Abstract
Cancer is one of the most feared diseases globally and there has been a sustained rise in its incidence in both developing and developed countries. Despite the growing therapeutic options for patients with cancer, their efficacy is time-limited and non-curative. Hence to overcome these drawbacks, an incessant screening for superior and safer drugs has been ongoing for numerous decades, resulting in the detection of anti-cancer properties of several phytochemicals. Chemoprevention using readily available natural substances from vegetables, fruits, herbs and spices is one of the significantly important approaches for cancer prevention in the present era. Among the spices, Crocus sativus L. (saffron; 番紅花 fān hóng huā) has generated interest because pharmacological experiments have established numerous beneficial properties including radical scavenging, anti-mutagenic and immuno-modulating effects. The more powerful components of saffron are crocin, crocetin and safranal. Studies in animal models and with cultured human malignant cell lines have demonstrated antitumor and cancer preventive activities of saffron and its main ingredients. This review provides a brief insight into the anticancer properties of saffron and its components.
Keywords: Crocus sativus, Saffron, Cancer, Anticancer, Chemoprevention, Pharmacology
Graphical abstract
1. Introduction
Cancer is one of the most feared diseases globally and there has been a sustained rise in its incidence in both developing and developed countries. It is one of the major noncommunicable diseases posing a threat to world health. Unfortunately, enhancements in socioeconomic circumstances are generally associated with increased cancer incidence. China, India, and Russia, which share rapidly rising cancer incidence, have cancer mortality rates that are nearly twice as high as in the UK or the USA. Vast geographies, growing economies, aging populations, increasingly westernized lifestyles, relatively disenfranchised subpopulations, serious contamination of the environment, and uncontrolled cancer-causing communicable infections have all contributed to its rapid rise in incidence.1 Under Indian circumstances cancer could lead to severe social and economic consequences, frequently causing family hardships and societal inequity. In a population of ∼1.2 billion, nearly > 1 million new cases of cancer are diagnosed every year causing ∼600,000–700,000 deaths in 2012.2 An increasing proportion of the burden is falling on low- and middle-income countries because of not only demographic change, but also a transition in risk factors, whereby the consequences of the globalization of economies and behaviors are adding to an existing burden of cancers of infectious origin.3 The increasing burden of cancers in low- and middle-income countries (LMICs) is attributable in part to increasing urbanization, expansion of the adult population at risk, and increasing or persistent exposures to infectious agents, tobacco, and dietary deficiencies. Based on the present knowledge of the risk factors it seems that in about 33–50% of cancers primary prevention is principally a helpful method.4
2. Cancer projections
A study conducted by D'Souza et al5 revealed that ∼0.44 million died due to cancer during the year 2011, whereas 0.51 million and 0.60 million persons are projected to die from cancer in 2016 and 2021. The projected cancer mortality would escalate to 0.70 million by 2026 because of the change in size and composition of the population. Among women, cancer of the breast, cervix, and ovary account for 34% of all cancer deaths. The leading sites of cancer mortality in males are the lung, esophagus, prostrate, and stomach. The above results show a need for commitment for tackling cancer by reducing risk factors and strengthening the existing screening and treatment facilities.5 Major contributors to the increase in the prevalence of cancer over the years are an increase in the population size as well as an increase in the proportion of elderly population, urbanization, and globalization. The cancer incidence results show an urgent need for strengthening and augmenting the existing diagnostic/treatment facilities, which are inadequate even to tackle the present load.6
Since time immemorial cancer has been a topic of exhaustive research both at the molecular as well as pharmaceutical level. Unfortunately, a major concern in successful treatment of cancer is a lack of understanding of the underlying molecular signaling and the probable targets of therapeutics. Despite the growing therapeutic options for patients with cancer, their efficacy is time-limited and noncurative.7 Since the early 1940s to the present > 50 chemotherapeutic drugs have been discovered for the treatment of cancer. Despite their efficacy the majority of these drugs are associated with severe toxicity leading to physical and mental trauma to patients. Hence, to overcome these drawbacks, an incessant screening for superior and safer drugs has been ongoing for numerous decades, resulting in the detection of anticancer properties of several phytochemicals.8
Since millennia nature has been providing us with a vast variety of therapeutic agents. Furthermore, scientists have been able to exploit these natural sources to isolate numerous modern drugs based on their usage pattern of plants in traditional medicine. For cancer prevention and therapeutics, anticancer agents derived from plants and their derivatives have been proven to be effective. For a long time vinca alkaloids either alone or in combination have been used for the treatment of various types of cancers.
Approximately 50–60% cancer patients in the United States utilize agents derived from different parts of plants or nutrients (complementary and alternative medicine), exclusively or concurrently with traditional therapeutic regimes such as chemotherapy and/or radiation therapy. The need for new drugs has prompted studies evaluating possible anticancer agents in fruits, vegetables, herbs, and spices.7 Chemoprevention using readily available natural substances from vegetables, fruits, herbs, and spices is one of the significantly important approaches for cancer prevention in the present era.
