Abstract
Trastuzumab has led to improved survival rates of HER2+ breast cancer patients. However, acquired resistance remains a problem in the majority of cases. t-Darpp is over-expressed in trastuzumab-resistant cell lines and its over-expression is sufficient for conferring the resistance phenotype. Although its mechanism of action is unknown, t-Darpp has been shown to increase cellular proliferation and inhibit apoptosis. We have reported that trastuzumab-resistant BT.HerR cells that over-express endogenous t-Darpp are sensitized to EGFR inhibition in the presence (but not the absence) of trastuzumab. The purpose of the current study was to determine if t-Darpp might modulate sensitivity to EGFR inhibitors in trastuzumab-resistant cells. Using EGFR tyrosine kinase inhibitors AG1478, gefitinib and erlotinib, we found that trastuzumab-resistant SK.HerR cells were sensitized to EGFR inhibition, compared to SK-Br-3 controls, even in the absence of trastuzumab. t-Darpp knock-down in SK.HerR cells reversed their sensitivity to EGFR inhibition. Increased EGFR sensitivity was also noted in SK.tDp cells that stably over-express t-Darpp. High levels of synergy between trastuzumab and the EGFR inhibitors were observed in all cell lines with high t-Darpp expression. These cells also demonstrated more robust activation of EGFR signaling and showed greater EGFR stability than parental cells. The T75A phosphorylation mutant of t-Darpp did not confer sensitivity to EGFR inhibition nor activation of EGFR signaling. The over-expression of t-Darpp might facilitate enhanced EGFR signaling as part of the trastuzumab resistance phenotype. This study suggests that the presence of t-Darpp in HER2+ cancers might predict the enhanced response to dual HER2/EGFR targeting.
Introduction
Breast cancer represents the most common cancer in women worldwide with an estimated 1.6 million new cases diagnosed each year [1, 2]. Approximately 25–30% of these women present with an over-expression of human epidermal growth factor receptor 2 (HER2) [3]. The amplification of HER2, a receptor tyrosine kinase encoded by the ERBB2 oncogene, correlates with a poor prognosis and a poor response to chemotherapy [4]. Trastuzumab, a humanized monoclonal antibody targeting the extracellular region of HER2, remains the primary treatment for HER2+ breast cancer patients. Despite the specificity and efficacy of trastuzumab, trastuzumab monotherapy is only effective in about 30–45% of patients. Response rates are improved by the addition of chemotherapy to the treatment regimen, but approximately 75% of patients treated with trastuzumab will still develop resistance within one year [5, 6]. Although the mechanism of resistance is still largely unknown, in vitro and in vivo data have confirmed that sustained signaling through the PI3K/Akt signaling pathway and phosphorylation of Akt are largely responsible for the resistance phenotype [7, 8].
One potential mechanism for sustained downstream signaling in the presence of trastuzumab is by compensatory signaling using a different HER family receptor, such as EGFR or HER3. Co-expression of EGFR occurs in 35–65% of HER2+ breast cancer and is associated with a worse clinical prognosis than for breast cancers that don’t express EGFR [9–12]. We have previously shown that trastuzumab-resistant BT.HerR cells are more sensitive to an EGFR tyrosine kinase inhibitor (TKI) in the presence of trastuzumab than in its absence, suggesting that those cells gain a dependence on EGFR when HER2 signaling is shut down [13]. More recent work has demonstrated that EGFR inhibitors are synergistic with trastuzumab in models of HER2+ breast cancer [14–16], again suggesting that EGFR is important as an alternative pathway when HER2 is inhibited.
We and others have reported that upregulation of the PPP1R1B gene plays a role in the trastuzumab resistance mechanism [17–20]. PPP1R1B codes for the 32kDa dopamine and cAMP-regulated phosphoprotein, Darpp-32, and its amino-truncated isoform, t-Darpp. Although Darpp-32 has been well characterized in neuronal cells as a dual-function phosphoprotein that inhibits protein kinase A (PKA) and protein phosphatase-1 (PP-1), its role in cancer has only been studied more recently [21, 22]. t-Darpp is frequently over-expressed in human adenocarcinomas of the esophagus, prostate, stomach, colon and breast [23] and over-expression of t-Darpp is sufficient to confer resistance to trastuzumab in HER2+ breast cancer [17, 18, 24]. Although the mechanism by which this occurs remains unclear, several groups have shown that t-Darpp upregulates cell growth through activation of the PI3K/Akt pathway and increased anti-apoptotic response through upregulation of Bcl-2 [18, 19, 24]. In this study, we examine the possible role of t-Darpp in conferring trastuzumab resistance via an effect on EGFR signaling. Our results suggest a novel function for t-Darpp by sensitizing breast cancer cells to EGFR inhibition.
