Abstract
Cinnamomum osmophloeum ct. linalool (土肉桂 tǔ ròu guì) is one chemotype of the indigenous cinnamons in Taiwan. This study examined the anxiolytic potency of leaf essential oil (LEO) from C. osmophloeum ct. linalool and its main constituent on 4-week ICR mice using an open field test (OFT), a light–dark test (LDT) and an elevated plus maze test (EPT). After oral administration of corn oil, LEO (250 mg/kg and 500 mg/kg), S-(+)-linalool (500 mg/kg), R-(−)-linalool (500 mg/kg), and trazodone hydrochloride (75 mg/kg) for 14 days, the anxiolytic effects on mice behavior were evaluated. The results showed that LEO from C. osmophloeum ct. linalool leaves and S-(+)-linalool can significantly increase the time mice remained in the center area of the OFT, the illuminated area of the LDT and the open arms of the EPT without any side effects affecting motor activity, indicating excellent anxiolytic responses. Furthermore, results from the measurements of monoamines in mice brain revealed decreases in serotonin, dopamine, and norepinephrine, which are consistent with their anxiolytic effects in animal models. The findings obtained suggest that LEO from C. osmophloeum ct. linalool and its major compound, S-(+)-linalool, possess anxiolytic properties without any side effects and thus support their potential use in treatment of anxiety disorders.
Keywords: anxiolytic activity, Cinnamomum osmophloeum ct. linalool, leaf essential oil, S-(+)-linalool, monoamines
Graphical abstract
1. Introduction
Rapid changes in the global environment have caused tremendous stress to many people, and in several cases has resulted in mental disorders or mental illness. Anxiety is one of the mental disorders caused by worries, fears, and tension. Mental disorders can influence physical or mental health, and also affects social, family, and job responsibilities.1 Generally, anxiety disorders are treated with medication, specific types of psychotherapy, or both. However, the medications have several side effects, including sweating, headache, unusual tiredness, weakness, irritability, changes in sexual desire or ability, drowsiness, and unusual involuntary movement.2 These side effects have a negative effect on the patient’s daily life; hence, it is both necessary and important to find a treatment with fewer side effects. As an alternative treatment approach to anxiety disorders, aromatherapy using leaf essential oils (LEOs) extracted from natural plants has fewer side effects.
As previously reported, many essential oils from plants can relieve stress and reducing anxiety.3 Two theories account for the effect of aromatic components in essential oils on physical and psychological functions of humans. One is that the aromatic components are directly linked to the odor receptors and release odor signals, which will be transmitted to the olfactory nerve. The other is a different subjective experience, which would lead to different physiological and psychological feelings. For example, essential oil from Acorus calamus has antidepressant and anxiolytic effects4; tea from Aloysia citriodora has good sedative and anxiolytic activities5; essential oil of ylang-ylang (Cananga odorata) helps decrease blood pressure and pulse rate and increase attentiveness and alertness6; essential oil of Citrus aurantium has demonstrated sedative effects7; essential oil of Citrus bergamia can relieve anxiety and calm fears8; aqueous extract of Coriandrum sativum seed has been recommended for relieving anxiety and insomnia in Iranian folk medicine9; and Salvia sclare essential oil can also ease states of anxiety such as fear and paranoia.10 In particular, essential oil from lavender (Lavandula angustifolia), which can reduce overexcitement, stabilize mood, and reduce anxiety and depression, is most commonly used in daily life. Linalool is the major compound of lavender, accounting for 23% of the content in essential oil.11 Psychopharmacological in vivo evaluation of linalool has shown that this compound has dose-dependent sedative effects on the central nervous system.
Cinnamomum osmophloeum ct. linalool (土肉桂 tǔ ròu guì) is one of the endemic hardwood species in Taiwan. In particular, it is one chemotype of the indigenous cinnamon, and our previous results revealed that essential oil of its leaves contains about 90% linalool; and it is an S-isomer.12 For these reasons, C. osmophloeum ct. linalool may be a promising commercial source of essential oil for aromatherapies. However, little is known about its anxiolytic effect. Therefore, in this study, the anxiolytic potency of LEO from C. osmophloeum ct. linalool and its main constituents were examined using the following animal models, including an open field test (OFT), a light–dark test (LDT), and an elevated plus maze test (EPT). It is expected that LEO from C. osmophloeum ct. linalool could be developed into cheap and safe food or health products with anxiolytic effects.
2. Materials and methods
2.1. Plant materials
C. osmophloeum ct. linalool (土肉桂 tǔ ròu guì) leaves were collected from Lienhuachih Research Center in Nantou County of central Taiwan. The species were identified by Mr. Yen-Ray Hsui of the Taiwan Forestry Research Institute. A voucher specimen (COLL) of each sample was deposited in the Laboratory of Wood Chemistry (School of Forestry and Resource Conservation, National Taiwan University). All samples were stored at 25°C until analysis.
2.2. Chemicals
Trazodone hydrochloride (Sigma, St. Louis, MO, USA) was dissolved in water (reverse osmosis H2O, RO H2O), while R-(−)-linalool (Sigma), S-(+)-linalool (separated and purified from LEO), and LEO were dissolved in corn oil (Sigma). The doses of trazodone hydrochloride used as positive controls were selected from the studies on which the methodologies were based.