3. Pharmacological properties of saffron
Among the spices, Crocus sativus L. (saffron; 番紅花 fān hóng huā) a member of the large family Iridaceae, has generated interest because, besides being used as a flavoring agent, pharmacological experiments have established numerous beneficial properties including radical scavenging, antimutagenic, and immuno-modulating effects.9 Saffron, a spice and a food colorant present in the dry stigmas of the plant Crocus sativus L., has been used as a herbal remedy for various ailments including cancer by the ancient Arabian, Indian, and Chinese cultures.7 In Ayurveda, saffron is used to cure chronic diseases such as asthma and arthritis. It is also useful in treating colds and coughs. Ayurvedic medicines containing saffron are used to treat acne and several skin diseases. A paste of the spice can be used as a dressing for bruises and superficial sores. Ancient texts on Ayurveda have information regarding the herb's use as an aphrodisiac. It is a stimulant. It is largely used as an indigenous medicine across India.10 The stigma of this plant is also a well known traditional Chinese medicine and is used as safflower to stimulate blood flow and relieve pain by removing stagnated blood.11
The pharmacological effects of aqueous or alcoholic extracts of C. sativus stigmas have been described in the literature and comprise a wide spectrum of activities, including anticonvulsant, antidepressant, antinociceptive and antiinflammatory, antioxidant, acetylcholinesterase inhibiting, antitussive, reducing withdrawal syndrome, improving male erectile dysfunction, enhancing spatial cognitive abilities after chronic cerebral hypoperfusion, hypotensive, and antisolar properties.12 Chemical analysis has shown the presence of > 150 components in saffron stigmas. The more powerful components of saffron are crocin, crocetin, and safranal. Studies in animal models and with cultured human malignant cell lines have demonstrated antitumor and cancer preventive activities of saffron and its main ingredients.13
The subsequent section provides a brief insight into the anticancer properties of saffron and its components.
4. Anticancer properties of saffron
4.1. Gastric cancer
Gastrointestinal cancers account for 20% of all incident cancers in the United States. The conventional treatment approaches for gastric cancer management include surgery, radiotherapy, and chemotherapy. Substantial amounts of investigations have been carried out to understand the role dietary factors play in the prevention of gastrointestinal cancers, but evidence regarding the potential preventive effect of antioxidants is inconsistent. In vitro and in vivo studies in animals continue to support the hypothesis that antioxidants reduce the risk of gastrointestinal cancers. However, evidence for the same in human populations is not as supportive.14
In the study undertaken by Bathaie et al,15 the beneficial effect of saffron aqueous extract (SAE) on 1-methyl-3-nitro-1-nitrosoguanidine (MNNG)-induced gastric cancer in rats was explored. SAE administration inhibited the progression of the cancer of the gastric tissue as evidenced by the pathologic data; so that, 20% of cancerous rats treated with higher doses of SAE was completely normal at the end of the experiment and there was no rat with adenoma in the SAE treated groups. Furthermore, the results of the flow cytometry/propidium iodide staining demonstrated that the apoptosis/proliferation ratio was augmented because of the SAE treatment of cancerous rats. Thus the investigators recommend crocin as a potential anticancer agent.15,16 In a study performed by Hoshyar et al,17 the mechanism of crocin action was investigated in the gastric adenocarcinoma (AGS) cells. Crocin revealed a dose- and time-dependent cytotoxic effect against an AGS cell line. Crocin-induced apoptosis was substantiated by flow cytometry and assessing caspase activity. The improved sub-G1 population and stimulated caspases in the treated AGS cells confirmed its anticancer effect. Apoptosis was markedly enhanced as demonstrated by raising the Bax/Bcl-2 ratio after crocin treatment.17
4.2. Colorectal cancer
In the Western hemisphere colorectal cancer (CRC) is the third most frequently encountered cancer and the incidence rise with increasing age. Surgery, radiation therapy, and chemotherapy are the key components of rectal cancer therapy.18 In the present era, preventive medicine is becoming a keystone in our model of health. CRC prevention, specially, has become one of the main goals for health care providers, clinicians, and the general public. CRC fits the norm of an ailment appropriate for chemopreventive interventions. It is a ubiquitous disease that is linked with substantial mortality and morbidity rates. CRC has a natural history of transition from precursor to malignant lesion that spans, on average, 15–20 years, providing a window of opportunity for effective interventions and prevention. Current advancement in molecular biology and pharmacology augments the probability that cancer prevention will progressively rely more on chemoprevention. Chemoprevention uses agents to inhibit, delay, or reverse carcinogenesis. The eventual aim of cancer treatment is suppression of the growth of precancerous and cancerous cells without affecting normal cells and is of particular importance in chemoprevention.19
Aung et al20 suggested that Crocus sativus L. (saffron; 番紅花 fān hóng huā) extract and its major constituent, crocin, significantly inhibited the growth of colorectal cancer cells while not affecting normal cells. They demonstrated major concentration-related inhibition effects of the extract on all three colorectal cancer cell lines. Marked antiproliferative effects were also seen in nonsmall cell lung cancer cells. However, Crocus sativus extract did not significantly affect the growth of noncancer young adult mouse colon cells. Hence, Crocus sativus extract should be investigated further as a viable option in the treatment of colorectal cancer.20,21
4.3. Hepatic cancer
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the third leading cause of cancer-related death and its incidence is increasing. The majority of HCC cases are associated with chronic viral hepatitis. With > 170 million individuals chronically infected with hepatitis C virus (HCV) worldwide, HCV is currently a serious global health concern, leading to chronic hepatitis, cirrhosis, and HCC, thereby causing significant morbidity and mortality. With the incidence of HCV infection increasing, the problem of HCV-associated HCC is expected to worsen as well, with the majority of HCCs developing in the setting of cirrhosis.22 Hepatocellular carcinoma diagnosis and treatment has witnessed many major changes and challenges in the past 2 decades.23 The limited effectiveness of chemotherapy and the high recurrence rate of cancers highlight the urgent need to identify new molecular targets and to develop new treatments. Numerous epidemiological studies have recently highlighted the existence of an inverse association between fruit and vegetable consumption, natural antioxidants, and cancer risk; in fact, antioxidant intake through diet or supplements of plant origin is strongly recommended for cancer prevention and cure. Hence, cancer can be prevented by the stimulation of the immune system to destroy cancer cells or to block their proliferation.24
