative diseases. The curcuminoid polyphenols, which are the primary polyphenols in the rhizome (underground stem) of the turmeric plant Curcuma longa L. (turmeric; 薑黃 jiāng huáng) and are responsible for its yellow color, have potent antioxidant, anti-inflammatory, and anticancer properties. These properties have led to investigations into the impact of curcumins in preventing cognitive decline relating to Alzheimer’s disease.15
The major sources of dietary polyphenols are cereals, legumes (barley, corn, nuts, oats, rice, sorghum, wheat, beans, and pulses), oilseeds (rapeseed, canola, flaxseed, and olive seeds), fruits, vegetables, and beverages (fruit juices, tea, coffee, cocoa, beer, and wine).16,17 Fruits such as apple, grape, pear, cherry, and various berries contain up to 200–300 mg polyphenols per 100 g fresh weight. Similarly, a glass of red wine or a cup of coffee or tea contains about 100 mg polyphenols. Their total dietary intake may be about 1 g/day, which is about 10 times higher than that of vitamin C and 100 times higher than those of vitamin E and carotenoids.18
The chief constituents of tea polyphenols are flavonols (catechin, epicatechin, catechingallate, and epigallocatechin gallate), flavanols (quercetin, kaempferol, and their glycosides), flavones (vitexin, isovintexin), and phenolic acids (gallic acid, chlorogenic acid). They constitute up to 30% of the dry weight of green leaves and 9–10% of the dry weight of black tea leaves. Ferulic acid is associated with dietary fiber linked with hemicellulose of the cell wall by means of ester bonds. Caffeic acid in the form of caffeoyl esters and coumaric acids are common in apples, pears, and grapes. Additionally, apples and pears are rich in chlorogenic acid and grapes in gallic acid. Apples contain the highest levels of quercetin among fruits. Grain-derived products are especially significant in human diet as they have higher concentration of phenolic acids in the outer layers of kernel that constitute the bran. Most of the phenolic acid derivatives are hydrolysable tannins and are usually esterified with glucose. Citrus fruits are major sources of flavonones and hesperidin is found in abundance (120–250 mg/L) in orange juice.
Green tea polyphenols have been shown to have powerful antioxidant, anti-inflammatory, and anticancer benefits. The most famous of these is epigallocatechin-3-gallate (EGCG). EGCG is found in high concentrations in green tea. As a member of the catechin family of compounds, it has antioxidant properties, but it also has other biochemical effects in cells. The majority of emphasis on the health promoting effects of EGCG has been related to its potential anticancer activities, particularly related to hormone-sensitive cancers.19,20 Cancer is the second leading cause of death in the elderly.
Grape seed extract (GSE) is a concentrated source of polyphenols. These resemble the catechins of green tea in basic molecular structure with the exception that components in GSE reach a larger molecular size. Although clinical research on GSE for inflammation and cancer is not as advanced as that for the curcumins, and green tea catechins, there is abundant animal and in vitro evidence suggesting that GSE also has efficacy in applications aimed at protecting against oxidative stress and aiding circulation, in addition to its general anti-inflammatory and anticancer effects.15
5.3. Carotenoids: lutein, zeaxanthin, and lycopene
Dietary carotenoids are obtained from a number of fruits and vegetables, such as green leafy vegetables, spinach, carrots, peaches, apricots, and sweet potatoes. Carotenoids are highly pigmented, yellow, orange and red, are present in fruits and vegetables, and when consumed by birds are incorporated into egg yolk. Carotenoids comprise two types of molecule: carotenes and xanthophylls. Lutein and zeaxanthin are members of the carotenoid family of compounds and are found abundantly in green leafy vegetables. These carotenoids have healthful properties and are found in high concentrations in the macula of the eye, which is responsible for central vision. Macular degeneration is a common problem in the elderly and is among the four leading eye diseases found in this population. Supplementation of patients with early signs of macular degeneration with lutein and zeaxanthin has been shown to be beneficial.21 In addition, consumption of diets rich in lutein and zeaxanthin have been found in a recent meta-analysis to be associated with a reduction in the risk of developing late stage macular degeneration.22 Lycopene is also a member of the carotenoid family and is responsible for the red to pink color found in tomatoes and watermelon and some other fruits and vegetables. Epidemiologic, animal, and cell culture evidence support a role for lycopene in cancer prevention.23
Based on epidemiological studies a positive link is suggested between higher dietary intake and tissue concentrations of carotenoids and lower risk of chronic diseases.24 Human diet supplemented with carotenoids is beneficial in reducing chronic conditions related to coronary heart diseases, certain cancers, and macular degeneration. β-carotene and lycopene have been shown to be inversely related to the risk of cardiovascular diseases and certain cancers where as lutein and zeaxanthin to the disorders related to the eye. Lutein protects against uterine, prostate, breast, colorectal, and lung cancers. They may also protect against risk of digestive tract cancer. The xanthophyll types of carotenoids offer protection to other antioxidants, and they may exhibit tissue specific protection. Zeaxanthin, cryptoxanthin, and astaxanthin are members of the xanthophyll group. The antioxidant properties of carotenoids have been suggested as being the main mechanism by which they afford their beneficial effects. Recent studies are also showing that carotenoids may mediate their effects via other mechanisms such as gap junction communication, cell growth regulation, modulating gene expression, immune response, and as modulators of Phase I and II drug metabolizing enzymes.24
The nutraceutical industry synthetically manufactures five major carotenoids on an industrial scale (lycopene, β-carotene, canthaxanthin, zeaxanthin, and astaxanthin) for use in a range of food products and cosmetics, such as vitamin supplements and health products and as feed additives for poultry, livestock, fish, and crustaceans.25 One of the most commercially valuable pigments, astaxanthin, is primarily synthesized by marine microorganisms, such as the green alga Haematococcus pluvialis and accumulates in fish such as salmon, coloring their flesh red. Astaxanthin has been implicated as a potential therapeutic agent treating cardiovascular disease and prostatic cancer.20,24,26
5.4. Plant stanols/sterols (phytosterols)
Plant sterols and stanols, an important terpene subclass, are naturally found in small amounts in many plant-based foods. The primary sources of phytosterols are vegetables, nuts, fruits, and seeds. Seeds contain an average of 120 mg of plant sterols/100 g wet weight; vegetables contain 20 mg/100 g of wet weight; and fruit about 15 mg/100 g wet weight. Sitosterol, campesterol, and stigmasterol are most abundant in nature comprising 65%, 30%, and 3% of dietary phytosterol intake, respectively.27 The primary phytosterols in the diet are sitosterol, stigmasterol, and campesterol and typical consumption of plant sterols is approximately 160–400 mg/day. The enrichment of foods with phytosterols is one of the recent developments in functional foods to enhance the cholesterol-lowering ability of traditional food products.28 Two sterol molecules that are synthesized by plants are β-sitosterol and its glycoside. These compounds have cholesterol-lowering properties resulting from the inhibition of cholesterol absorption.29 They are now widely used as food fortificants to help lower blood cholesterol and reduce the risk of heart disease. Since dyslipidemia is an important risk factor for heart disease and a common condition in older people, it would be prudent for this population to consider using plant stanol/sterol-enriched food products as part of a healthy diet.20
In recent studies, these two molecules have been shown to exhibit anti-inflammatory, antineoplastic, antipyretic, and immunomodulating activity in experimental animals. Phytosterols have been reported to block inflammatory enzymes, for example by modifying the prostaglandin pathways in a way that protected platelets. Phytosterols compete with cholesterol in the intestine for uptake, and aid in the elimination of cholesterol from the body. In the intestine, plant sterols are initially solubilized into a micelle form. These micelles interact with brush border cells and are transferred into enterocytes. Plant sterols are esterified within the enterocyte, assembled into chylomicrons and secreted into the lymphatics. They are excreted via the biliary system.