Materials and Methods
Cell culture and reagents
The human breast cancer cell lines BT474 and SK-Br-3 were obtained from the American Type Culture Collection (Rockville, MD). BT.HerR cells were generated as previously described through selection in the presence of trastuzumab [13]. BT474 cells and BT.HerR cells were maintained in DMEM with 10% FBS and 1% penicillin/streptomycin. SK.HerR cells were also generated through selection in the continuous presence of trastuzumab and were a kind gift from Rita Nahta [25]. Western hybridizations showing the relative levels of t-Darpp and Darpp-32 in parental and resistant BT474 and SK-Br-3 cells are shown in S1 Fig. Stably transfected SK-Br-3 cells expressing pcDNA3 empty vector (SK.empty), t-Darpp (SK.tDp), or t-Darpp plus flag-Darpp-32 (SK.dDp) were described previously [17]. SK-Br-3 cells were also stably transfected with a phosphorylation mutant of t-Darpp in which threonine 75 (T75) was mutated to an alanine (SK.tDp-T75A). SK-Br-3 cells, SK.HerR and the stably transfected clones were maintained in McCoys 5A media with 10% FBS, 1% penicillin/streptomycin and 1% L-glutamine. SK.empty, SK.tDp and SK.tDp-T75A cells were maintained in media containing 500 μg/ml G418. SK.dDp cells were maintained in media containing 500 μg/ml G418 and 200 μg/ml zeocin. Trastuzumab was purchased from the City of Hope Hospital Pharmacy. AG1478 (Calbiochem, Ballerica, MA), gefitinib and erlotinib (LC Laboratories, Woburn, MA) were dissolved in DMSO and further diluted with cell culture media to concentrations described in the figure legends. p-EGFR (Y1068) (#2234), EGFR (#2232) were obtained from Cell Signaling (Danvers, MA). H62, an antibody that recognizes both Darpp-32 and t-Darpp, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, sc-11365) and β-actin (A4700) and α-tubulin (T5168) antibodies were obtained from Sigma-Aldrich Corporation (St. Louis, MO).
Sulforhodamine B (SRB) assay
SRB assays were performed as previously described [13]. Briefly, cells were plated at a density of 4x103 per well in 96 well plates and allowed to attach overnight. On day 0, media containing 0.1% DMSO or increasing concentrations of EGFR inhibitor (AG1478, gefitinib or erlotinib) were added to the cells. After 5 or 7 days for SK-Br-3 or BT474 cells, respectively, cells were fixed with 10% cold trichloroacetic acid (Sigma) and stained with 0.4% SRB in 1% acetic acid. The SRB stain was released using 10mM Tris base. Absorbances at 565 nm and 695 nm were read using a spectrophotometer. IC50 values were determined using CalcuSyn. Each experiment was run in quadruplicate and repeated a minimum of three times.
siRNA gene knock-down
Cells were plated at a density of 2x105 cells per well in 6-well plates. After attaching overnight, cells were transfected with scrambled siRNA or t-Darpp siRNA (Santa Cruz Biotechnology) using Lipofectamine 2000 (Invitrogen by Life Technologies, Grand Island, NY). After 24 hours, media was changed and treatments were carried out as described in figure legends.
Western analysis
Cells were lysed using a modified RIPA buffer with 1mM Na3VO4 and a complete tablet of protease inhibitors (Roche, Indianapolis, IN). Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Proteins were separated on a 10 or 12% SDS-polyacrylamide gel or a precast 4–12% gradient NuPAGE Bis-Tris gel (Life Technologies) and transferred onto a nitrocellulose membrane. Membranes were probed with specific primary antibodies followed by horseradish peroxidase-coupled secondary antibodies (Cell Signaling). Chemiluminescence was detected using an ECL Plus kit (Thermo Fisher Scientific, Waltham, MA).
Drug combination analysis
Serial dilutions of a fixed 2:1 ratio of EGFR inhibitor (AG1478, gefitinib or erlotinib) and trastuzumab were used for the combination studies. Each drug was added alone and in combination for each experiment. Combination analysis was performed using the method of Chou and Talalay and the CalcuSyn software program [26]. Each experiment was run in quadruplicate and repeated three times. Combination index (C.I.) values of less than 0.9 are synergistic, 0.9–1.1 are additive, and greater than 1.1 are antagonistic.
Cycloheximide protein stability assay
Cells were plated at a density of 3.5x105 cells per well in 6-well plates and incubated with 80 μg/ml cycloheximide (Calbiochem) to inhibit new protein synthesis. Cells were harvested at 12, 24, 36 and 48 hours after the addition of cycloheximide. Protein lysates were collected and analyzed by Western hybridization as described above, probing for total EGFR. Protein bands were quantified using ImageJ software and the EGFR band intensities for each time point were normalized to α-tubulin. The protein degradation curve was produced by plotting the band intensity ratio (relative to time 0) as a function of cycloheximide treatment time. Protein half-life was calculated using the best-fit line and determining the time at which 50% of the original protein had degraded.
Colony formation assay
Cells were plated at a density of 500 cells per well in 6-well plates and allowed to attach overnight. Cells were treated with 0.1% DMSO or increasing concentrations of AG1478. Media was changed twice per week. Colonies, defined as having at least 50 cells, were counted after 14 days.
EGFR stimulation assay
Cells were plated at a density of 3.5x105 cells per well in 6-well plates and allowed to attach overnight. Cells were then serum-starved overnight (16 hours), followed by EGF (100 ng/ml) stimulation for the indicated time periods at 37°C. Protein lysates were collected and analyzed by Western hybridization as described previously to probe for p-EGFR (Y1068), total EGFR and α-tubulin. Protein bands were quantified using ImageJ Software. p-EGFR levels were normalized to α-tubulin and the relative p-EGFR activation levels were calculated relative to the zero time point for the appropriate parental cells in each case.
