Abstract
Background
Despite the well-established role of sex on the anterior cruciate ligament (ACL) injury risk, its effects on ACL surgical outcomes remain controversial. This is particularly critical when developing novel surgical techniques to treat the injury because there are limited data existing on how these procedures will respond in each sex. One such approach is bridge-enhanced ACL repair, in which primary suture repair of the ACL is augmented with a bioactive scaffold saturated with autologous blood. It has shown comparable biomechanical outcomes to ACL reconstruction in preclinical models.
Questions/purposes
We asked (1) whether sex affects the biomechanical outcomes of bridge-enhanced ACL repair; and (2) if suture type (absorbable or nonabsorbable), used to repair the torn ACL, can minimize the potential sex discrepancies in outcomes after 15 weeks of healing in a large animal preclinical model.
Methods
Seventeen (eight males, nine females) Yorkshire pigs (Parson’s Farms, Hadley, MA, USA) underwent bilateral ACL transection and received bridge-enhanced ACL repair with an absorbable suture (n = 17) on one side and with a nonabsorbable suture (n = 17) on the other side. The leg receiving the absorbable suture was randomized within each animal. ACL structural properties and AP knee laxity for each knee were measured after 15 weeks of healing. Mixed linear models were used to compare the biomechanical outcomes between sexes and suture groups.
Results
When treated with absorbable suture, females had a lower ACL linear stiffness (females, 11 N/mm [range, 8–42]; males, 31 N/mm [range, 12–56]; difference, 20 N/mm [95% confidence interval {CI}, 4–36]; p = 0.032), ACL yield (females, 121 N [range, 56–316]; males, 224 N [range, 55–538]; difference, 103 N [95% CI, 6–200]; p = 0.078), and maximum load (females, 128 N [range, 63–332]; males, 241 N [range, 82–538]; difference, 114 N [95% CI, 15–212]; p = 0.052) than males after 15 weeks of healing. Female knees treated with absorbable suture had a lower linear stiffness (absorbable, 11 N/mm [range, 8–42]; nonabsorbable, 25 N/mm [range, 8–64]; difference, 14 [95% CI, 2–26] N; p = 0.054), ACL yield (absorbable, 121 N [range, 56–316]; nonabsorbable, 230 N [range, 149–573]; difference, 109 N [95% CI, 56–162]; p = 0.002), and maximum load (absorbable, 128 N [range, 63–332]; nonabsorbable, 235 N [range, 151–593]; difference, 107 N [95% CI, 51–163]; p = 0.002) along with greater AP knee laxity at 30° (absorbable, 9 mm [range, 5–12]; nonabsorbable, 7 mm [range, 2–13]; difference, 2 mm [95% CI, 1–4]; p = 0.034) than females treated with nonabsorbable suture. When repaired using nonabsorbable suture, the biomechanical outcomes were similar between female and male knees (p > 0.10).
Conclusions
Females had significantly worse biomechanical outcomes than males when the repairs were performed using absorbable sutures. However, the use of nonabsorbable sutures ameliorated these differences between males and females.
Clinical Relevance
The current findings highlight the critical role of sex on the biomechanical outcomes of bridge-enhanced ACL repair in a relevant large animal model. Better understanding of the mechanisms responsible for these observations using preclinical models and concomitant clinical studies in human patients may allow for additional development of sex-specific surgical and rehabilitative strategies with potentially improved outcomes in women.
Introduction
Injuries to the anterior cruciate ligament (ACL) are common [31]. Women are at increased risk (up to 10-fold) for ACL injury in comparison to men when playing the same sport [29]. Despite reasonable success of ACL reconstruction, the current gold standard of treatment, in restoring the gross stability of the ACL-deficient knee, it fails to restore normal joint kinematics and kinetics [8, 22, 24, 25, 43, 52]. Moreover, ACL reconstruction is associated with reduced activity level [5], an increased rate of secondary injury [48], and high risk of posttraumatic osteoarthritis (OA), up to 74%, even with advanced anatomic reconstruction techniques [13, 32, 33, 40, 50, 56]. The associated complications with ACL reconstruction, in addition to the advent of functional tissue engineering, precipitated a move in ACL research from improving replacement techniques to developing procedures for biologically augmented repair of the ligament. One such approach, bridge-enhanced repair [34], has shown comparable biomechanical outcomes to ACL reconstruction in preclinical models [34, 35, 54]. This emerging surgical technique uses a combination of an extracellular matrix (ECM)-based scaffold saturated with autologous blood along with sutures to repair the torn ACL [34]. Most importantly, bridge-enhanced ACL repair has resulted in a substantial reduction of posttraumatic OA compared with ACL reconstruction in a 1-year followup preclinical study in a porcine large animal model [35].
The rapid rise in the incidence of ACL injuries among women, particularly in the young active population, has generated a substantial interest in studying the sexual dimorphism in the outcomes of ACL surgery [46, 53]. Despite recent findings of worse functional and biomechanical outcomes in women compared with men after ACL reconstruction [1, 9, 15, 17, 23, 41, 42, 44], the role of sex on the outcomes of recently developed surgical techniques such as bridge-enhanced ACL repair is not yet known. A clear understanding of how sex may affect the outcomes of such surgical techniques can help further optimize these approaches with improved outcomes for each sex. This is also necessary to comply with the current National Institutes of Health recommendations to study sex disparities in medical research and the inclusion of female animals in preclinical research to minimize the sex bias and further facilitate the translation from the preclinical stage to human trials [14].
