Abstract
Background
Despite widely reported success associated with hip and knee replacements, some patients remain dissatisfied with their outcomes. Patient activation, an individual’s propensity to engage in adaptive health behaviors, has been measured as a potentially important factor contributing to health outcomes, cost, and patient experience of care. However, to our knowledge, it has not been studied in patients undergoing total joint arthroplasties (TJAs).
Questions/purposes
We wanted to determine whether patients with higher activation scores would experience (1) greater resolution of pain and improved activity, (2) greater improvements in postoperative physical and mental health, and (3) greater patient satisfaction after primary THA or TKA.
Methods
We approached 174 patients and enrolled 135 who were undergoing primary THA or TKA at one of two hospitals between January 2013 and May 2014. Patient Activation Measure (PAM) scores were obtained preoperatively and patient-reported outcomes were assessed and completed for 125 patients pre- and postoperatively at the 6- or 12-month visit. We assessed pain and activity with the Hip Disability and Osteoarthritis Outcome Score (HOOS), Knee Injury and Osteoarthritis Outcome Score (KOOS), and University of California Los Angeles (UCLA) activity scores. We measured physical and mental health by calculating SF12v2® scores and measured patient satisfaction with the Hip and Knee Satisfaction Scale (HKSS). Linear regression models were used to test the association between baseline PAM and postoperative patient-reported outcomes.
Results
Overall, patients with a higher baseline PAM score experienced better pain relief using the HOOS/KOOS pain scores (R2 = 0.311, p = 0.048) and symptoms using the HOOS/KOOS symptom scores (R2 = 0.272, p = 0.021). In addition, higher PAM scores were associated with better postoperative mental health using the SF12v2® (R2 = 0.057, p < 0.001), but were not associated with higher physical health (R2 = 0.176, p = 0.173). Finally, higher PAM scores were associated with having greater postoperative satisfaction after surgery using the HKSS questionnaire (R2 = 0.048, p = 0.023).
Conclusions
Higher preoperative patient activation was associated with better pain relief, decreased symptoms, improved mental health, and greater satisfaction after TJA. Future efforts should be aimed at studying if improving patient activation before surgery results in better patient-reported outcomes after elective THA or TKA.
Level of Evidence
Level II, prognostic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4247-4) contains supplementary material, which is available to authorized users.
Introduction
It has been documented that a total joint arthroplasty (TJA) is an effective procedure for improving health-related quality-of-life dimensions for patients who have disabling arthritis of the hip and knee [10, 35]. However, despite these benefits, rates of patient satisfaction have been shown in some series to be less the 85% [1, 5, 9, 20, 36, 44]. Recently estimated projections for total joint replacements predict 511,837 (413,092–610,583) primary THAs and 1,375,574 (1,193,173–1,557,975) primary TKAs by 2020 [21], which could result in a substantial number of patients who are dissatisfied. Evidence to date shows the importance of biomedical, behavioral, and social factors on recovery after TJA, but these factors do not fully account for the variability in outcomes [25–27, 31, 35]. Patient activation has been defined as an individual’s propensity to engage in adaptive health behavior that may lead to improved outcomes. Patient activation has been measured as a potentially important factor in contributing to health outcomes, cost, and patient experience of care [14, 18, 42], however to our knowledge, it has not been studied as a possible predictor of postoperative patient-reported outcomes after TJAs. Patient activation differs from compliance as the patient is managing his or her own health a majority of the time. An activated patient is one who is armed with the skills, knowledge, and motivation to be an effective member of the healthcare team [42]. Using the Patient Activation Measure (PAM) we sought to understand if the influence of psychological factors (optimism, hope, self-efficacy, and locus of control) and personal competencies, such as condition-specific knowledge, result in individuals having better functional outcomes after TJA. The purpose of our study was to determine if an association existed between preoperative patient activation and patient-reported outcomes of pain relief, activity, physical and mental health, and satisfaction in a cohort of individuals after primary THA or TKA. We hypothesized that patients with a higher activation score would experience (1) greater resolution of pain and improved activity, (2) greater improvements in postoperative physical and mental health, and (3) greater patient satisfaction after primary THA or TKA.
Methods
All patients scheduled to undergo a primary THA or TKA at the University of California, San Francisco (UCSF) Medical Center from January 2013 through July 2013 and Harbor-UCLA Medical Center from March 2013 to January 2014 were eligible for recruitment in our study. Our inclusion criteria were that patients be at least 18 years old, with advanced arthritis refractory to conservative management, and who had no prior arthroplasty in the same joint. During the study period we approached 174 patients between the two sites to participate in the study. A total of 135 patients consented, completed our preoperative questionnaire, and met our inclusion criteria. Of the 135 initial patients, 125 completed followup 6- or 12-month patient-reported outcome surveys. A total of 7% of our patients (10 of 135) were lost to followup.
