Abstract
This study aimed to verify the effectiveness of an overall health care program (OHCP) for middle-aged Japanese women through assessing physical and physiological changes. The OHCP consisted of diet modification with natural alternative foods, walking and stretching exercises, and body massage and cupping treatments. Sixty-seven participants were assigned to one of three groups during a 3-year study period (2011–2013). The OHCP was performed for 3 months in each year. After the OHCP, most participants had significant decreases in the blood levels of triglycerides, low-density lipoprotein cholesterol, total cholesterol, alkaline phosphatase, γ-glutamyl transferase, and cholinesterase; body weight; body fat percentage; and body-mass index. The oxidative stress markers varied among the study years; however, a significant decrease in blood reactive oxygen-derived metabolites and a significant increase in the relative antioxidative potential were observed in 2013. In 2013, participants who were randomly selected for autonomic nervous activity measurements immediately before and after body massage and cupping treatments showed a significant predominance in parasympathetic nervous activity after the treatments. These results indicate that the OHCP in the present study is an effective and prompt method as a complementary treatment to improve the pre-obese or mild obese status without any noticeable physiological stress in most middle-aged women. However, because of the limitations of this study, the findings of this study need to be confirmed.
Keywords: autonomic nervous function, blood examination, body-mass index, middle-aged women, physiological stress
1. Introduction
In general, many middle-aged women tend to have lifestyle-related diseases (e.g., hyperlipidemia, diabetes, and obesity) with contributing factors such as insufficient exercise, poor quality of food intake, psychogenic stress, and endocrine system dysfunction or imbalance. Changes in the body shape and psychophysiological state that are accompanied by obesity lead to a loss of self-confidence and in decreased motivation to enjoy family life and social activities. Such undesirable changes in lifestyle lead to aging-related changes and poor health. Improving body weight, body-mass index (BMI), and body fat percentage (BFP) are important measures for promoting health care in middle-aged overweight women. However, an inappropriate health care program can lead to unfavorable changes in the physiological conditions of individuals or patients.1 Therefore, a careful program design is warranted. It is important to develop an ideal health care program with less physiological stress and to verify its effectiveness in middle-aged women at the “pre-obese” or “mild obese” stage before they become severely obese.
Many reports have been published on the effects of various health care programs or lifestyle in middle-aged women, and include physical exercise and dietary or nutritional conditions.2–8 Various methods for improving overweight-related health conditions have been proposed, although optimal health promotion should combine several types of treatments rather than focus only on one type of treatment. Including stress-reducing body treatments is also important for holistic health. Our OHCP is an integrated and complementary therapy for middle-aged women that has been performed every year for 10 years. The objective of an OHCP is weight control and promoting healthy condition inside the whole body. For this objective, the OHCP consisted of body treatments with cupping and massage, walking and stretching exercises, and diet treatments with special meals. To examine functional changes inside the body, we measured physiological parameters such as blood biochemical profiles and body composition in the study participants from 2011 to 2013. We performed the present study to clarify whether the proposed OHCP is a prompt and ideal method as a treatment in middle-aged Japanese women at the pre-obese or mild obese stage.
2. Materials and methods
2.1. Participants
Sixty-seven middle-aged women participated in the 3-year study (2011–2013). All participants arbitrarily applied to the overall health care program (OHCP), provided by Slim Beauty House Company (Shibuya Ward, Tokyo, Japan). Before the study, each participant provided informed, written consent. The OHCP included cupping treatments of the body surface, body massage, walking exercise, stretching, and dietary supplements that were based in Chinese herbal medicine. Of the 67 women, 17 women, 20 women, and 30 women participated in 2011, 2012, and 2013, respectively. Seventeen participants through the 3-year study were categorized as “obese” with a BMI >25 (range, 25.2–29.5). The BMI was calculated by the following equation: BMI = body weight (kg)/height2 (m2).
To avoid accidental health risks during OHCP, only participants who did not have underlying diseases such as allergies, serious inflammation or injury, scoliosis, low back pain, and hernia were allowed into the study. For this reason, all participants were required to provide a health certificate and receive interviews about their health conditions before undergoing the OHCP.
2.2. Contents of the OHCP
The research period of the OHCP was conducted for 3 months (from late July to early November) in each year of 2011–2013. The OHCP consisted of the health care treatments described below.
2.2.1. Cupping and vibration treatment
2.2.1.1. Cupping treatment
While the participant lay prone on a bed, 21 body surface regions were locally suctioned by a cupping cup and electrically powered suction equipment (Minipon; Origin Medical Instruments Co., Ltd., Tokyo, Japan). Cupping was performed with negative pressure of 66.5 kPa on 20 regions that were arranged symmetrically across the back midline from the upper scapular region to the lower limb and one region on the lumbar midline. A professional operator performed the cupping maneuver on each participant for approximately 15–20 minutes.
2.2.1.2. Vibration treatment
Each body portion from the shoulder to the foot of the participants was vibrated by a massage machine (Hot-viter VR-303; Meiko Tsusho, Tokyo, Japan) with an oscillation frequency of 50–60 Hz. In 2011 and 2012, participants were allowed to rent a similar type of vibration apparatus and use it in their own home whenever they wanted to use it. In 2013, the participants were not allowed to use this apparatus in their home.
2.2.2. Walking exercise
Participants were instructed to walk as daily exercise. For each study group, the total number of walking days was 78–91 days (mean, 87.0 days), 78–93 days (mean, 90.0 days) and 83–96 days (mean, 91.4 days) in 2011, 2012, and 2013, respectively. The daily average walking time was 34.4 minutes, 42.9 minutes, and 36.0 minutes in 2011, 2012, and 2013, respectively. There was no statistically significant difference (p > 0.05) between these walking times. In addition, there was no significant difference (p > 0.05) in total walking time between participants with a BMI <25 (mean, 3467 minutes; n = 50) and participants with BMI >25 (mean, 3149 minutes; n = 17).