5.5. B vitamins
The status of B-group vitamins is frequently inadequate in the elderly and recent studies have shown associations between loss of cognitive function or Alzheimer disease and inadequate B vitamin status. Evidence of the importance of the B vitamins folic acid, vitamin B-12, and vitamin B-6 for the well-being and normal function of the brain derives from data showing neurological and psychological dysfunction in vitamin deficiency states and in cases of congenital defects of one-carbon metabolism. These inadequacies could give rise to impairment of methylation reactions that are crucial to the health of brain tissue. In addition, these inadequacies could result in hyperhomocysteinemia, a recently identified risk factor for occlusive vascular disease, stroke, and thrombosis, any of which may result in brain ischemia. Advances in the understanding of this putative relation between inadequate vitamin status and loss of cognitive function in the elderly are likely to be slow and may depend on the outcomes of both prospective studies and longitudinal studies in which nutritional intervention is provided before cognitive decline occurs.30,31
Scientific studies have shown that B-vitamins have possible roles in heart disease and cognitive impairment.30 Vitamin B6, Vitamin B12, and folate are three important B-vitamins that are involved in metabolic cycles that supply the body with methyl groups (1-carbon metabolites) that are important to many functions in the body, including homocysteine metabolism, a potential risk factor for heart disease.31
5.6. Calcium and vitamin D
A recent study suggested that vitamin D when taken with calcium can reduce the rate of mortality in seniors, thereby providing a possible means of increasing life expectancy.32
The role of calcium and vitamin D has been associated with its important function in bone metabolism and the prevention of osteoporosis. However, in recent years, there has been increased research attention placed on the nonskeletal roles of these nutrients. For example, high calcium diets have been shown to have some efficacy in reducing the risk of colon cancer and the recurrence of colonic polyps, while vitamin D has been implicated in a variety of diseases including diabetes and various cancers.31
5.7. Omega-3 fatty acids
Omega-3 (n-3) fatty acids are found in fish oil and in some plants, such as flaxseed. N-3 fatty acids are known to have anti-inflammatory effects and to lower blood triglycerides33,34 and have also been suggested to have a positive effect in patients suffering from recent myocardial infarction (heart attack) or heart failure. The Japan EPA Lipid Intervention Study (JELIS) found a 19% reduction in the risk of coronary heart disease and a significant reduction in recurrent stroke after long-term use of pure eicosapentaenoic acid (EPA) in Japanese patients with hypercholesterolemia.35,36 Higher circulating long-chain omega-3 fatty acids have also been shown to be associated with a lower risk of congestive heart failure in a prospective cohort study.37 Omega-3 intake may also play a role in cancer development; for example, a recent study found that higher omega-3 intake was associated with a decrease in breast cancer risk in obese Mexican women.38 Currently, an ongoing study, called VITAL (Vitamin D and Omega-3 Trial), is investigating the effects of these compounds in a large randomized, double-blind, placebo-controlled study of primary cancer and cardiovascular disease prevention.39 Another interesting finding concerning omega-3 fatty acids is that female health professionals consuming higher intakes of EPA and docosahexaenoic acid had a lower incidence of age-related macular degeneration.20,40
A publication from the Food Agriculture Organization in 201041 contains dietary recommendations for total fat, saturated, trans, and polyunsaturated fatty acids, lipoic acid, α-lipoic acid, and their metabolites EPA and docosahexaenoic acid, for adults, pregnant and lactating women, infants aged 0–2 years, and children aged 2–18 years old. The recommendations are expressed as energy percentage. For adults, total fat intake is 20–35%,42 minimum intake for lipoic acid and α-lipoic acid to prevent deficiency is 2.5% plus 0.5% respectively; minimum total polyunsaturated fatty acids for reducing cardiovascular risk is 6%.34
5.8. Glucosamine, chondroitin, collagen, and quercetin
Glucosamine and chondroitin are part of normal cartilage. Cartilage acts as a cushion between the bones in a joint. During movement, the cartilage that surrounds the ends of bones in joints is subject to breaking down and must be repaired. Cartilage is composed of type II collagen. Studies have shown that oral consumption of these building blocks of cartilage is believed to be beneficial in reducing pain and protecting bone cartilage. A recent randomized, double-blind clinical trial in 40 Japanese patients with symptomatic knee osteoarthritis found an improvement in symptoms in those receiving a combination of glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides compared to the receiving placebo.43 Likewise, a study in Spain in 250 patients with knee osteoarthritis found that treatment with collagen hydrolysates resulted in a significant improvement in knee joint comfort.44 Moreover, a study in Belgium found in a follow-up of individuals who had previously been enrolled in clinical trials of glucosamine sulfate for knee osteoarthritis and had received treatment for at least 12 months that the glucosamine treatment group were 57% less likely to require total joint replacement surgery compared to the placebo group.45
Glucosamine, also called chitosamine, is a natural substance that is found in the covering of shellfish. It is available in different forms, including glucosamine hydrochloride, N-acetyl-glucosamine, and glucosamine sulfate, which is a combination of glucosamine and mineral salt.
