Abstract
Temporal integration (TI; threshold versus stimulus duration) functions and multipulse integration (MPI; threshold versus pulse rate) functions were measured behaviorally in guinea pigs and humans with cochlear implants. Thresholds decreased with stimulus duration at a fixed pulse rate and with pulse rate at a fixed stimulus duration. The rates of threshold decrease (slopes) of the TI and MPI functions were not statistically different between the guinea pig and human subject groups. A characteristic of the integration functions that the two groups shared was that the slopes of the TI functions were similar in magnitude to slopes of the MPI function only at low pulse rates (< approximately 300 pulses per second). This is consistent with the notion that the TI functions and the MPI functions at the low rates are mediated by a mechanism of long-term integration described in the statistical “multiple looks” model. Histological analysis of the guinea pig cochleae suggested that the slopes of both the MPI and the TI functions were dependent on sensory and neural health near the stimulated regions. The strongest predictor for spiral ganglion cell densities measured near the stimulation sites was the slope of the MPI functions below 1,000 pps. Several mechanisms may be considered to account for the association of shallow integration functions with poor sensory and neural status. These mechanisms are related to abnormal across-fiber synchronization, increased refractoriness and adaptation with impaired neural function, and steep growth of neural excitation with current level associated with neural pathology. The slope of the integration functions can potentially be used as a non-invasive measure for identifying stimulation sites with poor neural health and selecting those sites for removal or rehabilitation, but these applications remain to be tested.
Keywords: cochlear implants, cochlear health, temporal integration, multipulse integration
Introduction
It is well known that human and animal listeners show lower (better) detection thresholds for an auditory stimulus as its duration increases up to 300 ms, a phenomenon known as temporal integration (TI). Normal-hearing listeners show approximately a 2.5-dB decrease (improvement) in detection threshold for each doubling of stimulus duration (see review by Gerken et al. 1990). The slopes of temporal integration functions in listeners with sensorineural hearing loss are typically less than 1 dB/doubling (e.g., Florentine et al. 1988). Improvement in detection thresholds with stimulus duration has also been reported in humans and animals listening to electrical stimulation via cochlear implants, similar to the results for acoustic hearing (Eddington et al. 1978; Pfingst et al. 1991; Pfingst and Morris 1993; Donaldson et al. 1997). For an electrical pulse train delivered to a cochlear implant auditory prosthesis, stimulus energy is a function of the number of pulses, which in turn depends on both pulse rate and duration of the pulse train. With a fixed stimulation rate, detection of a pulse train increases with duration, which is described as classical TI. With the pulse train fixed in duration, detection of a pulse train can improve with stimulation rate, which we refer to as “multipulse integration“ (MPI).
There have been several classes of models that describe the processes that underlie temporal integration in human auditory systems. Older theories proposed either leaky integration or perfect integration of stimulus energy with either a long time constant on an order of a few hundreds of milliseconds (Plomp and Bouman 1959; Green 1960; Green and Swets 1966) or a short time constant of less than 10 ms, combined with a compressive nonlinearity (Penner 1978; Viemeister 1979; Moore et al. 1988; Oxenham and Moore 1994). A “multiple looks” model by Viemeister and Wakefield (1991) proposed a probabilistic mechanism, which argued against integration with a long time constant. In the probabilistic model, integration of energy occurs in a brief “look” at the stimulus, over approximately 3 ms. The improvement in performance with increases in stimulus duration up to 300 ms is postulated to be due to an increased number of short time-constant “looks,” each being statistically independent. The output of a look is said to be stored in short-term memory with a decay characteristic and a fairly long time constant of 300–500 ms. A greater number of samplings of the stimulus or “looks” then provides more information for making a decision.
In electrical stimulation of the auditory nerve via a cochlear implant, the mechanisms of temporal integration that involve the integration and combination of neural impulses are believed to be fundamentally similar to those in acoustic hearing. There are a few models that have accounted for temporal processing data in listeners with cochlear and midbrain implants that use pulsatile stimulation. Shannon (1989) described a model involving a parallel compressive and envelope process. Carlyon et al. (2005) used a simple linear model to predict data obtained with pulsatile stimulation based on the effects of the frequency of a sinusoid. Recently, McKay and colleagues (2013) described a more phenomenological approach that utilizes a sliding temporal integration window with a time constant of 3–10 ms and a decision device that acts upon the output of the window with different criterion matrices for loudness, threshold, modulation detection, or discrimination.
For detecting a pulse train with a fixed duration at various pulse rates (MPI functions), the existing models of temporal integration predict that the slopes of the MPI functions on a decibel vs. log-rate scale would increase with decreasing interpulse interval. Assuming that the most effective integration takes place within approximately 3 ms, for pulse trains below 300 pps, the interpulse intervals of the stimuli would be too long for the short-term integration mechanism to affect threshold. The MPI function would become steeper as the increase of pulse rate places multiple pulses in the short-term integration window. This prediction is consistent with previously reported data that showed steep threshold decreases only over the high pulse rate range (Shannon 1993). The slope of MPI functions becomes even steeper as the interpulse interval becomes shorter than 1 ms, enabling a facilitative mechanism where residual partial depolarization of the cell membrane from a sub-threshold pulse facilitates neuronal response to a following pulse (Middlebrooks 2004). Although integration of the pulses is expected to be more effective with the decrease of interpulse interval, it would be subject to the effect of neural refractoriness (McKay and McDermott 1998) and fatigue (adaptation) resulting from repeated stimulation (Kreft et al. 2004; McKay et al. 2013). The first objectives of the present study were to examine the MPI functions in guinea pig subjects with cochlear implants in relation to those measured in humans and to compare MPI slopes with the slopes of the classical TI (threshold versus stimulus duration) functions in both subject groups.
A second set of objectives concerned examination of biological conditions associated with the two integration functions. In our guinea pig studies, we have found that the MPI slopes below 1,000 pps were significantly correlated with the cochlear histopathology and electrical activity near the stimulation sites, which we refer to as “cochlear health.” In guinea pigs with preserved acoustic hearing in the implanted ear, where there were many hair cells and spiral ganglion cell bodies near the stimulation site, and ensemble spontaneous neural activity (ESA) could be recorded from the cochlear implant electrodes, thresholds decreased more steeply as a function of pulse rate than in deaf ears where there were no hair cells, low spiral ganglion cell densities, and little or no ESA (Kang et al. 2010; Pfingst et al. 2011). In the current study, we sought to determine if these measures of cochlear health in the implanted guinea pig cochleae had an equal effect on the slopes of the MPI function over different pulse-rate ranges, and whether this correlation also held true for temporal integration. Understanding the relationship between these integration measures and cochlear health would potentially allow us to use the integration measures in implanted human listeners as non-invasive psychophysical probes for identifying the health of the cochlea in localized areas near the stimulation sites. The knowledge could then potentially be used to optimize speech processing strategies for these listeners by selectively stimulating areas in the ear that are in good health.
