Abstract
Cutaneous leishmaniasis is a common parasitic disease that is endemic in some parts of Iran. The drugs of choice used for leishmaniasis therapy are associated with a risk of recurrence and serious adverse effects. Therefore, finding a safe and effective treatment is of great importance. In the present study, the effect of aloe-emodin on the growth of Leishmania major amastigotes was evaluated under in vitro conditions. In addition, the efficacy of a topical of aloe-emodin ointment was investigated in BALB/c mice with cutaneous leishmanial ulcers. Different concentrations (40 μg/mL, 80 μg/mL, 120 μg/mL, and 160 μg/mL) of aloe-emodin were tested on Leishmania amastigotes twice: 24 hours and 48 hours. The induced apoptosis and necrotic effects of two concentrations (40 μg/mL and 120 μg/mL) of aloe-emodin on promastigotes were investigated by flow cytometry. Under the in vivo condition, aloe-emodin ointment efficacy was evaluated at two concentrations (i.e., 0.1% and 1%). Serum indicator factors of the test and control groups were tested to evaluate the toxic effects of this compound on the liver and kidney. Results showed that aloe-emodin inhibited the growth of Leishmania amastigotes and induced apoptosis in promastigotes. Topical application of aloe-emodin ointment likewise reduced the ulcer size. No significant differences in biochemical analysis were observed between the control and treated groups. In conclusion, aloe-emodin showed antileishmanial effects under in vitro and in vivo conditions and may be used in clinical trials.
Keywords: aloe-emodin, apoptosis, flow cytometry, in vitro, in vivo, Leishmania major
1. Introduction
Leishmaniasis is a vector-born disease caused by protozoan parasites of the Leishmania genus and transmitted by the phlebotomine sandfly.1 The disease is normally localized to the skin and infects dermal macrophages, although metastasis to mucosal tissue and bone marrow can occur.2 Three forms of the disease are cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis. In CL, manifestations develop from a small nodule to ulcerative wounds.3 Cutaneous leishmaniasis is endemic in 98 countries and five continents.4 The five most affected countries are Afghanistan, Algeria, Brazil, Iran, and Syria.5 Throughout the world, 1–1.5 million new cases of the disease are reported annually.3 Different drugs such as miltefosin, liposomal amphotericin B, paromomycin, and allopurinol have been used to treat this disease,6 but the first-line drug treatment for CL is antimony compounds. Two popular types are sodium stibogluconate or pentostam and meglumine antimoniate; however, these compounds have adverse effects, and drug resistance and relapse after treatment can occur.5,7,8 Factors associated with unsuccessful treatment include the presence of more than three cutaneous lesions, previous treatment, body weight above 68 kg, and an incomplete treatment schedule.9 Aloe-emodin (1,8-dihydroxy-3-hydroxymethyl-anthraquinone; Fig. 1), an exudate from the aloe plant,10 is an anthraquinone in aloe vera and other species of the Asphodelaceae and Polygonaceae families. Some studies have indicated that aloe-emodin has antibacterial, antifungal, antiviral, diuretic, immunosuppressive, hepatoprotective, laxative, anti-inflammatory, and anticancer specificities.11–14 Aloe-emodin reportedly inhibits the replication of enveloped viruses such as herpes simplex virus, influenza virus, and human cytomegalovirus.15 Aloe-emodin induces apoptosis through the release of apoptosis-inducing factors and cytochrome c from the mitochondria in human gastric carcinoma cells.16 This compound has a considerable antimetastatic capability on a melanoma cell line, which is exerted by inducing cell differentiation.17 It also has anticancer activity against human promyelocytic leukemia cells18 and human hepatoma cells.19 The aim of the present study was to evaluate antileishmanial activity of aloe-emodin under in vitro and in vivo conditions.
Fig. 1.
Chemical structure of aloe-emodin.