2.2.3. Dietary supplements
Each subject was provided a special pack of alternative foods (i.e., “enzyme foods”) that contained more than 50 natural food components (Table 1). In the first month, two of three daily meals (i.e., breakfast and dinner) were replaced with the enzyme foods. In the second and third months, only the dinner meal was replaced with the enzyme foods. A 40-g pack of the enzyme foods contained 148–156 kcal. The enzyme foods were mixed with water or soy milk and consumed. Participants were instructed to consume approximately 1500 kcal per day.
Table 1.
Components of natural alternative “enzyme foods”.
| unpolished rice (15.8) | Plantago asiatica | corn |
| alfa- corn (9.5) | new leaves of barley | parsley |
| dry soy milk | cabbage | new leaves of wheat |
| barley | coix | Japanese mugwort |
| wheat | unpolished sticky rice | green tea- powder |
| alfa- unpolished rice | pine leaves | radish |
| soy protein (0.5) | apple | burdock |
| chicory fiber | shiitake mushroom | prune |
| erythritol | dried seaweed | Citrus junos |
| xylitol | kelp | Acanthopanax senticosus |
| rice bran | pumpkin | mulberry leaves |
| multi- grain malt | Spirulina | pomegranate extract |
| soy | green lavar | Garcinia cambogia |
| kale | yeast | black peas |
| cactus | Perilla | black sesame |
| lactic | bacteria malt (5.6) | |
| baked salt | black soy | |
| Bacillus natto | Angelica keiskei | |
| persimmon leaf | chestnut | |
| Bifidobacterium | germinating unpolished rice |
The weight (g) for the component with a relatively large amount of content is placed within parentheses.
2.3. Observation variables
2.3.1. Measurement of body composition
Body weight (kg) and BFP (%) were measured by a bioelectric impedance analysis scale(Inner Scan BC-621; Tanita Corporation, Tokyo, Japan). The circumference of the waist, lower limbs (i.e., thigh, calf, and ankle), arms, and lower thorax were also measured. The decrease rate (%) in body weight and BFP was determined as follows: (pre-OHCP - post-OHCP)/pre-OHCP × 100.
2.3.2. Blood biochemical examinations
Venous blood was collected from all participants before and after OHCP for biochemical examination of the plasma. The following blood levels were examined by researchers at a blood examination company (LSI Medience Corporation, Tokyo, Japan): creatine kinase (CK) and its isozymes (CK-MB and CK-MM), aspartate transaminase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GT), aldolase (ALD), choline esterase (ChE), leucine aminopeptidase (LAP), triglyceride (TG), total cholesterol (T-CHO), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, uric acid, creatinine, urea nitrogen, glucose, glycosylated hemoglobin A1c (HbA1c), total protein (TP), albumin, and the albumin/globulin (A/G) ratio. The reference range of these blood markers in Japanese women are as follows: CK, 40–150 IU/L; CK-MB, <5.2 IU/L; AST, 10–40 IU/L; ALT, 5–45 IU/L; LDH, 120–240 IU/L; ALP, 100–325 IU/L; γ-GT, <30 IU/L; ALD, 2.7–5.9 IU/L; ChE, 200–452 IU/L; LAP, 37–61 IU/L; TG, 30–149 mg/dL; T-CHO, 120–219 mg/dL; HDL, 40–95 mg/dL; LDL, 65–139 mg/dL; uric acid, 2.5–7.0 mg/dL; creatinine, 0.47–0.79 mg/dL; urea nitrogen, 8.0–20.0 mg/dL; glucose, 70–100 mg/dL; HbA1c, 4.6–6.2%; TP, 6.7–8.3 g/dL; and A/G ratio, 11–20.
2.3.3. Oxidative stress and antioxidative stress potential
In the present study, serum reactive oxygen metabolite-derived compounds (d-ROMs) and biological antioxidant potential (BAP) tests were used to evaluate oxidative stress and the antioxidative stress potential. These tests have been widely used as an accurate and convenient technique in clinical medicine.9–12
The d-ROMs test measures the level of hydroperoxide (ROOH–), which is a metabolite from the oxidation of lipids, proteins, amino acids, and nucleic acid. The blood d-ROMs values reflect the amount of reactive oxygen substances (ROS). The BAP was also determined by measuring the reduction potential of Fe3+ to Fe2+ by electrons in the blood. These measurements were based on color reaction by using a free radical analyzer (Free; Wismerll Co. Ltd., Tokyo, Japan).
2.3.4. Autonomic nervous function
Autonomic nervous activity was evaluated by power spectrum analysis of heart beat intervals, which were obtained by recording pulse waves from the finger in 13 women who were randomly recruited from among the study participants in 2013. Pulse waves were recorded while each participant sat in a chair for 5 minutes in a quiet room, after they had spent 5 minutes at rest in the waiting room immediately before and after undergoing body massage and cupping treatments, which were performed on each subject for 15–20 minutes.
Two major power components [i.e., low-frequency (LF; 0.014–0.15 Hz) power and high-frequency (HF; 0.15–0.4 Hz) power] were observed with the aid of a biophysical analyzer (ProComp, Thought Technology Ltd., Montreal, Canada). The LF power indicates sympathetic and parasympathetic autonomic nervous activities, whereas the HF power indicates only parasympathetic activity. The ratio of LF power to HF power shows the balance of autonomic nervous activity. The autonomic nervous activity was analyzed for the last 4 minutes in the pulse records.
2.4. Data analysis
All data were expressed as the mean value ± the standard deviation. Significant differences before and after the OHCP were statistically evaluated by the Wilcoxon signed-rank test. The Mann–Whitney U test was used for comparisons between the results of the body composition measures in participants with a BMI >25 or a BMI <25 in which a BMI value of 25 is borderline obese in Japanese adults. Spearman's rank correlation coefficient was used to examine the strength of the relationship between two sets of data such as BMI and BFP. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Body weight and body size
Table 2 shows the mean values and standard deviation of age, height, body weight, BFP, and BMI before the initiation of OHCP in each year. All study participants experienced a significant decrease in the body weight from pre-OHCP to post-OHCP (p < 0.001). As Table 2 shows, the mean decrease in body weight was 6.6 kg (11.1%), 7.0 kg (11.5%), and 6.1 kg (10.0%) in 2011, 2012, and 2013. There were individual differences in weight loss with a range of 1.8–12.3 kg among all 67 participants in the 3-year study. A concomitant reduction in the waist, thigh, and arm circumference measures was also observed after the OHCP, as Table 3 shows.