2.3. Sample preparation
Fresh mature C. osmophloeum ct. linalool leaves were cleaned with distilled water and air dried at 25°C. These samples (200 g each), in triplicate, were subjected to hydrodistillation for 6 hours using a Clevenger-type apparatus (Dinhaw Enterprise Co., New Taipei, Taiwan). Meanwhile, the yield of LEO after hydrodistillation was calculated.
2.4. Extraction and isolation
The S-(+)-linalool from C. osmophloeum ct. linalool LEO was separated and purified by 1100 series high-performance liquid chromatography (HPLC, Agilent, Alpharetta, GA, USA) on a model pump equipped with an ultraviolet detector (254 nm) and a 250 mm × 10 mm internal diameter, 5 μm silica gel column (Phenomenex, Torrance, CA, USA). The mobile phase was solvent A, 100% n-hexane; and solvent B, 100% ethyl acetate. Elution conditions were 0–8 minutes of 85% A; 8–10 minutes of 85–0% A to B (linear gradient) at flow rate of 4 mL/min. The resulting retention time of S-(+)-linalool was 6.05 minutes.
2.5. Animals
Four-week-old male ICR mice (28–36 g) used were purchased from BioLASCO Co. (Taipei, Taiwan) and groups of four mice each were housed in plastic cages. Mice were allowed 2 weeks to adapt to the environment before test. All mice were maintained under controlled temperature (22 ± 2°C), and illumination (12-hour light-dark cycle), with free access to food (LabDiet 5001 Rodent diet, Purina Mills LLC, St. Louis, MO, USA) and water. To reduce the influence of light variations, all assays were conducted in a special noise-free room with controlled illumination. Mice were treated orally with corn oil (controls) and different doses of LEO of C. osmophloeum ct. linalool (250 mg/kg and 500 mg/kg), 500 mg/kg S-(+)-linalool, and 500 mg/kg R-(−)-linalool, all of which were diluted in corn oil. Positive control groups were administered with 75 mg/kg of trazodone hydrochloride dissolved in water (RO H2O) (positive control, P.C.) (for OFT, LDT, and EPT). Mice undergoing various treatments were administered with a 0.2 mL sample for 14 days before the test.
2.6. Open field test
Following Woode et al,13 the OFT area in this study had black walls and floor (30 cm × 40 cm × 40 cm) (Fig. 1A) divided into 16 squares of equal area. LEO from C. osmophloeum ct. linalool (250 mg/kg and 500 mg/kg), 500 mg/kg S-(+)-linalool and 500 mg/kg R-(−)-linalool, and 75 mg/kg of trazodone hydrochloride (P.C.) were administered to mice 1 hour prior to the open field test. The open field was used to evaluate the exploratory activity of the animal. The observed parameters were the time mice stayed in the periphery, distance, and average speed of locomotor activity, which were recorded for 5 minutes.
Fig. 1.
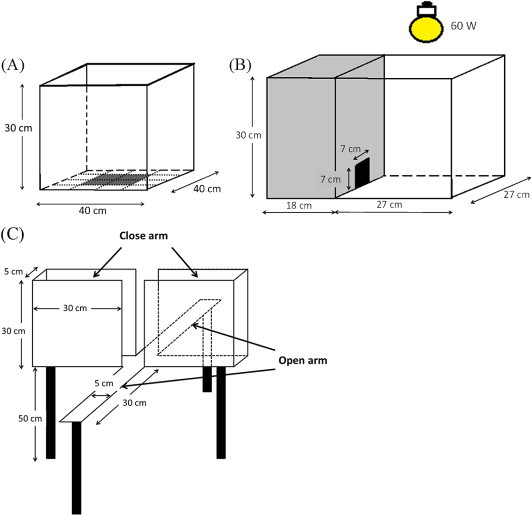
Three typical setups of anxiolytic animal model. (A) Open field test. (B) Light–dark test. (C) Elevated plus maze test.
2.7. Light–dark test
The apparatus (Fig. 1B) consisted of a box (45 cm × 27 cm × 30 cm) with two compartments, one small (18 cm × 27 cm) and one large (27 cm × 27 cm), with a door-like opening (7 cm × 7 cm) in between as the partition. The small compartment was painted black and was light-free, whereas the large one was illuminated with a white light and brightly lit with a 60-W light source. LEO from C. osmophloeum ct. linalool (250 mg/kg and 500 mg/kg), 500 mg/kg S-(+)-linalool and 500 mg/kg R-(−)-linalool, and 75 mg/kg of trazodone hydrochloride (P.C.) were administered to mice 1 hour prior to the LDT. When the test started, each animal was placed in the center of the illuminated compartment, facing the light area. The amount of time mice stayed in illuminated and dark places, and the number of times they got in and out of each compartment was recorded for 5 minutes.14 The test was performed in a quiet and darkened room, and mice were kept in this room for at least 1 hour before the test.
2.8. Elevated plus maze test
The method used was adopted from Pellow et al15 with some modifications for mice. The elevated plus maze (Fig. 1C) was made of opaque Plexiglas. It consisted of two opposite open arms (30 cm × 5 cm) without side walls and two close arms (30 cm × 5 cm × 30 cm), extending from a central square platform (5 cm × 5 cm). The maze was elevated to the height of 50 cm from the floor and placed in a lit room. The animals were divided into 10 groups of eight animals each and received treatments similar to those described for the OFT. LEO from C. osmophloeum ct. linalool (250 and 500 mg/kg), 500 mg/kg S-(+)-linalool and 500 mg/kg R-(−)-linalool, and 75 mg/kg of trazodone hydrochloride (P.C.) were administered to mice 1 hour prior to the EPT. Animals were placed individually in the central platform of the EPT for 5 minutes and the following behaviors were recorded on a videotape: (1) number of times mice got in and out of the closed and open arms (absolute value and percentage of the total); (2) time spent exploring the open and closed arms of the maze (absolute time and percentage of the total); (3) number of head-dips (absolute value and percentage of the total) with the head protruding over the edge of either an open (unprotected) or closed (protected) arm and down toward the floor; (4) number of stretch-attend postures (absolute value and percentage of the total) when the mouse stretches forward and retracts to its original position form an open (unprotected) or a closed (protected) arm. Entry into an arm was counted only when all four limbs of the mouse were within a given arm.
