Abstract
Background
One of the strongest predictors for osteoporosis is peak bone mass. Interventions to augment peak bone mass have yet to be developed. β-Ecdysone (βEcd), a natural steroid-like compound produced by arthropods to initiate metamorphosis, is believed to have androgenic effects and so may be used to augment bone mass.
Questions/purposes
The purpose of this study was to use both male and female (1) gonadal-sufficient; and (2) -insufficient mice to investigate sex differences in terms of bone development and structure after βEcd administration.
Methods
Two-month-old male and female Swiss-Webster mice were randomized to receive either vehicle or βEcd (0.5 mg/kg) for 3 weeks. In a separate experiment to evaluate the effects of βEcd on sex hormone-deficient mice, gonadectomy was performed in male (orchiectomy [ORX]) and female mice (ovariectomy [OVX]). Sham-operated and the ORX/OVX mice were then treated for 3 weeks with βEcd. Primary endpoints for the study were trabecular bone structure and bone strength.
Results
In male mice, the trabecular bone volume was 0.18 ± 0.02 in the placebo-treated (PL) and 0.23 ± 0.02 in the βEcd-treated group (p < 0.05 versus PL); and 0.09 ± 0.01 in the ORX group (p < 0.05 versus PL) and 0.12 ± 0.01 in the ORX + βEcd group. Vertebral bone strength (maximum load) was 43 ± 2 in PL and 51 ± 1 in the βEcd-treated group (p < 0.05 versus PL); and 30 ± 4 in the ORX group (p < 0.05 versus PL) and 37 ± 3 in the ORX + βEcd group. In female mice, trabecular bone volume was 0.23 ± 0.02 in PL and 0.26 ± 0.02 in the βEcd-treated group (p < 0.05 versus PL); and 0.15 ± 0.01 in the OVX group (p < 0.05 versus PL) and 0.14 ± 0.01 in the OVX + βEcd group. Maximum load of the vertebrae was 45 ± 2 in PL and 48 ± 4 in the βEcd-treated group; and 39 ± 4 in the OVX group (p < 0.05 versus PL) and 44 ± 4 in the OVX + βEcd group.
Conclusions
These findings suggest the potential use of βEcd in the augmentation of bone mass in growing male and female mice. It may also partially prevent the detrimental effects of gonadectomy on trabecular bone.
Clinical Relevance
Our results support the potential use of βEcd or nature products that are rich in βEcd to augment peak bone mass. βEcd may differ from the other anabolic hormone treatments that may have severe side effects such as serious cardiac complications. However, its effects on humans remain to be determined.
Introduction
Peak bone mass (PBM) is defined as the maximum amount of bone tissue that is presented at the end of skeletal maturation. PBM is dependent on the rate of bone growth, which is highest during infancy and the pubertal growth period. Adolescence is a particularly critical period of bone acquisition, because the rate of bone growth nearly doubles that of the earlier years, and approximately 40% of the new adolescent bone comes from periosteal expansion. At the end of puberty, the epiphyseal growth plates fuse and linear bone growth ends. However, bone growth continues at the endocortical and trabecular bone surfaces and 90% to 95% of the PBM is acquired by 20 years of age [22]. Epidemiological studies have shown that males have higher PBM than females, which may delay the osteoporosis development in males until their eighth decade of life [2, 5, 16]. Although sex differences in PBM are largely predisposed by genetic determinants in rodents [31] and humans [32], increased physical activities during the prepubertal period may alter PBM. In boys, regular exercise is associated with higher bone mineral density assessed at the spine, femoral neck, and total body, whereas in girls, bone mineral density is associated more with winter sunlight exposure [23]. Although weightbearing exercise and the intake of calcium and vitamin D intake through diet and supplements during puberty have a modest impact on the augmentation of PBM [6, 11, 29, 39], interventions with greater efficacy have yet to be developed.
During puberty, serum estrogen levels increase in females. Estrogen has a number of effects on bone cell activity including periosteal apposition inhibition, endosteal bone resorption, and chondrocyte remodeling. These effects result in epiphyseal closure and attenuate periosteal expansion [10]. During puberty in males, serum levels of estrogen also increase, resulting in periosteal expansion through estrogen receptor α, whereas androgen acts on osteoblasts and osteocytes through androgen receptor to increase trabecular bone formation [27]. Hormones largely regulate skeleton development, but the direct use of hormones in the growing skeleton is limited as a result of their negative effects such as tumorigenesis in the reproductive system [26, 33]. Additionally, there are other pitfalls associated with using anabolic hormones in adults such as severe cardiac and other complications [28, 34]. Therefore, we chose to investigate naturally derived products to increase PBM.
One of these naturally derived products is β-Ecdysone (βEcd), which is produced by arthropods to initiate metamorphosis, the so-called molting process [36]. A number of plants produce ecdysteroids that protect them from herbivores [14, 15]. Achyranthes root (Niu xi) is one of the Chinese medicinal herbs often used to treat low back pain, circulatory disorders, and aging [4, 8, 17]. Achyranthes extracts are highly rich in βEcd [7]. The latter is reported to stimulate muscular growth in rodents [3, 9, 18] and increase the growth plate width in estrogen-deficient rats [24].
