Abstract
Background
Osteogenesis imperfecta (OI) is a genetic disease characterized by skeletal fragility and deformity. There is extensive debate regarding treatment options in adults with OI. Antiresorptive treatment reduces the number of fractures in growing oim/oim mice, an animal model that reproducibly mimics the moderate-to-severe form of OI in humans. Effects of long-term treatments with antiresorptive agents, considered for treatment of older patients with OI with similar presentation (moderate-to-severe OI) are, to date, unknown.
Questions/purposes
Fourier transform infrared (FTIR) imaging, which produces a map of the spatial variation in chemical composition in thin sections of bone, was used to address the following questions: (1) do oim/oim mice show a sex dependence in compositional properties at 6.5 months of age; (2) is there a sex-dependent response to treatment with antiresorptive agents used in the treatment of OI in humans; and (3) are any compositional parameters in oim/oim mice corrected to wild-type (WT) values after treatment?
Methods
FTIR imaging data were collected from femurs from four to five mice per sex per genotype per treatment. Treatments were 24 weeks of saline, alendronate, or RANK-Fc; and 12 weeks of saline + 12 weeks RANK-Fc and 12 weeks of alendronate + RANK-Fc. FTIR imaging compositional parameters measured in cortical and cancellous bones were mineral-to-matrix ratio, carbonate-to-mineral ratio, crystal size/perfection, acid phosphate substitution, collagen maturity, and their respective distributions (heterogeneities). Because of the small sample size, nonparametric statistics (Mann-Whitney U- and Kruskal-Wallis tests with Bonferroni correction) were used to compare saline-treated male and female mice of different genotypes and treatment effects by sex and genotype, respectively. Statistical significance was defined as p < 0.05.
Results
At 6.5 months, saline-treated male cortical oim/oim bone had increased mineral-to-matrix ratio (p = 0.016), increased acid phosphate substitution (p = 0.032), and decreased carbonate-to-mineral ratio (p = 0.016) relative to WT. Cancellous bone in male oim/oim also had increased mineral-to-matrix ratio (p = 0.016) relative to male WT. Female oim/oim mouse bone composition for all cortical and cancellous bone parameters was comparable to WT (p > 0.05). Only the female WT mice showed a response of mean compositional properties to treatment, increasing mineral-to-matrix after RANK-Fc treatment in cancellous bone (p = 0.036) compared with saline-treated mice. Male oim/oim increased mineral-to-matrix cortical and cancellous bone heterogeneity in response to all long-term treatments except for saline + RANK-Fc (p < 0.04); female oim/oim cortical mineral-to-matrix bone heterogeneity increased with ALN + RANK-Fc and all treatments increased cancellous female oim/oim bone acid phosphate substitution heterogeneity (p < 0.04).
Conclusions
Both oim/oim and WT mice, which demonstrate sex-dependent differences in composition with saline treatment, showed few responses to long-term treatment with antiresorptive agents. Female WT mice appeared to be more responsive; male oim/oim mice showed more changes in compositional heterogeneity. Changes in bone composition caused by these agents may contribute to improved bone quality in oim/oim mice, because the treatments are known to reduce fracture incidence.
Clinical Relevance
The optimal drug therapy for long-term treatment of patients with moderate-to-severe OI is unknown. Based on bone compositional changes in mice, antiresorptive treatments are useful for continued treatment in OI. There is a reported sexual dimorphism in fracture incidence in adults with OI, but to date, no one has reported differences in response to pharmaceutical intervention. This study suggests that such an investigation is warranted.
Introduction
Osteogenesis imperfecta (OI) is a rare heritable skeletal dysplasia associated with fragile bones and skeletal deformities, in most cases caused by defects in the genes for Type I collagen [26]. Clinical treatments for OI include intramedullary rodding of long bones, surgical correction of scoliosis, physical therapy, and treatment with antiresorptive agents or other pharmaceuticals for variable periods of time, depending on the severity of the condition [25, 30]. Rodding and physical therapy lessen skeletal deformities, and antiresorptive agents increase bone mineral density. None of these treatments, however, succeeds in eliminating fractures in these patients. A recent report in the Cochran database [15] concluded that the optimal use and relative benefit of bisphosphonate therapy in OI were still uncertain. As a result of the existing therapies, however, patients with the moderate-to severe form of OI are living longer and thus may develop osteoporosis as adults. There is now concern about what therapy or combination of therapies to use after short-term treatment with bisphosphonates [7]. Our group has used the oim/oim mouse model as an analog for evaluating pharmaceutical therapies for moderate-to-severe OI [1–3, 9, 13, 27–29]. The oim/oim mice, as a result of an inappropriate stop codon in the collagen I alpha 2 chain’s gene, have a αI(I)3 triple helix instead of the expected αI(1)2αI(2) helix [11] and provide a reproducible model with a moderate-to-severe (Type III) form of OI [34], including the presence of spontaneous fractures. Both alendronate (a bisphosphonate) and RANK-Fc, an inhibitory antibody to RANKL (receptor activator of nuclear factor-κB ligand), tested in short-term (8-week) studies showed a comparable reduction in the number of fractures relative to saline-treated oim/oim mice [2]. The bone composition assessed by Fourier transform infrared (FTIR) imaging after short-term alendronate treatment of the oim/oim mouse, however, was unchanged relative to saline-treated mice [8]. RANK-Fc blocks the formation, activation, and survival of osteoclasts [2, 3, 13]. Because RANKL inhibitors have a shorter half-life in bone than do bisphosphonates, they are being investigated as an alternative therapy. Because of the reproducible number of fractures in the oim/oim mouse, this mouse has also been used by others to study the effect of whole-body vibration [37] and gene therapy [31] in preclinical models.
FTIR imaging is a vibrational spectroscopic method that produces chemical maps of thin sections of tissues [4]. Five compositional parameters are generally reported for bone: mineral-to-matrix ratio (corresponding to ash weight), carbonate-to-mineral ratio (extent of carbonate substitution for phosphate and hydroxide ions of the hydroxyapatite structure), crystallinity (crystal size and perfection in the c-axis direction as determined by line-broadening analysis of the xray diffraction 002 peak), acid phosphate substitution (the amount of acid phosphate ions found in the mineral; usually higher in more recently formed bone), and collagen maturity (the relative amount of enzymatic collagen crosslinks) [4]. These are reported in cortical and cancellous bones individually. The distribution (heterogeneity) of each parameter is calculated based on the full width at half maximum of the pixel histogram for spatial distribution in each individual image. Heterogeneity in adult human bone has been shown to decrease with bisphosphonate treatment [6, 14, 20]. These compositional parameters, taken together, provide an index of bone quality.