Statistical analysis
Statistical significance was calculated using the GraphPad Prism 6.0 statistical software. Differences between groups were determined by the two-tailed Student’s t-test or the one-way ANOVA with Dunnett’s post-test. p values less than 0.05 were considered significant.
Results
Sensitivity to EGFR inhibition in trastuzumab-resistant cell lines
We previously observed that trastuzumab-resistant BT474 (BT.HerR) cells are more sensitive to AG1478, an EGFR tyrosine kinase inhibitor, in the presence but not the absence of trastuzumab [13]. As a more rigorous determination of synergy, we used a combination index analysis to study the interaction of trastuzumab with EGFR-specific kinase inhibitors in parental and trastuzumab-resistant cell lines. The combination of either AG1478 or erlotinib with trastuzumab produced high levels of synergy in both the parental BT474 and the BT.HerR cells (S1 Table).
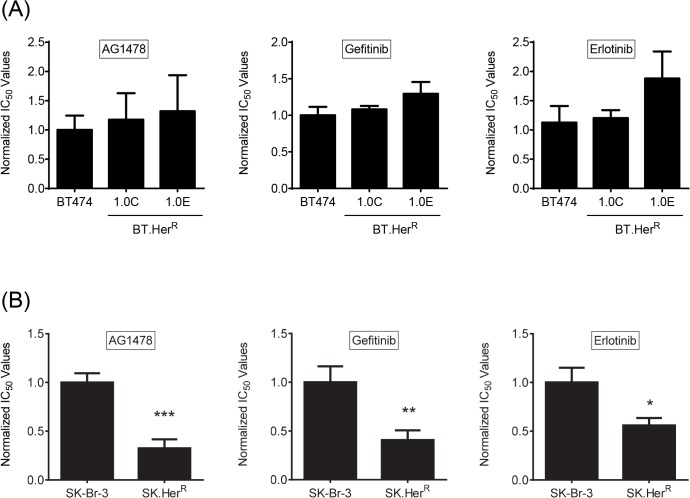
We next wanted to determine if this same phenotype would be observed in other trastuzumab-resistant cell lines. We first compared the sensitivity of BT.HerR and SK.HerR cells to three EGFR-specific tyrosine kinase inhibitors—AG1478, gefitinib and erlotinib—in the absence of trastuzumab. There was no significant difference in cell proliferation between the parental BT474 and resistant BT.HerR cell lines exposed to AG1478, gefitinib or erlotinib (Fig 1A), consistent with published data showing that enhanced sensitivity to EGFR inhibition in BT.HerR cells requires the presence of trastuzumab. SK.HerR cells, on the other hand, demonstrated a statistically significant two- to four-fold greater sensitivity to AG1478, gefitinib and erlotinib than parental SK-Br-3 cells, even in the absence of trastuzumab (Fig 1B), suggesting an inherent difference between BT.HerR and SK.HerR cells in their signaling through EGFR when Her2 is still active.
Fig 1. BT.HerR and SK.HerR cells demonstrate different sensitivities to EGFR inhibitors in the absence of trastuzumab.
Cell proliferation in AG1478 (left), gefitinib (middle) and erlotinib (right) was measured by SRB assay after exposure to 0.1% DMSO or increasing concentrations of the respective drugs. IC50 values were obtained using CalcuSyn and normalized to the average IC50 for the appropriate parental cell line. Each bar represents the mean of three independent experiments ±SD. (A) Relative sensitivity for BT474 parental and two BT.HerR clones (1.0C and 1.0E) treated with DMSO or drugs for 7 days. (B) Relative sensitivity for SK-Br-3 parental and SK.HerR cells treated with DMSO or drugs for 5 days. *, p<0.05; **, p<0.01; ***, p<0.001 compared to parental cells.