Methods
Guinea Pig Study
Guinea Pig Subjects
Data from 50 adult male pigmented-specific-pathogen-free guinea pigs were used. For 27 of these animals, some of the data have been published previously (Kang et al. 2010; Pfingst et al. 2011). Prior to the treatment procedure in the test ear, all guinea pigs were deafened in one ear (contralateral to the treatment ear) with either 5 or 10 % neomycin. The guinea pigs were behaviorally trained and then were divided into several groups, receiving in the treatment ear one of the following: (1) only a cochlear implant (n = 20); some residual hearing remained either in the area of the implant or apical to it; acoustic hearing thresholds for animals in this group are shown in Table 1; (2) deafening and cochlear implantation (n = 8); (3) deafening, AAV.Ntf3 or AAV.BDNF inoculation, and cochlear implantation (n = 17); the adeno-associated virus infects mesothelial cells and causes upregulation of the neurotrophin, NT3 or BDNF which helps to retard the degeneration of spiral ganglion neuron (SGN) cell bodies; or (4) deafening, AAV.empty inoculation and cochlear implantation (n = 5). These four treatment groups were used with the intention of achieving a large range of cochlear conditions across animals including the presence or absence of inner hair cells (IHCs), and a broad range of SGN densities, and levels of ESA. The use of guinea pig subjects was reviewed and approved by the University of Michigan Committee on the Use and Care of Animals.
TABLE 1.
Acoustic psychophysical detection thresholds. Means of thresholds measured over the time period when the MPI and TI data were collected and up to the time of sacrifice are shown. NR = no response at the highest levels of stimulation tested
| Subject | 8 kHz sinusoids (dB SPL) | 16 kHz sinusoids (dB SPL) | 24 kHz sinusoids(dB SPL) |
|---|---|---|---|
| 335L1 | 62.9 | 39.1 | 39.3 |
| 345L1 | 37.7 | 29.2 | 18.8 |
| 347L1 | 52.7 | 60.0 | 40.6 |
| 348L1 | 55.1 | 67.6 | 73.7 |
| 350L1 | 65.9 | 39.1 | 58.5 |
| 351L1 | 50.9 | 48.2 | 49.6 |
| 353L1 | 48.4 | 39.6 | 29.5 |
| 361L1 | 72.5 | 42.4 | 82.3 |
| 374L1 | 37.6 | 25.1 | 34.1 |
| 390L1 | 72.5 | 75.0 | 75.0 |
| 399L1 | 75.1 | 73.1 | NR |
| 407L1 | 80.9 | NR | NR |
| 411L2 | 60.7 | 55.0 | 49.9 |
| 412L1 | NR | NR | NR |
| 421L1 | 50.9 | 61.0 | 67.7 |
| 422L1 | 61.5 | 28.2 | 42.4 |
| 423L1 | 32.0 | 38.8 | 44.4 |
| 436L2 | 91.3 | NR | NR |
| 453L1 | 42.6 | 9.6 | 22.9 |
| 456L1 | 89.1 | NR | NR |
| Mean | 60.0 | 45.7 | 48.6 |
| STD | 17.2 | 18.5 | 19.4 |
| N | 19 | 16 | 15 |
Behavioral Training
Prior to implantation, guinea pigs were trained and tested in cages equipped with a response button, feeder, water, and a restraint system. Cages were located in sound attenuating booths. The guinea pigs were trained using positive reinforcement to perform a procedure in which they initiated a trial by pressing a response button and responded to a presented acoustic stimulus by releasing the button. To complete a trial and receive food reinforcement, the animals were required to wait through a variable observation period of 1 to 6 s while holding the button down and then release when the stimulus was presented. Once the guinea pigs could reliably respond to an audible stimulus level, the stimulus tables were adjusted to include supra- and sub-threshold levels. Psychophysical detection threshold was assessed using a method of constant stimuli with 10 stimulus presentations at each of 7 perithreshold stimulus levels including one clearly inaudible level used for measuring the animal’s guess rate. Threshold was defined as the level at which a correct detection was achieved on 50 % of the trials.
Deafening, Inoculation, and Implantation Procedures
Once the guinea pigs had learned to respond reliably to the threshold-level acoustic stimuli, depending on the group, they received one or more of the following deafening, inoculation, and implantation procedures.
Guinea pigs were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg). An incision was made post-auricularly, the temporal bone was exposed by blunt dissection, and the bulla was opened. In test ears receiving a deafening agent, either a cochleostomy was made in the basal turn of the cochlea or a small hole was made in the round window membrane and local deafening was achieved by infusion of a neomycin sulfate solution into the scala tympani. For ears receiving an inoculation, 5 μl of either AAV.Ntf3, AAV.BDNF, or AAV.empty (no neurotrophin) was infused into the scala tympani through a small cochleostomy using an infusion pump at a rate of 1 μl/min. All test ears were implanted with a multichannel cochlear implant electrode array (Cochlear Corporation, Englewood, CO) through a cochleostomy in the scala tympani and the bulla was then sealed. In a subset of guinea pigs, the cochleostomy and bulla were sealed without implantation and then reopened 2 weeks later for cochlear implantation.
The cochlear implants consisted of eight electrode bands surrounding a silicone carrier and spaced at 0.75 mm center to center. Typically only five to six electrodes could be safely inserted past the cochleostomy (4.0 to 4.5 mm). The primary electrode used for stimulation in these experiments was the second most apical electrode, which was located on average 2.7 mm apical to the cochleostomy (on average, in the 18 kHz region) and typically sat close to the modiolar wall.
Psychophysical MPI and TI Experiments
Following implantation surgery, testing with a standard electrical stimulus (200 ms trains of either 100 Hz sinusoids or 25 μs/phase biphasic pulses at 500 pps) began the first or second day post-implantation and continued until the guinea pig’s responses to this standard test stimulus were stable.