2.9. Quantification of monoamines
After the EPT session, the mice were sacrificed by cervical dislocation and then decapitated immediately. Their brains were rapidly removed, and the frontal cortex, striatum, and hippocampus were dissected on an ice-cold plate. The brain tissues were stored at −80°C for further analysis. All tissues were homogenized with 5 mL ice-cold homogenizing solution, containing 0.1N hydrochloric acid, 0.1μM ascorbic acid, 1.5 mg/100 mL pargyline, and 50 pg/μL isoproterenol, according to the method of Cheng et al16 with modifications. After homogenizing, the homogenate solution was centrifuged at 10,000 g for 20 minutes at 4°C. The supernatant was filtered (0.22 μm) and used for monoamine analysis.
The concentration of serotonin (5-hydroxytryptophan, 5-HT), 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and norepinephrine (NE) were measured by high-performance liquid chromatography (HPLC, Bioanalytical Systems Inc., West Lafayette, IN, USA) coupled with an electrochemical detector (ECD, Detector LC-4C, Bioanalytical Systems Inc., USA) and a pump (PM-92E, Bioanalytical Systems Inc., USA) with a column (Apollo C18 5 μm, 250 mm × 4.6 mm, Alltech Associates, Deerfield, IL, USA) and an automatic sample injector (Hitachi autosampler L-2200, Tokyo, Japan). The mobile phase contained 20.5 g NaH2PO4, EDTA 185 mg, sodium 1-octanesulfonic acid (SOS) 130 mg, methanol 200 mL, and was adjusted to pH 3.0 using H3PO4. The flow rate was 500 μL/min. The electrochemical detection condition was: range, 5 nA; filter, 0.1 Hz; and AppE cell, 0.75 V.
2.10. Statistical analysis
The statistically significant differences in cultivation on the essential oil yield and linalool content were evaluated with ordinary analysis of variance using the Statistics 17.0 package (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± standard error (n = 8). Different letters indicate significant difference at the level of p < 0.05 according to the Scheffe test.
3. Results
3.1. Effect of leaf essential oils from C. osmophloeum ct. linalool on locomotor activity in mice (OFT)
To examine the anxiolytic effect of LEO and its main constituent on mice, the OFT was carried out. The amount of time mice spent in the periphery served as a measure of their anxiety state, and their locomotor activity was assessed through measurements of distance traveled and average speed. As shown in Fig. 2, the time mice stayed in the periphery for the groups of COLL 250, COLL 500, S(+)-L 500, R(−)-L 500 and P.C. was 258.1, 265.7, 266.5, 261.1, and 262.0 seconds, respectively, times that were obviously shorter than that for the control group (285.0 seconds). This result revealed that LEO from C. osmophloeum ct. linalool, S-(+)-linalool and R-(−)-linalool are capable of reducing anxiety in mice.
Fig. 2.
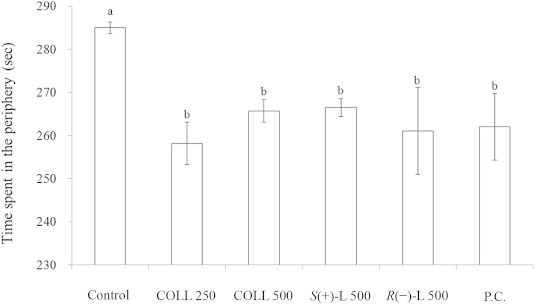
Effect of leaf essential oils from C. osmophloeum ct. linalool on the time spent in the periphery during the open field test in mice. Data are expressed as mean ± standard error (n = 8). The oral administration of corn oil (controls), 250 mg/kg and 500 mg/kg LEO of C. osmophloeum ct. linalool (COLL 250, COLL 500), 500 mg/kg S-(+)-linalool (S(+)-L 500), 500 mg/kg R-(−)-linalool (R(–)-L 500) and 75 mg/kg of the anxiolytic drug trazodone hydrochloride (P.C.). Different letters indicate significant difference at the level of p < 0.05 according to the Scheffe test.
In addition, the total distance traveled by COLL 250, COLL 500, S(+)-L 500, R(−)-L 500, and P.C. groups ranged from 19551.5 mm to 23831.1 mm, and the speed ranged from 65.1 to 79.4 mm/s. Both results indicated no statistically significant difference between the treated and control mice (total distance traveled and speed were 23735.9 mm and 79.1 mm/s, respectively). As a consequence, administration of LEO of C. osmophloeum ct. linalool, S-(+)-linalool, and R-(−)-linalool had no effect on locomotor activity. Rickels et al2 found that side effects of anxiolytic drugs could cause reduction of locomotor activity. Surprisingly, in our study, the locomotion of mice treated with S-(+)-linalool was not affected. In conclusion, LEOs from C. osmophloeum ct. linalool, S-(+)-linalool and R-(−)-linalool have not only anxiolytic effects, but also no negative effect on locomotor activity, unlike commercially available antianxiety drugs.