Thus, the current study was designed (1) to investigate the effect of sex on biomechanical outcomes of bridge-enhanced ACL repair; and (2) to determine if suture type (absorbable versus nonabsorbable) used to repair the torn ACL can alleviate the potential sex discrepancies in measured outcomes after 15 weeks of healing in a well-established porcine model with similar sex-specific knee anatomical and biomechanical properties as the human knee [30]. We hypothesized that there are sex differences in the biomechanical outcomes of bridge-enhanced ACL repair with lower ACL structural properties and greater AP knee laxity in females than males and that the choice of suture type used to repair the torn ACLs would affect the observed sex differences in measured biomechanical outcomes.
Materials and Methods
Experimental Design
Seventeen (eight males, nine females) Yorkshire pigs (Parson’s Farms, Hadley, MA, USA; age, 4 ± 1 months; weight, 28 ± 2 kg) underwent bilateral ACL transection and received bilateral bridge-enhanced ACL repair using an ECM-based bioactive scaffold (MIACH; Boston Children’s Hospital, Boston, MA, USA). This large animal model has been widely used in preclinical studies of ACL surgery [18–21, 27, 33, 35–37, 54, 55] and has recently been validated as a sex-specific large animal surrogate model to study the human knee [30]. The bridge-enhanced ACL repair procedures were performed using an absorbable suture stent (Vicryl®; Ethicon Inc, Somerville, NJ, USA; n = 17) on one hindlimb and using a nonabsorbable suture stent (Ethibond; Ethicon Inc; n = 17) on the other hindlimb. The animals were randomly selected. The legs receiving absorbable and nonabsorbable sutures within each animal were alternated across animals. This method of randomization was used to ensure equal distribution of suture type within the study population. Both knees were then harvested and subjected to biomechanical testing. ACL structural properties and AP knee laxity were measured and compared between males and females. Institutional Animal Care and Use Committee approvals were obtained before initiating this study.
Surgical Procedure
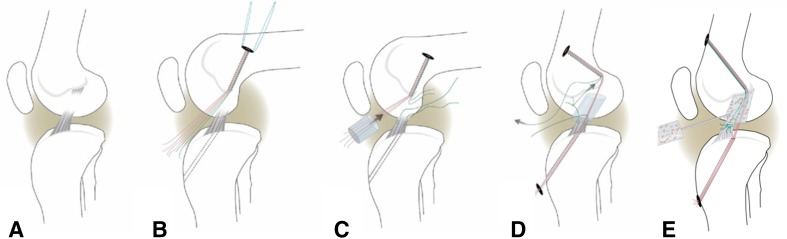
After general anesthesia, a medial arthrotomy was made to expose the ACL. The ACL was isolated and transected at its proximal third (Fig. 1A). The knee was irrigated with 500 mL saline solution. A Lachman test was performed to verify functional loss of the ACL. For all knees, a Kessler suture using No. 1 Vicryl® was placed in the tibial stump of the ACL to repair the transected ligament [20, 36, 37]. Femoral (4.5 mm) and tibial (2.4 mm) tunnels were then drilled in standard positions for ACL reconstruction with the tibial tunnel exiting in the center of the tibial attachment and the femoral tunnel placed at the center of the femoral ACL attachment site. An EndoButton (Smith & Nephew, Andover, MA, USA) loaded with three No. 1 sutures was passed through the femoral tunnel and flipped on the lateral cortex to provide femoral fixation of the sutures (Fig. 1B). Two of these three sutures were used to create the suture stent, which were either Vicryl® (absorbable) or Ethibond (nonabsorbable) based on the study group. The suture stent was then threaded through the ECM-based MIACH scaffold and the scaffold was introduced into the notch until femoral contact was visually verified (Fig. 1C). The suture stent was then passed through the tibial tunnel and tied over a button in front of the tibia with the knee at full extension. The remaining third suture (Vicryl®) from the femoral tunnel/EndoButton was tied to the Kessler suture in the tibial ACL stump to position the remaining ACL tissue in its anatomic orientation (Fig. 1D). Subsequently, the scaffold was saturated in situ with up to 5 mL of autologous blood (Fig. 1E). The knee was kept immobilized for 10 minutes to allow the implanted blood to clot in the scaffold before the incisions were closed in layers.
Fig. 1A–E.
(A) ACL injury was simulated by cutting the ACL in the midsubstance. (B) Femoral and tibial tunnels (dashed lines) were drilled and an EndoButton pulled through the femoral tunnel and engaged on the proximal femoral cortex. The EndoButton was loaded with three sutures, resulting in six free ends. (C) A Kessler suture was placed in the tibial ACL stump, and the MIACH scaffold was threaded onto four of the strands, which served as the suture stent (red). (D) The four suture strands running through the scaffold (red) were then passed through the tibial tunnel. (E) The transtibial sutures (red) were tightened and tied over an extracortical button. The scaffold was then saturated with 5 mL of autologous blood and placed into the notch. The free ends of the ACL suture pulley (green) were tied to secure the ACL stump in the blood-scaffold composite. Adapted and modified from Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672–680, with permission from Elsevier.