Once ears stabilized, MPI and TI tests were conducted using 25 μs/phase biphasic pulses, a monopolar electrode configuration, and the following stimulus durations and rates. For MPI testing, detection thresholds were measured for 200 ms pulse trains with pulse rates ranging from 5 to 5,000 pps in steps of doubling. All 50 guinea pigs completed the MPI experiment. Thirteen out of the 50 guinea pigs were only tested at 156 pps and above (Kang et al. 2010). For TI testing, detection thresholds were measured at a fixed stimulation rate of 400 pps, with durations ranging from 2.5 to 640 ms in steps of doubling. Twenty-nine of the 50 guinea pig subjects completed the TI experiment. For both experiments, each stimulus was tested three or more times until the average of the last three thresholds had a standard deviation ≤ 2.0 dB and there were no upward or downward trends in the threshold levels. Slopes for the MPI and TI functions were derived using a least-squares linear regression.
Ensemble Spontaneous Activity
The level of spontaneous activity in the auditory nerve was estimated by recording asynchronous electrical activity from the cochlear-implant electrode of interest (referenced to a skull screw) under quiet conditions (Dolan et al. 1990; Searchfield et al. 2004). Guinea pigs were tested at various time points throughout testing and immediately before perfusion. The ESA levels discussed in this paper were taken on or slightly before the animal’s last day of testing. A monopolar electrode configuration, usually the second most apical electrode referenced to a skull screw, was used. The recorded electrical waveform was filtered from 300 Hz to 3.0 kHz, amplified with a gain of 10,000, and transmitted to a spectrum analyzer with a sampling rate of 256 kHz (SR760, Stanford Research Systems, Sunnyvale, CA, USA). The SR760 collected 1,024 samples. A fast Fourier Transform was performed on each record using Blackman-Harris windowing. The frequency resolution of the FFT was 31.25 Hz. We chose a span bandwidth of 12.5 kHz, which corresponds to a 32-ms acquisition (for each average) with a 31.25-Hz resolution. One hundred fifty FFTs were acquired and averaged. Response levels were averaged across a frequency range (593.75–1,312.5 Hz) in which activity would normally be present in a cochlea with hair cells.
Histology
After the completion of psychophysical and electrophysiological data collection, guinea pigs were euthanized and fixed by vascular perfusion using a paraformaldehyde fixative. The temporal bones were removed with the implants intact. The tissues were decalcified in 3 % EDTA for 7–28 days until appropriate for sectioning. Before sectioning, the location of the primary test electrode (usually the second most apical electrode) was marked. The implant was then removed and tissues were embedded in JB-4 (Electron Microscopy Sciences, Hatfield, PA, USA) and sectioned with glass knives in a peri-midmodiolar plane centered at the mark indicating the location of the primary test electrode. Sections used for spiral ganglion cell counts were stained with Toluidine blue prior to assessment. Spiral ganglion cell body densities were estimated by counting the number of cells having a 12–25-μm diameter and a 5–9-μm nucleus diameter, and dividing the counts by the cross-sectional area of Rosenthal’s canal. IHC were counted if both the nucleus and stereocilia were present. The percentage of normal inner hair cells was determined by dividing the number of IHCs counted by the number known to be in a normal ear.
Human Study
Human Subjects
Twelve postlingually deaf subjects with nucleus cochlear implants (Cochlear Ltd.) participated in this study. All subjects had at least 12 months of experience using their implants. Subjects’ demographic information is provided in Table 2. Post-implantation acoustic threshold testing with narrow-band noises found that none of the subjects had measurable residual acoustic hearing in the implanted ear. The use of human subjects was reviewed and approved by the University of Michigan Medical School Institutional Review Board.
TABLE 2.
Demographics of the subjects
| Subject | Ear | Gender | Age | Onset of deafness (years) | Duration of deafness (years) | CI use (years) | Implant type | Biology of deafness |
|---|---|---|---|---|---|---|---|---|
| S52 | L | F | 59 | 2 | 57 | 3 | CI24R(CA) | Trauma |
| S60 | R | M | 72 | 60 | 12 | 2 | CI24R(CA) | Hereditary |
| S69 | R | M | 71 | 60 | 11 | 1 | CI512 | Noise exposure |
| S81 | R | F | 60 | 52 | 8 | 6 | C124RE(CA) | Hereditary |
| S84 | R | M | 52 | 26 | 26 | 6 | C124RE(CA) | Hereditary |
| S85 | R | F | 64 | 30 | 34 | 5 | C124RE(CA) | Hereditary |
| S86 | R | F | 65 | 60 | 5 | 4 | C124RE(CA) | Hereditary |
| S88 | L | M | 60 | 51 | 9 | 2 | C124RE(CA) | Progressive sensorineural |
| S89 | L | M | 66 | 64 | 2 | 2 | CI512 | Hereditary |
| S91 | L | F | 35 | 12 | 23 | 6 | C124RE(CA) | Meningitis |
| S92 | L | F | 28 | 1 | 27 | 6 | C124RE(CA) | Unknown |
| S93 | L | F | 64 | 38 | 26 | 6 | C124RE(CA) | Progressive sensorineural |
Choosing Two Stimulation Sites per Ear
Two of the 22 available stimulation sites were chosen from each ear to compare the temporal integration and multipulse integration functions. The two stimulation sites were one that showed relatively steep integration slope and one that showed relatively shallow integration slope from an initial screening of the whole electrode array for the ear. Screening of the electrode array involved measuring the threshold (T level) for each electrode at 80 and 640 pps. Stimuli were 250-ms trains of symmetric-biphasic pulses with a phase duration of 25 μs and an inter-phase interval of 8 μs, presented using a monopolar (MP1 + 2) electrode configuration. Psychophysical testing was performed using laboratory-owned Freedom processors (Cochlear Corporation, Englewood, CO). For this initial screening, a method of adjustment procedure was used for measuring the T levels, where the subjects adjusted the current level up and down until the stimulus was just audible. Thresholds were measured three times for each pulse rate condition and then averaged. For each ear, the stimulation site that showed the steepest and the one that showed the shallowest slope of threshold decrease as a function of pulse rate were selected for testing the integration functions.