Quercetin, a potent antioxidant, is found in red wine, onions, green tea, apples, berries and cruciferous vegetables and is a popular component in the herbal and supplemental marketplace due to its value in treating and preventing many illnesses. Studies have shown that quercetin-incorporated collagen matrix could be a novel dressing material for dermal wound healing. Quercetin, a flavonoid present in the human diet has been shown to inhibit platelet aggregation and signaling in vitro. Consequently, it has been proposed that quercetin may contribute to the protective effects against cardiovascular disease.46 The study showed relatively high systemic availability of quercetin in the form of quercetin-4′-O-b-D-glucoside by supplementation, and implicates quercetin as a dietary inhibitor of platelet cell signaling and thrombus formation.46
5.9. Dietary fiber
Dietary fiber intake is important from a metabolic viewpoint (lipid and glucose metabolism), acting as prebiotics on microbiota health, in preventing colonic cancer, in treating bowel diseases and symptoms, on mineral absorption. Fiber intake seems to be important in particular in the elderly to the point that all national dietary guidelines and food guide pyramids for elderly people underline the necessity to increase dietary fiber intake, and therefore fruits and vegetables.47 Dietary fiber can be classified into either soluble fiber or insoluble fiber. These two types of fiber have different effects metabolically due to their different chemical properties. Soluble dietary fiber, such as found in peas and soybeans, are soluble in water and have a gelling effect in the intestine and can thereby slow down the digestion of carbohydrates and flatten out the postprandial blood glucose curve. This metabolic effect of soluble fiber can be of benefit to help control blood glucose levels in diabetes. Insoluble dietary fiber is not water soluble and relatively indigestible, tending to increase the dry matter content of the stool and aiding in the prevention of constipation.48
5.10. Prebiotics and probiotics
Probiotics (particularly belonging to genera Lactobacillus and Bifidobacterium) and prebiotics (nondigestible oligosaccharides) have been shown to be useful in preventing certain disease conditions and promoting specific aspects of health particularly in the elderly population. Both probiotics and prebiotics are helpful in malnutrition, lactose intolerance, and calcium absorption and in constipation. Probiotics have also been shown to boost immunity in elderly people.49 It is believed that large populations of friendly bacteria aid in keeping the growth of unfriendly pathogenic bacteria and yeast at bay. An imbalance (dysbiosis) of intestinal bacteria can be caused by antibiotic treatment and result in disease, including antibiotic-induced diarrheal disease. Thus, it is believed that supplying the body with good bacteria (probiotics), such as those from the Lactobacillus and Bifidobacterium genera can help restore the correct bacterial balance. Prebiotics are nondigestible food carbohydrates that can enter the large intestine and act as nutritional supplements to stimulate the growth of certain intestinal bacteria. At present, a synbiotic formulation, consisting of a mixture of the above selected strains, oligosaccharides as prebiotic ingredients, glutamin, vitamin B6 and zinc, has been developed. The most used and already marketed synbiotics regard mixtures of oligofructose, fructo-oligosaccharides, galacto-oligosaccharides, with probiotic bacterial strains of Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus rhamnosus, Bifidobacterium bifidum, or Bifidobacterium lactis.
Prebiotics and lactic acid bacteria (probiotics) have demonstrated beneficial effects with respect to the function of innate immunity, intestinal barrier function, and increased resistance to disease. The gut mucosa and microbiota are intimately linked in the maintenance of a functional interface between the host and the external environment. The hope is that a combined supply of prebiotics and probiotics (synbiotics) shall have synergistic effects in enhancing immunity and facilitating intestinal barrier function.20,50
5.11. Potassium
When blood potassium levels exceed the normal range of 3.6–5.2 mmol/L, a condition called hyperkalemia develops. A high potassium level can lead to widespread muscle fatigue and weakness. If left untreated, it can cause muscle paralysis and potentially fatal problems with heart rhythm. The elderly are particularly at risk for hyperkalemia. High-potassium foods are often common components of an elderly person’s regular diet. Milk is a main source of calcium, but also provides plenty of potassium. For elderly patients with dental problems, high-potassium yogurt, boiled potatoes, tomatoes, and bananas are soft and easy to eat. Many older people also favor prunes or raisins, which are also very high in potassium. Additional dietary sources of potassium include meat, fish, broccoli, lima beans, citrus fruits, and apricots.51
However, potassium is an essential mineral nutrient that plays a number of critical roles in the body. Research has shown that diets high in fruits and vegetables are associated with a reduced risk of hypertension (high blood pressure), which may be due to the beneficial effects of dietary potassium on blood pressure.52 Low potassium levels produce an increased risk of death or hospitalization in patients with heart failure and chronic kidney disease.