A Norwegian database of adult humans with OI (age 44 ± 12 years) showed sexual dimorphism in fracture response with males having more fractures in adulthood than females [39]. Male and female oim/oim mice presented with different material and mechanical properties at 4 months of age [41] also showing sexual dimorphism. As a result of these facts, in an ongoing investigation, we are seeking to determine which therapy is best for adults with Type III OI treated as children with bisphosphonates and in need of additional treatment. We used FTIR imaging to determine: (1) do oim/oim mice show sex-dependence in compositional properties at 6.5 months of age; (2) is there a sex-dependent response to treatment with antiresorptive agents routinely used in humans with OI; and (3) do any of these therapies return compositional parameters in oim/oim mice to wild-type (WT) values?
Materials and Methods
Study Design
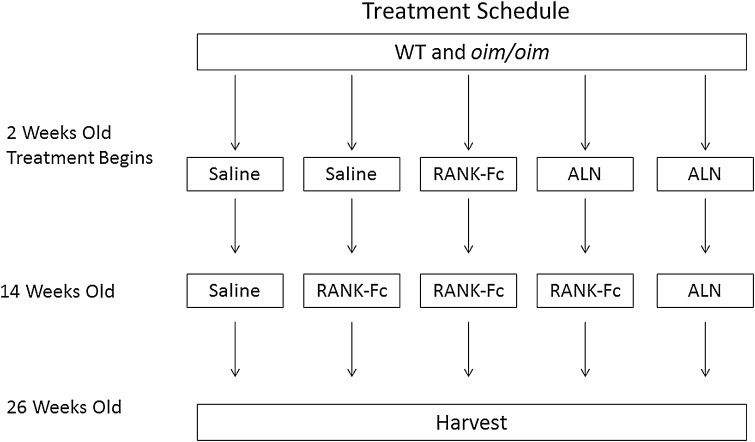
All procedures were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery. Homozygous oim/oim, heterozygous oim/+, and WT (+/+) control mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Homozygous oim/oim males were bred with heterozygous oim/+ females and offspring were tail-tipped at 2 weeks of age for genotyping (Transnetyx, Inc, Cordova, TN, USA). Animals were housed up to five mice per cage (according to genotype, sex, and treatment) in a light-controlled environment (12-hour light-dark cycles). They were given acidified water and fed a whole and powdered rodent diet mixture (Purina, St Louis, MO, USA). Starting at 2 weeks of age, male and female mice were typed and randomized to distribute litters within the five treatment groups of 15 to 23 (Fig. 1) and subcutaneously injected with soluble RANK-Fc (Amgen Inc, Thousand Oaks, CA, USA) at 1.5 mg/kg per dose biweekly, alendronate (ALN) at 0.21 mg/kg per dose weekly (Merck & Co, Whitehouse Station, NJ, USA), or saline weekly for 12 weeks. After 12 weeks of treatment (transition time point), half the mice previously receiving saline and half the mice previously receiving ALN began treatment with RANK-Fc. The remaining mice continued with their original therapy. Treatment ended 12 weeks after the transition time point. Doses of RANK-Fc and ALN were based on results of previous studies that showed increased bone mineral density without side effects using the same dose [1–3]. Weaning was carried out between 3 and 4 weeks. Animals were weighed before weekly or biweekly injections. After a total of 24 weeks of treatment, the mice were euthanized by carbon dioxide inhalation (2 L/min; 30% fill) and bones allocated for specific studies. Right femora were saved for FTIR imaging.
Fig. 1.
Schematic illustrating the timeline of the experiment.
Power Study
A power study with FTIR imaging of collagen maturity as the outcome selected, based on an earlier treatment study [1], indicated for α = 0.05, β = 80%, four mice per genotype per sex, were needed to address the question of whether oim/oim mice showed sexual dimorphism in compositional properties at 6.5 months of age. This calculation assumed comparisons would be of treated versus untreated animals of the same genotype. There were 20 animals per genotype for the treatment arms of the study, but these were not equally divided among sexes. We used four to five mice per genotype per sex per treatment for the FTIR imaging analyses.
Description of Experimental Methods and Outcome Parameters
Harvested femora, fixed in 100% ethanol and then embedded in PMMA, were cut into three to five longitudinal sections (1–2 μm) and the distal ends examined by FTIR imaging (Perkin Elmer model Spotlight 300 imaging system; Perkin Elmer, Waltham, MA, USA) as detailed elsewhere [1, 19]. Briefly, areas of bone corresponding to cortical or cancellous regions (approximately 60,000 um2 each) were imaged separately in three longitudinal sections, cut through the center of the shaft, with three to five different regions examined per section at 6.25-μm spatial resolution. The cortical regions scanned included the entire cortex on the medial side of the femur; cancellous regions were selected distal to the growth plate and included all trabeculae visible in the marrow cavity within the section. ISYS software (Spectral Dimensions, Olney, MD, USA) was used to process the data, including a subtraction of PMMA. Parameters calculated and exhibited as images were: (1) mineral-to-matrix ratios (a comparison of the relative ratios of the integrated intensities of the ν1,ν3 phosphate band, approximately 900 to 1200 cm−1 to that of the protein amide I band [centered at 1660 cm−1]); (2) carbonate (855–890 cm−1) to mineral band area ratio (indicating carbonate substitution for phosphate in the mineral); (3) crystallinity (an estimate of crystallite size and perfection based on the proportion of stoichiometric and nonstoichiometric apatite); (4) collagen maturity (a peak height ratio of subbands in the collagen amide I peak); and (5) acid phosphate substitution (1128 cm−1/1096 cm−1), an estimate of the amount of acid phosphate substitution in the mineral lattice. The heterogeneity of each parameter in each image was determined as the full width at half maximum of the pixel distribution, detailed elsewhere [14].
Statistics
The mean values and SDs for each tissue type and each parameter in each animal were calculated. The three sections examined in different areas for cortical and cancellous bone provided a single value for each animal. As a result of the small sample size, the oim/oim values were compared with WT (baseline only) for each sex and each parameter by sex and genotype using a Mann-Whitney U- and Wilcoxon test (SPSS Version 22.0; IBM Corp, Armonk, NY, USA). Further comparisons were done asking if there was a significant difference between treated oim/oim and the saline-treated oim/oim. Untreated versus treated, same sex, same genotype, and within treatment and genotype by sex were compared using the Kruskal-Wallis test with Bonferroni correction (SPSS Version 22.0; IBM Corp). Significance was determined as p < 0.05.