Comparison of Temporal and Multipulse Integration
In each ear, MPI and TI were compared for the two stimulation sites that had been chosen. Trains of 25 μs/phase biphasic pulses with an 8-μs inter-phase interval were presented in a monopolar configuration. For MPI, detection thresholds were measured for pulse trains with a fixed duration of 250 ms, with pulse rates ranging from 80 to 640 pps in steps of doubling. For TI testing, detection thresholds were measured for pulse trains at a fixed stimulation rate of 640 pps, with stimulus duration ranging from 31.25 to 250 ms in steps of doubling. For each stimulation site, dynamic range was first determined for each condition using the method of adjustment. T levels were determined as described above for each stimulus condition. The maximal comfortable level (C) was the level the subjects determined to be the loudest that they could tolerate for a long period of time. Detection threshold was measured three times for a given stimulus condition in a four-alternative forced-choice (4AFC) paradigm, where the subject was presented with four 500-ms sequential observation intervals separated by 500 ms silent intervals. One of the observation intervals, chosen at random, contained a stimulus while the other three intervals contained no stimulus. The stimulus level started at 50 % of the dynamic range and was changed from trial to trial using a two-down, one-up adaptive-tracking procedure (Levitt 1971). In the tracking procedure, the step size was 10 CLU (clinical level unit; 1 CLU ≈ 0.156 dB) until the first reversal, 5 and 2 CLU after the next two reversals, and 1 CLU after the remaining reversals. The mean of the levels at the last eight reversals was taken as the threshold. Feedback was presented after each trial by marking the chosen interval with a “C” for a correct response or an “X” for an incorrect response. Mean thresholds of the three repetitions were calculated for each condition. Slopes of the MPI and TI functions were calculated using least-squares linear regressions.
Results
In presenting the results of this study, we first consider the components of the MPI and TI functions, then show the relationships between these two functions, and finally show the relationship between the MPI- and TI-function slopes and anatomical and ESA measures of cochlear health.
Multipulse Integration Functions
Figure 1 shows individual and group mean MPI functions obtained from the guinea pig subjects (left panel) and the human subjects (right panel). Slopes of the MPI functions tended to be steeper as the interpulse interval of the stimuli became shorter. In guinea pigs, slopes were found to be significantly steeper for rates above 1,000 pps than those below 1,000 pps [t (49) = 10.04, p < 0.001)]. Human listeners were not tested at rates above 1,000 pps in the current study, but previous studies have found the slopes of the MPI functions above 1,000 pps to be significantly steeper than those below 1,000 pps (Shannon 1985; McKay and McDermott 1998; Zhou and Pfingst 2012). For the human data, a significant increase in slopes was observed above about 320 pps [t (23) = 5.78, p < 0.001)] (Fig. 2, right panel). In the guinea pigs, slopes from 313 to 625 pps were not consistently steeper than those below 313 pps (p > 0.05) (Fig. 2, left panel). The changes in the slopes were more apparent in subjects who showed integration at the lower pulse rate range. An independent samples T test with unequal N revealed that in the pulse range common to both subject groups, i.e., approximately 78–625 pps, the slopes of the MPI functions measured in humans were not statistically different than those measured in guinea pigs as a whole group [t (72) = −0.59, p = 0.55)] or guinea pigs with IHCs [t (40) = −2.02, p = 0.053)]. However, slopes of the MPI functions were independent of the detection threshold at 80 pps [r = 0.04, p = 0.84] in human subjects, whereas in guinea pigs, steeper MPI functions tended to have lower detection threshold at 78 pps [r = 0.32, p = 0.04].
FIG. 1.

Multipulse integration (MPI) functions measured from guinea pigs (left) and humans (right). Left panel: thresholds are shown for individual guinea pigs in different grey open symbols (see legend in Figure 2) and for group mean in black filled squares. Right panel: thresholds are shown for two individual stimulation sites measured in 12 ears in gray open circles and for group mean in black filled squares.
FIG. 2.
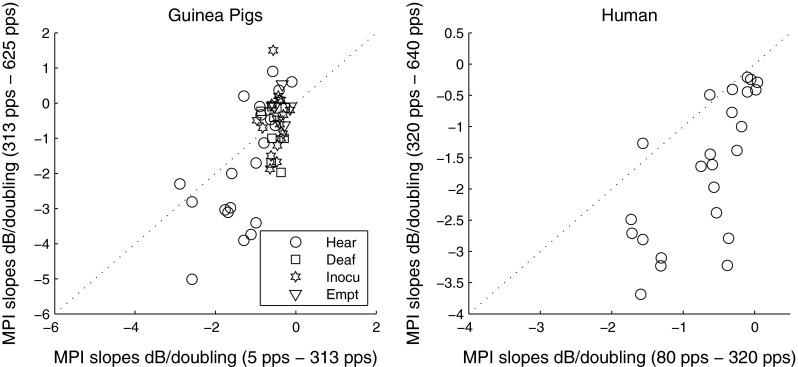
Scatter plot for the slopes of the first and second arm of the MPI functions measured in guinea pigs (left) and humans (right). In the left panel, different symbols represent different guinea pig groups: Hear = implanted in a hearing ear; Deaf = implanted in a neomycin-deafened ear; Inocu = implanted in a deaf ear that was inoculated with AAV.neurotrophin; Empt = implanted in a deaf ear that was inoculated with an empty AAV (please see “Methods” for further details of the groups).
Temporal Integration Functions
Figure 3 shows individual and group mean TI functions obtained from the guinea pig subjects (left panel) and the human subjects (right panel). The slopes of the TI functions measured in humans were not statistically different to those measured in guinea pigs in a similar stimulus-duration range, i.e., 40–320 ms [t (51) = −0.605, p = 0.55)]. Similar to MPI functions, slopes of the TI functions were independent of the detection threshold at the 31.25-ms pulse-train duration [r = 0.38, p = 0.06] in human subjects, whereas in guinea pigs, steeper TI functions tended to have lower detection threshold at the 40-ms pulse-train duration [r = 0.62, p = 0.002].
FIG. 3.