5.12. Whey protein
Whey protein is an important protein constituent of milk that may have health promoting properties. A recent study in elderly men found that consumption of a test meal that contained higher amounts of whey protein (35 g vs. 10 g) was associated with increased amino acid absorption and an increase in muscle protein synthesis.53 The same group had previously observed that feeding whey protein to elderly men had a more positive impact on muscle synthesis rates than feeding casein, the other major milk protein, as a protein source.54 These findings suggest that feeding whey protein may have a beneficial effect on building muscle mass in the elderly, which would be important because aging is associated with a loss of lean body mass (sarcopenia), which is an important cause of frailty and disability.55
Whey proteins are considered to have the highest nutritional values of all food proteins. They contain all the amino acids required by humans, in the right proportions. Whey proteins are rich in branched chain amino acids, components that provide energy for people undergoing intense or prolonged periods of exercise and help prevent loss of body mass and muscle. They are also readily digestible and completely bioavailable. Whey proteins supply additional nutritional benefits; for example, α-lactalbumin, the second most abundant whey protein, has a high content of the amino acid tryptophan, a precursor of the vitamin niacin.56,57
The health and nutritional value of the components of whey include: high quality nutritional source of amino acids; antimicrobial action; growth enhancement of beneficial gut microflora, such as bifidobacteria; immunoenhancing properties; control of specific diseases, including cancer; and antitoxin activity.57,58
5.13. Zinc
Zinc is an essential trace element that is biochemically involved in a wide variety of reactions and has important effects on DNA synthesis, cell proliferation, and differentiation. The trace element zinc is essential for the immune system, and zinc deficiency affects multiple aspects of innate and adaptive immunity. There are remarkable parallels in the immunological changes during aging and zinc deficiency, including a reduction in the activity of the thymus and thymic hormones, a shift of the T helper cell balance toward T helper type 2 cells, decreased response to vaccination, and impaired functions of innate immune cells. Many studies confirm a decline of zinc levels with age. Most of these studies do not classify the majority of elderly as zinc deficient, but even marginal zinc deprivation can affect immune function. Immune function is compromised in zinc deficiency59 and zinc supplementation along with antioxidants may play a role in protecting people from macular degeneration.60 Studies have shown that normal serum zinc concentrations in nursing home elderly were associated with decreased incidence and duration of pneumonia, and decreased use and duration of antimicrobial therapy. Zinc supplementation to maintain normal serum zinc concentrations in the elderly may help reduce pneumonia incidence and associated morbidity.61 Consequently, oral zinc supplementation demonstrates the potential to improve immunity and efficiently downregulates chronic inflammatory responses in the elderly. These data indicate that a wide prevalence of marginal zinc deficiency in elderly people may contribute to immuno-senescence.62
5.14. Coenzyme Q10
Coenzyme Q10 is a vitamin-like compound that plays an important role in aerobic respiration in the mitochondria of the cell and is involved in the generation of ATP, which is used as an energy source by the cell. Coenzyme Q10 is also a powerful antioxidant that can reduce oxidative stress. Supplementation with coenzyme Q10 has also been found to have an anti-inflammatory effect by reducing the inflammatory marker IL-6 in patients with coronary artery disease.63 Another study has found that coenzyme Q10 supplementation improves endothelial function in patients with heart disease.64 Coenzyme Q10 may also be implicated in cancer because a recent study in Chinese women observed that low plasma coenzyme Q10 levels are associated with an increased risk of breast cancer.65 It has also been shown that coenzyme Q10 affects creatine kinase activity and mood in geriatric bipolar depression.66
6. Supplementary dietary botanicals for geriatrics
6.1. Ginkgo biloba for neuroprotection
Ginkgo is widely known for its health benefits for the elderly population in Chinese medicine.67 Extract of Ginkgo biloba is well known to prevent Alzheimer’s disease (AD). The herbal extract EGb761 prepared from G. biloba is rich in phytochemicals such as flavonoids and terpenoids. These phytonutrients improve the functions of platelets and nerve cells and the blood flow to the nervous system and brain, due to their antioxidant properties.68
6.2. Vaccinium corymbosum (blueberry) for lifespan extension
Blueberries (Vaccinium corymbosum) are one of the richest sources of antioxidants. They contain a wide array of polyphenols that offer a variety of health benefits.69 Antioxidants optimize health by helping to combat the free radicals that can damage cellular structures as well as DNA. Animal studies have shown that supplementation of blueberry preserves learning and memory in experimental animals by improving neuronal function. Studies in Caenorhabditis elegans have also shown that polyphenols present in blueberries significantly increased the lifespan by decreasing age-related accumulation of the intracellular level of lipofuscin, a biomarker for age-related cellular damage, and reduces the level of 4-hydroxynonenal, a biomarker for lipid peroxidation.70 In addition, blueberry polyphenols improve the pharyngeal pumping rates of aged worms and increase thermo-tolerance, thereby improving the worms’ health span. A recent study has demonstrated that blueberry extract extends mean lifespan by approximately 10% in Drosophila.71 These research findings in invertebrates suggest the pivotal role of blueberries in mediating lifespan extension.
6.3. Cranberry and oregano for longevity promotion
Cranberry (Vaccinium oxycoccos) and oregano (Origanum vulgare) possess multiple medicinal properties, such as antimicrobial, antiviral, antimutagenic, antiangiogenic, and antioxidative functions.72 Scientists have shown that a mixture of oregano and cranberry (OC) extract increased lifespan in Mexican fruit flies (mexfly) in a diet composition dependent manner.73 In addition, OC supplementation in middle age was sufficient to promote longevity.74 However, lifespan was not increased when OC was supplemented only in young age or old age. These findings point out the importance of considering diet composition and implementing time in developing an efficacious aging intervention.
Scientists have also assessed the effect of cranberry extract alone on lifespan and health-span in C. elegans. The data indicate that the cranberry extract alone is sufficient to prolong lifespan in C. elegans.75 Cranberries are high in antioxidants and phytochemicals, including proanthocyanidins and vitamin C, which may neutralize free radicals and reduce oxidative damage, and, more importantly, modulate signaling transduction pathways.69,76
6.4. Nectarine and acai for life span extension
Nectarine (Prunus persica) is a globally consumed fruit77 and acai (Euterpe oleracea) is a fruit indigenous to the Amazon River area.78 Both fruits contain various kinds of bioactive phytochemicals.79,80 Studies have shown that nectarine supplementation can extend lifespan in flies.
There are several scientific studies carried out on experimental animals that show the role of nectarine and acai in extension of longevity of life. Research has shown that the lifespan extension induced by nectarine is associated with increased lifetime reproductive output and reduced lipid oxidation.81 In contrast, supplementation of acai pulp promotes survival in Drosophila fed with a high-fat but not a standard diet.82 The diet composition dependent effect of acai is also evident in the mexfly. Acai supplementation promotes the survival of the mexfly fed a high-fat and high sugar diet but not other nonfat diets.83 Along with the OC study described above, the importance of diet composition is also evident in aging intervention studies using pharmacological agents, such as resveratrol. Studies in Drosophila, mexfly, and mice have shown that the prolongevity effects of resveratrol depend on diet composition.84 These studies again stress the importance of diet composition in modulating the health benefits of nutraceuticals. Moreover, both nectarine and acai can promote the survival of flies with sod1 deficiency. Flies deficient in sod1 have a short lifespan and experience high levels of oxidative damage. These findings suggest that nectarine and acai possess antioxidant activities at the organism level.83,85
6.5. Rosa damascena for life span extension
A hybrid rose species, Rosa damascena, is well known for its use as rose oil and water in cosmetic and food industries.86 It is well documented that the extracts from R. damascene contains numerous volatile organic compounds including various terpenes such as citronellol, heneicosane, and disiloxane, and polyphenols, such as quercetin, myricetin, kaempferol, and gallic acid.87 R. damascena extracts have been shown to possess biological properties that are protective against microbial infection, seizures in rats, and toxicity of amyloid beta in neurons, a biomarker of AD.88,89 An R. damascena extract has been found to increase both mean and maximum lifespan in Drosophila. This extract also enhances flies’ resistance to oxidative stress and low iron stress. It has been proposed that R. damascena extract extends lifespan by protecting flies against iron-induced stress.