Results
Sexual Dependence of Compositional Properties
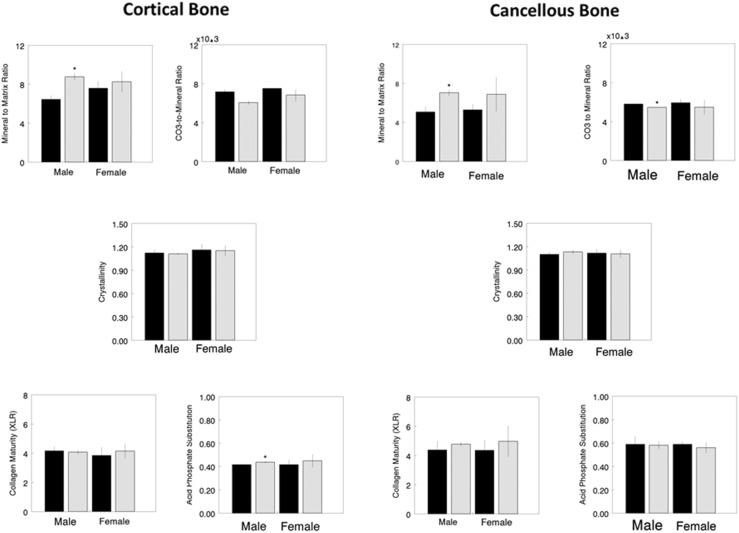
At 6.5 months of age, comparing saline-treated (baseline) genotypes of the same sex, male oim/oim had increased values for cortical mineral-to-matrix ratio (36%, p = 0.016), cortical acid phosphate substitution (5%, p = 0.032), cancellous mineral-to-matrix ratio (39%, p = 0.016), and decreased values for cancellous carbonate-to-mineral ratio (7%, p = 0.016) relative to male WT. Female oim/oim mice did not show such differences (Fig. 2). Higher relative intensities for baseline mineral-to-matrix ratio in oim/oim bones compared with the WT are seen in typical images of both males and females (Fig. 3). Heterogeneity was not significantly altered when saline-treated genotypes of the same sex were compared (Fig. 4).
Fig. 2.
FTIR imaging data for cortical (left) and cancellous (right) bone areas show differences between WT (solid bars) and oim/oim (hatched) of the same sex. Parameters shown are mineral-to-matrix ratio (M/M), carbonate-to-mineral ratio (C/P) × 103, crystal size and perfection (XST), collagen maturity (CM), and acid phosphate substitution (HPO4). *Statistical significance, based on Mann-Whitney U-test, indicates WT differs from oim/oim of the same sex with p < 0.05.
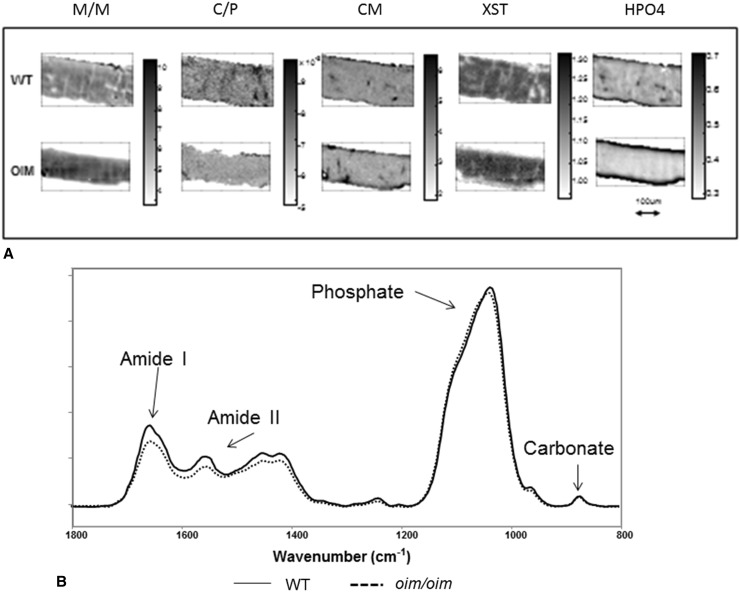
Fig. 3A–B.
(A) Typical FTIR images of WT and oim/oim cortical bone showing all parameters measured. All figures are to the same scale indicated by the arrow. Note the thinner cortex in the oim/oim. Each set of images shows the gray-scale scale bar for that parameter. Parameters are: mineral-to-matrix ratio (M/M), carbonate-to-mineral ratio (C/P), crystal size and perfection (XST), collagen maturity (CM), and acid phosphate substitution (HPO4). (B) Typical spectra were taken from the center of the cortex in WT and oim/oim bone normalized to the phosphate band intensity. Note the slight shift in the phosphate band shape and the major difference in amide I intensity, indicative of the decreased collagen content.
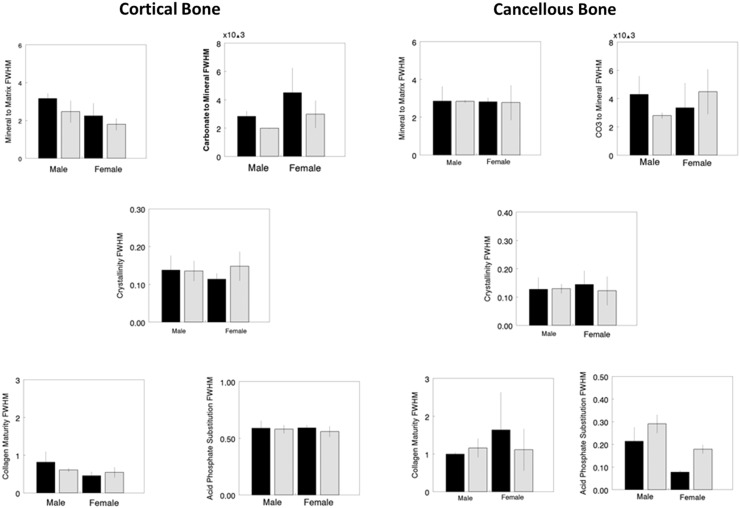
Fig. 4.
FTIR imaging heterogeneity (full width half maximum) data for cortical (left) and cancellous (right) bone areas shows differences between WT (solid bars) and oim/oim (hatched) of the same sex. Parameters shown are mineral-to-matrix ratio (M/M), carbonate-to-mineral ratio (C/P) × 103, crystal size and perfection (XST), collagen maturity (CM), and acid phosphate substitution (HPO4). *Statistical significance, based on Mann-Whitney U-test, indicates WT differs from oim/oim of the same sex with p < 0.05.