Saffron significantly reduced the diethylnitrosamine (DEN)-induced increase in the number and the incidence of hepatic dyschromatic nodules in rats. Saffron also diminished the number and the area of placental glutathione S-transferase-positive foci in livers of DEN-treated rats. Besides, saffron counteracted DEN-induced oxidative stress in rats as evaluated by restitution of superoxide dismutase, catalase, and glutathione-S-transferase levels, and decrease of myeloperoxidase activity, malondialdehyde, and protein carbonyl formation in liver. The result of immunohistochemical staining of rat liver demonstrated that saffron inhibited the DEN-mediated elevations in the numbers of cells positive for, cyclooxygenase 2, inducible nitric oxide synthase, nuclear factor-kappa B p-65, and phosphorylated tumor necrosis factor receptor. In vitro experiments carried out using HepG2 cells also confirmed these findings and showed inhibition of nuclear factor-kappa B activation, increased cleavage of caspase-3, as well as DNA damage and cell cycle arrest upon saffron treatment. Furthermore, telomerase activity of HepG2 cells reduces after treatment with crocin, which is probably caused by downregulation of the expression of the catalytic subunit of the enzyme. These investigations present verification that saffron exerts an important chemopreventive effect against liver cancer due to the inhibition of cell proliferation and induction of apoptosis. This study also confirms some evidence that saffron protects rat liver from cancer via modulating oxidative damage and suppressing inflammatory response.25,26
4.4. Pancreatic cancer
Pancreatic cancer is the fourth leading cause of cancer death with a median survival of 6 months and a dismal 5-year survival rate of 3–5%.27 Pancreatic cancer (PC) is a lethal disease with a low radical-resection rate and a poor prognosis.28 As the mortality rate for pancreatic cancer approaches the incidence rate with only 1–4% of all patients surviving 5 years, it would be of great value to provide chemopreventive treatment for high-risk individuals.29 In spite of recent advances in the current therapeutic modalities such as surgery, radiation, and chemotherapy patients, the average 5-year survival rate remains still < 5%. Recently, compounds from natural sources receive ample attention as anticancer agents. Many epidemiological studies published over the past few decades provide a strong correlation between consumption of vegetables, fruits, or plant derived products and reduced incidence of cancer.30 With the establishment of outstanding rodent models of pancreatic neoplasia and cancer, there are now systems available for evaluating the role diet, dietary supplements, and/or therapeutic compounds (which can be delivered in the diet) play in disease suppression. Several outstanding reports, which demonstrate clear inhibition or regression of pancreatic tumors following dietary manipulations, represent a noticeable advancement in the field by allowing for the contribution of diet and natural and synthetic compounds to be identified.31
A series of experiments were conducted to systematically establish whether crocetin significantly affects pancreatic cancer growth both in vitro and/or in vivo. For the in vitro studies, first, MIA-PaCa-2 cells were administered crocetin and in these sets of experiments, a proliferation assay using H(3)-thymidine incorporation and flow cytometric analysis imply that crocetin inhibited proliferation. Subsequently, cell cycle proteins were evaluated. Cdc-2, Cdc-25C, Cyclin-B1, and epidermal growth factor receptor were modified significantly by crocetin. For the in vivo studies, MIA-PaCa-2 as highly aggressive cells than other pancreatic cancer cells used in this study were injected into the right hind leg of the athymic nude mice and crocetin was given orally after the development of a palpable tumor. The in vivo results demonstrated significant decrease in tumor growth along with reduction of proliferation as estimated by proliferating cell nuclear antigen and epidermal growth factor receptor expression in the crocetin-treated animals compared with the controls. Both the in vitro pancreatic cancer cells and in vivo athymic nude mice tumor, apoptosis was significantly induced as pointed by Bax/Bcl-2 ratio. This study provides evidence that crocetin has a significant antitumorigenic effect in both in vitro and in vivo on pancreatic cancer.32,33
4.5. Prostate cancer
A high disease prevalence, the presentation in older age, a frequently slowly progressing course of disease, and high costs make diagnosis and therapy of prostate cancer a special challenge for urologists. Effective prevention of the disease may help to resolve some of the problems mentioned above. The individual risk/benefit ratio of prostate cancer screening is the focus of controversy and currently not in favor of a systematic screening program. Therefore, only prevention could reduce incidence, side effects of treatment, and related mortality. However, it could be expected that pharmaco- or diet-based prevention will be a huge tool for cancer control, even more for prostate cancer burden.34,35
In a study conducted by D'Alessandro et al36 the antiproliferative activity of saffron extract (SE) and its main constituent crocin on five different malignant and two nonmalignant prostate cancer cell lines were determined. Both SE and crocin decreased cell proliferation in all malignant cell lines in a time- and concentration-dependent manner. Nonmalignant cells were not affected. The majority of cells were arrested at G0/G1 phase with a noteworthy presence of apoptotic cells based on flow cytometry profiles. The expression of Bcl-2 was strikingly downregulated, whereas Bax was upregulated as per the Western blot analysis. Analysis of caspase activity indicated a caspase-dependent pathway with involvement of caspase-9 activation, suggesting an intrinsic pathway. Dependent on these results it is suggested that both SE and crocin can inhibit cell proliferation, arrest cell cycle progression, inducing apoptosis in prostate cancer. Based on these findings investigators recommend that these agents could potentially be used as a chemopreventive as well as a chemotherapeutic agent for prostate cancer management.36 Furthermore, a preclinical study by Samarghandian and Shabestari37 (2013) demonstrated a prostate cancer cell line to be highly sensitive to safranal-mediated growth inhibition and apoptotic cell death. Hence it appears to have potential as a therapeutic agent.