All animals were housed in individualized pens for 15 weeks postsurgery. The animals were allowed ad libitum activity during the 15-week postoperative period. They were then euthanized, and the hindlimbs were harvested and immediately frozen at −20 °C until the biomechanical evaluation.
Biomechanical Testing
The knees were thawed to room temperature 24 hours before the biomechanical testing. Specimens were sectioned at the proximal femur and distal tibia with all soft tissues external to the joint capsule removed. The distal tibia and proximal femur were potted for rigid attachment to the testing frame (MTS 810; Material Testing Systems, Prairie Eden, MN, USA) [21]. The joints were wrapped in towels saturated with physiologic saline to minimize dehydration before testing.
AP Knee Laxity
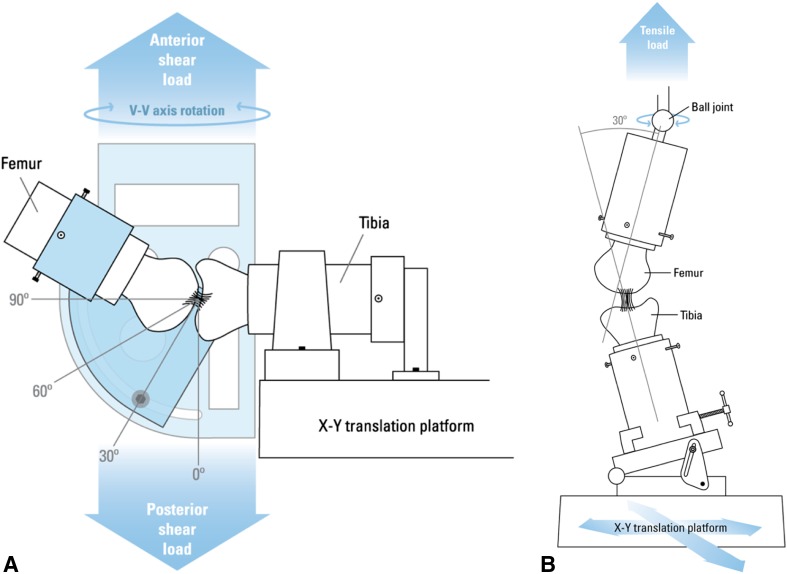
AP knee laxity values were measured at 30°, 60°, and 90° of knee flexion angle using a custom fixture within the testing frame (Fig. 2A) [18, 19]. With knees locked at each prespecified flexion angle, axial tibial rotation was constrained in the neutral position, whereas tibial translation/rotation remained unconstrained in the coronal plane [21]. The knees were subjected to 12 sinusoidal cycles of ± 40 N AP-directed shear loads at each knee flexion angle and the AP displacements were measured. The first three cycles were used to precondition the knees, whereas the data from the remaining nine cycles were averaged for final measurements. AP knee laxity was defined as the total femoral translation in the sagittal plane, with respect to the tibia, within the AP shear load limits of ± 30 N [19, 21].
Fig. 2A–B.
(A) AP knee laxity was measured with the a prescribed knee flexion angle, constrained axial tibial rotation in the neutral position, and the unconstrained translations in the coronal plane during the application of the cyclic AP shear loads. Adapted from Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27:639–645, with permission from John Wiley and Sons. (B) ACL structural properties were assessed with the knee flexion angle set at 30° initially. The tibia was mounted to the base of the MTS through a sliding X-Y platform while the femur was unconstrained to rotations so that the specimen could seek its own position to ensure that the load was distributed over the entire ACL cross-section. Adapted from Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563, with permission from Sage.
Anterior Cruciate Ligament Structural Properties
The structural properties of the repaired ACLs were determined using a tensile test to failure as previously described (Fig. 2B) [21]. After the laxity assessment, all remaining soft tissues were dissected from the joint leaving the femur-repaired ACL-tibia construct intact. All knees were grossly assessed for repair tissue integrity and the presence of remaining suture material within the repaired ACL during the dissection was noted. The femur-ACL-tibia constructs were secured in a custom-designed tensile testing fixture such that the mechanical axis of the ACL was collinear with the load axis of the test frame [21]. The femoral rotation was unconstrained with a ball joint and the tibia was connected to the test frame through a sliding X-Y table to enable the specimen to seek its own physiologic position as the tensile load was applied. Specimens were then loaded in tension to failure at 20 mm/min. This loading rate was adapted from previously established protocols for testing the tensile properties of bone-ligament-bone constructs in porcine and canine models [21, 26, 28, 57]. Linear stiffness, yield, and maximum loads of the repaired ACL were determined from the load displacement data [26, 28]. Energy to failure was also calculated as the area under the load displacement curve for each knee.