6.6. Cocoa polyphenols and longevity effect
Numerous polyphenols with high antioxidant activities, such as flavonoids, have been isolated from cocoa. Research has shown that flavonoid-enriched cocoa powder reduces oxidative stress in C. elegans.90 Cocoa polyphenols may promote longevity by reducing oxidative stress, influencing metabolism, and altering chromatin structure.
6.7. Green tea as health supplement
Green tea contains polyphenolic catechins that have been reported to have a number of health benefits, including prevention of AD, Parkinson’s disease, and heart disease.91 Green tea can protect against angiogenesis and tumor formation.92 The health benefits of green tea are due to bioactive properties of its phytochemical constituents. Green tea contains a number of polyphenolic catechins, such as epicatechin, epicatechin-3-gallate, epigallocatechin, EGCG, catechin, and gallocatechin.91 Among these, EGCG is perhaps the most abundant catechin in green tea, and has been reported to induce antioxidant enzymes, including glutathione peroxidase, catalase, and glutathione S-transferase, in mice. In addition, a recent study demonstrated that l-theanine, a unique amino acid particularly present in green tea promotes the survival of C. elegans in the presence of paraquat.93 It has been reported that l-theanine provides broad health benefits, such as antitumor, AD prevention, and blood pressure reduction.94,95 These findings suggest that green tea increases lifespan and stress resistance partially through its antioxidant properties.
6.8. Olive oil as a phenolics supplement
Scientific studies have shown that consumption of olive oil has beneficial effects on health and longevity in humans.96,97 This is due to the abundance of phenolic compounds present in olive oil. Preclinical studies have revealed that the tyrosol, one of the most abundant phenols in olive oil,98 significantly promotes the longevity of C. elegans, and also resistance to thermal and oxidative stress.99
6.9. HSP-12.6 for protein homeostasis
It has been reported that small heat shock proteins, including HSP-12.6, can extend lifespan and delay polyglutamine protein aggregation in C. elegans.100 Heat shock factor protein 1 is critical for maintaining protein homeostasis. Experimental studies suggest that tyrosol in olive oil extends lifespan by increasing oxidative stress resistance and thermo-tolerance as well as improves protein homeostasis.99
6.10. Quercetin and tannic acid as health supplement
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is one of the most important dietary flavonoids present in a wide array of foods such as fruits and vegetables and has numerous health benefits.101 Preclinical studies have shown that quercetin increases the lifespan of C. elegans.102,103 However, studies on the molecular basis have yielded conflicting results and the mechanisms underlying the prolongevity effect of quercetin are still unknown.
Tannic acid (TA) belongs to tannins, which are secondary metabolites of plants with many health benefits. TA possesses many phytochemicals that prevent neurodegeneration,104 pathogen infection,105 carcinogenesis, and oxidative damage.106 In an experimental study, it was found that TA significantly increases the lifespan of worms.102
Transcriptome studies indicate that quercetin affects expression of genes in the TGF-β signaling, insulin-like signaling, and p38 MAPK pathways, while TA changes expressions of genes in the TGF-β and the p38 MAPK pathways as well as the amino acid metabolism. Together, these studies suggest that TGF-β and p38 MAPK pathways play crucial roles in mediating the prolongevity effects of quercetin and TA.107
6.11. Caffeic acid and rosmarinic acid for lifespan extension
Caffeic acid [3-(3,4-dihydroxyphenyl)-2-propenoic acid; CA] and rosmarinic acid (α-o-caffeoyl-3, 4-dihydroxyphenyl lactic acid; RA) are abundantly present in a variety of fruits, vegetables, and herbs. CA and RA have anticarcinogenesis, antioxidant, antimicrobial, anti-inflammatory, and antirheumatic properties. CA and RA can prolong the healthy lifespan of C. elegans.108 Similar results have been obtained for CA mediated lifespan extension. The research findings suggest that CA and RA promote lifespan extension through overlapping pathways involved in metabolism and stress response.
6.12. Spermidine for prolonging lifespan
Spermidine is a type of polyamine present in citrus fruits and soybean, and has effects on epigenetic modifications, autophagy and necrosis.109,110 Polyamine concentrations and autophagy have been shown to decline in various organisms, including humans.111 Preclinical studies have shown that supplementation of spermidine prolongs the lifespan of C. elegans and Drosophila by 15% and 30%, respectively.109 More research is needed to see if spermidine and its derivatives can confer lifespan extension in humans by, at least in part, enhancing autophagy.
6.13. Curcumin and thioflavin T as health supplements
Curcumin (diferuloylmethane) is the pharmacologically active substance in turmeric (Curcuma longa), and has been widely used as an herbal medicine in Asia. It is well documented that curcumin possesses many biological activities, such as antioxidative, anti-inflammatory, anticancer, chemopreventive, and antineurodegenerative properties.112,113 With its pleiotropic activities, curcumin has been considered as a potential aging intervention compound. Studies in Drosophila and C. elegans have demonstrated that curcumin can delay aging and prolong the lifespan.114,115 Curcumin-treated flies exhibited enhanced resistance against oxidative stress, improved locomotor activity, and higher tolerance to chemotherapy drugs.