4.6. Cervical, ovarian, and breast cancer
The greatest potential for the chemoprevention of cervical cancer is in women with human papillomavirus (HPV) infection, an abnormal screening test, or a noninvasive neoplastic lesion. Potential chemopreventive agents include micronutrients, antiviral agents, and immune modifiers.38 To investigate the anticancer effect of crocetin, a major ingredient in saffron, and its underlying mechanisms cervical cancer cell line HeLa, nonsmall cell lung cancer cell line A549, and ovarian cancer cell line SKOV3 were treated with crocetin alone or in combination with vincristine. There was a significant inhibition of the proliferation of the three types of cancer cells with crocetin in a concentration-dependent manner. A noteworthy cell cycle arrest through p53-dependent and -independent mechanisms was induced by crocetin. Crocetin caused cytotoxicity by enhancing apoptosis in a time-dependent manner. It also significantly enhanced the cytotoxicity induced by vincristine. Additionally, this synergistic effect was also detected in the vincristine-resistant breast cancer cell line MCF-7/VCR. Thus the investigators suggest that crocetin is a potential anticancer agent, which may be used as a chemotherapeutic drug or as a chemosensitizer for vincristine.39 Breast cancer remains the leading cause of cancer death among females worldwide. It signifies a shift from the previous decade during which the most common cause of cancer death was cervical cancer. Current treatment modalities, including surgery, radiotherapy, and adjuvant chemotherapy or hormone therapy, have not been successful enough to impart significant improvement in the morbidity or mortality of breast cancer. This cancer is highly resistant to chemotherapy as no effective treatment exists for advanced disease conditions. Along with the existing therapeutic modalities of breast cancer the focus of researchers and clinicians have shifted towards the exploration of the preventive and therapeutic uses of natural products, including dietary phytoconstituents.40
Due to the high incidence of breast cancer, optimal strategies for its prevention are imperative. This entails identification of women who are at an increased risk for breast cancer and an integrative approach that includes effective screening methods as well as nutritional, pharmacologic, and surgical management.41 In India breast and cervical cancers are the most common cancers and have high annual age-adjusted rates in all the registries. In order to have significant improvement in cancer control in India there needs to be a disproportionate focus on women's breast and cervical cancer.42 In order to determine the effect of crocetin on breast cancer cells, the highly invasive MDA-MB-231 cells were used and their viability measured with the WST-1 assay and the invasiveness through a reconstituted basement membrane. Findings show that crocetin, the main metabolite of crocins, inhibits MDA-MB-231 cell invasiveness via downregulation of MMP expression.43
4.7. Skin cancer
The incidence of skin cancer is increasing worldwide. Over the past several decades, attention has been focused on understanding the molecular basis of skin carcinogenesis and identifying substances for use in chemoprevention of skin cancer. Reactive oxygen species generated by chemical carcinogens or UV irradiation play a key role in skin tumorigenesis. Multiple lines of evidence suggest that cellular antioxidant and/or phase-2 detoxification enzymes, collectively known as cytoprotective proteins, can protect against skin carcinogenesis.44
These days cancer chemoprevention is recognized as the most hopeful and novel approach to prevent, inhibit, or reverse the processes of carcinogenesis by intervention with natural products. Phytochemicals have antioxidant, antimutagenic, anticarcinogenic, and carcinogen detoxification capabilities thereby considered as efficient chemopreventive agents. Considerable efforts have been done to identify the phytochemicals which may possibly act on one or several molecular targets that modulate cellular processes such as inflammation, immunity, cell cycle progression, and apoptosis. To date, several phytochemicals in the light of chemoprevention have been studied by using suitable skin carcinogenic in vitro and in vivo models and proven as beneficial for prevention of skin cancer.45
The effects of an aqueous infusion of saffron on two stage skin papillogenesis/carcinogenesis in mice initiated by 7-12 dimethylbenz[a]anthracene (DMBA) and promoted with croton oil were evaluated. Marked inhibition in papilloma formation was demonstrated with saffron application in the pre- and postinitiation periods. The suppression could be attributed to the modulatory effects of saffron on some Phase II detoxifying enzymes such as glutathione-S-transferase (GST) and glutathione peroxidase (GPx), as well as catalase (CAT) and superoxide dismutase (SOD).7,46
4.8. Lung cancer