Table 2.
Basic characteristics of participants and changes in body weight, BFP, and BMI before and after OHCP.
| Year | Mean, S.D. | Age (years) | Height (cm) | Body Weight (kg) |
BFP (%) |
BMI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Rate (%) | Before | After | Rate (%) | Before | After | Rate(%) | ||||
| 2011 | Mean (n = 17) | 42.1 | 162.2 | 59.8 | * 53.2 | 11.1 | 30.9 | * 24.3 | 22.0 | 22.9 | * 20.3 | 11.3 |
| S.D. | 3.7 | 5.4 | 6.9 | 7.0 | 3.5 | 4.5 | 5.5 | 10.8 | 2.2 | 2.3 | 3.6 | |
| 2012 | Mean (n = 20) | 43.4 | 159.9 | 60.8 | * 53.8 | 11.5 | 33.0 | * 26.5 | 20.0 | 23.8 | * 21.0 | 12.2 |
| S.D. | 5.8 | 5.3 | 6.6 | 5.7 | 3.0 | 3.9 | 5.1 | 7.8 | 2.0 | 2.0 | 3.3 | |
| 2013 | Mean (n = 30) | 47.4 | 161.1 | 61.0 | * 54.9 | 10.0 | 30.1 | * 24.1 | 20.8 | 23.5 | * 21.1 | 10.5 |
| S.D. | 4.3 | 4.4 | 9.1 | 8.4 | 3.4 | 6.0 | 6.9 | 12.2 | 2.9 | 2.7 | 3.9 | |
The mean value and standard deviation in “rate”are obtained from the average decrease rate in each subject.
* Indicates a significant difference from the before-value (p < 0.01).
BFP = body fat percentage; BMI = body-mass index; n = number of subjects; OHCP = overall health care program; rate = decrease rate from pre-OHCP to post-OHCP; SD = standard deviation.
Table 3.
Changes in the circumference of body regions before and after the OHCP treatments.
| Year | Mean, S.D. | Waist |
Lower thorax |
Hip |
Thigh |
Calf |
Arm |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| 2011 | Mean | 75.0 | * 66.0 | 77.9 | * 72.7 | 96.0 | * 89.1 | 54.9 | * 49.5 | 35.8 | * 33.6 | 27.5 | * 24.8 |
| S.D. | 6.4 | 6.1 | 4.9 | 4.7 | 4.5 | 5.0 | 3.6 | 3.5 | 1.8 | 2.0 | 1.9 | 21 | |
| 2012 | Mean | 77.0 | * 68.1 | 79.8 | * 73.9 | 97.3 | * 89.6 | 55.5 | * 49.4 | 35.7 | * 33.3 | 28.5 | * 25.2 |
| S.D. | 5.4 | 4.9 | 5.8 | 5.0 | 4.7 | 4.1 | 3.9 | 3.2 | 2.2 | 1.9 | 2.4 | 20 | |
| 2013 | Mean | 77.6 | * 69.3 | 80.9 | * 75.8 | 95.7 | * 89.1 | 54.3 | * 49.4 | 36.0 | * 33.6 | 27.8 | * 25.1 |
| S.D. | 10.6 | 8.7 | 7.8 | 7.1 | 5.4 | 4.9 | 3.7 | 3.5 | 3.0 | 2.7 | 2.5 | 2.6 | |
* Indicates a significant difference from the before-value (p < 0.001).
OHCP = overall health care program.
3.2. BFP and BMI
All participants experienced significant decreases in the BFP (p < 0.001). In all participants, the mean rate of decrease was 22.0%, 20.0%, and 20.8% in 2011, 2012, and 2013, respectively (Table 2). The percent decrease in BFP in all participants (n = 67) was positively correlated with the percent decrease in the body weight (R2 = 0.27; p < 0.01) (Fig. 1A). As Table 2 shows, the BMI reduced by 11.3%, 12.2%, and 10.5% for each respective year during the 3-year period. Individual reductions in BMI ranged from 3.2–19.3% in all 67 participants. A high-level positive correlation existed between the percent decrease in BMI and the percent decrease in body weight (R2 = 0.89, p < 0.01), whereas a significant low-level positive correlation existed between the percent decrease in BMI and in BFP (R2 = 0.12, p < 0.01).
Fig. 1.
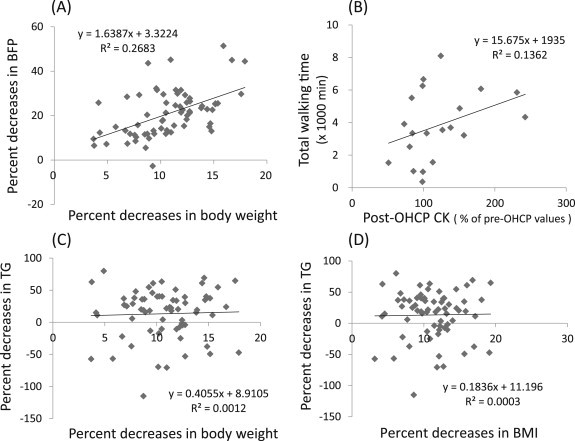
Correlations between (A) body weight and BFP, (B) CK level and total walking time, (C) body weight and TG level, and (D) BMI and TG level, with regard to changes in participants undergoing OHCP. BFP = body fat percentage; BMI = body-mass index; CK = creatine kinase; OHCP = overall health care program; TG = triglyceride.
Before initiating the OHCP, 17 participants had a BMI >25 (mean, 26.7) and 50 participants had a BMI <25 (mean, 22.3). After initiating the OHCP, the former group (i.e., participants with a high BMI) exhibited a significantly lower percent decrease in BFP (14.0%; p < 0.05), compared to the latter group (i.e., participants with low BMI; 23.2%; p < 0.05).
As Table 4 shows, no significant differences existed between these two BMI groups in percent body weight decrease and levels of T-CHO and TG, although the percentage of body weight loss in participants with BMI >25 was somewhat less than in the participants with BMI <25 (10.2% and 10.9%, respectively).