6.14. Formulation challenges
There are several formulation challenges while attempting to design fortified functional foods for geriatric individuals. Some of the issues include possible chemical interactions between nutrient ingredients, issues related to final product acceptance, product stability, taste and texture concerns, and product shelf-life.116
In the development of nutraceuticals for geriatrics, manufacturers should take into consideration that aging is associated with some notable physiological changes, including the loss of taste. Therefore, the incorporation of flavor enhancers and textual considerations should be carefully addressed in premix development for these products. As the taste of the product is of paramount importance for a product to be successful, it is important to pay close attention to flavor intensity, masking any off notes, and addition of colorants. Products can be flavored with herbs and spices, and a number of other ingredients can be included in a premix or finished product to intensify product color or enhance texture to increase product appeal. Additionally, as consumers age healthfully, many are more likely to be taking medications to address certain health conditions, so formulators need to consider potential interactions with common medicines. For example, within the juice category, the interaction between grapefruit juice and some immunosuppressant drugs (statins) used to lower blood cholesterol, and calcium-channel blockers used to treat high blood pressure would suggest that more attention should be placed on fortifying other types of juice applications for this population.116
7. Conclusion
From the above review it is evident that nutraceuticals made from widely-consumed plant products promote longevity, improve health-span, and protect against aging and stress. The diet composition-dependent effects will have a significant impact on the increasing demand for personalized nutritional intervention. However, the individual and synergistic effects of nutraceuticals as a component of dietary composition will require further study and scientific scrutiny. Some nutraceuticals and their synthetic derivatives are being tested for their therapeutic potential. Numerous promising results have been obtained in model organisms that suggest evolutionarily conserved mechanisms are involved in their beneficial effects. Much progress has been made to decipher the molecular mechanisms of aging shared among multiple species, which provide valuable guidance for aging interventions. However, further extensive studies will be required to demonstrate whether any nutraceuticals or pharmaceuticals can effectively delay aging or age-related disease in humans.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Rattan S.I.S., Sejersen H., Fernandes R.A., Luo W. Stress-mediated hormetic modulation of aging, wound healing, and angiogenesis in human cells. Ann N Y Acad Sci. 2007;1119:112–121. doi: 10.1196/annals.1404.005. [DOI] [PubMed] [Google Scholar]
- 2.De Luca d'Alessandro, Bonacci S., Giraldi G. Aging populations: the health and quality of life of the elderly. Clin Ter. 2011;162:e13–e18. [PubMed] [Google Scholar]
- 3.Fontana L., Partridge L., Longo V.D. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haigis M.C., Yankner B.A. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alic N., Partridge L. Death and dessert: nutrient signaling pathways and ageing. Curr Opin Cell Biol. 2011;23:738–743. doi: 10.1016/j.ceb.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon A.B., Richardson A., Pérez V.I. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Hazen R. March 2014. Strategic nutrition for healthy ageing.http://www.fortitechpremixes.com/wp-content/uploads/2014/02/Healthy_Aging_EN.pdf Technical Paper. Available at: [Google Scholar]
- 9.Sharma G., Prakash D., Gupta C. Phytochemicals of nutraceutical importance: do they defend against diseases? In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 1–19. [Google Scholar]
- 10.Sharma R. Nutraceuticals and nutraceutical supplementation criteria in cancer: a literature survey. Open Nutraceut J. 2009;2:92–106. [Google Scholar]
- 11.Kennedy D.O., Wightman E.L. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2:32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salminen A., Kauppinen A., Kaarniranta K. Phytochemicals suppress nuclear factor-kappaB signaling: impact on health span and the aging process. Curr Opin Clin Nutr Metab Care. 2012;15:23–28. doi: 10.1097/MCO.0b013e32834d3ae7. [DOI] [PubMed] [Google Scholar]
- 13.Prakash D., Gupta C. Role of antioxidant polyphenols in nutraceuticals and human health. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 208–228. [Google Scholar]
- 14.Obrenovich M.E., Li Y., Parvathaneni K. Antioxidants in health, disease and aging. CNS Neurol Disord Drug Targets. 2011;10:192–207. doi: 10.2174/187152711794480375. [DOI] [PubMed] [Google Scholar]
- 15.Kidd P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]
- 16.Katalinic V., Milos M., Kulisic T., Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- 17.Prakash D., Kumar N. Cost effective natural antioxidants. In: Watson R.R., Gerald J.K., Preedy V.R., editors. Nutrients, Dietary Supplements and Nutraceuticals. Humana Press; New York: 2011. pp. 163–188. [Google Scholar]
- 18.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Critical Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 19.Masson L. Phenolic acids as natural antioxidants. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 196–207. [Google Scholar]
- 20.Gupta C., Prakash D., Gupta S. Relationships between bioactive food components and their health benefits. In: Martirosyan D.M., editor. Introduction to Functional Food Science Textbook. CreateSpace Independent Publishing Platform; North Charleston: 2013. pp. 66–85. [Google Scholar]
- 21.Ma L., Dou H.L., Huang Y.M. Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol. 2012;154 doi: 10.1016/j.ajo.2012.04.014. 625–634 e1. [DOI] [PubMed] [Google Scholar]
- 22.Ma L., Dou H.L., Wu Y.Q. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. 2012;107:350–359. doi: 10.1017/S0007114511004260. [DOI] [PubMed] [Google Scholar]
- 23.Story E.N., Kopec R.E., Schwartz S.J., Harris G.K. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash D., Gupta C. Carotenoids: chemistry and health benefits. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 181–195. [Google Scholar]
- 25.Jackson H., Braun C.L., Ernst H. The chemistry of novel xanthophyll carotenoids. Am J Cardiol. 2008;101:50D–57D. doi: 10.1016/j.amjcard.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Fassett R.G., Coombes J.S. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011;9:447–465. doi: 10.3390/md9030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clifton P. Lowering cholesterol: a review on the role of plant sterols. Aust Fam Phys. 2009;38:218–221. [PubMed] [Google Scholar]
- 28.Masson L. Phytosterols and their healthy effects. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 173–180. [Google Scholar]
- 29.Musa-Veloso K., Poon T.H., Elliot J.A., Chung C. A comparison of the LDL-cholesterol lowering efficacy of plant stanols and plant sterols over a continuous dose range: results of a meta-analysis of randomized, placebo-controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2011;85:9–28. doi: 10.1016/j.plefa.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.M., Kim S.W., Shin I.S. Folate, vitamin b(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig. 2008;5:36–40. doi: 10.4306/pi.2008.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R.L., Vishwakarma S.P., Singh P. Vitamins and minerals: roles and plant sources. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 310–323. [Google Scholar]