2. Materials and methods
2.1. Preparation of aloe-emodin
Aloe-emodin powder was purchased from the Selleckchem Company (Houston, TX, USA) and dissolved in dimethyl sulfoxide (DMSO) at the concentration of 20 mg/mL. For the in vitro study, four concentrations were prepared: 40 μg/mL, 80 μg/mL, 120 μg/mL, and 160 μg/mL. Two concentrations (10 mg/mL and 1 mg/mL) were also prepared for the in vivo assay in an ointment base by Eucerin.
2.2. Leishmania culture
L. major (MRHO/IR/75/ER) promastigotes were cultivated in Roswell Park Memorial Institute (RPMI) 1640 medium, which contained penicillin (100 units/mL), streptomycin (100 μg/mL), and 20% fetal bovine serum, in an incubator 24 ± 2°C. The stationary phase of parasites was obtained by culture promastigotes in Novy–MacNeal–Nicolle medium.
2.3. Antiamastigotes assay
The drug susceptibility of amastigotes in BALB/c mouse macrophages was determined using the modified method by Love et al.20 In brief, peritoneal macrophages were isolated from the peritoneum of BALB/c mice. They were added onto a glass coverslip in tissue culture on 12-well plates and incubated for 24 hours at 37°C with 5% carbon dioxide. Nonadherent macrophages were removed by washing. Adherent macrophages were adjacent to the stationary growth phase of promastigotes at a parasite/macrophage ratio of 10:1. After 24 hours of incubation under the previous condition, washing was repeated and different concentrations (i.e., 40 μg/mL, 80 μg/mL, 120 μg/mL, and 160 μg/mL) of aloe-emodin were added to the infected macrophages in the wells and incubated separately for 24 hours and 48 hours. The coverslips were stained with Giemsa stain and the number of amastigotes inside the macrophages were counted (100 macrophages per coverslip).
2.4. Flow cytometry analysis
The promastigotes were cultured in 24-well plates (3 × 105 parasites per well) in the absence of aloe-emodin (i.e., negative control group) and in the presence of 40 μg/mL and 120 μg/mL of aloe-emodin. They were incubated at 24°C. The Annexin V FLUOS Staining Kit (Biovision, USA) was used to detect apoptotic and necrotic cells. In accordance with the kit instructions, the promastigotes were collected after a 24-hour incubation and a 48-hour incubation. They were centrifuged at 3000 rpm for 5 minutes. The supernatant was then discharged, and 500 μL binding buffer, 5 μL Annexin V, and 5 μL propidium iodide were added to the residue. The samples were incubated at room temperature and under a dark condition for 5 minutes. They were then obtained by BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and were analyzed by FlowJo Software (BD Biosciences).
2.5. In vivo assay
An ointment was prepared with 10 mg/mL (1%) and 1 mg/mL (0.1%) aloe-emodin in a standard ointment base (Eucerin). Forty female BALB/c mice that were 6–7 weeks old were used in this study. All mice were inoculated subcutaneously in a shaved area above the tail with approximately 2 × 106 stationary stages of Iranian strains of L. major promastigotes. Forty mice were divided into four groups with each group containing 10 mice. The groups were classified as follows: Group 1 was treated with the 1% aloe-emodin ointment; Group 2 was treated with the 0.1% aloe-emodin ointment; Group 3 was treated with only the ointment base (i.e., Eucerin); and Group 4 was untreated (i.e., control group). Treatment was initiated when local lesions were obvious. The mice were treated topically twice daily for 30 continuous days. Each week, the lesion size was measured before and after treatment by vernier calipers in two diameters (a, b). The lesion size was calculated by the formula:
| (1) |
2.6. Biochemical analysis
To evaluate the toxic effects of aloe-emodin in the livers and kidneys of the mice, serum samples were collected from a group of 12 mice. Aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatinine, urea, sodium and potassium were measured using Pars azmoon kit (Iran) and Hitachi Analyzer (Japan).
2.7. Statistical analysis
Statistical significance between groups was analyzed by one-way analysis of variance (ANOVA) using SPSS version 16 software (SPSS Inc., Chicago, IL, USA) and the obtained p < 0.05 were considered significant.