Table 4.
Comparison of changes in body weight, BFP, T-CHO, and TG between the two participant groups with a low or high BMI.
| Mean, S.D. | BMI > 25 (n = 17) |
BMI < 25 (n = 50) |
|||||
|---|---|---|---|---|---|---|---|
| Before | After | Rate (%) | Before | After | Rate (%) | ||
| Body weight (Kg) | Mean | 69.6 | * 62.5 | 10.2 | 57.6 | * 51.3 | 10.9 |
| S.D. | 5.7 | 5.8 | 3.3 | 5.8 | 5.3 | 3.4 | |
| BFP (%) | Mean | 36.2 | * 31.1 | # 14.0 | 29.4 | * 22.7 | # 23.2 |
| S.D. | 3.1 | 3.5 | 7.5 | 4.5 | 5.2 | 10.5 | |
| T-CHO (mg/dl) | Mean | 215.2 | * 195.9 | 8.2 | 220.2 | * 206.9 | 5.6 |
| S.D. | 42 | 33.9 | 9.0 | 43.4 | 53.7 | 14.4 | |
| TG (mg/dl) | Mean | 106.3 | *71.9 | 20.2 | 99.2 | * 83.6 | 10.9 |
| S.D. | 58.8 | 32.9 | 38.4 | 63.7 | 78.2 | 39.3 | |
* Indicates a significant difference from the before-value (p < 0.01 or 0.05).
# Indicates a significant difference between the same symbols (p < 0.05).
The mean value and standard deviation (SD) in “rate” are obtained from the average of the decrease rate in each subject.
BFP = body fat percentage; BMI = body-mass index; n = number of subjects; rate = decrease rate from pre-OHCP to post-OHCP; SD = standard deviation; T-CHO = total cholesterol; TG = triglyceride.
3.3. Blood biochemistry profiles
3.3.1. Muscle activity-related indexes (CK and its isozymes)
As Table 5 shows, there were significant increases in CK in the participants in 2011 (n = 17; p < 0.001). There was also an increase in CK-MM and CK-MB but within the normal range in healthy participants in 2012 (n = 20). There were no significant differences in these values in 2013 (n = 30). There was a positive correlation between the pre-OHCP and post-OHCP CK level in 2011 (R2 = 0.46, p < 0.01), whereas there was no significant positive correlation in 2012 (R2 = 0.23, p = 0.13) and in 2013 (R2 = 0.12, p = 0.38). There was no relationship between CK values or percent increase in CK values and total walking time during the OHCP in 2011 and 2013. In 2012, there was only a small degree of positive correlation between the percent increase in CK value and the total walking time during OHCP (R2 = 0.14, p = 0.09), as Fig. 1B shows.
Table 5.
Changes in blood biochemistry profiles before and after the OHCP treatments.
| Year | Mean, S.D. | CK (IU/L) |
CK- MB (IU/L) |
CK- MM (IU/L) |
AST (IU/L) |
ALT (IU/L) |
ALD (IU/L) |
LDH (IU/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| 2011 | Mean (n = 17) | 69.6 | * 116.0 | 1.20 | * 2.15 | 67.5 | * 97.7 | 19.4 | 19.6 | 17.2 | 15.8 | 4.52 | * 6.05 | 184.1 | * 210.2 |
| S.D. | 20.3 | 75.1 | 0.87 | 1.52 | 20.1 | 33.4 | 9.7 | 3.8 | 14.3 | 7.6 | 0.97 | 2.13 | 17.3 | 36.0 | |
| 2012 | Mean (n = 20) | 79.6 | * 91.7 | 0.86 | * 1.43 | 78.7 | 90.1 | 17.0 | 17.9 | 15.7 | 16.1 | 3.59 | 3.59 | 178.3 | 177.5 |
| S.D. | 27.4 | 35.8 | 0.50 | 0.89 | 26.8 | 35.1 | 4.0 | 5.4 | 8.8 | 11.8 | 0.83 | 0.90 | 32.3 | 25.2 | |
| 2013 | Mean (n = 30) | 97.4 | 103.1 | 1.64 | 2.06 | 95.8 | 101.1 | 19.4 | 19.1 | 17.4 | 16.2 | 3.71 | 3.91 | 182.7 | 185.4 |
| S.D. | 33.3 | 39.3 | 0.92 | 1.02 | 32.4 | 38.4 | 4.2 | 4.1 | 8.2 | 6.9 | 0.73 | 0.77 | 25.4 | 30.3 | |
| Year | Mean, S.D. | ALP (U/L) |
γ-GT (IU/L) |
ChE (IU/L) |
LAP (U/L) |
TG (mg/dL) |
T- CHO (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| 2011 | Mean (n = 17) | 170.2 | * 162.2 | 16.3 | * 14.9 | 286.6 | * 270.9 | 47.6 | * 50.8 | 90.7 | * 65.9 | 215.2 | 226.1 |
| S.D. | 32.0 | 24.4 | 7.6 | 4.8 | 46.3 | 57.0 | 5.5 | 5.4 | 43.0 | 29.2 | 54.7 | 81.2 | |
| 2012 | Mean (n = 20) | 182.6 | * 170.6 | 24.2 | * 19.5 | 327.5 | * 276.1 | 51.3 | 51.2 | 102.9 | * 93.4 | 219.3 | * 186.1 |
| S.D. | 41.6 | 43.5 | 14.7 | 12.8 | 54.7 | 52.3 | 7.0 | 10.2 | 71.4 | 114.1 | 41.9 | 32.4 | |
| 2013 | Mean (n = 30) | 189.7 | * 176.4 | 25.8 | * 20.8 | 322.6 | * 287.3 | 51.9 | * 48.9 | 105.6 | * 80.5 | 220.8 | * 203.7 |
| S.D. | 72.0 | 67.7 | 22.2 | 22.1 | 67.3 | 58.8 | 9.4 | 9.1 | 65.8 | 42.0 | 36.7 | 26.7 | |
| Year | Mean, S.D. | HDL (mg/dL) |
LDL (mg/dL) |
HDL/T- CHO |
LDL/T- CHO |
Creatinine (mg/dL) |
UA (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| 2013 | Mean (n = 30) | 72.3 | 71.1 | 124.3 | * 113.4 | 0.34 | * 0.36 | 0.56 | 0.55 | 0.68 | * 0.65 | 4.39 | 4.34 |
| S.D. | 16.4 | 14.9 | 35.1 | 27.9 | 0.10 | 0.09 | 0.09 | 0.07 | 0.09 | 0.08 | 1.01 | 1.01 | |
| Year | Mean, S.D. | UN (mg/dL) |
Glucose (mg/dL) |
HbA1c (%) |
TP (g/dL) |
Albumin (g/dL) |
A/G |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| 2013 | Mean (n = 30) | 12.3 | 12.7 | 83.8 | 83.2 | 5.29 | 5.32 | 7.27 | * 7.06 | 4.66 | 4.61 | 1.81 | * 1.90 |
| S.D. | 3.1 | 3.2 | 11.0 | 9.3 | 0.33 | 0.29 | 0.41 | 0.32 | 0.18 | 0.25 | 0.23 | 0.26 | |
n = number of subjects; OHCP = overall health care program; SD = standard deviation.