3.2. Anxiolytic effect of leaf essential oils from C. osmophloeum ct. linalool on mice (LDT)
The LDT is an animal model for identifying the potential of new anxiolytic drugs.14 In general, it is known that animals feel safe in a dark area but are afraid and anxious during exploration in light area. The length of time mice stay in the light area is generally used as an indication of anxiety or pharmacodynamics effects of anxiolytic drugs. Previous reports confirmed that the commercially available anxiolytic drugs could extend the stay of mice in the light area.17,18 In this study, the administration of LEO from C. osmophloeum ct. linalool (250 mg/kg and 500 mg/kg, p.o.), S-(+)-linalool (500 mg/kg, p.o.), R-(−)-linalool (500 mg/kg, p.o.) and trazodone hydrochloride (75 mg/kg, p.o.) (P.C.) in mice induced a significant increase in stay duration in the illuminated compartment of the light–dark apparatus (Fig. 3). The groups of mice administered with LEO, S-(+)-linalool, and R-(−)-linalool at the dose of 500 mg/kg spent a significantly longer time in the illuminated compartment (166.0, 162.1, and 155.0 seconds, respectively) than the control groups (126.0 seconds). The time 250 mg/kg LEO-treated mice spent in the illuminated compartment (138.2 seconds) also increased significantly, though it was shorter than that of the other groups. Mice treated with a standard anxiolytic drug (trazodone hydrochloride 75 mg/kg, p.o.) also extended the stay duration in the illuminated compartment (176.5 seconds). Thus, treatment with 500 mg/kg LEO from C. osmophloeum ct. linalool, S-(+)-linalool, and R-(−)-linalool produced greater anxiolytic effects in LDT than treatment with lower low doses.
Fig. 3.
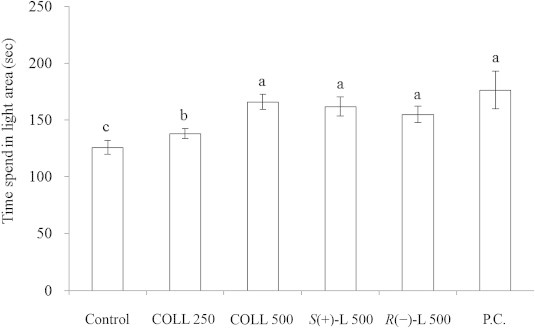
Effects of LEO from C. osmophloeum ct. linalool on the time spent in the light area during the light–dark test in mice. Data are expressed as mean ± standard error (n = 8). The oral administration of corn oil (control), 250 and 500 mg/kg LEO of C. osmophloeum ct. linalool (COLL 250, COLL 500), 500 mg/kg S-(+)-linalool (S(+)-L 500), 500 mg/kg R-(−)-linalool (R(–)-L 500) and 75 mg/kg of the anxiolytic drug trazodone hydrochloride (P.C.). Different letters are significantly different at the level of p < 0.05 according to the Scheffe test.
3.3. Anxiolytic effect of leaf essential oils from C. osmophloeum ct. linalool on mice (EPT)
The EPT is a well-established animal model19 extensively used to identify potential anxiolytic drugs.20 In this study, it is assumed that animals feel safe in the open arms but feel afraid and anxious during exploration in the closed arms. The time mice spent in open arms is generally taken to indicate the effects and properties of anxiolytic drugs. This study evaluated the effects of LEO from C. osmophloeum ct. linalool and its major compound, S-(+)-linalool, at graded doses using EPT. Mice administered with COLL 250, COLL 500, S(+)-L 500, and R(−)-L 500 increased significantly their stay duration in the open arms of the maze (122.8, 102.8, 124.9, and 121.7 seconds, respectively) as compared with the controls (49.4 seconds), but there was no significant difference in comparison with the positive controls (117.9 second) (Fig. 4A). Conversely, the mice in the groups of COLL 250, COLL 500, S(+)-L 500, R(−)-L 500 and positive controls spent shorter time in closed arms than the control groups (Fig. 4B). Identical results were observed regarding the number of entries into open and closed arms of the maze. Results from the current study indicated that LEO from C. osmophloeum ct. linalool and its major compound, S-(+)-linalool, have anxiolytic effects.
Fig. 4.
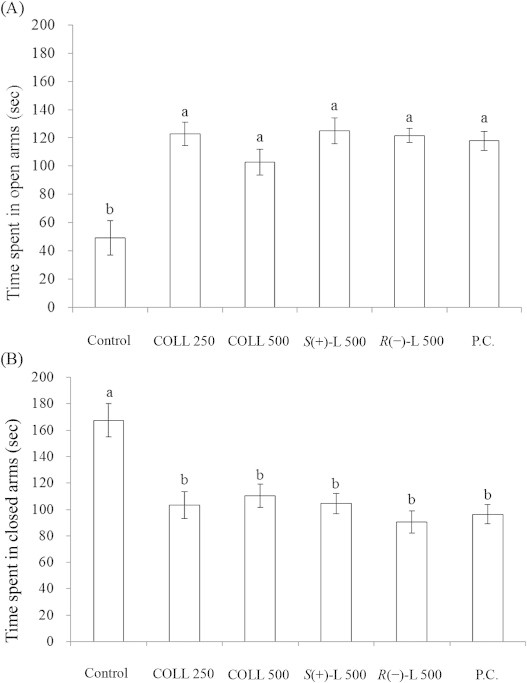
Effects of LEO from C. osmophloeum ct. linalool on the time spent in the (A) open arms (B) close arms during the elevated plus maze test in mice. Data are expressed as mean ± standard error (n = 8). The oral administration of corn oil (Control), 250 mg/kg and 500 mg/kg LEO of C. osmophloeum ct. linalool (COLL 250, COLL 500), 500 mg/kg S-(+)-linalool (S(+)-L 500), 500 mg/kg R-(−)-linalool (R(–)-L 500), and 75 mg/kg of the anxiolytic drug trazodone hydrochloride (P.C.). Different letters are significantly different at the level of p < 0.05 according to the Scheffe test.