Lung cancer is the leading cause of cancer related mortality worldwide largely because in the majority of patients it is at an advanced stage at the time it is discovered, when curative treatment is no longer feasible. Crocetin, saffron plant derivative is known to play a role in cancer chemoprevention. The effects of crocetin were evaluated against lung cancer-bearing mice in both the pre- and postinitiation periods. Crocein treatment normalized the raised levels of lipid peroxidation (LPO) and marker enzymes markedly in carcinogen administered animals. It also raised the activities of the enzymic antioxidants and glutathione metabolizing enzymes. The pathological changes seen in cancerous animals were notably normalized by Crocetin administration.47
Samarghandian et al48 investigated the potential of saffron to induce cytotoxic and apoptotic effects in lung cancer cells (A549). The investigators demonstrated that the proliferation of the A549 cells were reduced following saffron administration in a dose- and time-dependent manner. Furthermore there was an improvement in the percentage of apoptotic cells. The authors suggest that the anticancer activity of the aqueous extract of saffron could be attributed partly to its inhibition of the cell proliferation and induction of apoptosis in cancer cells through caspase-dependent pathways activation.48
The potential of the ethanolic extract of saffron to induce cytotoxic and apoptosis effects in carcinomic human alveolar basal epithelial cells (A549), a commonly used cell culture system for in vitro studies on lung cancer was evaluated. Saffron, in a concentration- and time-dependent manner could reduce the cell viability in the malignant cells. The extract exerts proapoptotic effects in a lung cancer-derived cell line and could be considered as a potential chemotherapeutic agent in lung cancer.49,50
4.9. Leukemia
Results of the study conducted by Sun et al51 demonstrated that crocin suppressed HL-60 cell proliferation and stimulated apoptosis and cell cycle arrest at G0/G1 phase, in a concentration and time-dependent manner. Furthermore, crocin reduced the tumor weight and size of HL-60 xenografts in nude mice, suppressed Bcl-2 expression, and enhanced Bax expression in xenografts.51 Cytotoxicity experiments conducted by Geromichalos et al52 demonstrated that safranal (SFR) and crocin mediate cytotoxic response to K562 cells (human chronic myelogenous leukemia cells). SFR and to a lesser extent imatinib mesylate (used in the treatment of human chronic myelogenous leukemia) suppressed the expression of Bcr-Abl gene expressing Bcr-Abl protein tyrosine kinase activity in in vitro studies. Additionally, in silico molecular docking experiments showed that SFR can be attached to Bcr-Abl protein, positioned within the protein's binding cavity at the same place with imatinib mesylate.52 Evaluation of the effects of crocin on human T-cell leukemia cell line, MOLT-4, by Rezaee et al53 demonstrated that crocin exhibited mild cytotoxic effects on a leukemia cell line which might be mediated through the increase of DNA fragmentation.
4.10. Anticancer toxicity
Anticancer treatments such as anthracyclines, though effective, induce cardiotoxicity by releasing radical oxygen species (ROS). ROS generated, respectively, by electrolysis and by Doxo considerably affects cardiovascular function; it reduces ventricular pressure, heart rate, and coronary flow. Augmentation of lipid peroxidation of the myocardium was also demonstrated, whereas superoxide dismutase activity diminished. The myocardial architecture was distorted and the intercellular spaces enlarged. Saffron perfused during electrolysis helps trap ROS and appreciably recovers myocardial function; however, saffron was less effective against Doxo, thus suggesting that mechanisms other than oxidative stress underlie Doxo cardiotoxicity.54
4.11. Toxicology
It is also worth noting that the crocin principle of saffron exhibited high efficacy along with no major toxicity in experimental models.55
According to LD50 values, safranal was low-toxic in acute intraperitoneal routes and practically nontoxic in acute oral administration in both mice and rats. In subacute toxicity, safranal changed some hematological and biochemical parameters. In hematological tests, a significant decrease in RBC counts, hematocrit, hemoglobin, and platelets were observed. Safranal decreased cholesterol, triglyceride, and alkaline phosphatase. Lactate dehydrogenase and serum urea nitrogen were increased by safranal. Histological studies indicated that safranal did not have any toxic effects on the heart, liver, and spleen. However, pathological changes were seen in the kidney and lung.56
5. Discussion and conclusion
Cancer is a major public health concern and its management is frequently unsuccessful. Hence, on-going efforts in the quest for novel agents and therapies to enhance survival are essential. Escalating recurrence of and toxic side-effects of chemotherapeutic agents decrease the clinical efficacy of a majority of anticancer agents that are presently being used. Accordingly, there is forever an invariable requisite to build up alternative or synergistic anticancer drugs with negligible side-effects.