* Indicates a significant difference from the before-value (p < 0.05 or 0.001).
3.3.2. Hepatic function-related indexes (AST, ALT, ALP, LDH, γ-GT, ALD, ChE, and LAP)
No significant changes were observed in the AST or ALT value throughout the 3 years (Table 5). One participant had relatively high levels of AST (55.0 IU/L) and ALT (71.0 IU/L) before the OHCP. However, this individual had a lower AST level (24.0 IU/L) and ALT level (37.0 IU/L) after OHCP.
There were significant decreases in ALP, γ-GT, and ChE after the OHCP in all 3 years (p < 0.05), whereas significant increases were present in ALD and LDH in 2011 (Table 5).
One participant exhibited a relatively high LDH value (255.0 IU/L) before the OHCP, although the value decreased to 203.0 IU/L after the OHCP. In 2011, the LDH value in only two participants was elevated to 267 IU/L and 284 IU/L after OHCP from 158 IU/L and 183 IU/L, respectively, before the OHCP. Significant increases in the LAP level occurred in 2011 and 2013. These aforementioned changes were all recognized as variations within normal ranges for healthy participants.
3.3.3. Lipid metabolism-related indexes (TG, T-CHO, LDL, and HDL)
A marked decrease in the TG level(p < 0.005)was observed after OHCP in all 3 years. As Table 5 shows, the mean TG values decreased by 27.3%, 9.2%, and 23.8% in 2011, 2012, and 2013, respectively. The TG level decreased on average by 34% (range, 3.3–79.8%) for 47 participants, whereas the TG level increased by 35.6% (range, 1.9–115.1%) for 20 participants. However, the pre-OHCP TG values were lower in the 20 participants (mean, 87.9 mg/dL) than in the 47 participants (mean, 106.6 mg/dL). There was a significant difference between the two groups in TG values pre-OHCP(p < 0.05).
There was no significant correlation between the decrease rate in the TG level and body weight or BMI (Fig. 1C and D, respectively). Twelve participants had pre-OHCP TG values greater than 150 mg/dL. Among these participants, 1 participant had an increase in the TG level from 369 mg/dL pre-OHCP to 565 mg/dL post-OHCP.
Significant decreases in T-CHO levels were also observed in 2012 and 2013. As Table 5 shows, the mean T-CHO value decreased from 219.3 mg/dL to 186.1 mg/dL in 2012 (15.1% decrease) and from 220.8 mg/dL to 203.7 mg/dL (7.7% decrease) in 2013. There was no significant difference in the T-CHO level before and after the OHCP in 2011. In 2013, a significant decrease in the LDL level and an increase in the HDL/T-CHO ratio were observed from pre-OHCP to post-OHCP.
3.3.4. Energy-related metabolites and renal function tests
In 2013, blood examination included energy metabolism and renal function. As Table 5 shows, significant decreases in the levels of creatinine and TP and a significant increase in the A/G ratio occurred after OHCP. No significant changes occurred in the levels of uric acid, urea nitrogen, glucose, HbA1c, or albumin.
3.3.5. Oxidative stress and antioxidative potential
As Table 6 shows, the serum d-ROMs and BAP values significantly increased post-OHCP in 2011(p < 0.001 and p < 0.005). In 2013, however, the serum d-ROMs levels significantly decreased (p < 0.001), whereas the BAP/d-ROMs ratio significantly increased (p < 0.001). No obvious changes in d-ROMs and BAP values occurred in 2012. In 67 participants, a significant positive correlation existed between the d-ROMs and BAP values post-OHCP (R2 = 0.19, p < 0.01), but no significant positive correlation existed pre-OHCP (R2 = 0.06, p > 0.05).
Table 6.
Changes in the parameters of blood oxidative and antioxidative potential before and after the OHCP treatments.
| Year | Mean, S.D. | d-ROMs (unit) |
BAP (μmol/L) |
BAP/d-ROMs |
|||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| 2011 | Mean (n = 17) | 282.5 | * 404.9 | 2050.6 | * 2532.3 | 7.43 | 6.36 |
| S.D. | 65.3 | 54.1 | 463.3 | 334.0 | 1.42 | 1.24 | |
| 2012 | Mean (n = 20) | 383.7 | 284.0 | 2517.4 | 1705.7 | 6.76 | 6.51 |
| S.D. | 60.7 | 84.2 | 242.3 | 444.3 | 1.46 | 2.58 | |
| 2013 | Mean (n = 30) | 426.9 | * 343.6 | 2157.0 | 2413.0 | 5.31 | * 7.18 |
| S.D. | 61.3 | 54.3 | 637.1 | 296.1 | 4.73 | 1.34 | |
| 2011–2013 | Mean (n = 30) | 375.2 | * 341.4 | 2256.3 | 2232.1 | 6.26 | 6.77 |
| S.D. | 82.4 | 78.0 | 510.9 | 494.4 | 1.76 | 1.79 | |
BAP = biological antioxidant potential; d-ROMs = reactive oxygen metabolites-derived compounds; n = number of subjects; SD = standard deviation.