The purpose of this study was to use both male and female (1) gonadal-sufficient; and (2) insufficient mice to investigate sex differences in terms of bone development and structure after βEcd administration.
Materials and Methods
Animals and Experimental Procedures
Two-month-old male and female Swiss-Webster mice were purchased from Jackson Laboratory and housed in the vivarium in UC Davis Medical Center. The mice were randomized into groups with eight to 11 per group: Group 1, male controls treated with phosphate-buffered saline (vehicle; Male); Group 2, males treated with βEcd (Male + βEcd 0.5 mg/kg, 5×/week); Group 3, female controls treated with phosphate-buffered saline (vehicle; Female); Group 4, females treated with βEcd (Female + βEcd 0.5 mg/kg, 5×/week). The β-Ecd dose was chosen from a pilot dose-response study we performed on male mice with doses of 0.25 to 1 mg/kg [12].
The mice were euthanized after 3 weeks of treatment at 3 months of age. All of the mice were maintained on commercial rodent chow (22/5 Rodent Diet; Teklad, Madison, WI, USA) ad libitum with 0.95% calcium and 0.67% phosphate. The mice were housed in a room that was maintained at 20 °C with a 12-hour light/dark cycle. In a separate experiment to evaluate the effects of βEcd on sex hormone-deficient mice, a gonadectomy was performed in male (orchiectomy [ORX]) and female (ovariectomy [OVX]) mice. Sham-operated and the ORX/OVX mice were then treated with βEcd 0.5 mg/kg, 5×/week for 3 weeks. βEcd was purchased from Sigma Aldrich (molecular weight = 480.6, with purity ≥ 98%). Calcein (Sigma Aldrich, St Louis, MO, USA) was injected at 20 mg/kg to all mice 7 and 2 days before euthanasia, respectively. All animals were treated according to the USDA animal care guidelines with the approval of the UC Davis Committee on Animal Research.
Micro-CT Measurements of Bone Architecture and Mineral Density of Bone Tissue
The fifth lumbar vertebrae were scanned and measured using the VivaCT 40 (Scanco Medical AG, Bassersdorf, Switzerland) at an energy level of 70 KeV and intensity of 145 μA with a voxel resolution of 10 μm in all three spatial dimensions. The entire lumbar vertebra was scanned and the trabecular bone juxtaposed to both the cranial and caudal growth plates and the cortex was evaluated. The gray-scale images were segmented using a constrained three-dimensional Gaussian filter (sigma, 0.8; support, 1.0; fixed threshold of 240) to extract the structure of the mineralized tissue to obtain trabecular bone volume and thickness [25, 42]. For the right midfemur, the scanning was performed at the middle femur continuing proximally 1 mm. All the slices were used to evaluate cortical bone volume and cortical bone thickness [19, 45, 46].
Biochemical Methods
Serum levels for bone turnover markers, osteoprotegerin, osteocalcin, and Dkk1 were measured using a luminex multiplex bone panel assay (EMD Millipore, Billerica, MA, USA) and serum CTX-1 was measured by enzyme-linked immunosorbent assay (Immunodiagnostic Systems Inc, Gaithersburg, MD, USA) following the manufacturer’s instructions.
Cryostat Section for Bone Formation Measurements
The first and second lumbar vertebrae were used for cryostat sectioning. The samples were fixed in fresh 4% paraformaldehyde on ice for 4 hours and then transferred to 30% sucrose overnight. The sections (8 µm) were obtained using a Leica microtome coupled with a tape transfer system (Leica Microsystems Inc, Buffalo Grove, IL, USA). The middle right femurs were embedded in methyl methacrylate and sectioned to 30 μm using a Leica SP1600 microtome (Leica Microsystems Inc). Bone histomorphometry was performed using the Bioquant imaging analysis system (Bioquant Image Analysis Corporation, Nashville, TN, USA) [19, 45, 46]. Lumbar vertebral samples from all groups and only FX from the intact groups were evaluated for bone histomorphometry. Bone histomorphometry was not performed for the cortical bone from the ORX and OVX + βEcd treatment groups as a result of the lack of structural and bone strength changes measured by micro-CT and mechanical testing.
Biomechanical Testing
The lumbar vertebral compression and the left femurs three-point bending tests were performed using an MTS 831 electro-servo-hydraulic testing system (MTS Systems Corp, Eden Prairie, MN, USA) at a displacement rate of 0.01 mm/s with a 90-N load cell. Values for the maximum load were determined. A measure of work to failure was estimated by calculating the area under the load versus displacement curve [19, 37, 44, 45].