Statistical Analysis
All quantified outcomes were grouped and compared based on sex (between subject) and suture type (within subject). Comparisons were conducted using multiple mixed linear models with hindlimb side as the repeating factor. Probability values were adjusted for multiple comparisons using post hoc Bonferroni correction. Results are reported as mean (range) and mean differences (95% confidence intervals); p ≤ 0.05 was considered statistically significant. Analyses were conducted using SPSS statistical package (Version 22.0; IBM Corp, Armonk, NY, USA).
Animal Welfare
All animals recovered well from surgery and survived the full 15-week followup with no signs of infections or other complications. Weightbearing status was achieved within 72 hours after surgery. All nonabsorbable sutures had ruptured by the 15-week time point with ruptures occurring in the distal half of the intraarticular portion. No residual suture material was found in any of the knees repaired with absorbable sutures as assessed by macroscopic evaluation before tensile testing.
Results
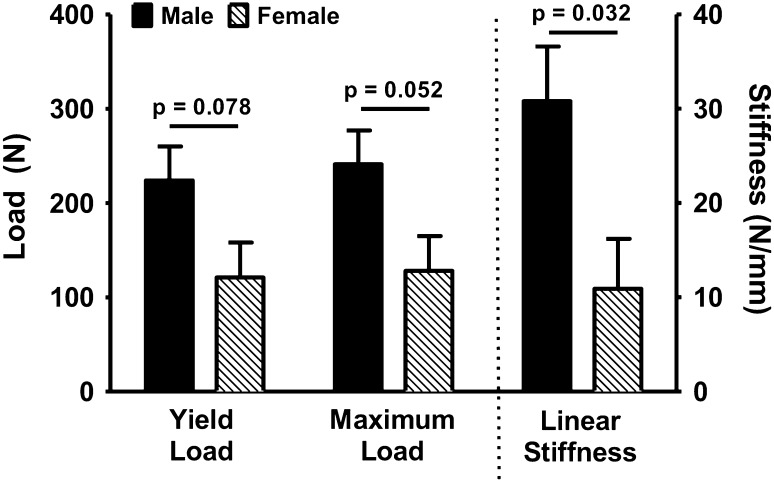
All the ACL ruptures occurred at the ligament midsubstance with no signs of bony avulsions. For knees undergoing bridge-enhanced ACL repair with an absorbable suture stent, females had a significantly lower ACL linear stiffness than their male counterparts after 15 weeks of healing (females, 11 N/mm [range, 8–42]; males, 31 N/mm [range, 12–56]; difference, 20 N/mm [95% confidence interval {CI}, 4–36]; p = 0.032). Females also had lower ACL yield (females, 121 N [range, 56–316]; males, 224 N [range, 55–538]; difference, 103 N [95% CI, 6–200]; p = 0.078) and maximum loads (females, 128 N [range, 63–332]; males, 241 N [range, 82–538]; difference, 114 N [95% CI, 15–212]; p = 0.052) compared with males, which approached statistical significance (Fig. 3). No differences were observed in ACL energy to failure and AP knee laxity between male and female knees treated with bridge-enhanced ACL repair with absorbable suture (p ≥ 0.70 for all comparisons; Table 1). For knees repaired with nonabsorbable sutures, there were no significant differences between males and females in any of the measured biomechanical outcomes (p > 0.10 for all outcomes; Table 1).
Fig. 3.
ACL structural properties for knees treated with bridge-enhanced ACL repair using absorbable suture were compared between sexes after 15 weeks of healing in a porcine model.
Table 1.
Quantified ACL structural properties and AP knee laxity in both the male (M) and female (F) pigs treated with absorbable (AB) and nonabsorbable (NA) sutures
| Parameter | Mean (Range) | Mean difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Absorbable | Nonabsorbable | p value* | ||||||
| Male | Female | Male | Female | AB (M versus F) | NA (M versus F) | M (AB versus NA) | F (AB versus NA) | |
| Yield load (N) | 224 (55–538) | 121 (56–316) | 235 (84–450) | 230 (149–573) | 103 (6–200) p = 0.078 |
11 (−45 to 67) p = 1.000 |
5 (−88 to 97) p = 1.000 |
109 (56–162) p = 0.002 |
| Maximum load (N) | 241 (82–538) | 128 (63–332) | 275 (123–469) | 235 (151–593) | 114 (15–212) p = 0.052 |
40 (−54 to 134) p = 0.772 |
33 (−25 to 92) p = 0.480 |
107 (51–163) p = 0.002 |
| Linear stiffness (N/mm) | 31 (12–56) | 11 (8–42) | 39 (21–75) | 25 (8–64) | 20 (4–36) p = 0.032 |
14 (−2 to 29) p = 0.166 |
8 (−5 to 21) p = 0.410 |
14 (2–26) p = 0.054 |
| Energy to failure (Nmm2) | 950 (272–1901) | 733 (173–1452) | 1194 (494–2268) | 1230 (742–2021) | 216 (−358 to 792) p = 0.888 |
36 (−516 to 588) p = 1.000 |
244 (−237 to 725) p = 0.588 |
497 (27–967) p = 0.08 |
| Laxity at 30° (mm) | 8 (1–14) | 9 (5–12) | 9 (5–12) | 7 (2–13) | 1 (−3 to 4) p = 1.000 |
2 (0–5) p = 0.158 |
1 (−1 to 3) p = 0.780 |
2 (1–4) p = 0.034 |
| Laxity at 60° (mm) | 14 (9–19) | 15 (8–20) | 12 (11–14) | 14 (8–21) | 1 (−1 to 4) p = 0.700 |
2 (−1 to 4) p = 0.422 |
2 (0–4) p = 0.216 |
1 (−1 to 3) p = 0.416 |
| Laxity at 90° (mm) | 10 (7–14) | 10 (8–14) | 9 (7–11) | 10 (7–12) | 0 (−2 to 2) p = 1.000 |
1 (−1 to 3) p = 0.544 |
1 (−1 to 3) p = 0.454 |
0 (−2 to 2) p = 1.000 |
*Significant differences (p ≤ 0.05) are shown in bold; ACL = anterior cruciate ligament.