3.4. Effect of LEO from C. osmophloeum ct. linalool on serotonin, dopamine, norepinephrine, and their metabolites in mice brain
Mood change and control are related to monoamine neurotransmitters serotonin, dopamine, and norepinephrine (5-HT, DA, and NE), and their metabolites (5-HIAA, HVA, and DOPAC) in brain.21 Thiebot et al20 reported that 5-HT injected into the brain could induce anxiety; by contrast, reduction of concentration of 5-HT would alleviate anxiety. The concentration of monoamines and their metabolites in brain played important roles in reducing and curing anxiety.22 Decreasing concentration of 5-HT, NE, and DA in the brain accompanied by increasing metabolites could produce effects of sedation.23 In addition, Charney and Redmond24 also found that noradrenergic hyperactivity was associated with some human anxiety states. Therefore, HPLC was used in this study to examine the effects of LEO from of C. osmophloeum ct. linalool on 5-HT, NE, DA, and their metabolites in brain of mice.
The results showed that concentrations of 5-HT in frontal cortex and hippocampus were significantly decreased in groups of COLL 250, COLL 500, S(+)-L 500, R(−)-L 500, and positive controls (frontal cortex [99.6 ± 4.5 – 125.0 ± 6.0 ng/g brain tissue] and hippocampus [83.7 ± 3.3 – 109.1 ± 2.8 ng/g brain tissue]) compared with controls (Table 1). However, in this study only high-dosage groups (LEO from C. osmophloeum ct. linalool, S-(+)-linalool and R-(−)-linalool 500 mg/kg) had significantly decreased concentration of 5-HT in striatum (COLL 500, S(+)-L 500, and R(−)-L 500) (188.3 ± 26.9 – 202.3 ± 26.1 ng/g brain tissue) compared with the control group (p < 0.05). In contrast, the concentrations of 5-HIAA in frontal cortex, hippocampus, and striatum were significantly increased in groups of COLL 250, COLL 500, S(+)-L 500, R(−)-L 500, and P.C. compared with the control group (Table 1). The concentration of DA in striatum increased in groups of COLL 500, S(+)-L 500, R(−)-L 500, and P.C. (1917.5 ± 73.5 – 2339.8 ± 329.1 ng/g brain tissue) compared with the control group (p < 0.05), suggesting that linalool enhances the release of dopamine from striatum of mice brain. These findings were similar to the results obtained by Okuyama et al,25 who confirmed the existence of dopamine in brain striatum slices of rat, and Yamada et al26 who observed changes in plasma dopamine levels of ovariectomized female rats. Meanwhile, it was reported that increasing the level of dopamine in striatum in mice brain could improve the symptom of Parkinson disease.27,28 Therefore, mice administered LEO from C. osmophloeum ct. linalool and S-(+)-linalool have increased levels of dopamine in the striatum, indicating that LEO from C. osmophloeum ct. linalool and S-(+)-linalool might be used as an alternative treatment for improving the symptoms of Parkinson disease.
Table 1.
Concentration of 5-HT, 5-HIAA, DA, DOPAC, HVA, and NE in three different sections of the brain after anxiety testing.