Statistical Methods
Sample size calculation was estimated from our published data on glucocorticoid-induced (GC) bone loss using trabecular bone volume as our primary endpoint. Mean trabecular bone volume for the placebo-treated (PL) group was 12%, in GC was 10%, and sigma was 2%. The effective sample size to have 80% power and alpha 0.05 was eight animals per group. Means and SDs were calculated for all parameters from all groups. Two-way analysis of variance was used to investigate the main effects of sex, treatments, and their interactions. Bonferroni post hoc tests were used to make comparisons between the groups (SPSS Version 18; SPSS Inc, Chicago, IL, USA; GraphPad Prism, La Jolla, CA, USA). p < 0.05 was considered significant.
Results
Does βEcd Treatment Affect Overall Well-being of the Mice?
Male mice weighed 31 g at the beginning (Day 0) of the study and were 36 g at the end (p < 0.05 from Day 0) when they were 11 weeks of age. Baseline body weight for the female mice was 25 g and they weighed 28 g at 11 weeks. ORXed mice did not gain body weight over the study period, whereas OVXed mice’s body weights increased from 25 g to 29 g and was similar to the sham-operated mice. βEcd treatment did not alter body mass changes in the male or female mice (Table 1).
Table 1.
Body weight changes (mean ± SD)
| Treatment groups | Day 0/8 weeks old (g) | Day 21/ 11 weeks old (g) |
|---|---|---|
| Male | ||
| Male + vehicle | 31 ± 1.7 | 36 ± 1.0 |
| Male + β-ecdysone | 32 ± 1.3 | 36 ± 1.6 |
| ORX + vehicle | 31 ± 1.2 | 31 ± 0.8* |
| ORX + β-ecdysone | 31 ± 1.3 | 30 ± 0.9* |
| Female | ||
| Female + vehicle | 25 ± 0.4† | 28 ± 0.5 |
| Female + β-ecdysone | 25 ± 0.3 | 28 ± 1.5 |
| OVX + vehicle | 25 ± 0.3 | 29 ± 0.8* |
| OVX + β-ecdysone | 25 ± 0.7 | 29 ± 0.9* |
* p < 0.05 for ORX/OVX versus sham; †p < 0.05 for sex difference; ORX = orchiectomy; OVX = ovariectomy.
Does βEcd Treatment Affect Bone Turnover and Bone Structures?
Serum Bone Turnover Marker Changes
In male mice, serum osteocalcin was 5.1 ± 1.6 in PL and 6.5 ± 1.5 in βEcd-treated group; and 13 ± 1.4 in the ORX group (p < 0.05 versus PL) and 15 ± 1.2 in the ORX + βEcd group. Serum CTX-1 did not change among the groups. In female mice, serum osteocalcin was 12 ± 2.6 in PL and 12 ± 1.5 in the βEcd-treated group; and 14 ± 3.1 in the OVX group and 14 ± 1.2 in the OVX + βEcd group. Serum CTX-1 was 19 ± 3.4 in PL and 16 ± 3.0 in the βEcd-treated group; and 24 ± 1.6 in the OVX group (p < 0.05 versus PL) and 20 ± 2.3 in the OVX + βEcd group (p < 0.05 versus OVX) (Table 2).
Table 2.
Bone turnover changes (mean ± SD)
| Treatment groups | Osteocalcin (µg/mL) | CTX-1 (ng/mL) | MS/BS (%) | BFR/BS (µm3/µm2/day) | Oc/BS (number/mm) |
|---|---|---|---|---|---|
| Male | |||||
| Male | 5.1 ± 1.6 | 23 ± 1.7 | 24 ± 6.9 | 0.45 ± 0.1 | 0.60 ± 0.1 |
| Male + β-ecdysone | 6.5 ± 1.5 | 21 ± 5.0 | 33 ± 1.1‡ | 0.61 ± 0.2‡ | 0.59 ± 0.1 |
| ORX + vehicle | 13 ± 1.4* | 26 ± 2.5 | 26 ± 4.1 | 0.61 ± 0.2* | 0.66 ± 0.1 |
| ORX + β-Ecdysone | 15 ± 1.2* | 25 ± 2.7 | 28 ± 4.3 | 0.69 ± 0.1 | 0.62 ± 0.1 |
| Female | |||||
| Female | 12 ± 2.6† | 19 ± 3.4 | 26 ± 4.8 | 0.64 ± 0.2† | 0.57 ± 0.1 |
| Female + β-ecdysone | 12 ± 1.5† | 16 ± 3.0 | 35 ± 10.3 | 0.86 ± 0.3 | 0.61 ± 0.1 |
| OVX + vehicle | 14 ± 3.1 | 24 ± 1.6* | 29 ± 8.3 | 0.66 ± 0.2 | 0.91 ± 0.1* |
| OVX + β-ecdysone | 14 ± 1.2 | 20 ± 2.3‡ | 34 ± 9.1 | 0.82 ± 0.2‡ | 0.78 ± 0.0‡ |
* p < 0.05 for ORX/OVX versus sham; †p < 0.05 for sex difference; ‡p < 0.05 treatment effect within the same sex; MS/BS = mineralizing surface; BFR/BS = surface-based bone formation rate; Oc/BS = osteoclast/bone surface; ORX = orchiectomy; OVX = ovariectomy.