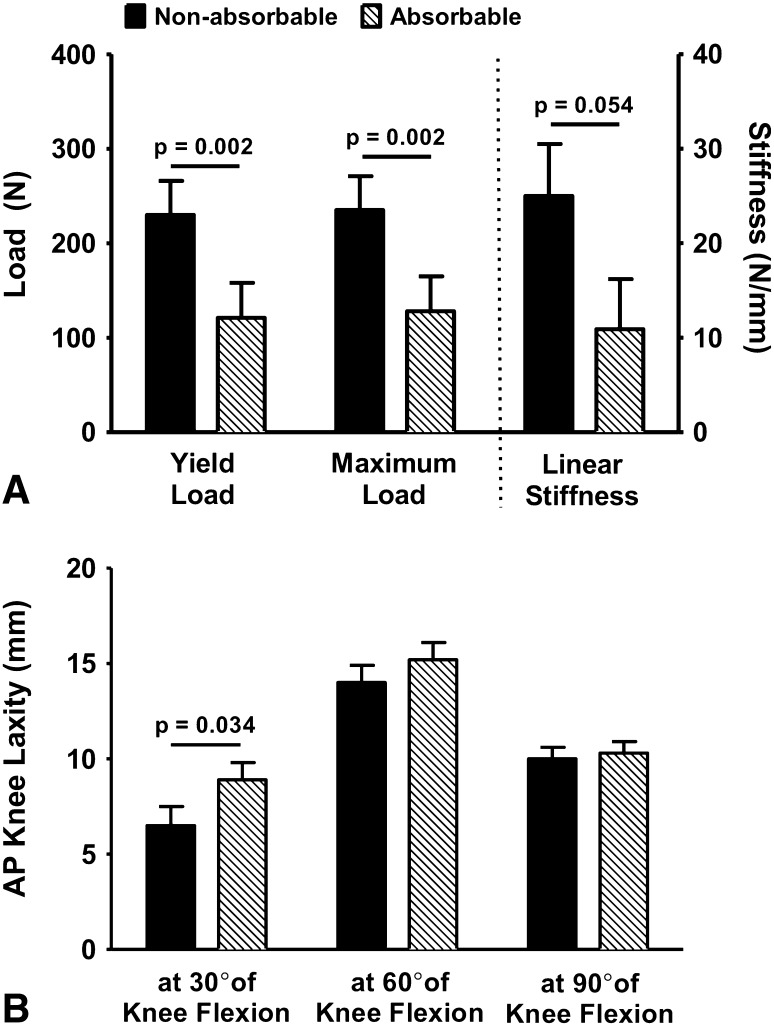
Female knees treated with absorbable suture had a significantly lower ACL yield (absorbable, 121 N [range, 56–316]; nonabsorbable, 230 N [range, 149–573]; difference, 109 N [95% CI, 56–162]; p = 0.002) and maximum loads (absorbable, 128 N [range, 63–332]; nonabsorbable, 235 N [range, 151–593]; difference, 107 N [95% CI, 51–163]; p = 0.002) than female knees repaired with nonabsorbable suture (Fig. 4A). There was a strong trend that female ACLs repaired with absorbable suture had a lower linear stiffness (absorbable, 11 N/mm [range, 8–42]; nonabsorbable, 25 N/mm [range, 8–64]; difference, 14 [95% CI, 2–26] N; p = 0.054) and failure energy (absorbable, 733 Nmm2 [range, 173–1452]; nonabsorbable, 1230 Nmm2 [range, 742–2021]; difference, 497 Nmm2 [95% CI, 27–967]; p = 0.08) than those treated with nonabsorbable suture (Fig. 4A). Female knees repaired with absorbable suture also had a significantly greater AP knee laxity at 30° of knee flexion (absorbable, 9 mm [range, 5–12]; nonabsorbable, 7 mm [range, 2–13]; difference, 2 mm [95% CI, 1–4]; p = 0.034) compared with female knees treated with nonabsorbable suture (Fig. 4B). No differences were observed in AP knee laxity at 60° and 90° of flexion between females treated with absorbable or nonabsorbable sutures (p > 0.20 for all comparisons; Table 1). All measured biomechanical outcomes were similar between male knees treated with either absorbable or nonabsorbable suture stents (p > 0.20 for all comparisons; Table 1).