3. Results
3.1. Antiamastigote effect
After adding 160 μg/mL aloe-emodin, the mean number of amastigotes/macrophage after 24 hours and 48 hours was 1.3 and 0.9, respectively, and in the negative control group the mean number was 4.3 and 4.8, respectively. Fig. 2 shows the other concentration results.
Fig. 2.
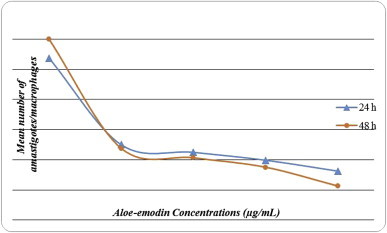
The mean number of amastigotes/macrophages at 24 hours and 48 hours after treatment with different concentrations of aloe-emodin.
3.2. Flow cytometry analysis
The treatment of promastigotes at two concentrations of aloe-emodin (i.e., 40 μg/mL and 120 μg/mL) for 24 hours and 48 hours resulted in necrotic and apoptotic effects in the parasite. The percent of apoptosis in promastigotes induced by 40 μg/mL and 120 μg/mL of aloe-emodin after 24 hours was 13.45% and 68.3%, respectively; after 48 hours, it was 15.43% and 70.2%, respectively (Fig. 3).
Fig. 3.
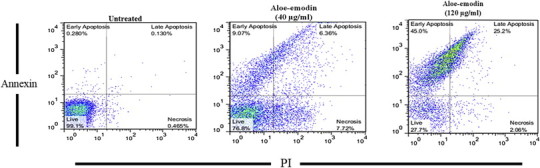
Flow cytometry result. Promastigotes were stained with Annexin V and propidium iodide 48 hours after treatment with aloe-emodin.
3.3. In vivo study
The mean LS decreased in the groups treated with aloe-emodin, compared to the control groups. The mean LS in the untreated groups was 14.1 mm, whereas it was 5.4 mm and 2.1 mm in groups treated with the 1 mg/mL and 10 mg/mL concentration of aloe-emodin, respectively. There was no significant difference in the mean LS between Groups 3 and 4, whereas the mean difference between Groups 1 and 2 versus the control groups was significant (p < 0.05; Table 1).
Table 1.
The mean lesion size in the test and control groups after treating the mice with aloe-emodin for 4 weeks.
| Group | Concentration (mg/mL) | Lesion size (mm) before treatment |
Lesion size (mm) after treatment |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| 1 | 1 | 6.1 ± 0.9 | 5.4 ± 0.6* |
| 2 | 10 | 5.8 ± 1.1 | 2.1 ± 1.9* |
| 3 | 0 | 6.1 ± 0.8 | 13.8 ± 1.6 |
| 4 | 0 | 5.23 ± 1.3 | 14.1 ± 1.3 |
* Indicates a significant difference with Groups 3 and 4 (p < 0.05).
3.4. Biochemical analysis
No significant differences were observed between the control groups and the treated groups (p > 0.05; Table 2). This result showed that the cutaneous application of aloe-emodin has no adverse effects on liver and kidney factors.
Table 2.
Serum biochemical analysis of mice treated with aloe-emodin for 4 weeks.
| Biochemical Factors | Aloe-emodin 0.1% | Aloe-emodin 1% | Control |
|---|---|---|---|
| ALT (IU/L) | 57 ± 1.5 | 60 ± 1.7 | 56.2 ± 2.2* |
| AST (IU/L) | 84 ± 2.9 | 86 ± 4.2 | 87.1 ± 4.1* |
| ALKP (IU/L) | 101 ± 6.3 | 98.7 ± 4.7 | 97 ± 8.1* |
| Cr (mg/dL) | 0.36 ± 0.21 | 0.38 ± 0.3 | 0.37 ± 0.32* |
| Urea (mg/dL) | 31 ± 1.1 | 30 ± 3.5 | 27.1 ± 2.4* |
| Na (mg/dL) | 166 ± 4.3 | 167 ± 6.8 | 165 ± 5.3* |
| K (mg/dL) | 5.5 ± 0.9 | 6 ± 1.1 | 6.1 ± 0.8* |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; Cr = creatinine; K = potassium; Na = sodium.