A general demographic questionnaire was used to assess age, sex, education, employment status, and medical/surgical history of the participating patients. The 125 patients were predominately women 60% (75 of 125), with a mean age of 64 (± 10) years, 56% (71 of 125) had a TKA, and 64% (72 of 125) were currently not working or were retired (Table 1).
Table 1.
Patient demographics
| Parameter | Overall values (n = 125) |
|---|---|
| Age, mean years ± SD | 63.5 ± 9.7 |
| Sex, % (n) | |
| Females | 60.0 (75) |
| Males | 40.0 (50) |
| THA | 43.2% (54) |
| TKA | 56.8% (71) |
| Charlson Comorbidity Index, grade, % (n) | |
| 0 | 58.5 (72) |
| 1 | 19.5 (24) |
| 2 | 11.4 (14) |
| 3 | 4.9 (6) |
| 4 | 2.4 (3) |
| 5 | 2.4 (3) |
| 6 | 0.8 (1) |
| Education, % (n) | |
| 8th grade or less | 6.9 (7) |
| Some high school | 8.8 (9) |
| High school degree | 13.7 (14) |
| Some college | 20.6 (21) |
| College degree (4-year) | 19.6 (20) |
| Postgraduate degree | 30.4 (31) |
| Employed, % (n) | 35.7 (40) |
| Unemployed, % (n) | 64.3 (72) |
| UCSF Medical Center | 73.6% (92) |
| Harbor-UCLA Medical Center | 26.4% (33) |
| Active smoker | 96.0% (5) |
UCSF = University of California San Francisco; UCLA = University of California Los Angeles.
Patient Activation
Patient activation was measured using the PAM, a 13-item, patient-completed questionnaire for evaluating self-concept as a manager of one’s own health [17]. The PAM addresses key psychological factors and personal competencies. The validity of the scale has been established through correlation with key clinical indicators, such as overall health status and self-management behaviors [18]. Scores on the PAM are continuous measures ranging from 0 (no activation) to 100 (high activation) [18]. The questionnaire was given to patients during their preoperative clinic visit. We used the Charlson Comorbidity Index as a scoring tool to evaluate patients’ overall health and to assist with risk adjustments for comorbid conditions [3, 7, 32]. Pain, activity, and physical and mental health were evaluated with the Hip Disability and Osteoarthritis Outcome Score (HOOS) [30], or Knee Injury and Osteoarthritis Outcome Score (KOOS) [34], the University of California, Los Angeles (UCLA) activity score [41], and the SF12v2® [19].
Patient pain and activity were assessed with the HOOS and KOOS questionnaires and the UCLA activity scores. The HOOS and KOOS questionnaires measure patient-reported outcomes in five separate subscales related to the hip and knee (pain, symptoms, activity of daily living, sport and recreation function, and hip or knee-related quality of life) [30, 33]. The UCLA activity score was used to assess patient self-reported level of activity [41, 46].
Physical and mental health of the patient were assessed with the SF12v2® [29]. The SF12v2® is a generic measure of health-related quality of life that measures how individuals value their current physical and emotional health states [29].
Patient recovery after surgery was assessed with the same measures of pain, activity level, and physical and mental health used preoperatively. In addition, at postoperative visits, we measured patient satisfaction using the Hip and Knee Satisfaction Scale (HKSS) which evaluates patient satisfaction through questions regarding pain relief and function [5, 22, 24]. All patients enrolled in the study were given the above-mentioned questionnaires and asked to complete them at their 6-week, 6-month, or 12-month followup.
Data were analyzed to compare changes in disability and functional status as a function of patient activation. Demographics and initial functional scores were compared across the activation groups at baseline using chi-square tests for categorical variables and ANOVA with multiple comparisons for continuous variables (Appendix 1. Supplemental materials are available with the online version of CORR®). Correlations between activation scores and the outcome variables were calculated. Patient outcomes (HOOS, KOOS, SF12v2®, UCLA activity score, and HKSS questionnaires) at baseline and 6- or 12-month followup were compared using ANOVA with multiple comparisons (Table 2). We found differences in the patient-reported outcomes between the two study sites and between THA and TKA (Appendix 1. Supplemental materials are available with the online version of CORR®), however the study was not designed or powered to detect any difference, therefore we pooled the groups. General linear models with repeated measures were used to test the association between patient activation and patient-reported pain and functional outcomes with time. We identified the Charlson Comorbidity Index as a confounding variable and controlled for it in our general linear model. We assessed hip and knee disability, activity level, and physical and mental health compared with patient activation. Statistical significance was based on an α level of 0.05, corrected for multiple comparisons when more than two groups were compared.