* Indicates a significant difference from the before-value (p < 0.01).
There were no noticeable correlations between the values of d-ROMs and CK pre-OHCP or post-OHCP (R2 = 0.02, 0.06, and 0.04 in 2011, 2012, and 2013, respectively). There were no significant correlations between the d-ROMs or BAP post-OHCP values and total walking time during OHCP in any study year from 2011 to 2013. No positive correlation existed between d-ROMs and BMI in 67 participants (Fig. 2A). However, there was a significant difference (p < 0.05) in the level of d-ROMs between the high BMI and low BMI groups. The mean d-ROMs value was 409.9 units (n = 17) in the high BMI group (BMI >25) and 363.4 units (n = 50) in the low BMI group (BMI <25) (Fig. 2B).
Fig. 2.
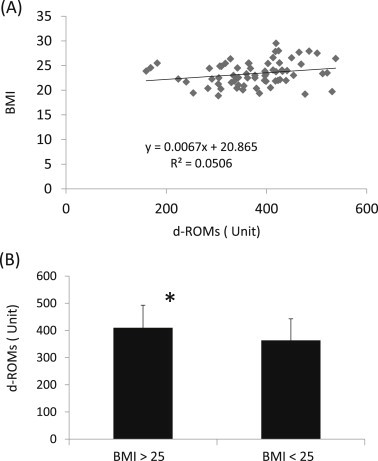
The correlation between (A) the values of d-ROMs and BMI. (B) A comparison of d-ROMs values between the two participant groups with a low BMI or high BMI. BMI = body-mass index; d-ROMs = reactive oxygen metabolites-derived compounds. * Indicates a significant difference (p < 0.05), compared to the value in participants with a BMI < 25.
3.4. Autonomic nervous function
In 2013, pulse wave recordings from the fingers of 13 participants were obtained immediately before and after the body massage and cupping treatments. As Table 7 shows, a significant increase (p < 0.01) in HF power and a significant decrease (p < 0.05) in the LF/HF ratio occurred after the treatments. As Fig. 3 shows, nine of the 13 participants experienced a decrease in the LF/HF ratio. A significant decrease in the heart rate was moreover observed after the treatments (p < 0.01) (Table 7).
Table 7.
Changes in autonomic nervous activities before and after the treatments.
| Year | Mean, S.D. | LF power |
HF power |
LF/HF |
Heart Rate |
||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | ||
| 2013 | mean (n = 13) | 75.2 | 56.0 | 53.7 | * 82.3 | 1.73 | * 0.94 | 77.2 | * 71.3 |
| SD | 66.6 | 25.8 | 52.9 | 58.7 | 1.45 | 0.76 | 8.1 | 6.9 | |
HF = high-frequency power; LF = low-frequency power; LF/HF = ratio of LF and HF; (n) = number of subjects; SD = standard deviation.
* Indicates a significant difference from the before-value (p < 0.01 or p < 0.05).
Fig. 3.
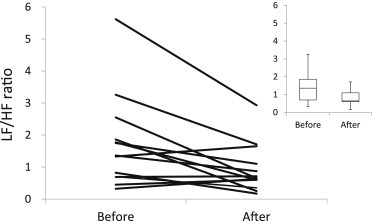
Changes in autonomic nervous balance in 13 subjects who received the body massage and cupping treatments. The upper-right graph shows a box plot of the LF/HF ratio before and after the treatments. HF = high-frequency power; LF = low-frequency power; LF/HF = ratio of LF power to HF power.
4. Discussion
4.1. Body weight, BFP, and BMI
All participants in this 3-year study had a marked decrease in body weight and a mean percent decrease of 10.0–11.5% from the pre-OHCP value. The body weight loss is primarily attributed to the effect of dietary control. However, cupping treatments, walking exercise, and body massage may also have promoted the weight loss. Such a decrease in body weight may be associated with a decrease in body fat—in our study, the percent decrease in body weight was correlated with the percent decrease in the BFP. The dietary modification, which involved replacing two daily meals (the first month) or one daily meal (the second and third months) with natural alternative foods, may have reduced the stomach capacity while maintaining nutritional balance and promoting fat consumption in the body. The alternative enzyme foods used in this study had 148–156 kcal per pack and contained various nutritional components that originated from crops, fruits, vegetables, and yeasts in a nutritionally adequate balance. Some components such as unpolished rice, rice bran, green tea leaves, and Bifidobacterium facilitate anti-inflammatory, antioxidant, and/or gastrointestinal actions,13–16 and reduce total cholesterol and LDL cholesterol.17 The green tea components (e.g., catechins) reportedly improve obesity-related hypertension.14
Walking exercise is an effective means of reducing body fat and improving body composition variables.7 The stretching, body massage, body vibration, and cupping treatments also may facilitate fat reduction by their mechanical and physical stimulation of the body. These stimuli to the body can improve blood and lymphatic circulation,18–21 and thereby may result in fat loss from the subcutaneous and visceral regions of the body.
A BMI >25 is considered obese in Japanese people. Before initiating OHCP, 17 of the 67 participants had a BMI >25. In Japan, 22.0% of women aged 40–49 years are obese (Japan Ministry of Health, Labour and Welfare, 2012). In the present study, 25.4% of participants were classified as “obese” and their mean BMI was 26.7.
The percent decrease in BFP after OHCP was unexpectedly larger in participants with a BMI <25 than in participants with a BMI >25. The reason for this difference was not evident in the present study. However, it is possible that participants with a larger BMI may be less susceptible to mechanical stimuli such as massage, cupping, and vibration. The dietary modification may have been less effective in participants with a high BMI because they received nutritional therapy through the meal replacement and the same volume of alternative enzyme foods as participants with the low BMI. Walking time did not differ significantly between the high BMI and low BMI groups. Therefore, it is unlikely that walking time factored into the BFP loss differential between the two groups.