Sex Dependence of Treatment Response
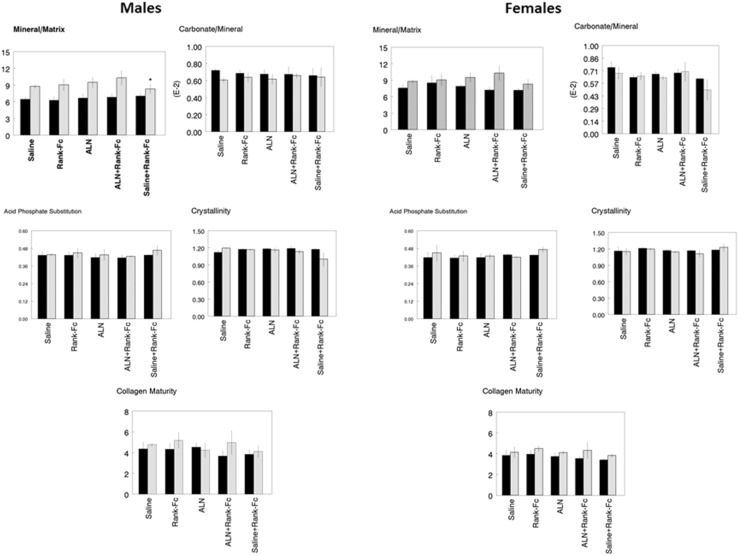
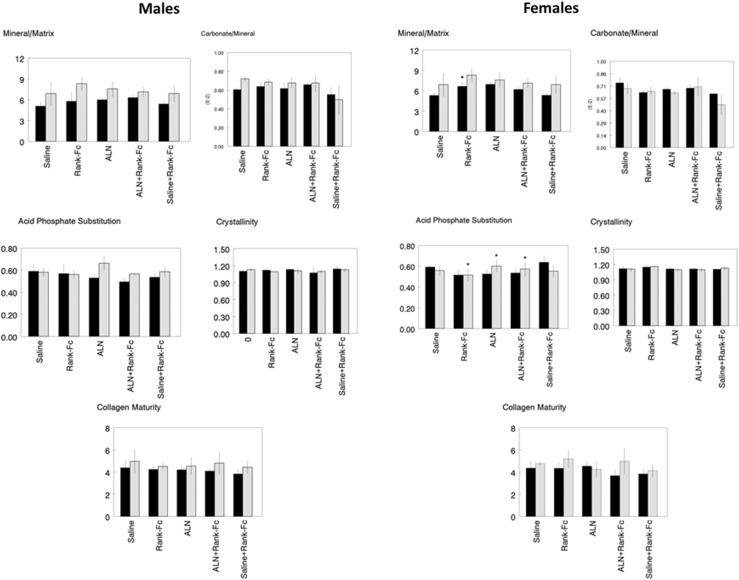
We next focused on the effect of each treatment in the same genotype with respect to the saline-treated group. In cortical bone (Fig. 5), there were no changes in average bone composition as a result of treatment for either WT or oim/oim for either sex. In cancellous bone, only female WT showed a significant response to treatment (Fig. 6). Treatment with RANK-Fc increased mineral-to-matrix average values in female WT (37%, p = 0.036). Increased acid phosphate substitution was also noted in female WT treated with saline + RANK-Fc (14%, p = 0.037) with decreases for alendronate + RANK-Fc (10%, p = 0.038) and RANK-Fc (16%, p = 0.032) treatments. There were no significant changes in the average FTIR imaging parameters in treated oim/oim mice of either sex. There were, however, changes in the heterogeneities of the FTIR imaging parameters (Tables 1, 2). In the male oim/oim cortical bone, heterogeneity of mineral-to-matrix ratio was increased by all long-term treatment modalities (alendronate, 51%, p = 0.035; alendronate + RANK-Fc, 132%, p = 0.032; RANK-Fc, 41%, p = 0.043). Female oim/oim cortical mineral-to-matrix heterogeneity was increased by alendronate + RANK-Fc treatment (173%, p = 0.0015). Cancellous heterogeneity (Table 2) was increased for acid phosphate substitution relative to saline treatment in female WT treated with RANK-Fc (192%, p = 0.005); female oim/oim cancellous bone had increased acid phosphate substitution heterogeneity after treatment with alendronate (30%, p = 0.026) and alendronate + RANK-Fc (3%, p = 0.034) and a decreased heterogeneity of this parameter with RANK-Fc treatment (13%, p = 0.041).
Fig. 5.
The response of male and female WT (solid bars) and oim/oim (hatched bars) cortical bone to treatment with ALN, ALN + RANK-Fc, RANK-Fc, or saline + RANK-Fc is compared with the same genotype and same sex treated with Saline based on a Kruskal-Wallis test with Bonferroni correction (*p < 0.05). Parameters illustrated in individual graphs are: mineral-to-matrix ratio (M/M), carbonate-to-mineral ratio (C/P) × 103, crystal size and perfection (XST), collagen maturity (CM), and acid phosphate substitution (HPO4).
Fig. 6.
The response of male and female WT (solid bars) and oim/oim (hatched bars) cancellous bone to treatment with ALN, ALN + RANK-Fc, RANK-Fc, or saline + RANK-Fc is compared with the same genotype and same sex treated with saline based on a Kruskal-Wallis test with Bonferroni correction (*p < 0.05). Parameters illustrated in individual graphs are: mineral-to-matrix ratio (M/M), carbonate-to-mineral ratio (C/P) × 103, crystal size and perfection (XST), collagen maturity (CM), and acid phosphate substitution (HPO4).
Table 1.