Effects of βEcd on Trabecular Bone Formation and Resorption
In male mice, the mineralizing surface was 24 ± 6.9 in PL and 33 ± 1.1 in the βEcd-treated group (p < 0.05 versus PL); and 26 ± 4.1 in the ORX group and 28 ± 4.3 in the ORX + βEcd group. In female mice, MS/BS did not change among the groups.
In male mice, the surface-based bone formation rate was 0.45 ± 0.1 in PL and 0.61 ± 0.2 in the βEcd-treated group (p < 0.05 versus PL); and 0.61 ± 0.2 in the ORX group (p < 0.05 versus PL) and 0.69 ± 0.1 in the ORX + βEcd group. In female mice, the BFR/BS was 0.64 ± 0.2 in PL and 0.86 ± 0.3 in the βEcd-treated group; and 0.66 ± 0.2 in the OVX group and 0.82 ± 0.2 in the OVX + βEcd group (p < 0.05 versus OVX) (Table 2).
The osteoclast surface did not change among the treatment groups in male mice. Oc/BS was 0.57 ± 0.1 in PL and 0.61 ± 0.1 in the βEcd-treated group; and 0.91 ± 0.1 in the OVX group (p < 0.05 versus PL) and 0.78 ± 0.01 in the OVX + βEcd group (p < 0.05 versus OVX) (Table 2).
Effects of βEcd on Trabecular Bone Structures
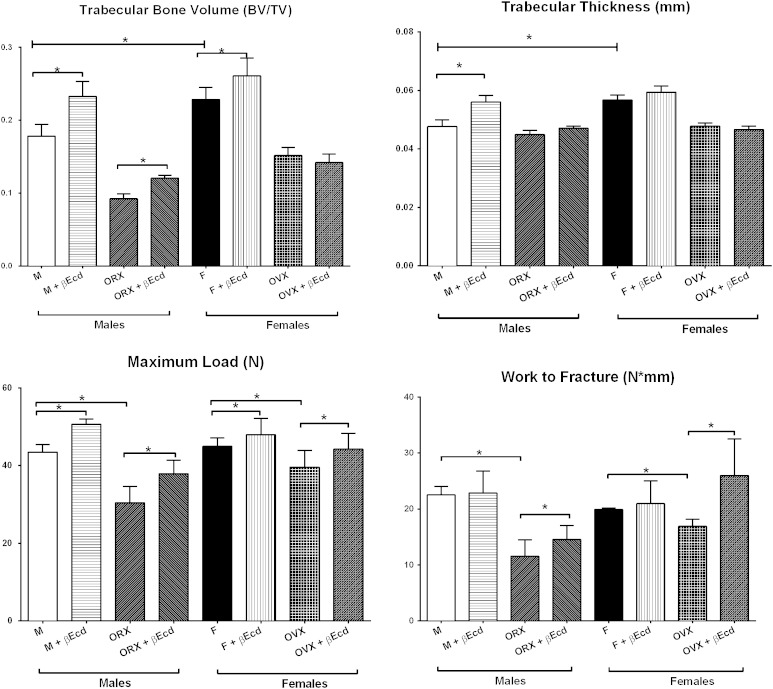
In male mice, the BV/TV was 0.18 ± 0.02 in PL and 0.23 ± 0.02 in the βEcd-treated group (p < 0.05 versus PL); and 0.09 ± 0.01 in the ORX group (p < 0.05 versus PL) and 0.12 ± 0.01 in the ORX + βEcd group. In female mice, BV/TV was 0.23 ± 0.02 in PL and 0.26 ± 0.02 in the βEcd-treated group (p < 0.05 versus PL); and 0.15 ± 0.01 in the OVX group (p < 0.05 versus PL) and 0.14 ± 0.01 in the OVX + βEcd group (Fig. 1).
Fig. 1.
βEcd treatment increases vertebral trabecular bone volume and bone strength. Two-month-old male or female mice were treated with βEcd for 21 days. Lumbar vertebral trabecular bone (LVB) structure measured by micro-CT and bone strength was measured by compression test. *p < 0.05 between indicated groups. M = male; F = female.