- 32.Rejnmark L., Avenell A., Masud T. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metabol. 2012;97:2670–2681. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Hwang B.S., Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masson L. Omega-3 and omega-6 fatty acids in human health. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 116–131. [Google Scholar]
- 35.Yokoyama M., Origasa H., Matsuzaki M. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K., Ishikawa Y., Yokoyama M. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: sub-analysis of the JELIS trial. Stroke. 2008;39:2052–2058. doi: 10.1161/STROKEAHA.107.509455. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D., Lemaitre R.N., King I.B. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–170. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chajès V., Torres-Mejia G., Biessy C. Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev. 2012;21:319–326. doi: 10.1158/1055-9965.EPI-11-0896. [DOI] [PubMed] [Google Scholar]
- 39.Manson J.E., Bassuk S.S., Lee I.M. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christen W.G., Schaumberg D.A., Glynn R.J., Buring J.E. Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol. 2011;129:921–929. doi: 10.1001/archophthalmol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Food Agriculture Organization (FAO) FAO of the United Nations; Rome: 2010. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO, Food Nutrition Paper 91. [PubMed] [Google Scholar]
- 42.Elmadfa I., Kornstainer M. Fats and fatty acids requirements for adults. Ann Nutr Metabol. 2009;55:57–75. doi: 10.1159/000228996. [DOI] [PubMed] [Google Scholar]
- 43.Kanzaki N., Saito K., Maeda A. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled study. J Sci Food Agric. 2012;92:862–869. doi: 10.1002/jsfa.4660. [DOI] [PubMed] [Google Scholar]
- 44.Benito-Ruiz P., Camacho-Zambrano M.M., Carrillo-Arcentales J.N. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int J Food Sci Nutr. 2009;60(suppl 2):99–113. doi: 10.1080/09637480802498820. [DOI] [PubMed] [Google Scholar]
- 45.Bruyere O., Pavelka K., Rovati L.C. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomized, placebo-controlled trials. Osteoarthritis Cartilage. 2008;16:254–260. doi: 10.1016/j.joca.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Hubbard G.P., Wolffram S., Lovegrove J.A., Gibbins J.M. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 47.Donini L.M., Savina C., Cannella C. Nutrition in the elderly: role of fiber. Arch Gerontol Geriatr. 2009;49(suppl 1):61–69. doi: 10.1016/j.archger.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Yang J., Wang H.P., Zhou L., Xu C.F. Effect of dietary fiber on constipation: a meta analysis. World J Gastroenterol. 2012;18:7378–7383. doi: 10.3748/wjg.v18.i48.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hempel S., Newberry S.J., Maher A.R. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 50.Gupta C., Prakash D., Rostagno M.H., Callaway T.R. Synbiotics: promoting gastro-intestinal health. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 61–78. [Google Scholar]
- 51.Perazella M.A., Mahnensmith R.L. Drugs exacerbate impaired potassium homeostasis. J Gen Intern Med. 1997;12:646–656. doi: 10.1046/j.1525-1497.1997.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fotherby M.D., Potter J.F. Long-term potassium supplementation lowers blood pressure in elderly hypertensive subjects. Int J Clin Pract. 1997;51:219–222. [PubMed] [Google Scholar]
- 53.Pennings B., Groen B., de Lange A. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 54.Pennings B., Boirie Y., Senden J.M., Gijsen A.P., Kuipers H., van Loon L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 55.Morley J.E. Sarcopenia in the elderly. Fam Pract. 2012;29(suppl 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 56.Somaye F., Marzieh M.N., Lale N. 18th National Congress on Food Technology. October 15–16, 2008. Single cell protein (SCP) production from UF cheese whey by Kluyveromyces marxianus. [Google Scholar]
- 57.Gupta C., Prakash D., Garg A.P., Gupta S. Whey proteins: a novel source of bioceuticals. Middle-East J Sci Res. 2012;12:365–375. [Google Scholar]
- 58.Thomä-Worringer C., Sørensen J., López-Fandiño R. Health effects and technological features of caseinomacropeptide. Int Dairy J. 2006;16:1324–1333. [Google Scholar]
- 59.Prasad A.S. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 60.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meydani S.N., Barnett J.B., Dallal G.E. Serum zinc and pneumonia in nursing home elderly. Am J Clin Nutr. 2007;86:1167–1173. doi: 10.1093/ajcn/86.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haase H., Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee B.J., Huang Y.C., Chen S.J., Lin P.T. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012;28:250–255. doi: 10.1016/j.nut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Dai Y.L., Luk T.H., Yiu K.H. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Cooney R.V., Dai Q., Gao Y.T. Low plasma coenzyme Q (10) levels and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2011;20:1124–1130. doi: 10.1158/1055-9965.EPI-10-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forester B.P., Zuo C.S., Ravichandran C. Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J Geriatr Psychiatry Neurol. 2012;25:43–50. doi: 10.1177/0891988712436688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu T.Y., Chen C.P., Jinn T.R. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50:131–135. doi: 10.1016/j.tjog.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Abdel-Wahab B.A., Abd El-Aziz S.M. Ginkgo biloba protects against intermittent hypoxia-induced memory deficits and hippocampal DNA damage in rats. Phytomedicine. 2012;19:444–450. doi: 10.1016/j.phymed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Neto C.C. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 70.Wilson M.A., Shukitt-Hale B., Kalt W., Ingram D.K., Joseph J.A., Wolkow C.A. Blueberry polyphenols increase lifespan and thermo-tolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng C., Zuo Y., Kwan K.M. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp Gerontol. 2012;47:170–178. doi: 10.1016/j.exger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Babili F.E., Bouajila J., Souchard J.P. Oregano: chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J Food Sci. 2011;76:C512–C518. doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 73.Zou S., Carey J.R., Liedo P., Ingram D.K., Yu B., Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol. 2010;65:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou S., Carey J.R., Liedo P., Ingram D.K., Yu B., Ghaedian R. Prolongevity effects of a botanical with oregano and cranberry extracts in Mexican fruit flies: examining interactions of diet restriction and age. Age. 2012;34:269–279. doi: 10.1007/s11357-011-9230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen E., Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nature Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X., Rui H.L. Cranberry phytochemicals: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2006;54:7069–7074. doi: 10.1021/jf061058l. [DOI] [PubMed] [Google Scholar]