Table 2.
Postoperative change in scores
| Scoring system | Mean difference | 95% CI of the difference | p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| UCLA Activity Score | −0.991 | −1.275 | −0.707 | < 0.001 |
| SF12v2® Physical Score | −10.672 | −12.601 | −8.743 | < 0.001 |
| SF12v2® Mental Score | −1.952 | −4.216 | 0.313 | 0.090 |
| HOOS/KOOS Symptom Score | −36.283 | −40.467 | −32.099 | < 0.001 |
| HOOS/KOOS Pain Score | −40.135 | −44.234 | −36.036 | < 0.001 |
| HOOS/KOOS Sports and Recreation score | −34.658 | −40.703 | −28.613 | < 0.001 |
| HOOS/KOOS QOL Score | −42.521 | −47.274 | −37.769 | < 0.001 |
| HOOS/KOOS WOMAC Score | −37.268 | −41.043 | −33.493 | < 0.001 |
UCLA = University of California Los Angeles; HOOS = Hip Disability and Osteoarthritis Score; KOOS = Knee Injury and Osteoarthritis Outcome Score; QOL = Quality-of-Life; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
The preoperative PAM scores did not follow the normal distribution seen in previous studies [17, 37, 38] which reported, on average, a mean of 60 ± 18 and range of 20−100; our average PAM score was 80 ± 16 (range, 19−100).
Our study was reviewed and approved by the Institutional Review Board at UCSF and Harbor-UCLA Medical Center as a joint study. All research occurred in private settings and information that was shared was anonymous with deidentified patient information.
Results
Pain and Activity
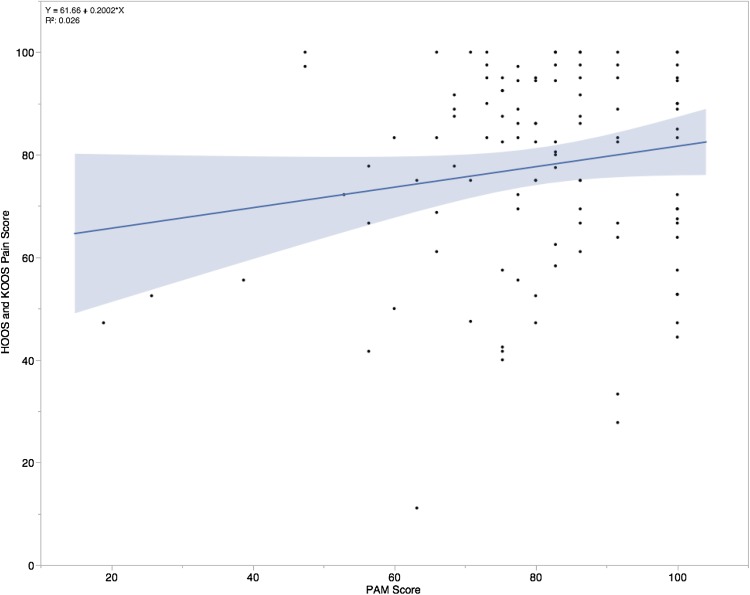
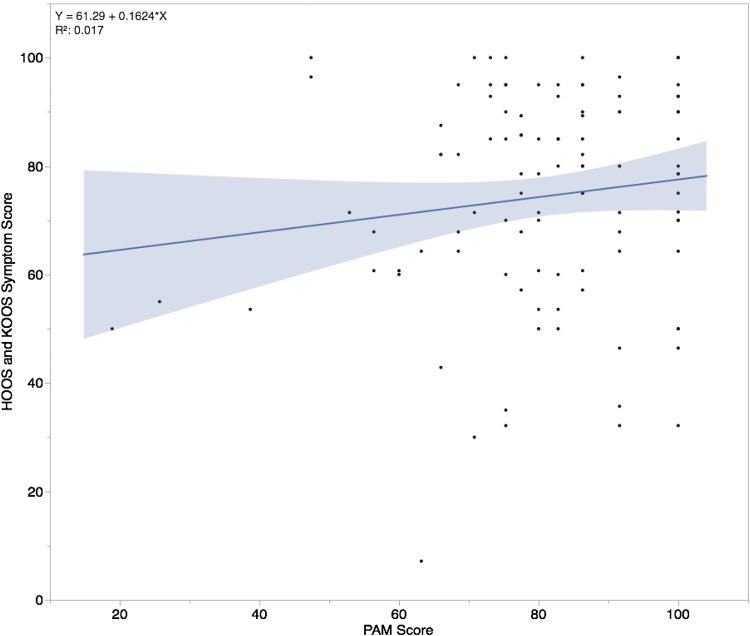
Higher PAM scores were associated with greater pain relief and improved activity using HOOS and KOOS pain scores and HOOS and KOOS symptom scores. The change in preoperative HOOS and KOOS pain subscale to postoperative pain subscale score was mean 40 (95% CI, 36–44, p < 0.001) (Table 2). In our multiple linear regression model patients with higher PAM scores had higher HOOS and KOOS pain scores (R2 = 0.311, p = 0.048; Table 3) at their 6- or 12-month followup. For every 1 unit increase in PAM score, the pain score improved by 0.20 (Fig. 1). Higher PAM scores also were associated with fewer patient-reported difficulties with walking and grinding of the joint using the HOOS and KOOS symptoms score. The change from preoperative HOOS and KOOS symptom score to postoperative symptom score was mean 36 (95% CI, 32–40; p < 0.001) (Table 2). In our multiple linear regression model, the PAM was associated with higher HOOS and KOOS symptom scores (R2 = 0.272, p = 0.021) (Table 3). For every 1 unit increase in PAM score, HOOS and KOOS symptom scores increased by 0.16 (Fig. 2). However, we did not find higher PAM scores were associated with an