One important approach to extend effective anticancer armamentarium is to investigate the anticancer agents derived from natural sources. Natural compounds, particularly those obtained from plants, have been best explored for their anticancer properties and most of them have been efficient against the known molecular targets of cancer. A substantial number of plant extracts and isolated compounds possess significant antiproliferative or proapoptotic effects. Thus the screening of medicinal plants for anticancer drugs has offered modern medicine with useful cytotoxic pharmaceuticals.
There is better approval by the scientific community that dietary phytochemicals could be promising agents in the fight against cancer. Emerging information has provided fresh insights into the molecular and cellular framework required to set up innovative mechanism-based approaches for cancer prevention by selective bioactive food components.
Cancer prevention by nutraceuticals present in fruits, vegetables, and spices has garnered extensive consideration due to their low cost and wide safety margin. A significant amount of verification from human, animal, and cell culture studies has shown cancer chemopreventive effects from these natural products.
Allopathic medicine commonly practiced currently is only 100 years old. Although traditional medicine has been around for thousands of years, no integration exists between it and allopathic medicine. Ayurveda, the science of long life and one of the most ancient medical systems still practiced on the Indian subcontinent, can be used in combination with modern medicine to provide better treatment of cancer.
Saffron, a spice obtained from the flower of Crocus sativus, is rich in carotenoids. Two major natural carotenoids of saffron, crocin and crocetin, are responsible for its color. Preclinical evidence has demonstrated that dietary intake of some carotenoids have potent antitumor effects both in vitro and in vivo, signifying their potential preventive and/or therapeutic roles in several tissues. The reports represent that the use of carotenoids without the potential for conversion to vitamin A may provide further protection and avoid toxicity.
The mechanisms underlying cancer chemo-preventive activities of carotenoids include modulation of carcinogen metabolism, regulation of cell growth and cell cycle progression, inhibition of cell proliferation, antioxidant activity, immune modulation, enhancement of cell differentiation, stimulation of cell-to-cell gap junction communication, apoptosis, and retinoid-dependent signaling. Various hypotheses for the antitumor actions of saffron and its components have been proposed such as: (1) the inhibitory effect on cellular DNA and RNA synthesis, but not on protein synthesis; (2) the inhibitory effect on free radical chain reactions; (3) the metabolic conversion of naturally occurring carotenoids to retinoids; and (4) the interaction of carotenoids with topoisomerase II, an enzyme involved in cellular DNA-protein interaction (Fig. 1). Additionally, the immunomodulatory activity on driving toward T Helper Cell Type 1 (Th)1 and Th2 limbs of the immune system of saffron was also demonstrated.
Fig. 1.
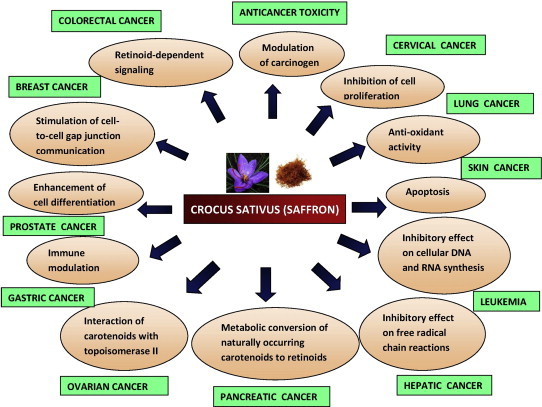
Crocus sativus Anticancer Mechanisms.
Given its therapeutic and economic importance, its natural abundance, in addition to its common usage in ethnic medicine, saffron provides a varied and accessible platform for phytochemical-based drug discovery. A consolidation of its traditional usage as well as its chemical and pharmacological profiles will thus guide efforts aimed at maximizing this potential. A stronger focus on clinical studies and phytochemical definition will be essential for future research efforts. Taken together, results and literature data indicate that saffron could be used as a potential cancer chemopreventive agent in clinical trials. However, further direct evidence of anticancer efficacy of saffron as a chemo-preventive agent could be obtained from trials that use actual reduction of cancer incidence as the primary endpoint. Hence, it is suggested that future research be warranted that will define the possible use of saffron as an effective anticancer and chemopreventive agent in clinical trials. Although data from in vitro studies look convincing, well designed clinical trials in humans are needed to ascertain whether saffron can become part of our armamentarium against cancer.
Promising results in in vitro and in vivo experiments could open up new avenues for the discovery of the ideal drug combinations of such phytochemicals with synthetic antineoplastic drugs over the conventional combinations of antineoplastic drugs and the possible interventions in the clinical settings.