| Region | Groups | Dose (mg/kg body weight) | 5-HT |
5-HIAA |
DA |
DOPAC |
HVA |
NE |
|---|---|---|---|---|---|---|---|---|
| (ng/g brain tissue) | ||||||||
| Frontal cortex | Control | 0 | 140.3 ± 6.0a | 58.6 ± 6.5b | 93.7 ± 7.9a | 55.7 ± 6.1a | 123.0 ± 15.0a | 447.0 ± 36.6a |
| COLL | 250 | 116.5 ± 5.2b | 92.8 ± 18.1ab | 65.2 ± 1.9b | 50.5 ± 2.4a | 108.4 ± 19.9ab | 299.9 ± 12.8b | |
| 500 | 99.6 ± 4.5c | 97.9 ± 19.8a | 61.2 ± 1.1b | 48.6 ± 0.8a | 76.8 ± 15.8b,c | 324.7 ± 18.1b | ||
| S(+)-L | 500 | 125.0 ± 6.0b | 113.7 ± 5.4a | 64.8 ± 2.2b | 50.4 ± 1.2a | 58.7 ± 9.0c | 267.9 ± 28.4b | |
| R(−)-L | 500 | 116.6 ± 4.8b | 103.6 ± 11.2a | 68.1 ± 3.0b | 56.7 ± 2.6a | 62.7 ± 3.8c | 341.6 ± 32.6b | |
| P.C. | 115.9 ± 4.8b | 106.9 ± 9.9a | 61.8 ± 1.2b | 50.7 ± 1.5a | 52.3 ± 10.0c | 320.9 ± 11.8b | ||
| Hippocampus | Control | 0 | 128.1 ± 5.8a | 43.0 ± 5.3b | 184.2 ± 19.4a | 100.2 ± 11.9a | 768.3 ± 78.8a | 217.1 ± 31.3a |
| COLL | 250 | 98.0 ± 8.2bc | 60.3 ± 12.0ab | 171.2 ± 12.1b | 121.2 ± 15.3a | 515.2 ± 37.7b | 84.4 ± 24.7bc | |
| 500 | 83.7 ± 3.3c | 49.6 ± 7.1ab | 133.8 ± 19.5c | 75.4 ± 6.9a | 491.1 ± 110.0b | 66.4 ± 31.1c | ||
| S(+)-L | 500 | 93.5 ± 4.2bc | 55.5 ± 15.4ab | 157.3 ± 16.2b | 85.6 ± 23.8a | 353.2 ± 115.0bc | 62.8 ± 8.9c | |
| R(−)-L | 500 | 109.1 ± 2.8b | 75.5 ± 6.1a | 160.9 ± 8.1b | 97.2 ± 14.4a | 350.7 ± 45.6bc | 91.3 ± 7.3c | |
| P.C. | 90.1 ± 5.1c | 71.9 ± 8.0ab | 144.4 ± 17.4bc | 81.9 ± 11.9a | 234.2 ± 20.4c | 103.4 ± 8.6b | ||
| Striatum | Control | 0 | 311.5 ± 18.2a | 117.5 ± 12.7c | 719.5 ± 137.63b | 366.5 ± 52.8b | 1891.3 ± 234.6a | 903.1 ± 111.4a |
| COLL | 250 | 241.9 ± 29.3ab | 226.3 ± 27.1ab | 1510.2 ± 206.4a | 593.7 ± 99.8ab | 1328.1 ± 58.3b | 575.5 ± 96.9b | |
| 500 | 202.3 ± 26.1b | 270.8 ± 29.9a | 1926.5 ± 208.6a | 659.7 ± 85.2ab | 1137.3 ± 42.1b | 407.7 ± 65.7b | ||
| S(+)-L | 500 | 188.3 ± 26.9b | 191.4 ± 9.8b | 2139.7 ± 720.3a | 420.6 ± 43.4b | 968.6 ± 59.6b | 414.5 ± 64.5b | |
| R(−)-L | 500 | 200.5 ± 21.2b | 227.7 ± 22.6ab | 2339.8 ± 329.1a | 792.7 ± 184.3a | 1037.4 ± 67.4b | 369.8 ± 52.9b | |
| P.C. | 284.7 ± 19.7a | 224.7 ± 21.7ab | 1917.5 ± 73.5a | 804.0 ± 103.4a | 617.4 ± 47.3c | 620.2 ± 36.1b | ||
Data are expressed as mean ± standard error. n = 8. Observations were made 60 minutes following the oral administration of corn oil (controls), 250 mg/kg and 500 mg/kg LEO of C. osmophloeum ct. linalool (COLL 250, COLL 500), 500 mg/kg S-(+)-linalool (S(+)-L 500), 500 mg/kg R-(−)-linalool (R(–)-L 500), and 75 mg/kg the anxiolytic drug trazodone hydrochloride (P.C.). Different superscript letters indicate significant differences at the level of p < 0.05 according to the Scheffe test.
5-HT = 5-hydroxytryptophan 5-HIAA = 5-hydroxyindoleacetic acid; DA = dopamine; DOPAC 3,4-dihydroxyphenylacetic acid; HVA = homovanillic acid; NE = norepinephrine.
The concentrations of DA in frontal cortex (61.2 ± 1.1 – 68.1 ± 3.0 ng/g brain tissue) and in hippocampus (133.8 ± 19.5 – 171.2 ± 12.1 ng/g brain tissue) (Table 1) were decreased in all treatment groups. The concentration of HVA in frontal cortex, hippocampus, and striatum decreased in all groups compared with the control group, but the concentration of DOPAC showed no significant difference among all groups in the test (Table 1).
In addition, compared with that of the control group, the concentration of NE decreased significantly in groups of COLL 250, COLL 500, S(+)-L 500, R(−)-L 500, and P.C. in the frontal cortex (267.9 ± 28.4 – 341.6 ± 32.6 ng/g brain tissue), hippocampus (62.8 ± 8.9 – 103.4 ± 8.6 ng/g brain tissue), and striatum (369.8 ± 52.9 – 620.2 ± 36.1 ng/g brain tissue) (p < 0.05) (Table 1). Fukumoto et al29 used LEO and its major component to assess the effects of flavor components on physical and psychological stress. It was shown that the anxiolytic effects obtained were attributed to the decrease in concentrations of 5-HT, NE, and DA by supplementation with LEO and its major component. According to the results obtained, including changes in concentration of monoamines in brains and behaviors observed in OFT, LDT, and EPT, it was concluded that LEO from C. osmophloeum ct. linalool and its major component, S-(+)-linalool, have anxiolytic effects.