FTIR imaging heterogeneities (FWHM*) for cortical bone
| Tissue source | M/M | p value | C/P | XST | CM | HPO4 |
|---|---|---|---|---|---|---|
| Saline WT | ||||||
| Male | 3.02 ± 0.30 | 0.0028 ± 0.00037 | 0.157 ± 0.041 | 0.83 ± 0.28 | 0.083 ± 0.031 | |
| Female | 2.31 ± 0.67 | 0.0026 ± 0.00060 | 0.114 ± 0.016 | 0.46 ± 0.11 | 0.067 ± 0.014 | |
| Saline oim/oim | ||||||
| Male | 2.47 ± 0.59 | 0.0020 ± 2.4E-05 | 0.156 ± 0.027 | 0.61 ± 0.048 | 0.070 ± 0.0064 | |
| Female | 1.80 ± 0.32 | 0.0029 ± 0.00035 | 0.148 ± 0.039 | 0.54 ± 0.14 | 0.095 ± 0.042 | |
| Rank-Fc WT | ||||||
| Male | 2.58 ± 0.28 | 0.0028 ± 0.00041 | 0.176 ± 0.010 | 0.55 ± 0.17 | 0.066 ± 0.016 | |
| Female | 2.46 ± 0.12 | 0.0020 ± 0.00062 | 0.123 ± 0.040 | 0.53 ± 0.22 | 0.87 ± 0.05 | |
| Rank-FC oim/oim | ||||||
| Male | 3.60 ± 0.79 | 0.043 | 0.0025 ± 0.00031 | 0.137 ± 0.017 | 0.58 ± 0.16 | 0.090 ± 0.054 |
| Female | 3.27 ± 0.19 | 0.00032 ± 0.0013 | 0.089 ± 0.046 | 0.50 ± 0.56 | 0.041 ± 0.050 | |
| ALN WT | ||||||
| Male | 3.01 ± 0.37 | 0.0026 ± 0.00024 | 0.144 ± 0.014 | 0.45 ± 0.12 | 0.088 ± 0.016 | |
| Female | 2.86 ± 0.24 | 0.0013 ± 0.00048 | 0.157 ± 0.058 | 0.46 ± 0.17 | 0.069 ± 0.022 | |
| ALN oim/oim | ||||||
| Male | 3.65 ± 0.12 | 0.035 | 0.002 ± 0.00097 | 0.158 ± 0.055 | 0.68 ± 0.19 | 0.083 ± 0.014 |
| Female | 3.05 ± 0.29 | 0.0025 ± 0.00046 | 0.153 ± 0.048 | 0.66 ± 0.39 | 0.065 ± 0.0092 | |
| ALN + Rank-Fc WT | ||||||
| Male | 2.46 ± 0.15 | 0.0024 ± 0.00023 | 0.138 ± 0.020 | 0.42 ± 0.13 | 0.054 ± 0.013 | |
| Female | 2.60 ± .423 | 0.0018 ± 0.00075 | 0.123 ± 0.033 | 0.28 ± 0.14 | 0.064 ± 0.013 | |
| ALN + Rank-Fc oim/oim | ||||||
| Male | 3.66 ± 0.18 | 0.032 | 0.0025 ± 0.00083 | 0.156 ± 0.034 | 0.71 ± 0.34 | 0.090 ± 0.021 |
| Female | 2.58 ± 0.42 | 0.0015 | 0.0033 ± 0.0012 | 0.171 ± 0.044 | 0.68 ± 0.23 | 0.10 ± 0.022 |
| Saline + Rank-Fc WT | ||||||
| Male | 2.68 ± 0.33 | 0.0023 ± 0.00068 | 0.150 ± 0.037 | 0.38 ± 0.089 | 0.064 ± 0.009 | |
| Female | 2.09 ± 0.42 | 0.0017 ± 0.00069 | 0.135 ± 0.033 | 0.31 ± 0.12 | 0.066 ± 0.013 | |
| Saline + Rank-Fc oim/oim | ||||||
| Male | 2.83 ± 0.35 | 0.0027 ± 0.0008 | 0.144 ± 0.057 | 0.52 ± I0.18 | 0.093 ± 0.037 | |
| Female | 2.61 ± 0.13 | 0.00238 ± 0.0005 | 0.155 ± 0.045 | 0.49 ± 0.26 | 0.084 ± 0.020 | |
Values are mean ± SD; *line width at half-maximum for images of FTIR imaging parameters: mineral-to-matrix (M/M), carbonate-to-mineral (C/P), crystallinity (XST), collagen maturity (CM), and acid phosphate substitution (HPO4); comparisons are untreated (saline) versus treatment, same sex, same genotype, p values shown if chi square showed significance and Kuskal-Wallis with Bonferroni correction had p < 0.05; FTIR = Fourier transform infrared; FWHM = full width half maximum; WT = wild-type; ALN = alendronate.
Table 2.
FTIR imaging heterogeneities (FWHM*) for cancellous bone
| Tissue source | M/M | p value | C/P | p value | XST | p value | CM | p value | HPO4 | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Saline WT | ||||||||||
| Male | 2.86 ± 0.78 | 0.0043 ± 0.0013 | 0.128 ± 0.042 | 1.00 ± 0.65 | 0.21 ± 0.063 | |||||
| Female | 2.82 ± 0.22 | 0.0034 ± 0.0002 | 0.145 ± 0.049 | 1.65 ± 1.00 | 0.078 ± 0.008 | |||||
| Saline oim/oim | ||||||||||
| Male | 2.84 ± 0.088 | 0.0028 ± 0.00022 | 0.129 ± 0.017 | 1.16 ± 0.025 | 0.294 ± 0.041 | |||||
| Female | 2.68 ± 0.83 | 0.0045 ± 0.0016 | 0.122 ± 0.051 | 1.11 ± 0.56 | 0.18 ± 0.020 | |||||
| Rank-Fc WT | ||||||||||
| Male | 3.55 ± 1.10 | 0.0042 ± 0.0008 | 0.127 ± 0.053 | 0.80 ± 0.44 | 0.24 ± 0.099 | |||||
| Female | 3.30 ± 0.84 | 0.0028 ± 0.0008 | 0.142 ± 0.023 | 0.83 ± 0.31 | 0.22 ± 0.028 | |||||
| Rank-Fc oim/oim | ||||||||||
| Male | 5.36 ± 1.12 | 0.041 | 0.0046 ± 0.0005 | 0.119 ± 0.040 | 1.94 ± 0.94 | 0.27 ± 0.085 | ||||
| Female | 4.43 ± 0.82 | 0.0034 ± 0.0014 | 0.144 ± 0.042 | 1.09 ± 0.08 | 0.16 ± 0.026 | |||||
| ALN WT | ||||||||||
| Male | 3.59 ± 0.31 | 0.0044 ± 0.0012 | 0.117 ± 0.028 | 0.85 ± 0.60 | 0.19 ± 0.040 | |||||
| Female | 3.32 ± 0.65 | 0.0025 ± 0.0004 | 0.235 ± 0.053 | 0.51 ± 0.26 | 0.17 ± 0.17 | |||||
| ALN oim/oim | ||||||||||
| Male | 4.08 ± 1.08 | 0.026 | 0.0034 ± 0.0008 | 0.112 ± 0.029 | 0.68 ± 0.12 | 0.31 ± 0.074 | ||||
| Female | 4.09 ± 0.31 | 0.0035 ± 0.0009 | 0.139 ± 0.027 | 1.55 ± 0.95 | 0.24 ± 0.057 | |||||
| ALN + Rank-Fc WT | ||||||||||
| Male | 3.74 ± 0.19 | 0.0046 ± 0.0002 | 0.107 ± 0.006 | 0.68 ± 0.19 | 0.19 ± 0.039 | 0.005 | ||||
| Female | 2.77 ± 0.64 | 0.0028 ± 0.0010 | 0.129 ± 0.033 | 0.49 ± 0.12 | 0.19 ± 0.040 | |||||
| ALN + Rank-Fc oim/oim | ||||||||||
| Male | 5.50 ± 0.62 | 0.034 | 0.0056 ± 0.0032‡ | 0.121 ± 0.030 | 2.15 ± 0.98 | 0.32 ± 0.036 | ||||
| Female | 4.12 ± 0.51 | 0.0041 ± 0.0012 | 0.117 ± 0.025 | 2.15 ± 0.92 | 0.21 ± 0.071 | |||||
| Saline + Rank-Fc WT | ||||||||||
| Male | 2.19 ± 0.36 | 0.0057 ± 0.0020 | 0.123 ± 0.057 | 0.47 ± 0.077 | 0.19 ± 0.052 | |||||
| Female | 2.95 ± 0.47 | 0.0028 ± 0.0009 | 0.15 ± 0.016 | 0.53 ± 0.36 | 0.11 ± 0.056 | |||||
| Saline + Rank-Fc oim/oim | ||||||||||
| Male | 3.83 ± 1.31 | 0.0034 ± 0.0009 | 0.133 ± 0.048 | 1.05 ± 0.33 | 0.28 ± 0.063 | |||||
| Female | 2.93 ± 0.54 | 0.0033 ± 0.0006 | 0.13 1 ± 0.049 | 1.18 ± 0.36 | 0.21 ± 0.11 | |||||
Values are mean ± SD; *line width at half-maximum for images of FTIR imaging parameters: mineral-to-matrix (M/M), carbonate-to-mineral (C/P), crystallinity (XST), collagen maturity (CM), and acid phosphate substitution (HPO4); comparisons are untreated (saline) versus treatment, same sex, same genotype, p values shown if chi square showed significance and Kruskal-Wallis with Bonferroni correction had p < 0.05; FTIR = Fourier transform infrared; FWHM = full width half maximum; WT = wild-type; ALN = alendronate.