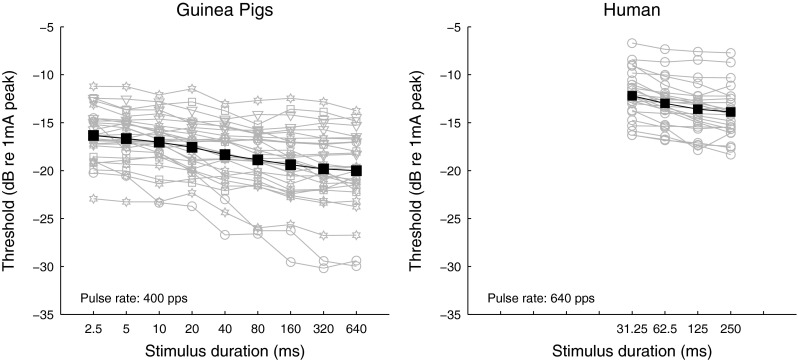
Temporal integration (TI) functions measured from guinea pigs (left) and humans (right). Left panel: thresholds are shown for individual guinea pigs in different grey open symbols (see legend in Figure 2) and for group mean in black filled squares. Right panel: thresholds are shown for two individual stimulation sites measured in 12 ears in gray open circles and for group mean in black filled squares.
Relationship Between the Two Integration Functions
The magnitudes of the TI and MPI function slopes were compared for each subject group. For guinea pigs, slopes of the MPI functions were significantly steeper than those of the TI functions [t (27) = 9.6, p < 0.001]. This difference was attributed to the MPI functions being steeper than the TI functions in the 313–625-pps range [t (27) = 2.16, p < 0.05] and above 1,000 pps [t (27) = 14.75, p < 0.001]. In humans, slopes of the MPI functions were also significantly steeper than those of the TI functions [t (23) = 4.69, p < 0.001)], due to the MPI functions being steeper than the TI functions in the 313–625-pps range [t (23) = 6.9, p < 0.001]. Slopes above 1,000 pps were not assessed in humans in this study.
Analyses were then performed to examine if the slopes of the two integration functions were correlated. In guinea pigs, a strong correlation between the slopes of the MPI below 1,000 pps and TI functions was observed [r = 0.84, p = 0.001]. For this result, one data point (Fig. 4, left panel, gray symbol) was omitted based on a Cook’s distance test that revealed a statistically significant leverage in regression when that point was included. However, the correlation remained strong, when the identified outlier was included in the analysis [r = 0.79, p < 0.001]. Interestingly, the slopes of the MPI functions above 1,000 pps were not related to those of the TI functions [r = 0.04, p = 0.82]. For human data, since the 640-pps 250-ms threshold was a common data point of the two functions, the data point was removed from the TI function for performing the correlational analysis. An univariate ANOVA with MPI slopes as the dependent variable, subject as the fixed factor, and TI slopes as the covariate showed that both the between-site [F (1) = 18.76, p < 0.001] and between-subject variance [F (1) = 8.863, p = 0.007] in the MPI slopes were accounted for by the TI slopes. In addition, the integration slopes were averaged between the two stimulation sites in each ear and the averaged MPI slopes were correlated with those of the TI slopes across subjects [r = 0.62, p < 0.001]. These results indicate that within an ear, stimulation sites with steeper MPI slopes tended to also have steeper TI slopes. Across ears, subjects with steeper MPI slopes tended to also have steeper TI slopes.
FIG. 4.
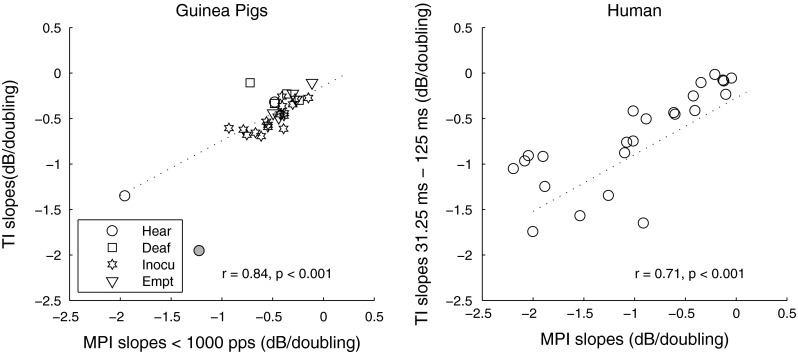
Correlation between slopes of the MPI functions and TI functions in guinea pigs (left) and humans (right). Different symbols represent different guinea pig groups (please see “Methods” for details of the groups). The one guinea pig data point that showed statistically significant Cook’s distance is shown in gray and was removed from the statistical analysis for this comparison. The regression lines show linear fit to the data. Correlation coefficients and p values are shown in each panel.
Relationship Between the Integration Measures and Cochlear Health
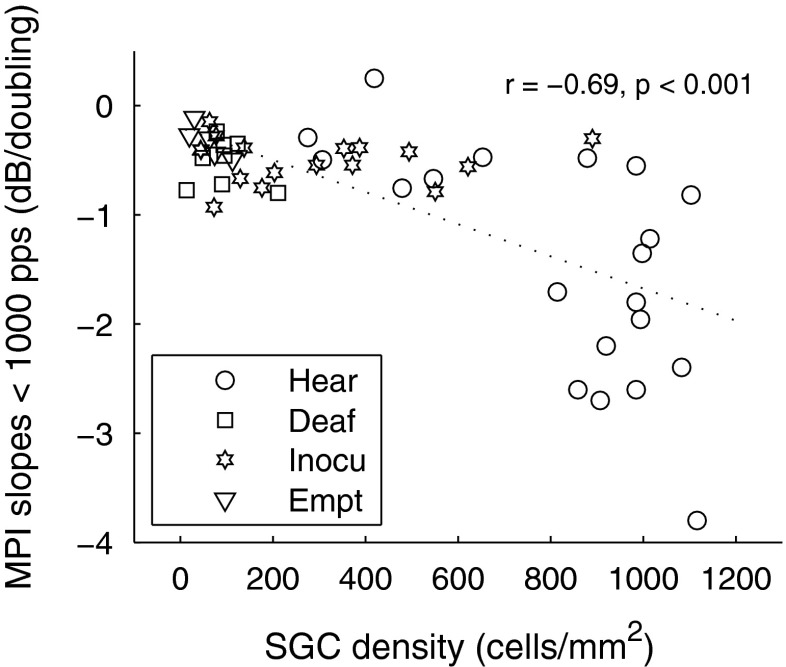
Histological and electrophysiological data from the guinea pigs included SGN density, IHC counts, and ESA levels recorded from the electrode of interest. Collectively, we refer to these data as measures of cochlear health. Correlations between each pair of the three cochlear health variables and their marginal correlations (correlations ignoring other variables) with the various integration slopes are shown in Table 3 (note that the sign of the correlations was negative indicating more negative slopes predicting healthier cochlea). All marginal correlations were significant except for MPI slopes above 1,000 pps with SGN density, and TI slopes with ESA levels. A regression analysis revealed that for the first arm of the MPI function (<313 pps), IHC survival was found to be the strongest predictor [F (1) = 45.76, p < 0.001] and explained 48.8 % of the variance. For the second arm of the MPI function (313–625 pps), SGN density was found to be the strongest predictor [F (1) = 23.72, p < 0.001] and explained 33.1 % of the variance. For the MPI slopes below 1,000 pps as a single segment, SGN density as well as IHCs were found to be the significant predictor variables, and jointly explained 51.6 % of the variance [F (2) = 25.10, p < 0.001]. For MPI slopes above 1,000 pps, cochlear health was a weaker predictor, with ESA and SGN density jointly explaining only 26.6 % of the variance [F (2) = 8.51, p = 0.001]. For TI slopes, similar to the first arm of the MPI functions, IHC was the strongest predictor [F (1) = 15.16, p = 0.001] and explained 34.4 % of the variance. Using SGN density as the dependent variable, regression analysis revealed that, among the MPI slopes in various pulse-rate ranges, the strongest predictor for SGN density was MPI slopes below 1,000 pps as a single segment [F (1) = 43.14, p < 0.001]. The marginal correlation is shown in Figure 5.