4. Discussion
Linalool is the major compound of flavor additives and fragrance oil in perfume industry. Psychopharmacological in vivo evaluation has shown that linalool has dose-dependent effects on the central nervous system, including sedation, hypnotic, anticonvulsant, and anxiolytic effects.30–32
On the basis of previous results, C. osmophloeum ct. linalool is the best plant for obtaining essential oil, because its yield and quality are better than those of other plants. LEO from C. osmophloeum ct. linalool is an easily obtainable source of S-(+)-linalool.12
This study compared S-(+)-linalool with R-(−)-linalool in terms of anxiolytic effects and their side effects on locomotor activity were also evaluated. Previous literature reported that these isomers existed in the essential oil of various plant species. They have shown different effects on physiological activities such as odor threshold33 and alarm pheromone activity.34
In this study, it has also been proven that LEO from C. osmophloeum ct. linalool and its main constituent have anxiolytic potencies in the three animal models, including OFT, LDT, and EPT. In these animal models, the anxiety-related behaviors in mice were decreased, meaning that anxiety in mice was relieved after treatment with LEO (250 and 500 mg/kg), S-(+)-linalool (500 mg/kg), and R-(−)-linalool (500 mg/kg), which were as effective as the reference drug, trazodone hydrochloride. Unlike the contrasting effects of S-(+)-linalool and R-(−)-linalool on mood states demonstrated by Kuroda et al,35 there is no difference in anxiolytic effect between S-(+)-linalool and R-(−)-linalool.
Furthermore, the dosage of sample administered has no side effects on OFT, LDT, and EPT, which were confirmed by the locomotor activity. Our results are consistent with an earlier report that indicated R-(−)-linalool has anxiolytic effects.32
In addition, release of monoamine from mice brain frontal cortex, hippocampus, and striatum were evaluated. The striatum containing numerous DA neurons is an important region of emotions36 and Parkinson disease,27,28 acting as a useful area for analysis of DA release. In previous research, striatum is sensitive to monoterpenes, and the amount of DA released depends on the structures of monoterpene compounds.25 The frontal cortex is the largest mice brain tissue, with few DA and 5-HT neurons. The methods of slicing brain are useful for discerning compounds acting on neurotransmitter release, but they cannot recognize interaction among various regions of brain. The results from quantification of monoamine indicated that the enantiomers of linalool and LEO from C. osmophloeum ct. linalool have similar effects on monoamine release from mice brain tissue. Consequently, LEO from C. osmophloeum ct. linalool and its major component, S-(+)-linalool, might act as an anxiolytic agent via modulation of 5-HT.
5. Conclusion
In conclusion, the results showed that LEO from C. osmophloeum ct. linalool and its major compound, S-(+)-linalool, have anxiolytic effects as good as R-(−)-linalool. LEO from C. osmophloeum ct. linalool and S-(+)-linalool reduced the amount of 5-HT, DA, and NE released from mice brain frontal cortex and hippocampus. It was also found that mice administered LEO from C. osmophloeum ct. linalool and S-(+)-linalool have increased the level of dopamine in striatum, indicating that LEO from C. osmophloeum ct. linalool and S-(+)-linalool might have the potential to be used as reagents to improve Parkinson disease.
The data indicated that the frontal cortex, hippocampus, and striatum were affected by LEO of C. osmophloeum ct. linalool and S-(+)-linalool, and they use other neurotransmitters and brain functions to acquire anxiolytic activity. In addition, no side effects affecting locomotor activity, including drowsiness and unusual involuntary movement, were observed. Therefore, LEO from C. osmophloeum ct. linalool has the potential to be developed into cheap and safe additives and health products with anxiolytic effects.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by a grant (102AS-13.2.4-FB-e1) from the Forestry Bureau, Council of Agriculture, Executive Yuan, Taiwan. The authors thank Mr Yen-Ray Hsui and Dr Jeen-Lian Hwong, of the Taiwan Forestry Research Institute, Council of Agriculture, Executive Yuan, Taiwan and the Lienhuachih Research Center in Nantou County for providing C. osmophloeum ct. linalool materials.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Lee-Yan Sheen, Email: lysheen@ntu.edu.tw.
Shang-Tzen Chang, Email: peter@ntu.edu.tw.