Effects of βEcd on Cortical Bone Formation in Gonadal-sufficient Mice
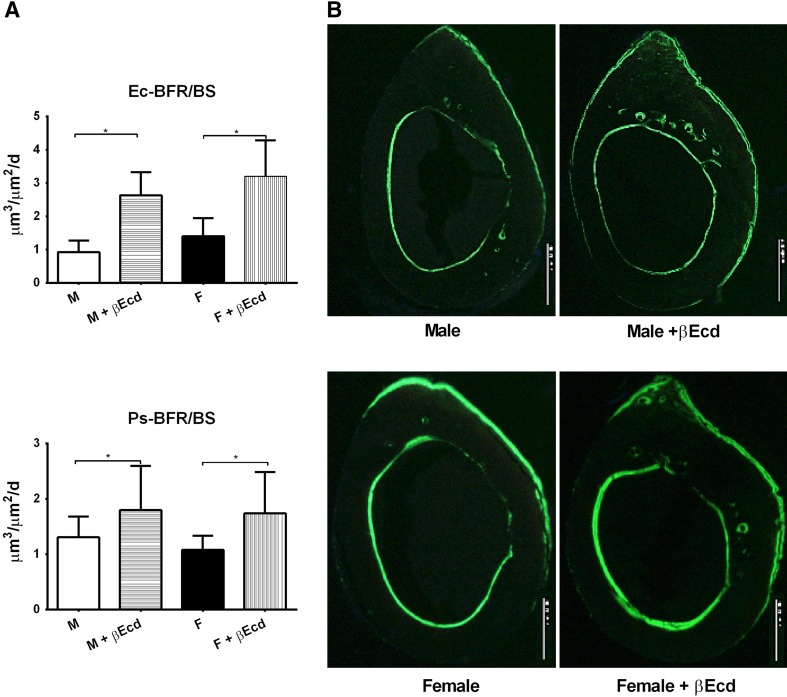
In male mice, bone formation at the periosteal bone surface was 1.31 ± 0.1 in PL and 1.80 ± 0.3 in the βEcd-treated group (p < 0.05 versus PL). In female mice, Ps-BFR was 1.1 ± 0.1 in PL and 1.7 ± 0.2 in the βEcd-treated group (p < 0.05 versus PL) (Fig. 2). In male mice, bone formation at the endocortical bone surface was 0.9 ± 0.1 in PL and 2.6 ± 0.3 in the βEcd-treated group (p < 0.05 versus PL). In female mice, Ec-BFR was 1.4 ± 0.1 in PL and 3.2 ± 0.4 in the βEcd-treated group (p < 0.05 versus PL) (Fig. 2).
Fig. 2A–B.
βEcd treatment increases cortical bone formation. (A) Bone formation rate measured at the periosteal and endocortical bone surfaces by bone histomorphometry. (B) Representative cross-sectional cortical bone sections from the midfemurs in intact male and female mice that were treated with βEcd for 21 days. *p < 0.05 between indicated treatment groups. M = male; F = female. Scale bar = 100 micron.
Effects of βEcd on Cortical Bone Structure
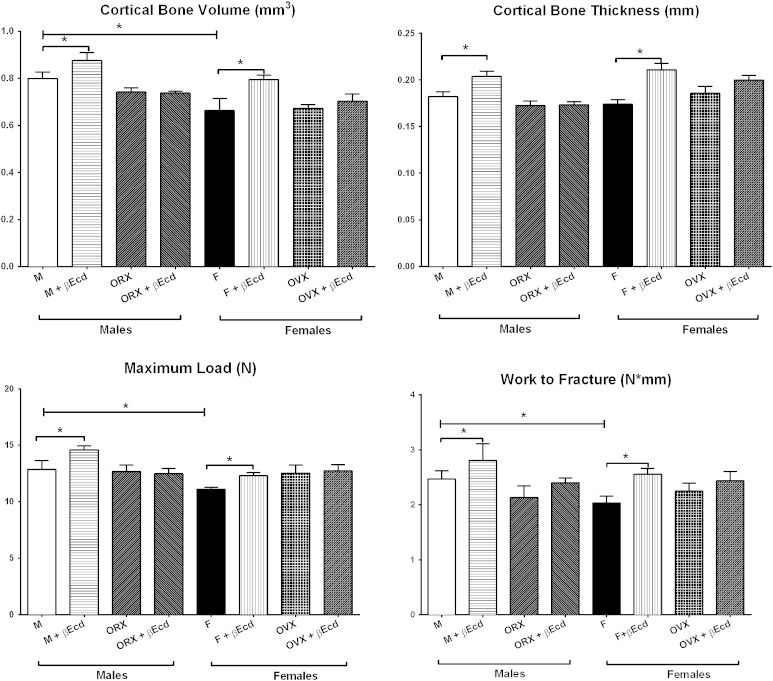
In male mice, cortical bone volume was 0.80 ± 0.02 in PL and 0.88 ± 0.03 in the βEcd-treated group (p < 0.05 versus PL); and 0.74 ± 0.02 in the ORX group (p < 0.05 versus PL) and 0.74 ± 0.01 in the ORX + βEcd group (p < 0.05 versus PL). In female mice, cortical BV was 0.66 ± 0.1 in PL and 0.79 ± 0.2 in the βEcd-treated group (p < 0.05 versus PL); and 0.67 ± 0.01 in the OVX group and 0.70 ± 0.03 in the OVX + βEcd group.
In male mice, cortical bone thickness was 0.182 ± 0.005 in PL and 0.204 ± 0.06 in the βEcd-treated group (p < 0.05 versus PL); and 0.173 ± 0.005 in the ORX group (p < 0.05 versus PL) and 0.173 ± 0.003 in the ORX + βEcd group (p < 0.05 versus PL). In female mice, Ct.Th was 0.174 ± 0.005 in PL and 0.211 ± 0.007 in the βEcd-treated group (p < 0.05 versus PL); and 0.186 ± 0.007 in the OVX group and 0.200 ± 0.005 in the OVX + βEcd group (Fig. 3).