t-Darpp over-expression confers sensitivity to EGFR inhibition
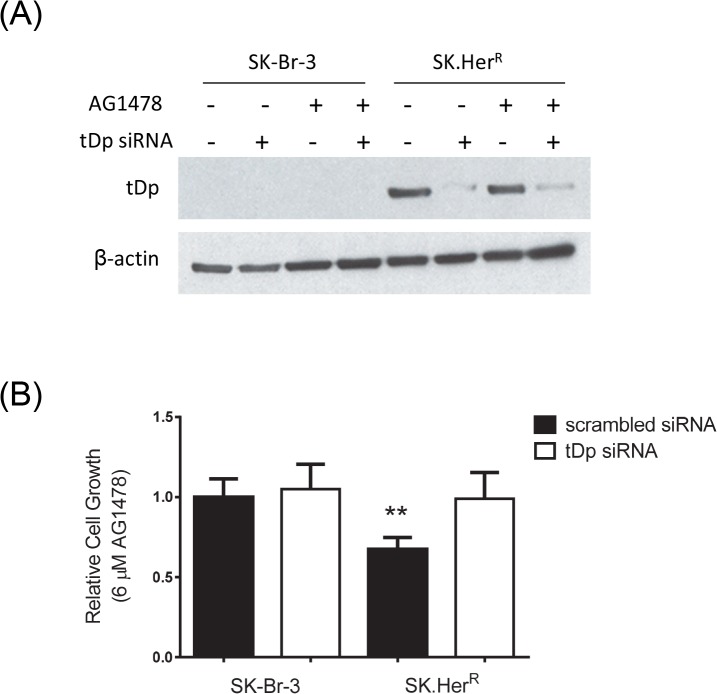
The difference in response to EGFR inhibitors between BT.HerR and SK.HerR cell lines led us to investigate the mechanism for the increased sensitivity in SK.HerR cells. We and others have previously reported that over-expression of t-Darpp is sufficient to confer resistance to trastuzumab [17–19], so we wanted to determine if t-Darpp over-expression was responsible for the sensitization to EGFR inhibitors as well. Because SK.HerR cells over-express endogenous t-Darpp, we first asked if t-Darpp was necessary for the enhanced sensitivity to EGFR inhibition in these cells. We performed siRNA knock-down of t-Darpp in SK.HerR and SK-Br-3 cells and examined cell growth in 6μM AG1478 (the approximate IC50 for the parental cells), relative to cells treated with control siRNA. The sensitivity to EGFR inhibition in SK.HerR cells was abrogated by t-Darpp knock-down (Fig 2), suggesting that t-Darpp is essential for increased sensitivity to EGFR inhibition.
Fig 2. t-Darpp is responsible for increased EGFR sensitivity in SK.HerR cells.
SK-Br-3 and SK.HerR cells were transfected with scrambled (-) or tDp siRNA (+). 24 hours later, cells were treated with DMSO or AG1478 for an additional 24 hours. (A) Western analysis to confirm siRNA knockdown. (B) SRB cell proliferation assay to determine relative sensitivity to AG1478. Shown are the averages (± S.D.) of four experiments, with normalization to DMSO-treated controls in all cases. **, p<0.01.
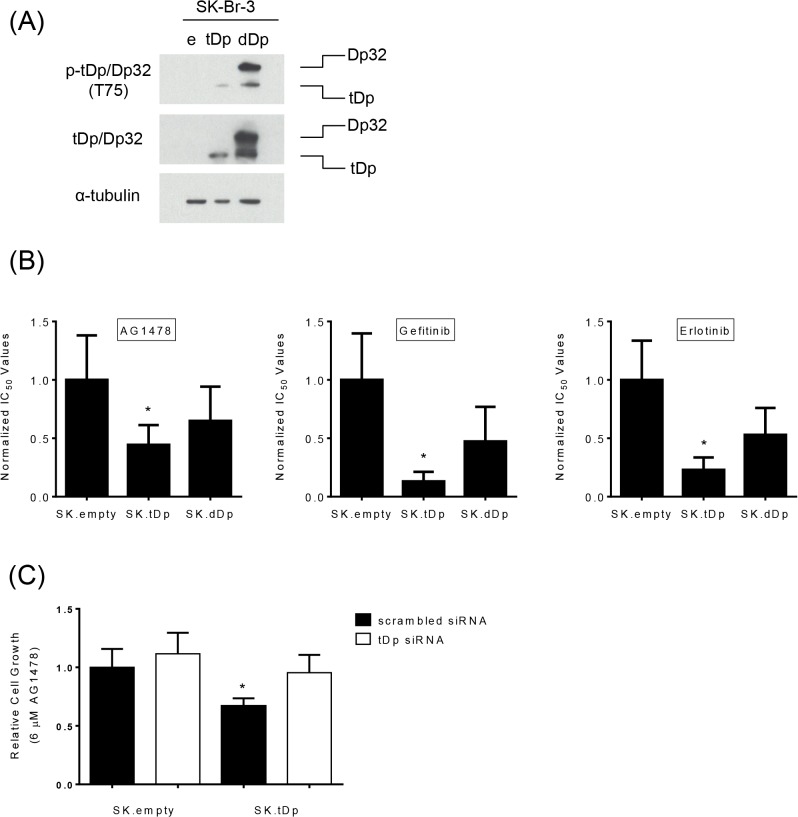
To determine if t-Darpp was sufficient for conferring sensitivity to EGFR inhibition in cells that were not previously selected for trastuzumab resistance, we used SK.tDp cells stably transfected with t-Darpp cDNA and compared with SK.empty cells transfected with an empty vector (SK.empty). As an additional control, we used cells that had been transfected with both t-Darpp and Darpp-32 (SK.dDp cells). We have previously reported that SK.tDp cells are resistant to trastuzumab and SK.dDp cells have lost that resistance [17].
We first confirmed that the over-expression of t-Darpp alone caused increased cell proliferation and resistance to trastuzumab in the current study and that knock-down of t-Darpp by siRNA reversed these effects in SK.tDp cells (S2 Fig). We next evaluated the sensitivity of SK.tDp, SK.empty and SK.dDp cells to EGFR inhibition. In SRB cell proliferation assays, SK.tDp cells were 3- to 5-fold more sensitive than SK.empty cells to AG1478, gefitinib and erlotinib, whereas SK.dDp cells demonstrated an intermediate sensitivity that did not reach statistical significance (Fig 3B). Colony formation assays using 6μM AG1478 also indicated that SK.tDp cells were more sensitive to AG1478 compared to SK.empty cells (S3 Fig). Moreover, siRNA knock-down of t-Darpp in SK.tDp cells reversed the sensitivity to AG1478 (Fig 3C). Taken together, these results demonstrate that t-Darpp is sufficient to confer sensitivity to EGFR inhibition in SK-Br-3 cells and they extend previous findings that Darpp-32 over-expression reverses the t-Darpp phenotype [17].