References
- 1.Baldwin D., Bobes J., Stein D.J., Scharwächter I., Faure M. Paroxetine in social phobia/social anxiety disorder: randomised, double-blind, placebo-controlled study. Br J Psychiatry. 1999;175:120–126. doi: 10.1192/bjp.175.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Rickels K., Downing R., Schweizer E. Antidepressants for the treatment of generalized anxiety disorder – a placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50:884–895. doi: 10.1001/archpsyc.1993.01820230054005. [DOI] [PubMed] [Google Scholar]
- 3.William N.S. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4:1305–1316. [PubMed] [Google Scholar]
- 4.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acorus calamus: scientific validation of Ayurvedic tradition from natural resources. Pharmaceutical Biol. 2007;45:651–666. [Google Scholar]
- 5.Carnat A., Carnat A.P., Fraisse D., Lamaison J.L. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia. 1999;70:44–49. doi: 10.1016/j.fitote.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Gaydou E.M., Randriamiharisoa R., Bianchini J.P. Composition of the essential oil of ylang-ylang (Cananga odorata Hook Fil. et Thomson forma genuina) from Madagascar. J Agr Food Chem. 1986;34:481–487. [Google Scholar]
- 7.Jäger W., Buchbauer G., Jirovetz L., Dietrich H., Plank C. Evidence of the sedative effect of neroli oil, citronellal and phenylethyl acetate on mice. J Essential Oil Res. 1992;4:387–394. [Google Scholar]
- 8.Verzera A., Lamonica G., Mondello L., Trozzi A., Dugo G. The composition of bergamot oil. Perfumer and Flavorist. 1996;21:19–34. [Google Scholar]
- 9.Emamghoreishi M., Khasaki M., Aazam M.F. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–370. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Peana A.T., Moretti M.D.L. Pharmacological activities and applications of Salvia sclarea and Salvia desoleana essential oils. Stud Nat Prod Chem. 2002;26:391–423. [Google Scholar]
- 11.Romeo F.V., De Luca S., Piscopo A., Poiana M. Antimicrobial effect of some essential oils. J Essential Oil Res. 2008;20:373–379. [Google Scholar]
- 12.Cheng B.H., Lin C.Y., Yeh T.F., Cheng S.S., Chang S.T. Potential source of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaf: essential oil profile and the enantiomeric purity. J Agr Food Chem. 2012;60:7623–7628. doi: 10.1021/jf302248w. [DOI] [PubMed] [Google Scholar]
- 13.Woode E., Boakye-Gyasi E., Amidu N., Duwiejua M. Anxiolytic and antidepressant effects of a leaf extract of Palisota hirsuta K. Schum. (commelinaceae) in mice. Int J Pharmacol. 2010;6:1–17. [Google Scholar]
- 14.Crawley J.N., Goodwin F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 15.Pellow S., Chopin P., File S.E., Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng F.C., Kuo J.S., Shih Y., Lai J.S., Ni D.R., Chia L.G. Simultaneous measurement of serotonin, catecholamines and their metabolites in mouse-brain homogenates by high-performance liquid-chromatography with a microbore column and dual electrochemical detection. J Chromatogr. 1993;615:225–236. doi: 10.1016/0378-4347(93)80336-3. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez C. Serotonergic mechanisms involved in the exploratory behaviour of mice in a fully automated two-compartment black and white text box. Pharmacol Toxicol. 1995;77:71–78. doi: 10.1111/j.1600-0773.1995.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 18.Costall B., Jones B.J., Kelly M.E., Naylor R.J., Tomkins D.M. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 19.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 20.Thiebot M.H., Soubrie P., Hamon M., Simon P. Evidence against the involvement of serotonergic neurons in the anti-punishment activity of diazepam in the rat. Psychopharmacology. 1984;82:355–359. doi: 10.1007/BF00427685. [DOI] [PubMed] [Google Scholar]
- 21.Ruhé H.G., Mason N.S., Schene A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 22.Beninger R.J. The role of dopamine in locomotor-activity and learning. Bruin Res Rev. 1983;6:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh M.T., Peng W.H., Hsieh C.C. Effects of DL-tetrahydropalmatine on motor activity and the brain monoamine concentration in rats. Chin J Physiol. 1994;37:79–82. [PubMed] [Google Scholar]
- 24.Charney D.S., Redmond D.E. Neurobiological mechanisms in human anxiety. Neuropharmacology. 1983;22:1531–1536. doi: 10.1016/0028-3908(83)90122-3. [DOI] [PubMed] [Google Scholar]
- 25.Okuyama S., Sawasaki E., Yokogoshi H. Conductor compounds of phenylpentane in mycoleptodonoides aitchisonii mycelium enhance the release of dopamine from rat brain striatum slices. Nutr Neurosci. 2004;7:107–111. doi: 10.1080/10284150410001710429. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K., Mimaki Y., Sashida Y. Effects of inhaling the vapor of Lavandula burnatii super-derived essential oil and linalool on plasma adrenocorticotropic hormone (ACTH), catecholamine and gonadotropin levels in experimental menopausal female rats. Biol Pharm Bull. 2005;28:378–379. doi: 10.1248/bpb.28.378. [DOI] [PubMed] [Google Scholar]
- 27.Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 28.Dauer W., Przedborski S. Parkinson's disease: mechanisms and model. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 29.Fukumoto S., Morishita A., Furutachi K., Terashima T., Nakayama T., Yokogoshi H. Effect of flavour components in lemon essential oil on physical or psychological stress. Stress Health. 2008;24:3–12. [Google Scholar]
- 30.Elisabetsky E., Coelho De Souza G.P., Dos Santos M.A.C., Siquieira I.R., Amador T.A. Sedative properties of linalool. Fitoterapia. 1995;66:407–414. [Google Scholar]
- 31.Elisabetsky E., Coelho De Souza G.P. Anticonvulsant properties of linalool and g-decanolactone in mice. Acta Horticulturae. 1999;501:227–234. [Google Scholar]
- 32.Linck V., Da Silva M.A.L., Figueiró M., Caramão E.B., Moreno P.H.R., Elisabetsky E. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17:679–683. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Schieberle P., Grosch W. Quantitative analysis of important volatile flavour compounds in fresh and stored lemon oil/citric acid emulsions. Lebensmittel-Wissenschaft Technologie. 1988;21:158–162. [Google Scholar]
- 34.Kuwahara Y., Suzuki H., Matsumoto K., Wada Y. Pheromone study on acarid mites. XI. Function of mite body as geometrical isomerization and reduction of citral (the alarm pheromone) Appl Entomol Zool. 1983;18:30–39. [Google Scholar]
- 35.Kuroda K., Inoue N., Ito Y., Kubota K., Sugimoto A., Kakuda T. Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur J Appl Physiol. 2005;95:107–114. doi: 10.1007/s00421-005-1402-8. [DOI] [PubMed] [Google Scholar]
- 36.Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]