Restoration of Composition to Control Values
None of the treatment regimens studied affected the mean compositional parameters in the oim/oim mice. In either sex, there was no decrease in mineral-to-matrix ratio or collagen maturity, increase in carbonate-to-phosphate ratio, acid phosphate substitution, or crystallinity that would make these bones more like those of WT. Heterogeneity (full width half maximum) of the FTIR imaging parameters (Tables 1, 2) did show increases for male oim/oim cortical bone mineral-to-matrix ratio in animals treated with all drugs except saline + Rank-Fc. Female oim/oim showed increases in mineral-to-matrix heterogeneity for treatment with alendronate + Rank-Fc. Unexpectedly, long-term treatment with any of the antiresorptive agents caused excessive trabeculation of the cortical bone (or corticalization of trabecular bone) within the marrow cavity of both WT and oim/oim bones treated with any of the long-term antiresorptive agents. This area had a different FTIR imaging appearance and was excluded from the analyses (approximately 0.4% of files) reported here. This finding seemed random and was not limited to either sex or genotype.
Discussion
Recent human data indicate that antiresorptive agents are equally effective in restoring and increasing bone mineral density in men and women with osteoporosis [24]. No comparative data exist for children or patients with OI. Because fracture incidence in adults within OI does show sexual dimorphism [35] and, as yet, no study has considered sex dependence of these commonly used drugs in juveniles or adults with OI, we addressed that question in a mouse model, oim/oim. Sexual dimorphism in oim/oim material properties was previously reported for 4-month-old mice (on a C57/BL background) based on measurements of micro-CT, Raman, and mechanical properties [41]. At age 4 months, male mice were stronger and larger. Raman mineral-to-matrix values, however, were independent of sex. The oim/oim mice in a B6C3Fe background (as used in the current study), age 2 months, showed a sex-dependent increase in mineral density as measured by quantitative backscatter electron imaging.. Females had greater bone density than did males [35]. FTIR imaging has previously been used to characterize oim/oim mice [8, 9]; however, in those studies, data from both sexes were pooled as a result of the small sample size. The present study used FTIR imaging to investigate questions about sexual dimorphism in response to long-term (6-month) treatment in 6.5-month-old mice. This study was part of a larger ongoing investigation into appropriate therapy for adult patients with Type III OI, previously untreated or treated with alendronate. Composition in male but not female oim/oim did differ in comparison to WT mice of the same sex. There were no treatment-induced changes in mean composition of oim/oim bones exposed to long-term treatment. Female, but not male, WT mice did show a treatment response based on the FTIR imaging analysis. There were changes in heterogeneity in response to several treatments of oim/oim mice. None of the treatments appeared to make the bone composition similar to that of WT. Most treatments increased heterogeneity. Of interest, in this study, male oim/oim mice tended to have more radiographic fractures (4 ± 2 at 14 weeks; 4 ± 2 at 26 weeks; n = 7) than did females (2 ± 2 at 14 weeks, 3 ± 2 at 26 weeks; n = 14).
The present study was limited by the small number of mice (four to five per sex per genotype); however, for each mouse, multiple cortical and cancellous regions were examined. Values within a given individual mouse were reproducible to 5% or less. As a result of sample size, a nonparametric statistical test was used. Ideally having 10 to 15 mice per group would have eliminated this problem and perhaps shown more significance, especially because many parameters had p values between 0.06 and 0.08. The second limitation concerns the use of the oim/oim mouse as a model of Type III OI in humans. The mutation is extremely rare in humans; on the other hand, the oim/oim mice have a reproducible fracture pattern that is not seen in any of the other animal models of OI [5]. The oim/oim mice, having fewer trabeculae than WT mice of the same sex, presented with larger SDs, which, in turn, limited the significance of the cancellous data. Ultimately, it would have been interesting to give the mice a short “drug holiday” before switching treatments, making the schedule more comparable to that in humans with OI. This was not economically feasible.
Referring to the first question concerning sexual dimorphism in the oim/oim mice, we found that at 6.5 months, saline-treated oim/oim mice had an increased mineral-to-matrix ratio compared with WT and that increase in males was statistically significant. Such was not the case in females. This is not the first report of sexual dimorphism in the oim/oim mouse. We are, however, the first to report differences in FTIR imaging properties as a function of both sex and treatment. Yao et al [41] reported sex-dependent differences in 4-month-old oim/oim bone density and trabecular morphology (determined by micro-CT) in a congenic C57BL/6 J background as opposed to the B6C3Fe background studied here. The same authors also reported sex-dependent differences in mechanical strength. No differences were noted in cortical composition (Raman spectroscopy) and cancellous bone was not studied. Sex-dependent differences in skeletal muscle development in these mice were also noted [18]. Increased mineral-to-matrix ratio in the males is consistent with the increased density reported in younger oim/oim males in the same background [36], studied here. Increased mineral-to-matrix ratio observed in both genders of oim/oim mice indicates a lower matrix content rather than excessive mineralization. An increase in cancellous mineral-to-matrix ratio at 6 months (approximately the age of the mice in our study) was previously noted in a pooled sex group of oim/oim mice [12]. Failure to observe a change in size/perfection of mineral crystals in the oim/oim mice does not agree with small angle-scattering reports that oim/oim mice bones had mineral crystals that were thinner and less well oriented on collagen fibrils [21]. These features were confirmed in a recent transmission electron microscopic study [35]. This discrepancy may be the result of the older age of our mice, the small sample size in each group, or a lack of sensitivity of the FTIR imaging methodology.