Fig. 4A–B.
(A) Biomechanical outcomes in female pig knees using absorbable suture were compared with those treated with nonabsorbable suture with regard to (A) ACL structural properties and (B) AP knee laxity after 15 weeks of healing.
Discussion
Despite reported sex differences in ACL reconstruction outcomes [1, 9, 15, 17, 23, 41, 42, 44], no data exist on potential sexual dimorphism in outcomes of recently developed surgical treatments for ACL injuries such as bridge-enhanced ACL repair. In this study we examined the effect of sex on the biomechanical outcomes of bridge-enhanced ACL repair in a validated sex-specific large animal preclinical model. We also investigated whether the choice of suture type affects the sex differences in biomechanical outcomes after 15 weeks of healing. Female knees showed worse outcomes than males with regard to ACL structural properties when repaired with absorbable suture; however, the use of nonabsorbable suture resulted in a significant improvement in the biomechanical outcomes of the repairs in females, to the point where there was no longer a significant difference in outcomes between the male and female knees after 15 weeks of healing. The choice of suture type did not have any effect on the biomechanical outcomes of bridge-enhanced ACL repair among male pigs.
Our study has several limitations to consider. The pig is a quadruped and postoperative rehabilitation is difficult to control. It therefore does not fully represent the human condition. However, similar anatomical and biomechanical features between pigs and humans have been noted in terms of biomechanics of the knee, hematology, and wound healing [11, 45, 58]. Moreover, the porcine large animal model has been previously shown to be a valid surrogate model for the human knee in the study of sex disparities thought to increase risk for ACL injury [30]. The investigation was conducted on juvenile, skeletally immature pigs. Although they were sexually immature at the time of surgery, they were approximately 7 months old and sexually mature [10] at the time the biomechanical analyses were performed. Thus, the observed sex differences may have been influenced as well by hormonal factors specific to adolescence. These findings underscore the need for further investigation of the mechanisms through which sex affects the biomechanical outcomes of ACL surgeries including hormonal influences in maturing and sexually mature animals. Future studies are essential to determine whether the current findings also translate to older animals (adolescents and adults). The two-factor study design (ie, sex and suture type) with a sample size of eight or nine per group may limit the statistically significant differences in some of the biomechanical outcomes. However a post hoc power analysis, using a fixed-effects F-test, indicated a power of 0.80, 0.83, 0.90, and 0.87 for ACL yield load, maximum load, linear stiffness, and failure energy, respectively, with a nominal α of 0.05 based on observed mean values for all four groups. Assuming a clinically relevant minimal detectable difference of 3 mm in AP knee laxity [38, 49, 54], the study was powered to 0.89, 0.81, and 0.91 at 30°, 60°, and 90°, respectively. To reduce the potential for bias, all investigators were blinded to the sex during surgeries (MMM), harvest (MMM, BCF), and mechanical testing (BCF). Future studies with higher sample sizes and a more specific study design, focused on the sex effect, are required to address these limitations and build on the current findings.
Another shortcoming of this study is that the ACL injuries were produced in the midsubstance of the ligament using a surgical blade, which does not truly replicate a clinical tear. This may have affected the outcomes of ACL repair; however, it is less likely that the observed sex differences in this study would be affected by this limitation because all animals received the same injury. Additionally, animals underwent bilateral surgery, which may have resulted in lower repaired ACL structural properties compared with a unilateral procedure in which the contralateral knee remains intact and the animal can better protect the healing knee [54]. However, because animals in both groups had bilateral procedures, it is less likely that this variation in healing ACL properties significantly affected the observed sex differences in the biomechanical outcomes of ACL repair. Future studies using a unilateral approach are required to further address this issue. Lastly, animals were followed for only 15 weeks after surgery. However, our prior studies in this model suggest that the initial 15 weeks are when the greatest improvements in ligament biomechanical properties occur [12, 16, 27, 35] and this time point has shown to be a reasonable predictor of long-term healing response in the porcine model [20]. Further investigations are required to determine whether the sex differences in the outcomes of bridge-enhanced ACL repair exist in later stages of ACL healing.
Although no frank ligament failures were noted among the pigs after surgical repair in the current study, female pigs repaired with absorbable suture showed a lower mean ACL linear stiffness than males (by 65%) after 15 weeks of healing (Fig. 2). A poorly healed ACL can present with inferior structural properties with no signs of gross failure, which can lead to lowered functional outcomes. This is in consistent with prior reports of higher rates of graft failure [41] and worse patient-reported outcomes [1, 17, 23, 42] in women compared with men after ACL reconstruction. In a recent study of 375 patients, Teitsma and colleagues [53] have shown time-dependent differences in clinical outcomes between males and females for up to 12 months after ACL surgery. Significantly worse outcomes were reported in females when measured using the Knee Injury and Osteoarthritis Outcome Score, Lysholm score, and Tegner score at several intervals within the first 9 months after ACL reconstruction, whereas both sexes showed comparable outcomes at 12 months [53]. These findings along with the observed sex differences in the current work suggest that there are sex differences in ACL (or graft) healing rates with slower healing in females. Interestingly, in the current study, the use of nonabsorbable suture stents was able to diminish these sex differences.