Fig. 3.
βEcd treatment increases cortical bone mass and bone strength. Middle femoral bone structure measured by micro-CT and bone strength was measured by femoral three-point ending test. *p < 0.05 between indicated treatment groups. M = male; F = female.
Does βEcd Treatment Affect Bone Strength?
Effects of βEcd Treatment on Vertebral Bone Strength
In male mice, the vertebral maximum load was 43 ± 2 in PL and 51 ± 1 in the βEcd-treated group (p < 0.05 versus PL); and 30 ± 4 in the ORX group (p < 0.05 versus PL) and 37 ± 3 in the ORX + βEcd group (p < 0.05 versus ORX). The vertebral work to failure was 22 ± 1 in PL and 23 ± 4 in the βEcd-treated group; and 11 ± 3 in the ORX group (p < 0.05 versus PL) and 15 ± 2 in the ORX + βEcd group (p < 0.05 versus ORX) (Fig. 2).
In female mice, the maximum load of the vertebrae was 45 ± 2 in PL and 48 ± 4 in the βEcd-treated group; and 39 ± 4 in the OVX group (p < 0.05 versus PL) and 44 ± 4 in the OVX + βEcd group. The vertebral work to failure was 20 ± 1 in PL and 21 ± 4 in the βEcd-treated group; and 17 ± 1 in the OVX group (p < 0.05 versus PL) and 26 ± 6 in the ORX + βEcd group (p < 0.05 versus OVX) (Fig. 2).
Effects of βEcd Treatment on Cortical Bone Strength
In male mice, the femoral maximum load was 13 ± 0.8 in PL and 15 ± 0.3 in the βEcd-treated group (p < 0.05 versus PL); and 13 ± 0.5 in the ORX group and 13 ± 0.5 in the ORX + βEcd group. The femoral work to failure was 2.5 ± 0.2 in PL and 2.8 ± 0.3 in the βEcd-treated group (p < 0.05 versus PL); and 2.1 ± 0.2 in the ORX group and 2.4 ± 0.1 in the ORX + βEcd group (Fig. 3).
In female mice, the maximum load of the femurs was 11 ± 0.5 in PL and 12 ± 0.5 in the βEcd-treated group (p < 0.05 versus PL); and 12 ± 0.5 in the OVX group and 13 ± 0.5 in the OVX + βEcd group. The femoral work to failure was 2.0 ± 0.1 in PL and 2.6 ± 0.1 in the βEcd-treated group (p < 0.05 versus PL); and 2.2 ± 0.2 in the OVX group and 2.4 ± 0.2 in the ORX + βEcd group (Fig. 3).
Discussion
Treatment with βEcd increased both trabecular and cortical bone volume and bone strength in both sexes through anabolic mechanisms in gonadal-sufficient mice. In gonadal-insufficient mice, trabecular bone loss was partially prevented with concurrent βEcd treatment in the ORX and OVX animals.
This study had a number of limitations. First, we only used one dose of β-ecdysone and only included one time point. Additionally, we did not evaluate the potential activation or deactivation of hormonal receptors that include estrogen and androgen receptors after βEcd treatment or if β-Ecd would alter the sensitivities of the hormonal receptors. Because βEcd is structurally similar to testosterone or estrogen, it is thought to have steroid hormone-like effects including stimulating protein synthesis, promoting carbohydrate and lipid metabolism, alleviating hyperglycemia and hyperlipidemia, having immunologic modulation effects, and protecting endothelial cells from apoptosis. However, it was reported that βEcd did not bind to the estrogen receptor [35], and its pharmacological effects may not involve estrogen receptor activation. To further examine whether hormones play a role in the effects of βEcd on bone, we treated the ORX male and OVX female mice with βEcd. βEcd treatment partially prevented trabecular bone loss primarily through further increases in tissue-level bone formation markers and bone formation rates. However, βEcd treatment at the current dose and treatment length failed to have beneficial effects on the cortical bone in hormone-deficient mice. A longer treatment period or dose-dependent study may be necessary to further elucidate whether sex steroid status altered βEcd treatment efficacy as far as the skeletal changes are concerned.
Sex Differences in Skeletal Geometry and Bone Strength
The mice we used gained approximately 10% body mass during the study period. These Swiss Webster mice normally obtain their peak bone mass by 4 months of age [25, 40, 41, 43]. For this reason, the bone development in mice in this study might resemble late pubertal human bone development. We found that female mice had similar vertebral bone mass and strength as the male mice at 3 months of age. These observations were similar to the results of another study performed in C57BL/6 mice [1]. Although the females had higher trabecular bone volume within the vertebral body, the smaller bone size in female mice compared with the male mice may correspond to a lower maximum compression load and the overall bone strength (work to failure) for both trabecular bone (vertebrae) and cortical bone (femoral shafts).