TABLE 3.
Correlation coefficients (r)
| SGN density | IHC | ESA | |
|---|---|---|---|
| MPI slopes (5–156 pps) | −0.67* | −0.70* | −0.58* |
| MPI slopes (313–625 pps) | −0.58* | −0.53* | −0.49* |
| MPI slopes <1,000 pps | −0.69* | −0.68* | −0.57* |
| MPI slopes >1,000 pps | −0.19 | −0.40* | −0.43* |
| TI slopes | −0.51* | −0.60* | −0.40 |
| SGN | 1 | 0.82* | 0.83* |
| IHC | 1 | 0.85* | |
| ESA | 1 |
*p < 0.0063 (Bonferonni corrected criterion)
FIG. 5.
Correlation between MPI slopes below 1,000 pps and SGN density (marginal correlation ignoring other variables). Each data point represents one guinea pig. Different symbols represent different guinea pig groups (please see Figure 2 caption and “Methods” for details of the groups). The regression line represents the linear fit to the data. The correlation coefficient and p value are shown in top right corner of the figure.
Discussion
The present study examined detection threshold versus pulse rate (MPI) functions and detection threshold versus stimulus duration (TI) functions in guinea pigs and humans with cochlear implants. In order to understand whether the effect of cochlear health on the integration slopes in guinea pigs reported previously (Kang et al. 2010; Pfingst et al. 2011) and here can be extrapolated in human subjects, we compared the characteristics of the two integration functions between the two subject groups. Magnitude of the slopes for the MPI functions was not different between the two subject groups in the pulse rate range common to both groups, and so was the magnitude of the TI functions in the common stimulus duration range.
The two subject groups shared another characteristic in the integration functions. That is, the magnitude of the TI function slopes was similar to that of the MPI function slopes only in the low pulse rate range. This was due to a trend that the MPI functions became progressively steeper from below approximately 300 pps, to between 300 and 600 pps, and steeper again above 1,000 pps. The slope increase at 1,000 pps has been well documented in both guinea pig and human studies (Pfingst et al. 2011; Zhou et al. 2012) and is thought to reflect a facilitative neural mechanism where residual depolarization on the cell membrane following sub-threshold pulses helps reduce neural thresholds to subsequent pulses in pulse trains with interpulse intervals smaller than 1 ms (Middlebrooks 2004). The slope change observed at approximately 300 pps, which was more apparent in human subjects than in guinea pigs in this study, could also be attributed to the effect of interpulse interval. Assuming that the most effective integration occurs within 3 ms, the doubling of pulse rate from 300 to 600 pps places two pulses within the short-term integration window, and the threshold decrease is roughly equivalent to the threshold decrease for detecting a pulse pair close in time (within 3 ms) relative to detecting a single pulse. Below 300 pps, the observed decreases in threshold are consistent with a model in which the probability that the integration-window outputs exceed threshold increases as the number of looks at the stimulus increases (Viemeister and Wakefield 1991). The rate of threshold decrease with stimulus duration (i.e., TI function slope) appears to be mediated by a similar mechanism as that for MPI at these low rates. It is important to note that the absolute thresholds of TI functions studied in the current experiment do reflect a mechanism of short-term integration, since the stimuli used for the TI functions were greater than 300 pps for the guinea pig group (400 pps) and for the human group (640 pps). Nonetheless, the rate of threshold change with stimulus duration (slopes of the TI functions) was significantly correlated across ears with MPI function slopes only at pulse rates below 300 pps, suggesting a mechanism based on increased looks at the stimulus. Interestingly, while the magnitude of the TI function slopes were only similar to those for MPI functions at the low rates, they were correlated with the slopes of the MPI function below 1,000 pps as one segment for both groups of subjects (Fig. 4). This indicates that while the various integration mechanisms produce different slopes for the two functions, or different slopes for different arms of the same function, these mechanisms seem to produce correlated threshold differences.
One characteristic of the human integration functions that was different from the guinea pigs was that the integration slopes were independent of detection thresholds when compared to thresholds measured at low pulse rates or low stimulus durations where integration was minimal. Detection threshold is presumably a function of both neural survival and factors related to electro-neuron interface such as distance of the electrodes from the neurons (Long et al. 2014). In guinea pigs, the electrode array at the apical end typically fills the whole width of the scala and thus the distance from the test electrodes to the modiolus in this study tended to be uniform. Variation in the detection thresholds therefore was likely related to neural status alone. In humans, the distance between the electrodes and the neural elements is more variable across stimulation sites and ears, which might explain why thresholds did not necessarily co-vary with the slopes of the MPI functions or slopes of the TI functions. The lack of correlation between integration and detection thresholds at low rates was also reported in Zhou et al. (2012).