improvement in HOOS and KOOS activities of daily living score (R2 = 0.255, p = 0.079), sports and recreation score (R2 = 0.193, p = 0.985, or quality of life score (R2 = 0.293, p = 0.988). A higher PAM was not associated with patients having higher patient-reported activity with the UCLA activity score (R2 = 0.062, p = 0.099). There was an overall improvement in the UCLA activity score between preoperative and postoperative scores (1.0; 95% CI, 1.3–0.71; p < 0.001) (Table 2). A portion of the variation seen in the HOOS and KOOS pain and symptom scores is attributable to preoperative PAM scores.
Table 3.
General linear model of patient activation
| Dependent variables | SD | p value | R-square |
|---|---|---|---|
| Satisfaction score | |||
| Charlson Comorbidity Index | 13.872 | 0.311 | |
| Patient Activation Measure Score | 1.099 | 0.023 | 0.048 |
| HOOS/KOOS WOMAC Score | |||
| Charlson Comorbidity Index | 16.247 | 0.084 | |
| Patient Activation Measure Score | 1.275 | 0.078 | 0.294 |
| HOOS/KOOS Quality of Life Score | |||
| Charlson Comorbidity Index | 18.680 | 0.798 | |
| Patient Activation Measure Score | 1.472 | 0.988 | 0.293 |
| HOOS/KOOS Sports and Recreation Score | |||
| Charlson Comorbidity Index | 21.466 | 0.919 | |
| Patient Activation Measure Score | 1.687 | 0.985 | 0.193 |
| HOOS/KOOS ADL Score | |||
| Charlson Comorbidity Index | 17.169 | 0.294 | |
| Patient Activation Measure Score | 1.334 | 0.079 | 0.255 |
| HOOS/KOOS Pain Score | |||
| Charlson Comorbidity Index | 16.855 | 0.080 | |
| Patient Activation Measure Score | 1.315 | 0.048 | 0.311 |
| HOOS/KOOS Symptoms Score | |||
| Charlson Comorbidity Index | 16.678 | 0.079 | |
| Patient Activation Measure Score | 1.315 | 0.021 | 0.272 |
| SF 12v2® Mental Score | |||
| Charlson Comorbidity Index | 8.889 | 0.515 | |
| Patient Activation Measure Score | 0.687 | < 0.001 | 0.057 |
| SF12v2® Physical Score | |||
| Charlson Comorbidity Index | 7.947 | 0.033 | |
| Patient Activation Measure Score | 0.628 | 0.173 | 0.176 |
| UCLA Activity Score | |||
| Charlson Comorbidity Index | −1.432 | 0.942 | |
| Patient Activation Measure Score | 32.454 | 0.099 | 0.062 |
PAM = Patient Activation Measure Score; ADL = activities of daily living; HOOS = Hip Disability and Osteoarthritis Outcome Score; KOOS = Knee Injury and Osteoarthritis Outcome Score; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Fig. 1.
The independent effect of the PAM as a function of the HOOS and KOOS Pain Scores is shown. PAM = Patient Activation Measure; HOOS = Hip Disability and Osteoarthritis Score; KOOS = Knee and Osteoarthritis Outcome Score; blue line = Regression model.