4.2. Blood biochemistry profiles
No significant changes occurred in the AST and ALT throughout the 3-year study, which indicated that hepatic functions remained normal. Furthermore, significant decreases in ALP and γ-GT values within normal ranges suggest that hepatic functions actually improved from pre-OHCP to post-OHCP.
Significant increases in CK, CK-MB, and CK-MM were within their normal ranges in 2011 and 2012. The levels of CK and its isozymes are increased by exercise, which suggests that the increased muscle work from walking, massage, and stretching during the OHCP influenced the blood levels of CK. The increase in CK blood level is induced by regional muscle works and by local or whole body vibration,19,22–26 which suggests that passive-mechanical stimuli alone on the skeletal muscle can induce an elevation in blood CK. Therefore, we have attributed the increase of CK and its isozymes in the present study to the walking exercise, body vibration and/or body massage that was performed 1 day or more before the blood sampling. In the present study, there was no evidence of a correlation between the total walking time and the CK values. Because CK is an “escape enzyme” derived from skeletal muscle contraction, the blood CK level may be influenced by the intensity of the muscle activity rather than by the walking time.
The consistent and significant decreases in ALP and γ-GT indicated that the OHCP maintained the liver functions (including the biliary system) in good condition. The significant increase within normal ranges in ALD and LDH in 2011 suggests facilitation of glycolysis metabolism during the OHCP. In addition, the significant decrease in ChE that occurred every year may be associated with lipid metabolism because ChE (e.g., butylcholinesterase) tends to increase in obese humans and dogs,27,28 and decreased ChE improves lipid profiles after physical exercise.27
In the present study, TG decreased considerably every year, as did T-CHO in 2012 and 2013. These positive lipid changes, along with the post-OHCP changes in the body weight and BMI, clearly show the effectiveness of the OHCP among the study participants. The positive outcomes may be derived from the total integrated program, which includes walking exercise, body massage and cupping treatments, and basal calorie control through meal replacement for 3 months.
The one participant who had high pre-OHCP and post-OHCP TG levels (369 mg/dL and 565 mg/dL, respectively) also had high pre-OHCP and post-OHCP T-CHO levels (273 mg/dL and 249 mg/dL, respectively), and her BMI slightly decreased from 25.2 to 24.2. This “low responder,” although a rare case, may denote a lack of effectiveness of OHCP in participants with high levels of TG and T-CHO. Further research is warranted for this specific population.
4.3. Oxidative stress and antioxidant potential
In our study, the mean d-ROMs value before OHCP was between 282.5 units and 426.9 units, and the mean BAP value was between 2050.6 μmol/L and 2517.4 μmol/L during the 3 years of measurements. These values are nearly consistent with the values in middle-aged Japanese healthy women (30–49 years old) reported by Komatsu et al.29 The d-ROMs and BAP tests have been validated in Japanese participants with metabolic syndromes and lifestyle-related diseases; a significant correlation was observed between oxidative stress (i.e., the d-ROMs levels) and these abnormalities.10,30 The d-ROMs levels were higher in obese participants than in overweight and normal weight participants, and there is a positive correlation between d-ROMs levels and BMI.12 Our study also showed a significant difference in the d-ROMs level between subject groups with a BMI higher than 25 or lower than 25. Therefore, d-ROMs seem to reflect the overweight condition. However, the present study failed to show a clear positive correlation between the d-ROMs level and BMI because of the small number of obese participants and participants with less extensive obesity, compared to participants included in the study by Vassalle et al.12
In 2011, significant increases in the post-OHCP d-ROMs and BAP levels suggested that oxidative stress by ROS and the elevation of antioxidant potential were concurrently induced. It is generally known that physical exercise provokes an increase in ROS in the blood and skeletal muscle and that ROS is associated with intracellular signaling.31,32 The walking time in the participants in 2011 was not different from the other 2 years; however, it is possible that exercise and/or muscle activation by the other treatments that preceded the blood sampling after OHCP were somewhat intensive in 2011. In 2013, on the other hand, the d-ROMs level significantly decreased after OHCP, and was accompanied by a significant increase in the BAP/d-ROMs ratio. Therefore, the OHCP in 2013 resulted in less oxidative stress for participants, but more antioxidative stress potential.
4.4. Autonomic nervous functions
In 2013, 13 randomized participants were assigned to have their autonomic nervous function measured by power spectrum analysis of pulse intervals immediately before and after body cupping and massage treatments. A significant increase in HF power and a significant decrease in the LF/HF ratio, along with a significant decrease in the heart rate, suggested that the autonomic nervous function shifted to a predominantly parasympathetic nervous tone rather than a sympathetic tone. This shift provided evidence that body cupping and massage in this program produced the desired physical and mental relaxation. Such a change in autonomic nervous tone in healthy participants may contribute to the recovery of the physiological condition from overall body fatigue.
5. Conclusions
Our study results indicated that OHCP effectively improved the physiological state and the body composition of middle-aged women without noticeable physiological stress. The OHCP may aid in the prevention of lifestyle-related diseases such as hyperlipidemia and obesity in middle-aged, Japanese women. However, further studies are required because of the limitations in the present study resulting from a relatively small number of participants, lack of a control group that had not undergone OHCP, lack of measurements of blood hormonal activities, and the exclusion of participants with severe obesity.
Conflicts of interest
A part of this study was financially supported by Slim Beauty House Company (Tokyo, Japan).