Sex Differences in Response to βEcd Treatment
Sex is a significant contributing factor to the different therapeutic responses observed between females and males. For example, muscle-derived mesenchymal stem cells obtained from female mouse donors have superior myogenic ability to those from males [13] but lower chondrogenic potential [30]. Supplementation of the culture medium with estrogen increases the osteogenic differentiation of the bone marrow-derived stromal cells from both male and female rat donors [20]. In humans, the prevalence of colony-forming osteogenic units cultured from bone marrow aspirates is significantly reduced with age for women but not for men [20]. One of the main growth factors for peak bone mass acquisition, insulin-like growth factor, is associated with bone mineral density of the spine and hip in postmenopausal women (≥ 55 years) who were not on estrogen but not in aged-matched men [21]. In patients with growth hormone deficiency, men had significantly higher bone gain than women after recombined human growth hormone treatment [21]. In skeletally mature mice, parathyroid hormone has similar osteogenic effects on osteogenesis in vitro but exerts a greater anabolic effect on the trabecular and cortical bone in male mice than in female mice [38].
In the current study, we found βEcd increased bone formation rates to similar degrees in male and female mice at the trabecular bone site. The increase in bone formation rate at the trabecular bone surface resulted primarily from greater the osteoblast-forming surface (mineralized surface). Similarly, the percentage increases in cortical bone volume and bone strength (maximum load and work to failure) were similar in intact male mice than in intact female mice that were treated with βEcd.
In summary, we found that the short-term administration of βEcd in growing intact mice resulted in marked enhancements in both trabecular and cortical bone formation that was associated with significantly increased trabecular and cortical bone volume. These findings imply the potential use of βEcd in the bone mass augmentation in growing males and females. Our results indicate that βEcd may partially prevent the detrimental effects of gonadectomy on trabecular bone but not on cortical bone. βEcd may differ from the other anabolic hormone treatments that have caused some real problems when taken from the laboratory to human models such as serious cardiac complications. However, its efficacy in humans awaits further investigations.
Acknowledgments
We would like to thank Dr. Qiujuan Xing from the Department of Science and Technology, Integrative Medicine, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai, PR China and funding sources from Natural Science Foundation of Shanghai, China 15ZR1441600 (to WD) and the National Natural Science Foundation of China, 81102606 (to QX) for laying the foundation work for this report.
Footnotes
Two of the authors (WD, HZ) contributed equally to this manuscript.
This work was funded by a National Institutes of Health grant R01 AR061366 (WY), 1P50AR063043 (NEL), K24 AR048841 (NEL), and the endowed chair for aging at UC Davis (NEL).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the UC Davis Medical Center, Sacramento, CA, USA.
References
- 1.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 3.Bathori M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev Med Chem. 2002;2:285–293. doi: 10.2174/1389557023406269. [DOI] [PubMed] [Google Scholar]
- 4.Bathori M, Toth N, Hunyadi A, Marki A, Zador E. Phytoecdysteroids and anabolic-androgenic steroids—structure and effects on humans. Curr Med Chem. 2008;15:75–91. doi: 10.2174/092986708783330674. [DOI] [PubMed] [Google Scholar]
- 5.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–444. doi: 10.1016/S8756-3282(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 6.Bonjour JP, Chevalley T, Ammann P, Slosman D, Rizzoli R. Gain in bone mineral mass in prepubertal girls 3.5 years after discontinuation of calcium supplementation: a follow-up study. Lancet. 2001;358:1208–1212. doi: 10.1016/S0140-6736(01)06342-5. [DOI] [PubMed] [Google Scholar]
- 7.Boo KH, Lee D, Jeon GL, Ko SH, Cho SK, Kim JH, Park SP, Hong Q, Lee SH, Lee DS, Riu KZ. Distribution and biosynthesis of 20-hydroxyecdysone in plants of Achyranthes japonica Nakai. Biosci Biotechnol Biochem. 2010;74:2226–2231. doi: 10.1271/bbb.100410. [DOI] [PubMed] [Google Scholar]
- 8.Cahlikova L, Macakova K, Chlebek J, Host’alkova A, Kulhankova A, Opletal L. Ecdysterone and its activity on some degenerative diseases. Nat Prod Commun. 2011;6:707–718. [PubMed] [Google Scholar]
- 9.Cheng DM, Kutzler LW, Boler DD, Drnevich J, Killefer J, Lila MA. Continuous infusion of 20-hydroxyecdysone increased mass of triceps brachii in C57BL/6 mice. Phytother Res. 2013;27:107–111. doi: 10.1002/ptr.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chokalingam K, Roforth MM, Nicks KM, McGregor U, Fraser D, Khosla S, Monroe DG. Examination of ERalpha signaling pathways in bone of mutant mouse models reveals the importance of ERE-dependent signaling. Endocrinology. 2012;153:5325–5333. doi: 10.1210/en.2012-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courteix D, Jaffre C, Lespessailles E, Benhamou L. Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. Int J Sports Med. 2005;26:332–338. doi: 10.1055/s-2004-821040. [DOI] [PubMed] [Google Scholar]