* No significant differences were observed between control and treated groups (p > 0.05).
4. Discussion
Drug resistance and the adverse effects of common drugs used in the treatment of CL and relapses in improved patients increases the need for pharmacological studies to achieve effective and safe drugs. The enhanced anti microorganism capability of aloe vera is attributed to aloe-emodin, which has antibacterial, antifungal, and anticancer effects.12–14 The results of our work showed that aloe-emodin reduces the number of L. major amastigotes in macrophages. A comparison of the amastigotes number in the control and test groups at two different times showed that the efficacy of aloe-emodin is dose- and time-dependent, and that aloe-emodin reduces the number of parasites with increasing concentration of aloe-emodin. Results showed aloe-emodin induces apoptosis in Lesihmania major promastigotes in a dose- and time-dependent manner so that the rate of apoptosis increased with increasing aloe-emodin concentration and time.
Aloe-emodin induces apoptosis in human gastric carcinoma cells and glioma cells.16,21 Aloe vera leaf exudate induces a caspase-independent cell death in Leishmania donovani promastigotes.22 The mechanism of apoptosis induced in L. major promastigotes by aloe-emodin is unclear. Topical application of aloe-emodin showed that using this compound on a CL wound reduces the size of the lesion. The mean LS reduced to 2.1 mm after using 1% aloe-emodin ointment. This difference was statistically significant with the control group (14.1 mm). The number of amastigotes in macrophage was reduced under the in vitro condition. The in vivo study showed that aloe-emodin has a favorable effect on wound healing; however, the wound did not heal completely. Based on previous studies, aloe-emodin has many effects on different microorganisms such as viruses, fungi, and bacteria, and it reportedly exhibits anticancer activity on neuroectodermal tumors, lung squamous cell carcinomas, and hepatoma cells.16 No study has been performed using aloe-emodin on CL; however, useful studies that have evaluated the therapeutic effects of aloe vera on cutaneous and visceral leishmaniasis show that aloe vera has antileishmanial activity by activation of host macrophages and that it reduces parasitemia by >90% in the liver, spleen, and bone marrow.23,24 Selecting effective compounds with no potential toxicity has an important role in introducing new treatments for CL. With regard to the aforementioned issue, the serum biochemical factors were analyzed. The results indicated no significant differences between the test and control groups, and aloe-emodin has no adverse effects on liver and renal factors. However, more biochemical analysis and pathological investigation are needed to survey the adverse effects of aloe-emodin. It may be that using aloe-emodin with other skin repair compounds considerably contributes to wound healing.
5. Conclusion
In general, the results of this study demonstrated for the first time that aloe-emodin exhibits antileishmanial activity under in vitro and in vivo conditions. Different concentrations of aloe-emodin and various using methods are required before any conclusion can be formed about the efficacy of aloe-emodin in the treatment and healing of human CL wounds.
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
This research was conducted as a PhD thesis and was financially supported by Tarbiat Modares University (Tehran, Iran).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Balasegaram M., Ritmeijer K., Lima M.A. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerging Drugs. 2012;17:493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero G.A., Vinitius De Farias G.M., Gomes P.M., de Oliveira M. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L (V) guyanensis in Brazil: clinical findings and diagnostic approach. Clin Infect Dis. 2001;32:1304–1312. doi: 10.1086/319990. [DOI] [PubMed] [Google Scholar]
- 3.Garnier T., Croft S.L. Topical treatment for cutaneous leishmaniasis. Curr Opin Invest Drugs. 2002;3(4) [PubMed] [Google Scholar]