Acknowledgments
The authors appreciate the many individuals (i.e., study participants and operators) who cooperated in this study for 3 years.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Mohan A., Lahiri A. Herbal medications and plastic surgery: a hidden danger. Aesthetic Plast Surg. 2014;38:479–481. doi: 10.1007/s00266-013-0250-x. [DOI] [PubMed] [Google Scholar]
- 2.Kamijo T., Murakami M. Regular physical exercise improves physical motor functions and biochemical markers in middle-age and elderly women. J Phys Act Health. 2009;6:55–62. doi: 10.1123/jpah.6.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Ingram D., Wilbur J., McDevitt J., Buchholz S. Women's walking program for African American women: expectations and recommendations from participants as experts. Women Health. 2011;51:566–582. doi: 10.1080/03630242.2011.606357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemmingsson E., Johansson K., Eriksson J., Sundström J., Neovius M., Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr. 2012;96:953–961. doi: 10.3945/ajcn.112.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morimoto A., Ohno Y., Tatsumi Y., Mizuno S., Watanabe S. Effects of healthy dietary pattern and other lifestyle factors on incidence of diabetes in a rural Japanese population. Asia Pac J Clin Nutr. 2012;21:601–608. [PubMed] [Google Scholar]
- 6.Salehpour A., Hosseinpanah F., Shidfar F. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.H., Seo B.D., Chung S.M. The effect of walking exercise on physical fitness and serum lipids in obese middle-aged women: pilot study. J Phys Ther Sci. 2013;25:1533–1536. doi: 10.1589/jpts.25.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulsen S.K., Due A., Jordy A.B. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr. 2014;99:35–45. doi: 10.3945/ajcn.113.069393. [DOI] [PubMed] [Google Scholar]
- 9.Vassalle C., Novembrino C., Maffei S. Determinants of oxidative stress related to gender: relevance of age and smoking habit. Clin Chem Lab Med. 2011;49:1509–1513. doi: 10.1515/CCLM.2011.622. [DOI] [PubMed] [Google Scholar]
- 10.Kotani K., Yamada T. Oxidative stress and metabolic syndrome in a Japanese female population. Australas J Ageing. 2012;31:124–127. doi: 10.1111/j.1741-6612.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M., Miyashita M., Park J.H. The association between physical activity and sex-specific oxidative stress in older adults. J Sports Sci Med. 2013;12:571–578. [PMC free article] [PubMed] [Google Scholar]
- 12.Vassalle C., Vigna L., Bianchi S. A biomarker of oxidative stress as a nontraditional risk factor in obese subjects. Biomark Med. 2013;7:633–639. doi: 10.2217/bmm.13.49. [DOI] [PubMed] [Google Scholar]
- 13.Islam M.S., Nagasaka R., Ohara K. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr Top Med Chem. 2011;11:1847–1853. doi: 10.2174/156802611796235099. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanski P., Suliburska J., Szulinska M., Stepien M., Pupek-Musialik D., Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Suliburska J., Bogdanski P., Szulinska M., Stepien M., Pupek-Musialik D., Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149:315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarowska J., Choroszy-Król I., Regulska-Ilow B., Frej-Mądrzak M., Jama-Kmiecik A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med. 2013;22:759–766. [PubMed] [Google Scholar]
- 17.Kim A., Chiu A., Barone M.K. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720–1729. doi: 10.1016/j.jada.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Stewart J.M., Karman C., Montgomery L.D., McLeod K.J. Plantar vibration improves leg fluid flow in perimenopausal women. Am J Physiol Regul Integr Comp Physiol. 2005;288:R623–R629. doi: 10.1152/ajpregu.00513.2004. [DOI] [PubMed] [Google Scholar]
- 19.Chen S. The clustered needling, massage and cupping used for treatment of obstinate myofascitis of the back—a report of 68 cases. J Trad Chin Med. 2007;27:113–114. [PubMed] [Google Scholar]
- 20.Duman I., Ozdemir A., Tan A.K., Dincer K. The efficacy of manual lymphatic drainage therapy in the management of limb edema secondary to reflex sympathetic dystrophy. Rheumatol Int. 2009;2009(29):759–763. doi: 10.1007/s00296-008-0767-5. [DOI] [PubMed] [Google Scholar]
- 21.Khan A.A., Jahangir U., Urooj S. Management of knee osteoarthritis with cupping therapy. J Adv Pharm Technol Res. 2013;4:217–223. doi: 10.4103/2231-4040.121417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newham D.J., Jones D.A., Edwards R.H. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve. 1986;9:59–63. doi: 10.1002/mus.880090109. [DOI] [PubMed] [Google Scholar]
- 23.Ispirlidis I., Fatouros I.G., Jamurtas A.Z. Time-course of changes in inflammatory and performance responses following a soccer game. Clin J Sport Med. 2008;18:423–431. doi: 10.1097/JSM.0b013e3181818e0b. [DOI] [PubMed] [Google Scholar]
- 24.Iodice P., Bellomo R.G., Gialluca G., Fanò G., Saggini R. Acute and cumulative effects of focused high-frequency vibrations on the endocrine system and muscle strength. Eur J Appl Physiol. 2011;111:897–904. doi: 10.1007/s00421-010-1677-2. [DOI] [PubMed] [Google Scholar]
- 25.Vanderthommen M., Triffaux M., Demoulin C., Crielaard J.M., Croisier J.L. Alteration of muscle function after electrical stimulation bout of knee extensors and flexors. J Sports Sci Med. 2012;11:592–599. [PMC free article] [PubMed] [Google Scholar]
- 26.Gojanovic B., Feihl F., Liaudet L., Gremion G., Waeber B. Whole-body vibration training elevates creatine kinase levels in sedentary subjects. Swiss Med Wkly. 2011;141:w13222. doi: 10.4414/smw.2011.13222. [DOI] [PubMed] [Google Scholar]
- 27.Silva I.M., Leite N., Boberg D. Effects of physical exercise on butyrylcholinesterase in obese adolescents. Genet Mol Biol. 2012;35:741–742. doi: 10.1590/S1415-47572012005000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tvarijonaviciute A., Ceron J.J., Tecles F. Acetylcholinesterase and butyrylcholinesterase activities in obese Beagle dogs before and after weight loss. Vet Clin Pathol. 2013;42:207–211. doi: 10.1111/vcp.12032. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu F., Kudoh H., Kagawa Y. Evaluation of oxidative stress and effectiveness of low-dose glucocorticoid therapy on exacerbation of chronic obstructive pulmonary disease. J Gerontol A Biol Sci Med Sci. 2007;62:459–464. doi: 10.1093/gerona/62.4.459. [DOI] [PubMed] [Google Scholar]
- 30.Fukui T., Yamauchi K., Maruyama M., Yasuda T., Kohno M., Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34:1041–1045. doi: 10.1038/hr.2011.76. [DOI] [PubMed] [Google Scholar]
- 31.Powers S.K., Nelson W.B., Hudson M.B. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]