Conflicts of interest
All authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Goss P.E., Strasser-Weippl K., Lee-Bychkovsky B.L. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 2.Mallath M.K., Taylor D.G., Badwe R.A. The growing burden of cancer in India: epidemiology and social context. Lancet Oncol. 2014;15:e205–e212. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- 3.Vineis P., Wild C.P. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 4.Schottenfeld D., Beebe-Dimmer J.L., Buffler P.A., Omenn G.S. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health. 2013;34:97–117. doi: 10.1146/annurev-publhealth-031912-114350. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza N.D., Murthy N.S., Aras R.Y. Projection of burden of cancer mortality for India, 2011−2026. Asian Pac J Cancer Prev. 2013;14:4387–4392. [PubMed] [Google Scholar]
- 6.D'Souza N.D., Murthy N.S., Aras R.Y. Projection of cancer incident cases for India -till 2026. Asian Pac J Cancer Prev. 2013;14:4379–4386. [PubMed] [Google Scholar]
- 7.Gutheil W.G., Reed G., Ray A., Anant S., Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13:173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan A., Narayanan S., Sethuraman S., Krishnan U.M. Combinations of plant polyphenols and anticancer molecules: a novel treatment strategy for cancer chemotherapy. Anticancer Agents Med Chem. 2013;13:281–295. doi: 10.2174/1871520611313020015. [DOI] [PubMed] [Google Scholar]
- 9.Das I., Chakrabarty R.N., Das S. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pac J Cancer Prev. 2004;5:70–76. [PubMed] [Google Scholar]
- 10.Champalal K.D., Nilakshi N., Gadiya R.V., Abhyankar M.M. Detailed profile of Crocus sativus. Int J Pharm Biol Sci. 2011;2:530–540. [Google Scholar]
- 11.Kumar V., Bhat Z.A., Kumar D., Khan N.A., Shah M.Y. Pharmacological profile of Crocus sativus – a comprehensive review. Pharmacologyonline. 2011;3:799–811. [Google Scholar]
- 12.Rezaee R., Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16:12–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Samarghandian S., Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams C.D. Antioxidants and prevention of gastrointestinal cancers. Curr Opin Gastroenterol. 2013;29:195–200. doi: 10.1097/MOG.0b013e32835c9d1b. [DOI] [PubMed] [Google Scholar]
- 15.Bathaie S.Z., Miri H., Mohagheghi M.A., Mokhtari-Dizaji M., Shahbazfar A.A., Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the Wistar albino rat. Iran J Basic Med Sci. 2013;16:27–38. [PMC free article] [PubMed] [Google Scholar]
- 16.Bathaie S.Z., Hoshyar R., Miri H., Sadeghizadeh M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol. 2013;91:397–403. doi: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- 17.Hoshyar R., Bathaie S.Z., Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013;32:50–57. doi: 10.1089/dna.2012.1866. [DOI] [PubMed] [Google Scholar]
- 18.Haraldsdottir S., Einarsdottir H.M., Smaradottir A., Gunnlaugsson A., Halfdanarson T.R. Colorectal cancer – review. Laeknabladid. 2014;100:75–82. doi: 10.17992/lbl.2014.02.531. [DOI] [PubMed] [Google Scholar]
- 19.N1 Arber, Levin B. Chemoprevention of colorectal cancer: ready for routine use? Recent Res Cancer. 2005;166:213–230. doi: 10.1007/3-540-26980-0_14. [DOI] [PubMed] [Google Scholar]
- 20.Aung H.H., Wang C.Z., Ni M. Crocin from Crocus sativus possesses significant antiproliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- 21.Bajbouj K., Schulze-Luehrmann J., Diermeier S., Amin A., Schneider-Stock R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement Altern Med. 2012;12:69. doi: 10.1186/1472-6882-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim E.J., Torresi J. Prevention of hepatitis C virus infection and liver cancer. Recent Res Cancer. 2014;193:113–133. doi: 10.1007/978-3-642-38965-8_7. [DOI] [PubMed] [Google Scholar]
- 23.Rasool M., Rashid S., Arooj M. New possibilities in hepatocellular carcinoma treatment. Anticancer Res. 2014;34:1563–1571. [PubMed] [Google Scholar]
- 24.Costantini S., Colonna G., Castello G. A holistic approach to study the effects of natural antioxidants on inflammation and liver cancer. Cancer Treat Res. 2014;159:311–323. doi: 10.1007/978-3-642-38007-5_18. [DOI] [PubMed] [Google Scholar]
- 25.Amin A., Hamza A.A., Bajbouj K., Ashraf S.S., Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- 26.Noureini S.K., Wink M. Antiproliferative effects of crocin in HepG2 cells by telomerase inhibition and hTERT downregulation. Asian Pac J Cancer Prev. 2012;13:2305–2309. doi: 10.7314/apjcp.2012.13.5.2305. [DOI] [PubMed] [Google Scholar]
- 27.Iovanna J., Mallmann M.C., Gonçalves A., Turrini O., Dagorn J.C. Current knowledge on pancreatic cancer. Front Oncol. 2012;2:6. doi: 10.3389/fonc.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling S., Feng T., Jia K., Tian Y., Li Y. Inflammation to cancer: the molecular biology in the pancreas (Review) Oncol Lett. 2014;7:1747–1754. doi: 10.3892/ol.2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fendrich V. Chemoprevention of pancreatic cancer-one step closer. Langenbecks Arch Surg. 2012;397:495–505. doi: 10.1007/s00423-012-0916-x. [DOI] [PubMed] [Google Scholar]