A number of studies have reported the effects of neural survival on electrophysiological measures in implanted guinea pigs (e.g., Prado-Guitierrez et al. 2006; Ramekers et al. 2014). Here, we report the effects of various aspects of cochlear health of the implanted ears on psychophysical integration of electrical pulses. Three aspects of cochlear health were considered in this study: IHC counts, SGN density, and the level of ESA of the surviving neurons near the electrode where the psychophysical data were obtained. Slopes of the TI functions and MPI functions in different pulse rate ranges were related to one or more features of cochlear health. The MPI slopes above 1,000 pps were only weakly associated with cochlear health. This might be due to the facilitative mechanism of partial depolarization occurring on a single-neuron level so that integration should be less dependent on neural density than is the case below 1,000 pps. Below 1,000 pps, each pulse in the stimulus might elicit responses from a different set of neurons depending on refractoriness, which demands a good quantity of available neurons. This mechanism however is not necessary for threshold decrease as a result of partial depolarization. The strongest predictor for SGN density was MPI slopes below 1,000 pps as one segment.
It is worth noting that IHC counts did explain a significant amount of variance in the MPI slopes at the low pulse rates and jointly with SGN density for rates below 1,000 pps as one segment. Unlike in humans, auditory nerve fibers in deafened guinea pigs degenerate fairly quickly. In guinea pigs studied in this experiment, good SGN density primarily depended on the presence of IHCs in the animal subjects as indicated by a high correlation between SGNs and IHCs. The only cases with steep MPI slopes were the ears from the hearing guinea pig group that had very high SGN densities accompanied by high IHC counts (Fig. 5). We cannot say on the basis of these data whether it was the IHCs or SGN density that was responsible for the steep MPI slopes. Mechanisms by which hair cells might contribute to the integration slopes, besides their supporting role in the preservation of the auditory nerve, include the creation of spontaneous activity in the auditory nerve or possibly an electrophonic effect (van den Honert and Stypulkowski 1984).
IHCs are known to be responsible for the generation of spontaneous activity in the auditory nerve. The ESA measure supports this interpretation. The lack of spontaneous activity in the auditory nerve leads to abnormally high across-fiber synchrony in response to electrical stimulation (Wilson et al. 1997; Matsuoka et al. 2000). If the fibers are all silent in the absence of stimulation, they all tend to respond in synchrony to the first pulse in an electrical pulse train and then go into a refractory state so that the response to a closely following electrical pulse produces little response. After recovery from refraction, the recovered fibers again tend to respond in synchrony to the next pulse in the train. This repeating sequence results in an alternating high and low neural response to sequential pulses at a particular rate for a few hundred milliseconds. At high stimulation rates, it is therefore likely that the electrical pulses were under sampled due to the refractory effects limiting increases in neural excitation for an increase in pulse rate. In addition, the highly synchronous response of the auditory nerve to some pulses might significantly alter the central processing of the auditory nerve input.
While the contribution of the IHCs to the integration slopes could be their effects on SGN survival or spontaneous activity, we cannot rule out the possibility that the IHCs were stimulated directly producing thresholds lower than the direct-membrane depolarization thresholds (Moxon 1971; van den Honert and Stypulkowski 1984). This might explain why in guinea pigs steeper MPI cases often tended to have lower absolute thresholds at the low rate or short duration, a trend not observed in human subjects.
Data from human subjects however suggest that the presence of IHCs or their supporting effects might not be necessary for integration and that SGN density might play a more important role. Zhou et al. (2012) and the results from the present study showed that a wide range of integration slopes can be found in human subjects without measurable acoustic hearing. In deaf human ears, many auditory nerve fibers survive for long periods after deafness, possibly due to neurotrophin support from supporting cells. SGN density levels in the absence of hair cells in humans can be much higher than those in neomycin-deafened guinea pigs (Hinojosa and Marion 1983; Nadol et al. 2001; Fayad and Linthicum 2006). It is unknown if the auditory nerve is spontaneously active in these human subjects, but it seems unlikely given what is known about the requirements for spontaneous activity in animals. The human data suggest that SGN alone might be a sufficient condition for integration.
One explanation for the correlation between SGN density and the integration slopes is that reduced neural responsiveness is rooted with the health of the surviving auditory nerves that might co-vary with their density. Animal studies have shown a high correlation across ears between the counts of spiral ganglion cells and the morphology of the surviving auditory neurons (Zhou et al. 1995a, b). In mice that have sparse neural survival, the surviving fibers also tend to have reduced myelination. These fibers with myelination deficit have been shown to have prolonged refractoriness, which could lead to reduced neural responsiveness at higher stimulation rates. It is possible that poor physiological status could result in greater neural adaptation, or fatigue, with an increase of stimulation rate or duration.
It has been suggested that with sparse neural survival, which possibly produces broader spread of excitation, the growth of neural excitation with stimulus level might be steeper than in cases with good neural survival. The steeper neural excitation versus level function could explain shallow integration slopes because, in order to compensate for an increase in pulse-train duration or stimulation rate, a smaller change in stimulus level would be required to maintain stimulus detection. This has been used to partially explain shallow TI functions in cases of sensory hearing impairments (Carlyon et al. 1990), as well as in cochlear implant users (Donaldson et al. 1997).
The hypotheses that are proposed above to account for the association between slopes of the integration measures and various features of cochlear health remain to be explicitly tested, as do the clinical applications of these measures. In a recent study using human subjects with cochlear implants, we found that the slopes of the MPI functions predicted sentence recognition performance in noise as well as the transmission of spectrally dependent articulatory features such as place of articulation of consonants (Zhou and Pfingst 2014b). However, it remains to be evaluated if these measures can potentially be used in place of other psychophysical measures to guide site-selection (e.g., Garadat et al. 2013; Zhou and Pfingst 2012) or rehabilitation strategies (e.g., Zhou and Pfingst 2014a) for improving speech recognition with cochlear implants.