- 12.Dai W, Jiang L, Lay YA, Chen H, Jin G, Zhang H, Kot A, Ritchie RO, Lane NE, Yao W. Prevention of glucocorticoid induced bone changes with beta-ecdysone. Bone. 2015;74C:48–57. doi: 10.1016/j.bone.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Dinan L, Lecture Karlson. Phytoecdysteroids: what use are they? Arch Insect Biochem Physiol. 2009;72:126–141. doi: 10.1002/arch.20334. [DOI] [PubMed] [Google Scholar]
- 15.Dinan L, Lafont R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J Endocrinol. 2006;191:1–8. doi: 10.1677/joe.1.06900. [DOI] [PubMed] [Google Scholar]
- 16.Duan Y, Turner CH, Kim BT, Seeman E. Sexual dimorphism in vertebral fragility is more the result of gender differences in age-related bone gain than bone loss. J Bone Miner Res. 2001;16:2267–2275. doi: 10.1359/jbmr.2001.16.12.2267. [DOI] [PubMed] [Google Scholar]
- 17.Esposito D, Komarnytsky S, Shapses S, Raskin I. Anabolic effect of plant brassinosteroid. FASEB J. 2011;25:3708–3719. doi: 10.1096/fj.11-181271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorelick-Feldman J, MacLean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agr Food Chem. 2008;56:3532–3537. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- 19.Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong L, Sultana H, Paulius K, Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol. 2009;114:180–185. doi: 10.1016/j.jsbmb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson AG, Engstrom BE, Ljunghall S, Karlsson FA, Burman P. Gender differences in the effects of long term growth hormone (GH) treatment on bone in adults with GH deficiency. J Clin Endocrinol Metab. 1999;84:2002–2007. doi: 10.1210/jcem.84.6.5743. [DOI] [PubMed] [Google Scholar]
- 22.Johnston CC, Jr, Slemenda CW. Determinants of peak bone mass. Osteoporos Int. 1993;3(Suppl 1):54–55. doi: 10.1007/BF01621864. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Dwyer T. Bone mass in prepubertal children: gender differences and the role of physical activity and sunlight exposure. J Clin Endocrinol Metab. 1998;83:4274–4279. doi: 10.1210/jcem.83.12.5353. [DOI] [PubMed] [Google Scholar]
- 24.Kapur P, Wuttke W, Jarry H, Seidlova-Wuttke D. Beneficial effects of beta-Ecdysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomedicine. 2010;17:350–355. doi: 10.1016/j.phymed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao DJ, Dickson RB. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol. 2002;80:175–189. doi: 10.1016/S0960-0760(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 27.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ, Skugor M, Nagode LA, Mobley SL, Ha EJ, Hangartner TN, Clairmont A. Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr. 2005;81:175–188. doi: 10.1093/ajcn/81.1.175. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, Kubo S, Meszaros LB, Corsi KA, Cooper GM, Li G, Usas A, Osawa A, Fu FH, Huard J. The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum. 2008;58:3809–3819. doi: 10.1002/art.24125. [DOI] [PubMed] [Google Scholar]
- 31.Orwoll ES, Belknap JK, Klein RF. Gender specificity in the genetic determinants of peak bone mass. J Bone Miner Res. 2001;16:1962–1971. doi: 10.1359/jbmr.2001.16.11.1962. [DOI] [PubMed] [Google Scholar]
- 32.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 33.Roehm E. A reappraisal of women’s health initiative estrogen-alone trial: long-term outcomes in women 50–59 years of age. Obstet Gynecol Int. 2015;2015:713295. doi: 10.1155/2015/713295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 35.Seidlova-Wuttke D, Christel D, Kapur P, Nguyen BT, Jarry H, Wuttke W. Beta-ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine. 2010;17:884–889. doi: 10.1016/j.phymed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- 37.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-K. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Sakata T, Elalieh HZ, Munson SJ, Burghardt A, Majumdar S, Halloran BP, Bikle DD. Gender differences in the response of CD-1 mouse bone to parathyroid hormone: potential role of IGF-I. J Endocrinol. 2006;189:279–287. doi: 10.1677/joe.1.06351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward KA, Roberts SA, Adams JE, Lanham-New S, Mughal MZ. Calcium supplementation and weight bearing physical activity—do they have a combined effect on the bone density of pre-pubertal children? Bone. 2007;41:496–504. doi: 10.1016/j.bone.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145:1980–1987. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao W, Cheng Z, Koester KJ, Ager JW, Balooch M, Pham A, Chefo S, Busse C, Ritchie RO, Lane NE. The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone. 2007;41:804–812. doi: 10.1016/j.bone.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao W, Cheng Z, Pham A, Busse C, Zimmermann EA, Ritchie RO, Lane NE. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 2008;58:3485–3497. doi: 10.1002/art.23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010;25:190–199. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, Guan M, Lane NE. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PloS One. 2010;5:e11410. doi: 10.1371/journal.pone.0011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam KS, Nolta J, Olvera D, Ritchie RO, Lane NE. Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013;31:2003–2014. doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]