The use of a nonabsorbable suture stent (as opposed to a absorbable suture stent) significantly improved the structural properties of the healing ACL in females with a 56% increase in ACL linear stiffness and 2 mm decrease in AP knee laxity. The fact that all sutures were already disrupted at 15 weeks after surgery makes it less likely that the reported differences are the result of retained suture material. The suture stent used in the bridge-enhanced ACL repair procedure provides initial biomechanical stability and mechanical protection, in particular in early phases of ACL healing and remodeling [55]. Although both the absorbable and nonabsorbable sutures used in this study have similar structural properties at time zero [39], their stress shielding and mechanical protection for repaired ACL may vary as a result of the absorption rate or time until suture failure. Ethibond is a nonabsorbable, braided poly-(ethylene, terephthalate) suture, which does not lose strength as long as it remains intact. In contrast, Vicryl® is an absorbable, polyglactin suture that loses approximately 25% of its strength each week after implantation and completely dissolves in almost 63 days [47, 51]. This may result in exposure of the repaired tissue to higher loads at earlier stages of healing than the nonabsorbable Ethibond suture. It is noteworthy to mention that excessive stress shielding of a healing ligament could also be detrimental and lead to lower outcomes [4]. Furthermore, suture absorption results in release of breakdown products such as glycolic acid (from polyglycolic acid) or lactic acid (from polylactic acid), which reduce tissue pH and inhibit cell growth [2, 3, 6, 7], thus potentially affecting tissue healing. All of these may in turn preclude the repaired ACL to heal properly.
The findings support our hypothesis that sex significantly affects the biomechanical outcomes of bridge-enhanced ACL repair. Inferior mechanical properties were found for females after 15 weeks of repair using absorbable sutures. Surprisingly, the use of nonabsorbable sutures significantly improved outcomes in female knees. Although more research is needed, these results highlight the potential need to further define sex-specific healing patterns to aid in the development of sex-specific surgical and rehabilitative interventions to match the sex-specific healing requirements.
Acknowledgments
We thank Arthur Nedder DVM, and Mark Kelly from the Animal Resources at Children’s Hospital for their help with animal handling and veterinary care.
Footnotes
Funding for this study was received from the National Institutes of Health (RO1-AR054099 [MMM], RO1-AR056834 [BCF, MMM], and RO1-AR052772 [MMM]) and the Lucy Lippitt Endowment (BCF). Funding was also provided by the Translational Research Program at Boston Children’s Hospital (MMM).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Sports Medicine Research Laboratory, Department of Orthopaedic Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA, and the Department of Orthopaedics, Rhode Island Hospital, Providence, RI, USA.
References
- 1.Ageberg E, Forssblad M, Herbertsson P, Roos EM. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med. 2010;38:1334–1342. doi: 10.1177/0363546510361218. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38:105–114. doi: 10.1002/(SICI)1097-4636(199722)38:2<105::AID-JBM4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.An YH, Woolf SK, Friedman RJ. Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials. 2000;21:2635–2652. doi: 10.1016/S0142-9612(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 4.Andrish JT, Woods LD. Dacron augmentation in anterior cruciate ligament reconstruction in dogs. Clin Orthop Relat Res. 1984;183:298–302. [PubMed] [Google Scholar]
- 5.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med. 2011;39:538–543. doi: 10.1177/0363546510384798. [DOI] [PubMed] [Google Scholar]
- 6.Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14:726–737. doi: 10.1016/S0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- 7.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 8.Beard DJ, Murray DW, Gill HS, Price AJ, Rees JL, Alfaro-Adrian J, Dodd CAF. Reconstruction does not reduce tibial translation in the cruciate- deficient knee–an in vivo study. J Bone Joint Surg Br. 2001;83:1098–1103. doi: 10.1302/0301-620X.83B8.11320. [DOI] [PubMed] [Google Scholar]
- 9.Bizzini M, Gorelick M, Munzinger U, Drobny T. Joint laxity and isokinetic thigh muscle strength characteristics after anterior cruciate ligament reconstruction: bone patellar tendon bone versus quadrupled hamstring autografts. Clin J Sport Med. 2006;16:4–9. doi: 10.1097/01.jsm.0000188040.97135.43. [DOI] [PubMed] [Google Scholar]
- 10.Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J. Steering Group of the RETHINK Project. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29:641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 12.Butler DL. Kappa Delta Award paper. Anterior cruciate ligament: its normal response and replacement. J Orthop Res. 1989;7:910–921. doi: 10.1002/jor.1100070618. [DOI] [PubMed] [Google Scholar]
- 13.Chu CR, Beynnon BD, Buckwalter JA, Garrett WE, Jr, Katz JN, Rodeo SA, Spindler KP, Stanton RA. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39:1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27:444–454. doi: 10.1177/03635465990270040701. [DOI] [PubMed] [Google Scholar]
- 16.Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16:360–369. doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari JD, Bach BR, Jr, Bush-Joseph CA, Wang T, Bojchuk J. Anterior cruciate ligament reconstruction in men and women: an outcome analysis comparing gender. Arthroscopy. 2001;17:588–596. doi: 10.1053/jars.2001.24686. [DOI] [PubMed] [Google Scholar]