Relative to the second question regarding sex-dependent response to treatment with antiresorptive agents, female WT responded to a greater degree compared with saline treatment in terms of mean compositional parameters than male WT mice. The antiresorptive-treated male oim/oim mice did show greater responses to treatment compared with saline treatment in terms of heterogeneity. Treatment with antiresorptive agents increased mineral content but had no effect on other parameters. The increases in heterogeneity in the oim/oim animals imply that more new bone has been deposited and existing bone has increased or maintained its properties. This may be related to the increased amount of bone visibly noted in most treatment groups; because male mice expand their bone endosteally [40], this could explain the greater change in oim/oim male heterogeneity. It was previously reported [16] that high-dose alendronate treatments reduced bone length only in male oim/oim mice. Rao et al [33] also found gender differences in response of pamidronate-treated oim/oim mice with females showing a greater reduction in bone length in response to higher doses. Vanleene et al [36] also found that female human fetal bone stem/stromal cell transplantation into male and female oim/oim mice responded differently with females showing an increase in carbonate content and males an increase in crystallinity.
Lastly, in so far as the ability of the antiresorptive treatments to return compositional parameters in oim/oim mice to that of WT, none corrected the increased mineral-to-matrix ratio. Most antiresorptive treatments caused additional increases in this value, significant only in the females treated with RANK-Fc. Without a complex statistical analysis requiring larger numbers, we can only suggest that no treatment corrected any FTIR-determined material property parameters in oim/oim of either sex to WT values. The increased heterogeneity discussed may be beneficial, because increased heterogeneity has been correlated with reduced fracture risk in untreated osteoporotic humans [6, 14, 20]. The lack of change in collagen maturity, noted to be slightly elevated in untreated male and female oim/oim cortical and cancellous bone, merits comment. Increased values of collagen crosslinks do not agree with thermal analysis of collagen in oim/oim bones [23] where reduced stabilization was explained by an increase in the water content of the collagen trimer fibrils rather than a distortion in molecular structure [29]. In studies of hearts of pooled male and female oim/oim, WT, and heterozygous mice, decreased levels of myocardial collagen and thinner fibrils in oim/oim mice were associated with decreased fiber and chamber stiffness despite modestly increased collagen crosslinking [32, 38] as we and others [10] previously noted in oim/oim bone. We speculate that the increased separation of the thinner fibrils might facilitate increased inter- rather than intrafibrillar collagen crosslinking. FTIR imaging measurements only provide an estimate of enzymatic crosslinks. Glycated chains formed by nonenzymatic reactions [22] are not detected by this method. There are data suggesting that crosslinking estimated by FTIR imaging is related to bone strength [17] with higher values found in more brittle bones; thus, this finding merits further investigation.
In conclusion, these data combined suggest that effective treatment of oim/oim mice with antiresorptive agents and, by analogy, patients with Type III OI with similar agents, may depend on their sex. Sex-dependent changes in compositional parameters noted in WT (with a trend in oim/oim) appear to vary in intensity by treatment with antiresorptive agents. This may be related to differences in sizes of male versus female animals and their patterns of bone growth [40]. None of the treatment regimens appeared optimal for correcting the compositional differences between WT and oim/oim. Additional studies that include a gap between treatments and the use of agents that stimulate new bone formation are needed. In human patients, studies examining sex-specific response to therapy in patients with different forms of OI are also needed. Sex-specific therapies in human patients will be more of a challenge when these concepts are recognized.
Acknowledgments
We thank Dr J. Gerstein for his critical review of this manuscript and his suggestions and Joseph Nyugen for his assistance with the statistical evaluations.
Footnotes
One or more of the authors (CLR) has received research funding from Amgen (Thousand Oaks, CA, USA) in partial support of this study in an amount less than USD 10,000. One or more of the authors (ALB) owns stock in Amgen (Thousand Oaks, CA) in an amount less than USD 10,000 and in Merck (Kenilworth, NJ, USA) in an amount less than USD 10,000.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Bargman R, Huang A, Boskey AL, Raggio C, Pleshko N. RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect Tissue Res. 2010;51:123–131. doi: 10.3109/03008200903108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargman R, Posham R, Boskey A, Carter E, DiCarlo E, Verdelis K, Raggio C, Pleshko N. High- and low-dose OPG-Fc cause osteopetrosis-like changes in infant mice. Pediatr Res. 2012;72:495–501. doi: 10.1038/pr.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargman R, Posham R, Boskey AL, DiCarlo E, Raggio C, Pleshko N. Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta. Osteoporos Int. 2012;23:1141–1150. doi: 10.1007/s00198-011-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boskey AL. Infrared spectra and imaging. In: DiMasi E, Gower LB, editors. Biomineralization Sourcebook: Characterization of Biominerals and Biomimetic Materials. Boca Raton, FL, USA: Taylor and Frances Inc; 2014. pp. 47–58. [Google Scholar]
- 5.Boskey AL, Doty SB. Mineralized tissue: histology, biology and biochemistry. In: Shapiro, JR. Byers, PH, Sponseller, PD. Glorieux F, eds. Osteogenesis Imperfecta: A Translational Approach to Brittle Bone Disease. New York, NY, USA: Academic Press; 2013:31–44.