Fig. 2.
The independent effect of the PAM as a function of the HOOS and KOOS Symptom Scores is shown. PAM = Patient Activation Measure; HOOS = Hip Disability and Osteoarthritis Score; KOOS = Knee and Osteoarthritis Outcome Score; blue line = Regression model.
Physical and Mental Health
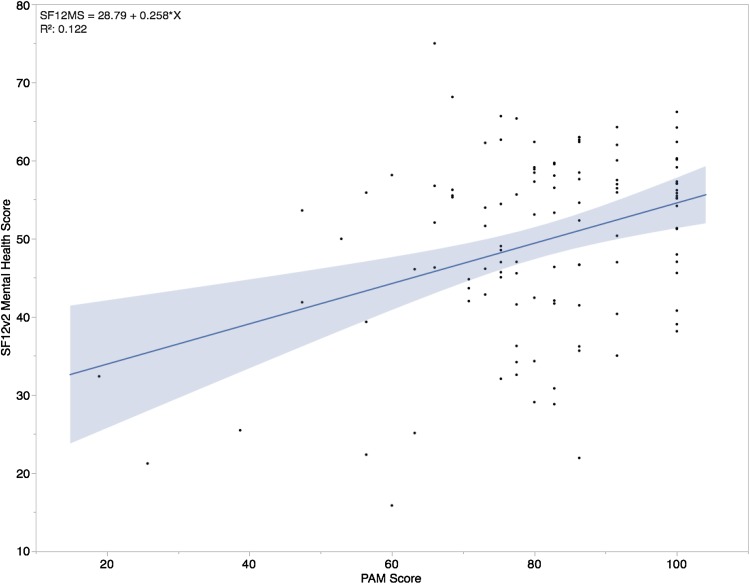
Higher patient activation scores were associated with higher postoperative mental health scores using the SF12v2® mental health score, however we did not find an association with an improvement in postoperative physical health on the SF12v2® physical health score. The mean change in SF12v2® mental health score from preoperative to postoperative was 2.0 (95% CI, 0.3–4.2; p = 0.09) (Table 2). Higher PAM scores were associated with higher SF12v2® mental health scores at 6 or 12 months postoperatively (R2 = 0.057, p < 0.001) (Table 3). For every 1 unit increase in PAM scores, mental health scores improved by 0.26 (Fig. 3). A portion of the variation seen in SF12v2® scores is attributable to preoperative PAM scores. The mean change in preoperative SF12v2® physical health score to postoperative health score was 10.6 (95% CI, 8.7–12.6; p < 0.001) (Table 2). Higher PAM scores were not associated with better physical health using the SF12v2® physical health score (R2 = 0.176, p = 0.173) (Table 3). Therefore, patients with a higher patient-activation score did not have a higher postoperative physical health score at their 6- or 12-month followup.
Fig. 3.
The independent effect of the PAM as a function of the SF12v2®Mental Health Score is shown. PAM = Patient Activation Measure; blue line = Regression model.
Patient Satisfaction
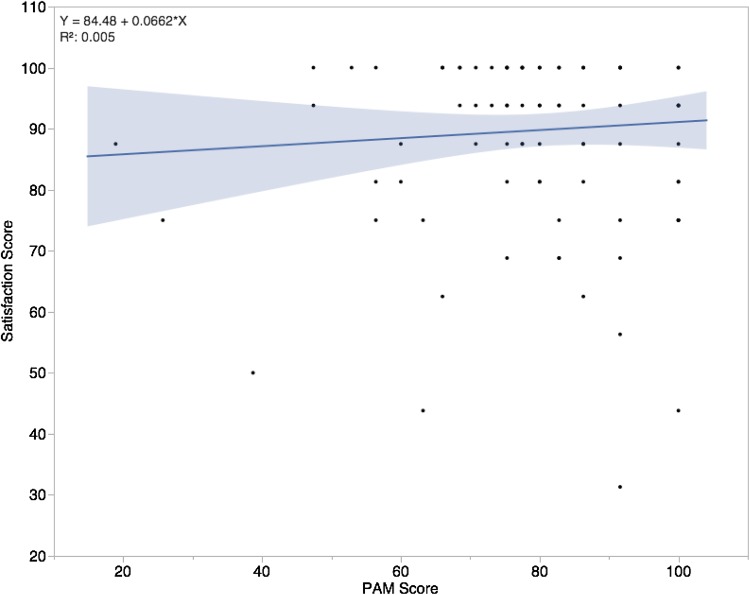
Higher patient activation scores were associated with higher postoperative patient satisfaction using the HKSS questionnaire. The average patient satisfaction score postoperatively was 91(± 13). In our multiple linear regression model higher PAM scores were associated with higher patient satisfaction (R2 = 0.048, p = 0.023) (Table 3). For every 1 unit increase in PAM, satisfaction scores increased by 0.06 (Fig 4). A small portion of the variation seen in the HKSS is explainable by the preoperative PAM score.