- 77.Konopacka D., Jesionkowska K., Kruczyńska D. Apple and peach consumption habits across European countries. Appetite. 2010;55:478–483. doi: 10.1016/j.appet.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Schauss A.G., Wu X., Prior R.L. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (Acai) J Agric Food Chem. 2006;54:8598–8603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- 79.Pizza V., Agresta A., Acunto C.W.D., Festa M., Capasso A. Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets. 2011;10:621–634. doi: 10.2174/187152711796235014. [DOI] [PubMed] [Google Scholar]
- 80.Fontana L. Modulating human aging and age-associated diseases. Biochim Biophys Acta. 2009;1790:1133–1138. doi: 10.1016/j.bbagen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyd O., Weng P., Sun X. Nectarine promotes longevity in Drosophila melanogaster. Free Rad Biol Med. 2011;50:1669–1678. doi: 10.1016/j.freeradbiomed.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun X., Seeberger J., Alberico T. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liedo P., Carey J.R., Ingram D.K., Zou S. The interplay among dietary fat, sugar, protein and acai (Euterpe oleracea Mart.) pulp in modulating lifespan and reproduction in a Tephritid fruit fly. Exp Gerontol. 2012;47:536–539. doi: 10.1016/j.exger.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zou S., Carey J.R., Liedo P. The prolongevity effect of resveratrol depends on dietary composition and calorie intake in a tephritid fruit fly. Exp Gerontol. 2009;44:472–476. doi: 10.1016/j.exger.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piper M.D.W., Bartke A. Diet and aging. Cell Metabol. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Hongratanaworakit T. Relaxing effect of rose oil on humans. Nat Prod Commun. 2009;4:291–296. [PubMed] [Google Scholar]
- 87.Kumar N., Bhandari P., Singh B., Gupta A.P., Kaul V.K. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J Sep Sci. 2008;31:262–267. doi: 10.1002/jssc.200700372. [DOI] [PubMed] [Google Scholar]
- 88.Shokouhinejad N., Emaneini M., Aligholi M., Jabalameli F. Antimicrobial effect of Rosa damascena extract on selected endodontic pathogens. J Calif Dent Assoc. 2010;38:123–126. [PubMed] [Google Scholar]
- 89.Awale S., Tohda C., Tezuka Y., Miyazaki M., Katoda S. Protective effects of Rosa damascena and its active constituent on Aβ(25–35)-induced neuritic atrophy. Evid Based Complement Alternat Med. 2011;2011:131042. doi: 10.1093/ecam/nep149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martorell P., Forment J.V., De Llanos R. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J Agric Food Chem. 2011;59:2077–2085. doi: 10.1021/jf104217g. [DOI] [PubMed] [Google Scholar]
- 91.Zaveri N.T. Green tea and its polyphenolic catechins: medicinal uses in cancer and non-cancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 92.Sagar S.M., Yance D., Wong R.K. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-part 1. Curr Oncol. 2006;13:14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zarse K., Jabin S., Ristow M. L-Theanine extends lifespan of adult Caenorhabditis elegans. Eur J Nutr. 2012;51:765–768. doi: 10.1007/s00394-012-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryan J. Psychological effects of dietary components of tea: caffeine and L-theanine. Nutr Rev. 2008;66:82–90. doi: 10.1111/j.1753-4887.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- 95.Kim T.I., Lee Y.K., Park S.G. l-Theanine, an amino acid in green tea, attenuates β-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-κB pathways. Free Radic Biol Med. 2009;47:1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Lagiou P., Trichopoulos D., Sandin S. Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr. 2006;96:384–392. doi: 10.1079/bjn20061824. [DOI] [PubMed] [Google Scholar]
- 97.Buckland G., Agudo A., Travier N. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br J Nutr. 2011;106:1581–1591. doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
- 98.Bendini A., Cerretani L., Carrasco-Pancorbo A. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods: An overview of the last decade. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cañuelo A., Gilbert-López B., Pacheco-Liñán P., Martínez-Lara E., Siles E., Miranda-Vizuete A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech Ageing Dev. 2012;133:563–574. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Hsu A.L., Murphy C.T., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 101.Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 102.Saul N., Pietsch K., Menzel R., Stürzenbaum S.R., Steinberg C.E.W. The longevity effect of tannic acid in Caenorhabditis elegans: disposable soma meets hormesis. J Gerontol. 2010;65:626–635. doi: 10.1093/gerona/glq051. [DOI] [PubMed] [Google Scholar]
- 103.Surco-Laos F., Cabello J., Gómez-Orte E. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011;2:445–456. doi: 10.1039/c1fo10049a. [DOI] [PubMed] [Google Scholar]
- 104.Yazawa K., Kihara T., Shen H., Shimmyo Y., Niidome T., Sugimoto H. Distinct mechanisms underlie distinct polyphenol-induced neuroprotection. FEBS Lett. 2006;580:6623–6628. doi: 10.1016/j.febslet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Kim T.J., Silva J.L., Jung Y.S. Antibacterial activity of fresh and processed red muscadine juice and the role of their polar compounds on Escherichia coli O157:H7. J Appl Microbiol. 2009;107:533–539. doi: 10.1111/j.1365-2672.2009.04239.x. [DOI] [PubMed] [Google Scholar]
- 106.Andrade R.G., Jr., Ginani J.S., Lopes G.K.B., Dutra F., Alonso A., Hermes-Lima M. Tannic acid inhibits in vitro iron-dependent free radical formation. Biochimie. 2006;88:1287–1296. doi: 10.1016/j.biochi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Pietsch K., Saul N., Swain S.C., Menzel R., Steinberg C.E., Stürzenbaum S.R. Meta-analysis of global transcriptomics suggests that conserved genetic pathways are responsible for quercetin and tannic acid mediated longevity in. C. elegans. Front Genet. 2012;3:48. doi: 10.3389/fgene.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pietsch K., Saul N., Chakrabarti S., Stürzenbaum S.R., Menzel R., Steinberg C.E.W. Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology. 2011;12:329–347. doi: 10.1007/s10522-011-9334-7. [DOI] [PubMed] [Google Scholar]
- 109.Eisenberg T., Knauer H., Schauer A. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 110.Morselli E., Mariño G., Bennetzen M.V. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prakash D., Gupta C. Phytochemicals of nutraceutical importance from Curcuma longa L. and their role in human health. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI International Publishers; Wallingford: 2014. pp. 266–287. [Google Scholar]
- 114.Lee K.S., Lee B.S., Semnani S. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13:561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 115.Liao V.H., Yu C.W., Chu Y.J., Li W.H., Hsieh Y.C., Wang T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 2011;132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Dong Y., Guha S., Sun X., Cao M., Wang X., Zou S. Nutraceutical Interventions for Promoting Healthy Aging in Invertebrate Models. Oxid Med Cell Longev. 2012;2012:718491. doi: 10.1155/2012/718491. [DOI] [PMC free article] [PubMed] [Google Scholar]