- 6.Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 2009;20:793–800. doi: 10.1007/s00198-008-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyce AM, Tosi LL, Paul SM. Bisphosphonate treatment for children with disabling conditions. PM R. 2014;6:427–436. doi: 10.1016/j.pmrj.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho NP, Carroll P, Raggio CL. Fourier transform infrared imaging spectroscopy (FT-IRIS) of mineralization in bisphosphonate-treated oim/oim mice. Calcif Tissue Int. 2003;72:604–609. doi: 10.1007/s00223-002-1038-1. [DOI] [PubMed] [Google Scholar]
- 9.Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, Toledano TR, Boskey AL. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif Tissue Int. 2001;69:94–101. doi: 10.1007/s002230010045. [DOI] [PubMed] [Google Scholar]
- 10.Chang SW, Shefelbine SJ, Buehler MJ. Structural and mechanical differences between collagen homo- and heterotrimers. Relevance for the molecular origin of brittle bone disease. Biophys J. 2012;102:640–648. doi: 10.1016/j.bpj.2011.11.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chipman SD, Sweet HO, McBride DJ Jr, Davisson MT, Marks SC Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice. a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–1705. [DOI] [PMC free article] [PubMed]
- 12.Coleman RM, Aguilera L, Quinones L, Lukashova L, Poirier C, Boskey A. Comparison of bone tissue properties in mouse models with collagenous and non-collagenous genetic mutations using FTIRI. Bone. 2012;51:920–928. doi: 10.1016/j.bone.2012.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delos D, Yang X, Ricciardi BF, Myers ER, Bostrom MPG, Pleshko Camacho N. The effects of RANKL inhibition on fracture healing and bone strength in a mouse model of osteogenesis imperfecta. J Orthop Res. 2008;26:153–164. doi: 10.1002/jor.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD, Lorich DG, Lane JM, Boskey AL. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res. 2012;27:672–678. doi: 10.1002/jbmr.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2014;7:CD005088. [DOI] [PubMed]
- 16.Evans KD, Lau ST, Oberbauer AM, Martin RB. Alendronate affects long bone length and growth plate morphology in the oim mouse model for osteogenesis imperfecta. Bone. 2003;32:268–274. doi: 10.1016/S8756-3282(02)00974-2. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P. The contribution of collagen crosslinks to bone strength. Bonekey Rep. 2012;1:182–185. doi: 10.1038/bonekey.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010;29:638–644. doi: 10.1016/j.matbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46:666–672. doi: 10.1016/j.bone.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourion-Arsiquaud S, Lukashova L, Power J, Loveridge N, Reeve J, Boskey AL. Fourier transform infrared imaging of femoral neck bone: reduced heterogeneity of mineral-to-matrix and carbonate-to-phosphate and more variable crystallinity in treatment-naive fracture cases compared with fracture-free controls. J Bone Miner Res. 2013;28:150–161. doi: 10.1002/jbmr.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabner B, Landis WJ, Roschger P, Rinnerthaler S, Peterlik H, Klaushofer K, Fratzl P. Age- and genotype-dependence of bone material properties in the osteogenesis imperfecta murine model (oim) Bone. 2001;29:453–457. doi: 10.1016/S8756-3282(01)00594-4. [DOI] [PubMed] [Google Scholar]
- 22.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuznetsova NV, McBride DJ, Leikin S. Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. J Mol Biol. 2003;331:191–200. doi: 10.1016/S0022-2836(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 24.Laurent M, Gielen E, Claessens F, Boonen S, Vanderschueren D. Osteoporosis in older men: recent advances in pathophysiology and treatment. Best Pract Res Clin Endocrinol Metab. 2013;27:527–539. doi: 10.1016/j.beem.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Lindahl K, Langdahl B, Ljunggren O, Kindmark A. Therapy of endocrine disease. Treatment of osteogenesis imperfecta in adults. Eur J Endocrinol. 2014;171:R79–R90. doi: 10.1530/EJE-14-0017. [DOI] [PubMed] [Google Scholar]
- 26.Marini JC, Reich A, Smith SM. Osteogenesis imperfect due to mutations in non-collagenous genes. lessons in the biology of bone formation. Curr Opin Pediatr. 2014;26:500–507. doi: 10.1097/MOP.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy EA, Raggio CL, Hossack MD, Miller EA, Jain S, Boskey AL, Camacho NP. Alendronate treatment for infants with osteogenesis imperfecta. Demonstration of efficacy in a mouse model. Pediatr Res. 2002;52:660–670. doi: 10.1203/00006450-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Miles CA, Sims TJ, Camacho NP, Bailey AJ. The role of the alpha2 chain in the stabilization of the collagen type I heterotrimer. A study of the type I homotrimer in oim mouse tissues. J Mol Biol. 2002;321:797–805. doi: 10.1016/S0022-2836(02)00703-9. [DOI] [PubMed] [Google Scholar]
- 29.Misof BM, Roschger P, Baldini T, Raggio CL, Zraick V, Root L, Boskey AL, Klaushofer K, Fratzl P, Camacho NP. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone. 2005;36:150–158. doi: 10.1016/j.bone.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Monti E, Mottes M, Fraschini P, Brunelli P, Forlino A, Venturi G, Doro F, Perlini S, Cavarzere P, Antoniazzi F. Current and emerging treatments for the management of osteogenesis imperfecta. Ther Clin Risk Manag. 2010;6:367–381. doi: 10.2147/tcrm.s5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niyibizi C, Smith P, Mi Z, Phillips CL, Robbins P. Transfer of proalpha2(I) cDNA into cells of a murine model of human osteogenesis imperfecta restores synthesis of type I collagen comprised of alpha1(I) and alpha2(I) heterotrimers in vitro and in vivo. J Cell Biochem. 2001;83:84–91. doi: 10.1002/jcb.1209. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer BJ, Franklin CL, Hsieh FH, Bank RA, Phillips CL. Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol. 2005;24:451–458. doi: 10.1016/j.matbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Rao SH, Evans KD, Oberbauer AM, Martin RB. Bisphosphonate treatment in the oim mouse model alters bone modeling during growth. J Biomech. 2008;41:3371–3376. doi: 10.1016/j.jbiomech.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro JR, McBride DJ, Jr, Fedarko NS. OIM and related animal models of osteogenesis imperfecta. Connect Tissue Res. 1995;31:265–268. doi: 10.3109/03008209509010820. [DOI] [PubMed] [Google Scholar]
- 35.Vannleene M, Porter A, Guillot PV, Boyde A, Oyen M, Shefelbine S. Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone. 2012;50:1317–1323. doi: 10.1016/j.bone.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanleene M, Saldanha Z, Cloyd KL, Jell G, Bou-Gharios G, Bassett JH, Williams GR, Fisk NM, Oyen ML, Stevens MM, Guillot PV, Shefelbine SJ. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood. 2011;117:1053–1060. doi: 10.1182/blood-2010-05-287565. [DOI] [PubMed] [Google Scholar]
- 37.Vanleene M, Shefelbine SJ. Therapeutic impact of low amplitude high frequency whole body vibrations on the osteogenesis imperfecta mouse bone. Bone. 2013;53:507–514. doi: 10.1016/j.bone.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weis SM, Emery JL, Becker KD, McBride DJ, Jr, Omens JH, McCulloch AD. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim) Circ Res. 2000;87:663–669. doi: 10.1161/01.RES.87.8.663. [DOI] [PubMed] [Google Scholar]
- 39.Wekre LL, Eriksen EF, Falch JA. Bone mass, bone markers and prevalence of fractures in adults with osteogenesis imperfecta. Arch Osteoporos. 2011;6:31–38. doi: 10.1007/s11657-011-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. 2010;86:470–483. doi: 10.1007/s00223-010-9359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao X, Carleton SM, Kettle AD, Melander J, Phillips CL, Wang Y. Gender-dependence of bone structure and properties in adult osteogenesis imperfecta murine model. Ann Biomed Eng. 2013;41:1139–1149. doi: 10.1007/s10439-013-0793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]