Fig 3. Increased sensitivity to EGFR inhibition in SK.tDp cells.
(A) Western analysis showing relative amounts of Darpp-32 and t-Darpp in stably transfected SK-Br-3 cells: SK.empty (e), SK.tDp (tDp) and SK.flag-Dp32. tDp (dDp). (B) Normalized IC50 values for transfected SK-Br-3 cells treated with AG1478 (left), gefitinib (middle) or erlotinib (right). Each bar represents the mean of three independent experiments ± S.D. (C) SK.empty and SK.tDp cells were transfected with scrambled or tDp siRNA. After 24 hours, cells were treated with DMSO or AG1478 for an additional 48 hours and an SRB cell proliferation assay was performed to determine relative sensitivity to AG1478. Shown are averages (± S.D.) of three experiments normalized to DMSO-treated controls in all cases. Error bars indicate SD. *, p<0.05.
Dual EGFR and HER2 inhibition is synergistic in cells over-expressing t-Darpp
Given the enhanced sensitivity of SK.HerR and SK.tDp cells to EGFR inhibition, we wanted to determine if trastuzumab and EGFR inhibitors would have synergistic effects on these cells. We used combination index analysis to study the interaction of trastuzumab with AG1478 and erlotinib in SK.empty and SK.tDp cell lines. The AG1478 + trastuzumab combination was highly synergistic in SK.tDp cells but only additive in SK.empty cells (Table 1). The combination of erlotinib + trastuzumab was highly synergistic in SK.tDp cells (ED50 of 0.39 ± 0.10, ED75 of 0.50 ± 0.10) and moderately synergistic in SK.empty cells (ED50 of 0.56 ± 0.12, ED75 of 0.72 ± 0.12) (Table 1). Taken together, these results suggest that t-Darpp over-expression can, at a minimum, enhances the synergistic effect between Her2 and EGFR inhibitors.
Table 1. Combination Index.
| Combination Index 1 | ||||
|---|---|---|---|---|
| Cell Line | AG1478 + Trastuzumab | Erlotinib + Trastuzumab | ||
| ED50 2 | ED75 2 | ED50 | ED75 | |
| SK.empty | 1.00 ± 0.14 | 1.21 ± 0.09 | 0.56 ± 0.12 | 0.72 ± 0.12 |
| SK.tDp | 0.39 ± 0.10** | 0.62 ± 0.05*** | 0.39 ± 0.10 | 0.50 ± 0.10 |
1Combination Index values indicate the degree of synergy (CI <0.9), additivity (CI 0.9–1.1) or antagonism (CI >1.1) for two drugs maintained in a fixed ratio.
2ED50 and ED75 are the effective doses at which 50% and 75% of cell killing occurred, respectively.
**, p<0.01
***, p<0.005
T75 residue of t-Darpp may be responsible for conferring increased EGFR sensitivity
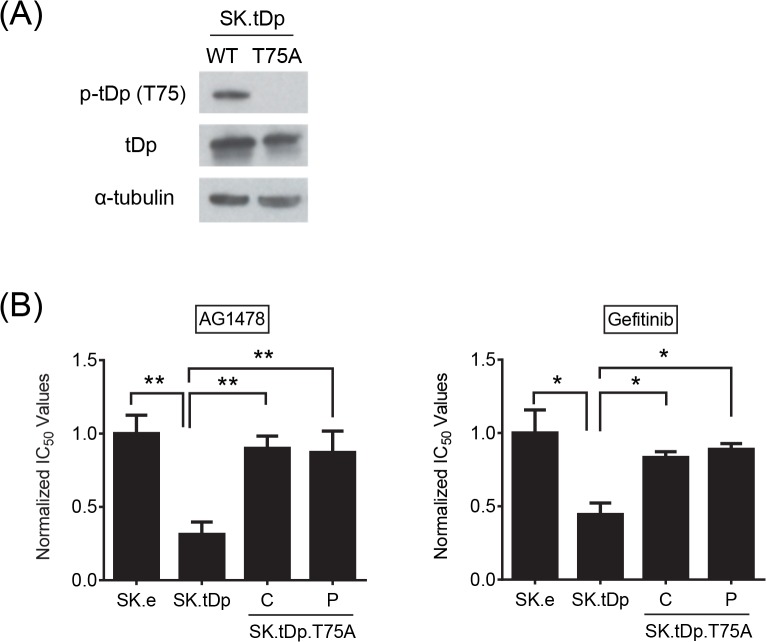
Although t-Darpp regulation has yet to be studied in-depth, phosphorylation at an internal threonine residue (T75, using the numbering scheme of Darpp-32) appears to be required for t-Darpp to confer trastuzumab resistance [19]. This same T75 site is important for regulating Darpp-32’s function as a PKA inhibitor [22]. To determine if phosphorylation at T75 is required for t-Darpp’s effects on EGFR, we stably transfected SK-Br-3 cells with t-Darpp cDNA encoding an alanine substitution at residue T75 (SK.tDp-T75A) and confirmed that these cells expressed t-Darpp lacking T75 phosphorylation (Fig 4A). In cell proliferation assays, SK.tDp cells again exhibited a 3-fold increased sensitivity to AG1478, relative to SK.empty controls, but SK.tDp-T75A cells were not more sensitive to AG1478 than parental cells (Fig 4B). Similar results were obtained with gefitinib (Fig 4B). These results suggest that the T75 residue might play an important role in t-Darpp’s ability to confer EGFR sensitivity.