Fig. 4.
The independent effect of the PAM as a function of the Satisfaction Score is shown. PAM = Patient Activation Measure; blue line = Regression model.
Discussion
Some patients have unexpectedly poor self-reported outcomes and unmet expectations after TJA [25–27, 31, 35]. Patient activation is one measure that has been studied preliminarily in patients with chronic disease and found to influence patient outcomes and experiences, however, to our knowledge, it has not been studied in TJAs. The PAM is an easy-to-use survey that could help explain why some patients have better outcomes than others. In our study, we found that patients with higher PAM scores had better pain relief, decreased symptoms, improved mental health, and higher satisfaction after TJA.
There were several limitations to our study. First, our patients had a much higher patient-activation score mean and smaller variation than previously reported [17]. This small range of PAM scores ultimately limited our ability to perform a large multivariate regression model as the inclusion of every variable maximizes standard errors and masks the effect of the PAM. The PAM is a self-assessment product preliminarily validated in 2004 and was shown to be a good measure of psychometric properties [18]. However activation and psychometric testing may be difficult to assess in a preoperative surgical setting. It is possible our elevated PAM score mean was a result of our study design, as we administered the PAM questionnaire after patients were scheduled for surgery. It is possible that patients completed the questionnaire attempting to convince their surgeon or themselves of their personal health engagement. It also is possible that a physician may have referred patients whom they considered better candidates for surgery (that is, patients with higher activation). Our results did not follow the typical four stages of activation seen in other studies [14, 34, 35], although our regression model showed patients with higher activation had higher postoperative patient-reported outcomes. Second, there are multiple confounding variables we did not measure that could influence postoperative patient-reported outcomes. Total joint replacements are influenced by many complex factors including demographic or comorbid conditions, preoperative joint conditions, surgical factors, postoperative rehabilitation, and complications after surgery. We measured multiple demographic characteristics in our analysis and used the Charlson Comorbidity Index as a tool to risk adjust for comorbid conditions. This study was performed at two medical centers by five different arthroplasty surgeons, thus we could not control surgical factors (ie, implants, surgical time, resident involvement) or postoperative rehabilitation protocols. In addition, 6 to 12 months followup may be considered too early to see many of the long-term complications that occur after a TJA. However, our model fit was good despite omissions indicating that our simplified model adequately explained a portion of the variability for our postoperative outcomes. Third, although the self-administered HKSS is validated [24], the methods used in its validation study were not strong and its correlations with the WOMAC were not robust. Nonetheless the HKSS has been reported in multiple studies and seems to be accepted [5, 13, 24, 39, 43]. Fourth, our primary endpoint was measured at 6 or 12 months; a 6-month followup could be too early in terms of patient rehabilitation and recovery to see full improvements after surgery. This may be one of the reasons we did not observe an improvement in UCLA activity score and HOOS and KOOS scores for activities of daily living and sports and recreation. In the shorter term, patients are likely to have pain relief but it may take 6 months to a year or longer to regain full function, completely return to work, and resume normal activities of daily living. Finally, our sample was drawn from two academic institutions in their specialty-specific arthroplasty clinics. Patients who present to these institutions and subspecialty clinics may not be typical of patients from a community setting.
Patient activation has been shown to improve an individual’s health outcome in medical or nonsurgical fields, as individuals with higher activation are more likely to avoid health-damaging behavior, engage in regular self-monitoring at home, and have increased medication adherence [12, 16, 17, 28, 42]. In 2011, Skolasky et al. [38] reported that patient activation was associated with better recovery after an orthopaedic procedure (lumbar spine surgery) and suggested that the PAM may be used as a tool to identify patients who are at risk for a poor outcome. Patient activation also has been shown to correlate with increased participation and engagement in physical therapy [37], an important component of postoperative THA- and TKA-care protocols. Previous investigators have reported that patients who are highly activated endorse better care experiences compared with their less-activated colleagues who see the same provider [14]. It is thought that highly activated patients will have the skills and confidence to get the outcomes they need from their physician. Thus, our study further strengthens the relationship between patient activation and postoperative patient-reported outcomes for orthopaedic procedures such as TJA.