Acknowledgments
We thank our dedicated subjects with cochlear implants. The research was supported by NIH-NIDCD R01 DC010786, R01 DC 007634, R01 DC010412, T32 DC00011, P30 DC05188, the U. of M. Center for Organogenesis, and a contract from MED-El. We thank Jennifer M. Benson, Lisa L. Kabara, and Melissa M. Watts for their assistance with data collection and the laboratory of Dr. Yehoash Raphael for contributions to the gene therapy and histological work in guinea pigs.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Carlyon RP, Buus S, Florentine M. Temporal integration of trains of tone pulses by normal and by cochlearly impaired listeners. J Acoust Soc Am. 1990;87:260–268. doi: 10.1121/1.399293. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Wieringen AV, Deeks JM, Long CJ, Lyzenga J, Wouters J. Effect of inter-phase gap on the sensitivity of cochlear implant users to electrical stimulation. Hear Res. 2005;205:210–224. doi: 10.1016/j.heares.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Dolan FD, Alfred NL, Gopal A. Asynchronous neural activity recorded from the round window. J Acoust Soc Am. 1990;87:2621–2627. doi: 10.1121/1.399054. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Viemeister NF, Nelson DA. Psychometric functions and temporal integration in electric hearing. J Acoust Soc Am. 1997;101:3706–3721. doi: 10.1121/1.418330. [DOI] [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, Mladejovsky MG, Parkin JL. Auditory prostheses research with multiple channel intracochlear stimulation in man. Ann Otol Rhinol Laryngol. 1978;87:1–39. [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Florentine M, Fastl H, Buus S. Temporal integration in normal hearing, cochlear impairment, and impairment simulated by masking. J Acoust Soc Am. 1988;84:195–203. doi: 10.1121/1.396964. [DOI] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, Pfingst BE. Using temporal modulation sensitivity to select stimulation sites for processor MAPs in cochlear implant listeners. Audiol Neurootol. 2013;184:247–260. doi: 10.1159/000351302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken GM, Bhat VK, Hutchison-Clutter M. Auditory temporal integration and the power function model. J Acoust Soc Am. 1990;88:767–778. doi: 10.1121/1.399726. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Green DM. Auditory detection of a noise signal. J Acoust Soc Am. 1960;32:121–131. doi: 10.1121/1.1907862. [DOI] [Google Scholar]
- Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann N Y Acad Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users. J Acoust Soc Am. 2004;115:1885–1888. doi: 10.1121/1.1701895. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Long CJ, Holden TA, McClelland GH, Parkinson WS, Shelton C, et al. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol. 2014;15:293–304. doi: 10.1007/s10162-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: additive Gaussian noise. Hear Res. 2000;149:129–137. doi: 10.1016/S0378-5955(00)00173-8. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. Loudness perception with pulsatile electrical stimulation: the effect of interpulse intervals. J Acoust Soc Am. 1998;1042:1061–1074. doi: 10.1121/1.423316. [DOI] [PubMed] [Google Scholar]
- McKay CM, Lim HH, Lenarz T. Temporal processing in the auditory system: insights from cochlear and auditory midbrain implantees. J Assoc Res Otolaryngol. 2013;14:103–124. doi: 10.1007/s10162-012-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR, Plack CJ, Biswas AK. The shape of the ear’s temporal window. J Acoust Soc Am. 1988;83:1102–1116. doi: 10.1121/1.396055. [DOI] [PubMed] [Google Scholar]
- Moxon ED (1971) Neural and mechanical responses to electric stimulation of the cat’s inner ear. MIT-Doctoral Dissertation, Cambridge
- Nadol JB Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT Jr, Shallop JK (2001) Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 110:883–891 [DOI] [PubMed]
- Oxenham AJ, Moore BC. Modeling the additivity of nonsimultaneous masking. Hear Res. 1994;80:105–118. doi: 10.1016/0378-5955(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Penner MJ. A power law transformation resulting in a class of short-term integrators that produce time-intensity trades for noise bursts. J Acoust Soc Am. 1978;63:195–201. doi: 10.1121/1.381712. [DOI] [PubMed] [Google Scholar]
- Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK. Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res. 2006;215:47–55. doi: 10.1016/j.heares.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL. Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am. 2011;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, DeHaan DR, Holloway LA. Stimulus features affecting psychophysical detection thresholds for electrical stimulation of the cochlea. I: phase duration and stimulus duration. J Acoust Soc Am. 1991;90:1857–1866. doi: 10.1121/1.401665. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Morris DJ. Stimulus features affecting psychophysical detection thresholds for electrical stimulation of the cochlea. II: frequency and interpulse interval. J Acoust Soc Am. 1993;94:1287–1294. doi: 10.1121/1.408155. [DOI] [PubMed] [Google Scholar]
- Plomp R, Bouman MA. Relation between hearing threshold and duration for tone pulses. J Acoust Soc Am. 1959;31:749–758. doi: 10.1121/1.1907781. [DOI] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, et al. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 2014;15:187–202. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searchfield GD, Munoz DJB, Thorne PR. Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear Res. 2004;192:23–35. doi: 10.1016/j.heares.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-X. [DOI] [PubMed] [Google Scholar]
- Shannon RV. A model of threshold for pulsatile electrical stimulation of cochlear implants. Hear Res. 1989;40:197–204. doi: 10.1016/0378-5955(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Psychophysics in cochlear implants. Audiological foundations edited by Tyler RS. San Diego: Singular; 1993. pp. 357–388. [Google Scholar]
- van den Honert C, Stypulkowski PH (1984) Physiological properties of the electrically stimulated auditory nerve. II. single fiber recordings. Hear Res 14:225–243 [DOI] [PubMed]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Viemeister NF, Wakefield GH. Temporal integration and multiple looks. J Acoust Soc Am. 1991;90:858–865. doi: 10.1121/1.401953. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. Am J Otol. 1997;18:S30–S34. [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Psychophysically based site selection coupled with dichotic stimulation improves speech recognition in noise with bilateral cochlear implants. J Acoust Soc Am. 2012;132:994–1008. doi: 10.1121/1.4730907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Effects of site-specific level adjustments on speech recognition with cochlear implants. Ear Hear. 2014;35:30–40. doi: 10.1097/AUD.0b013e31829d15cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Relationship between a psychophysical estimate of neural health and speech recognition with cochlear implants. J Acoust Soc Am. 2014;136:1257–1268. doi: 10.1121/1.4890640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Xu L, Pfingst BE. Characteristics of detection thresholds and maximum comfortable loudness levels as a function of pulse rate in human cochlear implant users. Hear Res. 2012;284:25–32. doi: 10.1016/j.heares.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Assouline JG, Abbas PJ, Messing A, Gantz BJ. Anatomical and physiological measures of auditory system in mice with peripheral myelin deficiency. Hear Res. 1995;88:87–97. doi: 10.1016/0378-5955(95)00104-C. [DOI] [PubMed] [Google Scholar]
- Zhou R, Abbas PJ, Assoulin JG. Electrically evoked auditory brainstem response in peripherally myelin-deficient mice. Hear Res. 1995;88:98–106. doi: 10.1016/0378-5955(95)00105-D. [DOI] [PubMed] [Google Scholar]