- 4.Ben Salah A., Ben Messaoud N., Guedri E. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- 5.Alvar J., Velez I.D., Bern C. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croft S.L., Seifert K., Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- 7.Mohammadzadeha M., Behnaz F., Golshan Z. Efficacy of glucantime for treatment of cutaneous leishmaniasis in Central Iran. J Infect Public Health. 2013;6:120–124. doi: 10.1016/j.jiph.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 8.William H.M., Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:455–460. [PubMed] [Google Scholar]
- 9.Alavi-Naini R., Fazaeli A., O'Dempsey T. Topical treatment modalities for Old World cutaneous leishmaniasis: a review. Prague Med Rep. 2012;113:105–118. doi: 10.14712/23362936.2015.26. [DOI] [PubMed] [Google Scholar]
- 10.Tian B., Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91:413–418. [Google Scholar]
- 11.Li J., Chen J., Zhang X.L., Lu C.H., Yang H.H. A novel sensitive detection platform for antitumor herbal drug aloe-emodin based on the graphene modified electrode. Talanta. 2010;83:553–558. doi: 10.1016/j.talanta.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 12.Mandrioli R., Mercolini L., Ferranti A., Fanali S., Augusta Raggi M. Determination of aloe emodin in aloe vera extracts and commercial formulations by HPLC with tandem UV absorption and fluorescence detection. Food Chem. 2011;126:387–393. [Google Scholar]
- 13.Radovic J., Maksimovic-Ivanic D., Timotijevic G., Popadic S., Ramic Z., Trajkovic V. Cell-type dependent response of melanoma cells to aloe emodin. Food Chem Toxicol. 2012;50:3181–3189. doi: 10.1016/j.fct.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 14.Shia D.H., Huang W., Lia C., Wang L.T., Wang S.F. Synthesis, biological evaluation and molecular modeling of aloe-emodin derivatives as new acetylcholinesterase inhibitors. Bioorg Med Chem. 2013;21:1064–1073. doi: 10.1016/j.bmc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Lin C.W., Wu C.F., Hsiao N.W. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and entero virus 71. Int J Antimicrob Agents. 2008;32:355–359. doi: 10.1016/j.ijantimicag.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S.H., Lin K.Y., Chang C.C., Fang C.L., Lin C.P. Aloe-emodin-induced apoptosis in human gastric carcinoma cells. Food Chem Toxicol. 2007;45 doi: 10.1016/j.fct.2007.06.005. 2296–230. [DOI] [PubMed] [Google Scholar]
- 17.Tabolacci C., Lentini A., Mattioli P. Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16-F10 melanoma cells. Lif Sci. 2010;87:316–324. doi: 10.1016/j.lfs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Chen H.C., Hsieh W.T., Chang W.C., Chung J.C. Aloe-emodin induced in vitro G2/M arrest of cell cycle in human promyelocytic leukemia HL-60 cells. Food Chem Toxicol. 2004;42:1251–1257. doi: 10.1016/j.fct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Kuo P., Lin T., Lin C. The antiproliferative activity of aloe–emodin is through p53 dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002;71:1879–1892. doi: 10.1016/s0024-3205(02)01900-8. [DOI] [PubMed] [Google Scholar]
- 20.Love D.C., Esko J.D., Mosser D.M. A heparin-binding activity on Leishmania amastigote which mediates adhesion to cellular proteoglycans. J Cell Biol. 1993;123:759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acevedo–Duncan M., Russell C., Patel S., Patel R. Aloe–emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int Immunopharmacol. 2004;4:1775–1784. doi: 10.1016/j.intimp.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Dutta A., Bandyopadhyay S., Mandal C., Chatterjee M. Aloe vera leaf exudate induces a caspase-independent cell death in Leishmania donovani promastigotes. J Med Microbiol. 2007;56:629–636. doi: 10.1099/jmm.0.47039-0. [DOI] [PubMed] [Google Scholar]
- 23.Dutta A., Mandal G., Mandal C., Chatterjee M. In vitro antileishmanial activity of Aloe vera leaf exudate: a potential herbal therapy in leishmaniasis. Glycoconj J. 2007;24:81–86. doi: 10.1007/s10719-006-9014-z. [DOI] [PubMed] [Google Scholar]
- 24.Dutta A., Sarkar D., Gurib-Fakim A., Mandal C., Chatterjee M. In vitro and in vivo activity of aloe vera leaf exudate in experimental visceral leishmaniasis. Parasitol Res. 2008;102:1235–1242. doi: 10.1007/s00436-008-0899-2. [DOI] [PubMed] [Google Scholar]