Fig 4. T75 residue of t-Darpp might be responsible for conferring increased EGFR sensitivity.
(A) Western analysis showing relative amounts of p-t-Darpp (p-T75) and t-Darpp (tDp) in stably transfected SK-Br-3 cells. α–tubulin was used as a loading control. (B) Normalized IC50 values for transfected SK-Br-3 cells treated with AG1478 (left) or gefitinib (right). A clone (C) and a pooled population (P) are shown for the SK.tDp-T75A cells. Each bar represents the mean (±S.D.) IC50 of three independent experiments, with each normalized to the average IC50 of the SK.empty cells. *, p<0.05; **, p<0.01.
EGFR signaling is enhanced in SK.HerR and SK.tDp cells
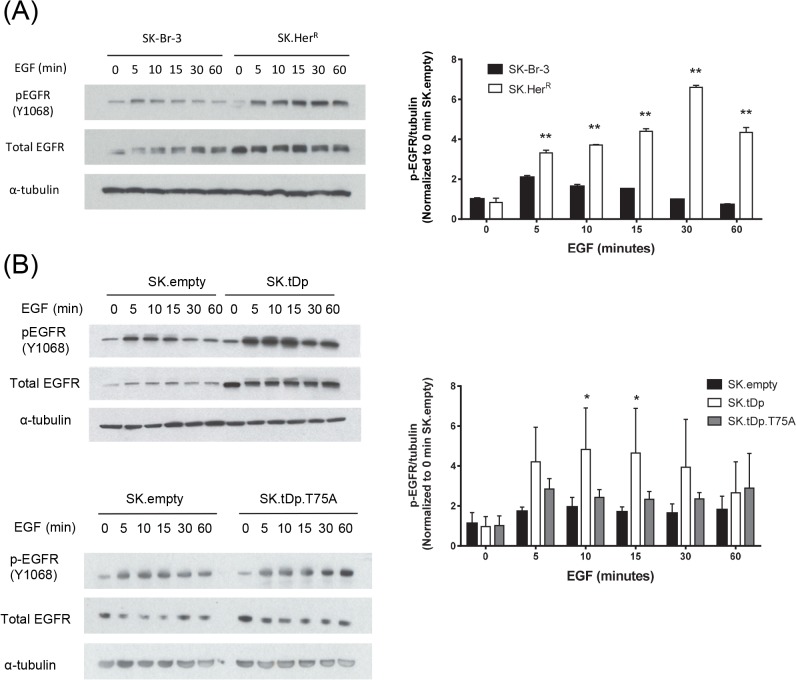
To investigate the possible role of EGFR activation in modulating the increased sensitivity to EGFR inhibition, we measured total EGFR levels and phosphorylation at tyrosine 1068 (Y1068) in serum-starved cells over the course of one hour of EGF stimulation (Fig 5). Both SK.HerR and SK.tDp cells had elevated EGFR protein, relative to their respective controls, even in the absence of EGF, and this remained steady over the course of EGF stimulation. We observed a significantly more dramatic increase in phospho-EGFR levels after the addition of EGF in both SK.HerR and SK.tDp cells, compared with their respective control cell lines. SK.tDp-T75A cells, on the other hand, did not have significantly elevated EGFR nor p-EGFR levels, compared to SK.empty controls, in the absence or presence of EGF (Fig 5B). These results suggest that t-Darpp promotes enhanced signaling through EGFR and that phosphorylation at the T75 residue may be required for this effect.
Fig 5. Increased EGFR activation in cells over-expressing t-Darpp.
Western analysis (left) and quantification (right) of cells serum-starved overnight (16 hours) followed by 100 ng/ml EGF stimulation for the indicated time points. α–tubulin was used as a loading control. Protein expression was quantified using the ImageJ software. p-EGFR data was normalized to α–tubulin in each lane and represented as the fold change relative to the zero time point p-EGFR signal for the SK-Br-3 or SK.empty cell line. (A) SK-Br-3 and SK.HerR cells (B) SK.empty, SK.tDp and SK.tDp-T75A cells. *, p <0.05; **, p<0.01.