- 30.Boreddy S.R., Srivastava S.K. Pancreatic cancer chemoprevention by phytochemicals. Cancer Lett. 2013;334:86–94. doi: 10.1016/j.canlet.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascariñas E., Eibl G., Grippo P.J. Evaluating dietary compounds in pancreatic cancer modeling systems. Methods Mol Biol. 2013;980:225–248. doi: 10.1007/978-1-62703-287-2_12. [DOI] [PubMed] [Google Scholar]
- 32.Dhar A., Mehta S., Dhar G. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther. 2009;8:315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- 33.Bakshi H., Sam S., Rozati R. DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev. 2010;11:675–679. [PubMed] [Google Scholar]
- 34.Schmitz-Dräger B.J., Schöffski O., Marberger M., Sahin S., Schmid H.P. Risk adapted chemoprevention for prostate cancer: an option? Recent Res Cancer. 2014;202:79–91. doi: 10.1007/978-3-642-45195-9_10. [DOI] [PubMed] [Google Scholar]
- 35.Eisinger F., Cancel-Tassin G., Azzouzi A.R., Gravis G., Rossi D., Cussenot O. Pharmaco and diet based prostate cancer prevention. Bull Cancer. 2013;100:497–507. doi: 10.1684/bdc.2013.1739. [DOI] [PubMed] [Google Scholar]
- 36.D'Alessandro A.M., Mancini A., Lizzi A.R. Crocus sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr Cancer. 2013;65:930–942. doi: 10.1080/01635581.2013.767368. [DOI] [PubMed] [Google Scholar]
- 37.Samarghandian S., Shabestari M.M. DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J Urol. 2013;29:177–183. doi: 10.4103/0970-1591.117278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasieni P. Chemoprevention of cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20:295–305. doi: 10.1016/j.bpobgyn.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhong Y.J., Shi F., Zheng X.L. Crocetin induces cytotoxicity and enhances vincristine-induced cancer cell death via p53-dependent and -independent mechanisms. Acta Pharmacol Sin. 2011;32:1529–1536. doi: 10.1038/aps.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha D., Biswas J., Sung B., Aggarwal B.B., Bishayee A. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr Drug Targets. 2012;13:1799–1819. doi: 10.2174/138945012804545632. [DOI] [PubMed] [Google Scholar]
- 41.Advani P., Moreno-Aspitia A. Current strategies for the prevention of breast cancer. Breast Cancer (Dove Med Press) 2014;6:59–71. doi: 10.2147/BCTT.S39114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh A.A. India can do more for breast and cervical cancer control. Asian Pac J Cancer Prev. 2009;10:527–530. [PubMed] [Google Scholar]
- 43.Chryssanthi D.G., Dedes P.G., Karamanos N.K., Cordopatis P., Lamari F.N. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011;77:146–151. doi: 10.1055/s-0030-1250178. [DOI] [PubMed] [Google Scholar]
- 44.Chun K.S., Kundu J., Kundu J.K., Surh Y.J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol Lett. 2014 doi: 10.1016/j.toxlet.2014.05.018. S0378–4274(14)00223−9. [DOI] [PubMed] [Google Scholar]
- 45.Singh M., Suman S., Shukla Y. New enlightenment of skin cancer chemoprevention through phytochemicals: in vitro and in vivo studies and the underlying mechanisms. Biomed Res Int. 2014;2014:243452. doi: 10.1155/2014/243452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das I., Das S., Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: a histopathological study. Acta Histochem. 2010;112:317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Magesh V., Singh J.P., Selvendiran K., Ekambaram G., Sakthisekaran D. Antitumor activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes, and histopathological studies. Mol Cell Biochem. 2006;287:127–135. doi: 10.1007/s11010-005-9088-0. [DOI] [PubMed] [Google Scholar]
- 48.Samarghandian S., Borji A., Farahmand S.K., Afshari R., Davoodi S. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. Biomed Res Int. 2013;2013:417928. doi: 10.1155/2013/417928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Samarghandian S., Tavakkol Afshari J., Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl Biochem Biotechnol. 2011;164:238–247. doi: 10.1007/s12010-010-9130-x. [DOI] [PubMed] [Google Scholar]
- 50.Samarghandian S., Boskabady M.H., Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y., Xu H.J., Zhao Y.X. Crocin exhibits antitumor effects on human leukemia HL-60 cells in vitro and in vivo. Evid Based Complement Alternat Med. 2013;2013:690164. doi: 10.1155/2013/690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geromichalos G.D., Papadopoulos T., Sahpazidou D., Sinakos Z. Safranal, a Crocus sativus L constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia. In silico and in vitro study. Food Chem Toxicol. 2014 Sep 17;74C:45–50. doi: 10.1016/j.fct.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Rezaee R., Mahmoudi M., Abnous K. Cytotoxic effects of crocin on MOLT-4 human leukemia cells. J Complement Integr Med. 2013:10. doi: 10.1515/jcim-2013-0011. [DOI] [PubMed] [Google Scholar]
- 54.Chahine N., Hanna J., Makhlouf H., Duca L., Martiny L., Chahine R. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm Biol. 2013;51:1564–1571. doi: 10.3109/13880209.2013.802812. [DOI] [PubMed] [Google Scholar]
- 55.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Hosseinzadeh H., Sadeghi Shakib S., Khadem Sameni A., Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]