- 18.Fleming BC, Abate JA, Peura GD, Beynnon BD. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 19.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28:703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 23.Gobbi A, Domzalski M, Pascual J. Comparison of anterior cruciate ligament reconstruction in male and female athletes using the patellar tendon and hamstring autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12:534–539. doi: 10.1007/s00167-003-0486-0. [DOI] [PubMed] [Google Scholar]
- 24.Hall M, Stevermer CA, Gillette JC. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture. 2012;36:56–60. doi: 10.1016/j.gaitpost.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino Y, Fu FH, Irrgang JJ, Tashman S. Can joint contact dynamics be restored by anterior cruciate ligament reconstruction? Clin Orthop Relat Res. 2013;471:2924–2931. doi: 10.1007/s11999-012-2761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt P, Scheffler SU, Unterhauser FN, Weiler A. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg. 2005;125:238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 27.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 29.Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiapour AM, Shalvoy MR, Murray MM, Fleming BC. Validation of porcine knee as a sex-specific model to study human anterior cruciate ligament disorders. Clin Orthop Relat Res. 2015;473:639–650. doi: 10.1007/s11999-014-3974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 33.Murray JR, Lindh AM, Hogan NA, Trezies AJ, Hutchinson JW, Parish E, Read JW, Cross MV. Does anterior cruciate ligament reconstruction lead to degenerative disease? Thirteen-year results after bone-patellar tendon-bone autograft. Am J Sports Med. 2012;40:404–413. doi: 10.1177/0363546511428580. [DOI] [PubMed] [Google Scholar]
- 34.Murray MM, Fleming BC. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J Orthop Res. 2013;31:1501–1506. doi: 10.1002/jor.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26:S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92:2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36:1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najibi S, Banglmeier R, Matta J, Tannast M. Material properties of common suture materials in orthopaedic surgery. Iowa Orthop J. 2010;30:84–88. [PMC free article] [PubMed] [Google Scholar]
- 40.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21:696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Noojin FK, Barrett GR, Hartzog CW, Nash CR. Clinical comparison of intraarticular anterior cruciate ligament reconstruction using autogenous semitendinosus and gracilis tendons in men versus women. Am J Sports Med. 2000;28:783–789. doi: 10.1177/03635465000280060301. [DOI] [PubMed] [Google Scholar]
- 42.Ott SM, Ireland ML, Ballantyne BT, Willson JD. McClay Davis IS. Comparison of outcomes between males and females after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2003;11:75–80. doi: 10.1007/s00167-003-0348-9. [DOI] [PubMed] [Google Scholar]
- 43.Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006–2012. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 44.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35:564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 45.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:469–476. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan J, Magnussen RA, Cox CL, Hurbanek JG, Flanigan DC, Kaeding CC. ACL reconstruction: do outcomes differ by sex? A systematic review. J Bone Joint Surg Am. 2014;96:507–512. doi: 10.2106/JBJS.M.00299. [DOI] [PubMed] [Google Scholar]
- 47.Sanz LE, Patterson JA, Kamath R, Willett G, Ahmed SW, Butterfield AB. Comparison of Maxon suture with Vicryl, chromic catgut, and PDS sutures in fascial closure in rats. Obstet Gynecol. 1988;71:418–422. [PubMed] [Google Scholar]
- 48.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37:246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 49.Snyder-Mackler L, Fitzgerald GK, Bartolozzi AR, 3rd, Ciccotti MG. The relationship between passive joint laxity and functional outcome after anterior cruciate ligament injury. Am J Sports Med. 1997;25:191–195. doi: 10.1177/036354659702500209. [DOI] [PubMed] [Google Scholar]
- 50.Song EK, Seon JK, Yim JH, Woo SH, Seo HY, Lee KB. Progression of osteoarthritis after double- and single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:2340–2346. doi: 10.1177/0363546513498998. [DOI] [PubMed] [Google Scholar]
- 51.Storch M, Scalzo H, Van Lue S, Jacinto G. Physical and functional comparison of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 suture with triclosan) with Coated VICRYL* Suture (coated polyglactin 910 suture) Surg Infect (Larchmt). 2002;3(Suppl 1):S65–S77. doi: 10.1089/sur.2002.3.s1-65. [DOI] [PubMed] [Google Scholar]
- 52.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 53.Teitsma XM, van der Hoeven H, Tamminga R, de Bie RA. Impact of patient sex on clinical outcomes data from an anterior cruciate ligament reconstruction registry, 2008–2013. Orthop J Sports Med. 2014;2:2325967114550638. doi: 10.1177/2325967114550638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672–680. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vavken P, Proffen B, Peterson C, Fleming BC, Machan JT, Murray MM. Effects of suture choice on biomechanics and physeal status after bioenhanced anterior cruciate ligament repair in skeletally immature patients: a large-animal study. Arthroscopy. 2013;29:122–132. doi: 10.1016/j.arthro.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1:22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 58.Xerogeanes JW, Fox RJ, Takeda Y, Kim HS, Ishibashi Y, Carlin GJ, Woo SL. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]