To examine further the observation of higher steady-state protein levels in the presence of wild type t-Darpp, we measured EGFR protein stability using cycloheximide. The average half-life of EGFR in SK.tDp cells was 42 ± 11 hours compared to 20 ± 5 hours in the SK.empty cells (p = 0.032). The average half-life in SK.HerR cells was 26 ± 7 hours compared to that in parental SK-Br-3 cells of 11 ± 3 hours (p = 0.023). A representative gel analysis and quantification of all cycloheximide experiments are shown in S4 Fig. These results support the idea that t-Darpp over-expression enhances signaling through EGFR, either by stabilizing the protein or by promoting its stimulation, and thereby sensitizes cells to EGFR inhibition.
Discussion
Although trastuzumab is useful in treating patients with HER2+ breast cancer, overcoming trastuzumab resistance remains an important challenge to improve patient outcomes. The mechanisms of acquired resistance are still being determined, but the upregulation of other HER family receptors as a compensatory signaling mechanism and sustained signaling through the PI3K/Akt pathway are well established components of resistance [27]. The combination of trastuzumab with EGFR inhibitors represents a viable strategy to address this resistance mechanism.
We demonstrate that t-Darpp over-expression sensitizes SK.HerR, but not BT.HerR cells, to EGFR inhibition when trastuzumab itself is not present. Although both cell lines have high HER2 expression levels, it is well accepted that BT474 and SK-Br-3 cells have different signaling patterns. The difference that may be most relevant to the current study is the inherent difference in receptor dimerization patterns between the two cell lines, with BT474 cells signaling primarily through HER2 homodimers and SK-Br-3 cells signaling through HER2 heterodimerization with EGFR and possibly HER3 [28]. We reported previously that BT.HerR are sensitized to AG1478 only in the presence of trastuzumab, which is consistent with a model in which EGFR can only compensate for HER2 signaling when HER2 homodimers are down-regulated by trastuzumab [28]. In SK.HerR cells, on the other hand, the greater reliance on EGFR as part of the normal signaling mechanism, coupled with enhanced EGFR levels, stability and/or activity in those cells (Fig 5 and S4 Fig), most likely leads to greater sensitivity to EGFR inhibition even in the absence of HER2 inhibition by trastuzumab (Fig 1). The fact that the same phenotype was observed in SK.tDp cells, was reversed by Darpp-32 (Fig 3), and was not observed in SK.tDp-T75A cells (Fig 4), suggests that the enhancing effect is mediated by t-Darpp itself and that T75 phosphorylation is required for t-Darpp to confer this effect.
Another reported signaling discrepancy is that BT474 cells express estrogen receptor (ER), whereas SK-Br-3 cells do not. Wang et al. recently found that upon sustained HER2 inhibition with trastuzumab, ER acts as a crucial and compensatory survival pathway in HER2+ and ER+ cancers [29]. Although we did not investigate changes in estrogen receptor signaling in trastuzumab resistance BT474 cells, it is possible that BT.HerR cells do not shift to EGFR signaling as a primary pathway (in the absence of trastuzumab) because they have the ER pathway available as a more robust back-up than EGFR. SK.HerR cells, on the other hand, which lack ER, might be more likely to upregulate other HER family members as a mechanism of resistance because they lack other significant signaling pathways.
The EGFR sensitizing effect also translates into greater synergy between EGFR inhibitors and trastuzumab in SK.HerR and SK.tDp cells compared with parental cells. An additivity or moderate synergy in parental cells is consistent with published reports demonstrating that gefitinib in combination with trastuzumab produces a small synergistic or additive effect in SK-Br-3 cells [30] and is perhaps reflective of the important role that EGFR plays in the normal signaling phenotype of these cells. The greater synergy in SK.HerR and SK.tDp cells might be explained by inhibition of stabilized HER2/EGFR heterodimers or combined inhibition of HER2/EGFR and EGFR/EGFR or EGFR/HER3 dimers that compensate for HER2/EGFR in SK.HerR and SK.tDp cells. Further investigation of receptor dimerization patterns in SK.HerR and SK.tDp cells could reveal important details about the role of dimerization in determining drug sensitivities in cell lines and clinical cases.
The mechanism by which t-Darpp over-expression promotes enhanced sensitivity to EGFR inhibition and synergism with HER2 inhibition remains unclear. We showed here that t-Darpp expression resulted in a more robust activation of the EGFR pathway, possibly by stabilizing EGFR protein and, by extension, the dimers in which it participates. El-Rifai et al. previously reported that t-Darpp can bind HER2 in trastuzumab-resistant HR5 cells, a BT474-derived cell line, in a complex with HSP90 [18]. The same group also reported that t-Darpp can bind and stabilize HER2 in a complex to inhibit trastuzumab from correctly binding with the HER2 receptor in esophageal cells [20]. We have not yet detected a direct interaction between t-Darpp and EGFR (G. Lenz, N. Brink-Goodman, E. Denny, unpublished observations), but additional experiments are needed to determine if t-Darpp confers its effects through such a physical interaction with EGFR or perhaps via HER2.
There might be a more mechanistic effect of t-Darpp on EGFR signaling, not just through formation or stabilization of complexes. This is evidenced by the lack of sensitization to EGFR inhibition and the lack of enhanced p-EGFR stimulation in SK.tDp-T75A cells. The T75 phosphorylation site is crucial for the full-length protein, Darpp-32, to modulate PKA signaling and Hamel et al. demonstrated that the T75A mutation in t-Darpp abolishes its ability to confer trastuzumab resistance in BT474 cells [19]. It is possible that T75 phosphorylation is required for physical interaction with EGFR and that this promotes enhanced EGFR signaling and trastuzumab resistance, but it is also possible that t-Darpp’s effect is manifested via an effect on PKA signaling, consistent with a model that we have previously proposed [17]. We are currently investigating the regulation of t-Darpp by T75 phosphorylation as well as the importance of this site to t-Darpp’s effects on PKA.