Patients who describe their health as fair or poor or with higher levels of depression are more likely to have worse outcomes and satisfaction after TJA [4, 13]. The additional information provided by patient activation and its association with functional outcomes after TJA is a novel contribution. The PAM has the potential to be incorporated in routine orthopaedic practices to assist with measuring the psychometric and personal competencies of a patient. These traits may affect willingness to participate in rehabilitation [37] and correlate with outcomes after surgery.
With more value being placed on patient-reported outcomes to measure quality and effectiveness of healthcare interventions [2, 6], orthopaedic surgeons may need to consider all variables that may affect patient-reported outcomes. In addition, the move toward new payment models that incentivize value over volume eventually might require orthopaedists to build shared decision making and other patient-focused care models to promote patient engagement in their practices [45]. New research has shown that interventions directly targeting patient activation have led to improved adoption of positive health behaviors in patients regardless of their socioeconomic status or medical comorbidites [8, 11, 15, 23, 40]. Thus, future research should be focused on evaluating if increasing a patient’s activation actually improves their recovery, behavior, and patient-reported outcomes after orthopaedic surgery.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Isabel E. Allen PhD. (Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, CA, USA) for statistical support; Long-Co Nguyen BS (University of California San Francisco, School of Medicine, San Francisco, CA, USA); Cory Pham BS (University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA); and Martina Shoukralla, Matthew Satyadi, Christina Mikhail, Daniel Alvarado, and Gavin Tsuchida (all from University of California Los Angeles, College of Letters and Science, Los Angeles, CA, USA) for assistance collecting the data.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Ethical review committee statement: Each author certifies that institution where the work was performed approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of California, San Francisco, CA, USA, and Harbor−University of California, Los Angeles, CA, USA.
References
- 1.Anakwe RE, Jenkins PJ, Moran M. Predicting dissatisfaction after total hip arthroplasty: a study of 850 patients. J Arthroplasty. 2011;26:209–213. doi: 10.1016/j.arth.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Andrawis JP, Chenok KE, Bozic KJ. Health policy implications of outcomes measurement in orthopaedics. Clin Orthop Relat Res. 2013;471:3475–3481. doi: 10.1007/s11999-013-3014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser score work. Med Care. 2013 May 23. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Baker PN, Rushton S, Jameson SS, Reed M, Gregg P, Deehan DJ. Patient satisfaction with total knee replacement cannot be predicted from pre-operative variables alone: a cohort study from the National Joint Registry for England and Wales. Bone Joint J. 2013;95:1359–1365. doi: 10.1302/0301-620X.95B10.32281. [DOI] [PubMed] [Google Scholar]
- 5.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bumpass DB, Samora JB, Butler CA, Jevsevar DS, Moffatt-Bruce SD, Bozic KJ. Orthopaedic quality reporting: a comprehensive review of the current landscape and a roadmap for progress. JBJS Reviews. 2014;2:e5. doi: 10.2106/JBJS.RVW.M.00126. [DOI] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Deen D, Lu WH, Rothstein D, Santana L, Gold MR. Asking questions: the effect of a brief intervention in community health centers on patient activation. Patient Educ Couns. 2011;84:257–260. doi: 10.1016/j.pec.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar MJ, Richardson G, Robertsson O. I can’t get no satisfaction after my total knee replacement: rhymes and reasons. Bone Joint J. 2013; 95(11 suppl A):148–152. [DOI] [PubMed]
- 10.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Frosch DL, Rincon D, Ochoa S, Mangione CM. Activating seniors to improve chronic disease care: results from a pilot intervention study. J Am Geriatr Soc. 2010;58:1496–1503. doi: 10.1111/j.1532-5415.2010.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27:520–526. doi: 10.1007/s11606-011-1931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanusch BC, O’Connor DB, Ions P, Scott A, Gregg PJ. Effects of psychological distress and perceptions of illness on recovery from total knee replacement. Bone Joint J. 2014;96:210–216. doi: 10.1302/0301-620X.96B2.31136. [DOI] [PubMed] [Google Scholar]
- 14.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207–214. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 15.Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient’s level of activation. Am J Manag Care. 2009;15:353–360. [PubMed] [Google Scholar]
- 16.Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst NP, Ruta DA, Kind P. Comparison of the MOS short form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritis. Br J Rheumatol. 1998;37:862–869. doi: 10.1093/rheumatology/37.8.862. [DOI] [PubMed] [Google Scholar]
- 20.Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, Dieppe PA. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford). 2012;51:1804–1813. [DOI] [PubMed]
- 21.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96:624–630. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 22.Lingard EA, Sledge CB, Learmonth ID. Kinemax Outcomes Group. Patient expectations regarding total knee arthroplasty: differences among the United States, United Kingdom, and Australia. J Bone Joint Surg Am. 2006;88:1201–1207. doi: 10.2106/JBJS.E.00147. [DOI] [PubMed] [Google Scholar]
- 23.Lorig K, Alvarez S. Re: Community-based diabetes education for Latinos. Diabetes Educ. 2011;37:128. doi: 10.1177/0145721710393089. [DOI] [PubMed] [Google Scholar]
- 24.Mahomed N, Gandhi R, Daltroy L, Katz JN. The self-administered patient satisfaction scale for primary hip and knee arthroplasty. Arthritis. 2011;2011:591253. doi: 10.1155/2011/591253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahomed NN, Liang MH, Cook EF, Daltroy LH, Fortin PR, Fossel AH, Katz JN. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J Rheumatol. 2002;29:1273–1279. [PubMed] [Google Scholar]
- 26.Mancuso CA, Jout J, Salvati EA, Sculco TP. Fulfillment of patients’ expectations for total hip arthroplasty. J Bone Joint Surg Am. 2009;91:2073–2078. doi: 10.2106/JBJS.H.01802. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso CA, Sculco TP, Wickiewicz TL, Jones EC, Robbins L, Warren RF, Williams-Russo P. Patients’ expectations of knee surgery. J Bone Joint Surg Am. 2001;83:1005–1012. doi: 10.1302/0301-620X.83B7.12105. [DOI] [PubMed] [Google Scholar]
- 28.Mosen DM, Schmittdiel J, Hibbard J, Sobel D, Remmers C, Bellows J. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage. 2007;30:21–29. doi: 10.1097/00004479-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Nordhorn J, Roll S, Willich S. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90:523–527. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS): validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsdotter AK, Toksvig-Larsen S, Roos EM. Knee arthroplasty: are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop. 2009;80:55–61. doi: 10.1080/17453670902805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell RL, Lim LL. Utility of the Charlson comorbidity index computed from routinely collected hospital discharge diagnosis codes. Methods Inf Med. 2000;39:7–11. [PubMed] [Google Scholar]
- 33.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS): validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santaguida PL, Hawker GA, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, Coyte PC, Wright JG. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg. 2008;51:428–436. [PMC free article] [PubMed] [Google Scholar]
- 36.Scott CE, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92:1253–1258. doi: 10.1302/0301-620X.92B9.24394. [DOI] [PubMed] [Google Scholar]
- 37.Skolasky RL, Mackenzie EJ, Wegener ST, Riley LH 3rd. Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine (Phila Pa 1976). 2008;33:E784–791. [DOI] [PMC free article] [PubMed]
- 38.Skolasky RL, Mackenzie EJ, Wegener ST, Riley LH., 3rd Patient activation and functional recovery in persons undergoing spine surgery. J Bone Joint Surg Am. 2011;93:1665–1671. doi: 10.2106/JBJS.J.00855. [DOI] [PubMed] [Google Scholar]
- 39.Smith AJ, Wylde V, Berstock JR, Maclean AD, Blom AW. Surgical approach and patient-reported outcomes after total hip replacement. Hip Int. 2012;22:355–361. doi: 10.5301/HIP.2012.9455. [DOI] [PubMed] [Google Scholar]
- 40.Solomon M, Wagner SL, Goes J. Effects of a Web-based intervention for adults with chronic conditions on patient activation: online randomized controlled trial. J Med Internet Res. 2012;14:e32. doi: 10.2196/jmir.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terwee C, Bouwmeester W, van Elsland S, de Vet H, Dekker J. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthritis Cartilage. 2011;19:620–633. doi: 10.1016/j.joca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 43.Wright RJ, Sledge CB, Poss R, Ewald FC, Walsh ME, Lingard EA. Patient-reported outcome and survivorship after Kinemax total knee arthroplasty. J Bone Joint Surg Am. 2004;86:2464–2470. doi: 10.2106/00004623-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Wylde V, Dieppe P, Hewlett S, Learmonth ID. Total knee replacement: is it really an effective procedure for all? Knee. 2007;14:417–423. doi: 10.1016/j.knee.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Youm J, Chenok K, Belkora J, Chan V, Bozic KJ. The emerging case for shared decision making in orthopaedics. J Bone Joint Surg Am. 2012;94:1907–1912. [Google Scholar]
- 46.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13:890–895. doi: 10.1016/S0883-5403(98)90195-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.