Our results reiterate the importance of molecular profiling to determine the most effective therapies. Pre-screening of EGFR status is frequently performed in lung and gastric cancers to determine the effectiveness of EGFR-targeted therapies including gefitinib and erlotinib. Our results suggest that t-Darpp over-expression in combination with EGFR activation and dimerization patterns might correlate with sensitivity to EGFR inhibition in trastuzumab-resistant tumors, and t-Darpp itself could potentially be pursued as a new molecular target. These findings may be indicative of a novel function for t-Darpp in trastuzumab-resistant breast cancers. Further studies are necessary to delineate the mechanism by which t-Darpp causes this effect.
Supporting Information
Phosphorylated t-Darpp and total t-Darpp levels were measured by Western analysis in a panel of breast cancer cell lines. BT 1.0C and BT 1.0E are trastuzumab resistant BT474 (BT.HerR) cells that were continuously selected in the presence of trastuzumab. β-actin was used as a protein loading control.
(PDF)
SK.empty and SK.tDp cells were transfected with scrambled or tDp siRNA for 24 hours and subsequently treated with DMSO or trastuzumab for an additional 24 hours. A SRB cell proliferation assay was used to determine relative sensitivity to trastuzumab. Shown are the averages ± SD of three experiments normalized to DMSO-treated controls. **, p<0.01; *, p<0.05.
(PDF)
Colony formation assay showing percent proliferation at various concentrations of AG1478. Colonies, defined as having at least 50 cells, were counted after 14 days treatment.
(PDF)
Western analysis (left) and quantification (right) showing (A) SK.empty and SK.tDp cells and (B) SK-Br-3 and SK.HerR cells treated with 80 μg/ml cycloheximide (CHX) over 72 or 48 hours, respectively. α–tubulin was used as a loading control. EGFR protein expression was normalized to α–tubulin and represented as the fold change relative to the untreated control for each cell line. Error bars represent the SD in three independent experiments.
(PDF)
Each tab in the spreadsheet contains raw data for an individual figure, as labeled. Data for Fig 1A and 1B are calculated IC50 values for the indicated cell lines and drugs, from three replicate experiments. Data for Fig 2B are average (±S.D.) cell proliferation read-outs from each of four experiments, each done in quadruplicate. Data for Fig 3B and 3C are calculated IC50 values and cell proliferation read-outs, respectively, for the indicated transfected cells lines. Data for Fig 4B are calculated IC50 values for the indicated transfected cells (wild type and mutant t-Darpp) from three replicate experiments. Data for Fig 5A and 5B are individual ImageJ readings of phospho-EGFR and tubulin band intensities in the indicated cell lines and EGF incubation times, taken from two to seven gel images.
(XLS)
Combination index analysis showing the interaction of trastuzumab with EGFR-specific kinase inhibitors AG1478 and erlotinib in parental and trastuzumab-resistant BT.HerR cell lines. The combination of either AG1478 or erlotinib with trastuzumab produced high levels of synergy in all cell lines.
(PDF)
Acknowledgments
The authors thank R. Nahta for providing cell lines and J. Christenson and G. Lenz for critical review of data.
Data Availability
All relevant data are within the paper and its Supporting Information files. Raw data from replicate experiments can be viewed in supplementary document S1 File.
Funding Statement
This study was supported by grants CA137914 (SEK), GM105898 (SEK) and CA33572 (Cancer Center Support Grant, S.T. Rosen) from the National Institutes of Health. The remainder of funding came from an internal discretionary account assigned to SEK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61: 69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378: 1461–1484. 10.1016/S0140-6736(11)61351-2 [DOI] [PubMed] [Google Scholar]
- 3. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: Ten years of targeted anti–HER-2 therapy and personalized medicine. Oncologist. 2009;14: 320–368. 10.1634/theoncologist.2008-0230 [DOI] [PubMed] [Google Scholar]
- 4. Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16: 413–428. [DOI] [PubMed] [Google Scholar]
- 5. Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26: 3637–3643. [DOI] [PubMed] [Google Scholar]
- 6. Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20: 719–726. [DOI] [PubMed] [Google Scholar]
- 7. Kute T, Lack CM, Willingham M, Bishwokama B, Williams H, Barrett K, et al. Development of Herceptin resistance in breast cancer cells. Cytometry. 2004;57: 86–93. [DOI] [PubMed] [Google Scholar]
- 8. Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12: 395–402. [DOI] [PubMed] [Google Scholar]
- 9. Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, et al. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol. 2002;196: 17–25. [DOI] [PubMed] [Google Scholar]
- 10. Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery. 2003;133: 219–221. [DOI] [PubMed] [Google Scholar]
- 11. DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, Moore D, Thor AD. Relationship of epidermal growth factor receptor expression to erbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23: 1152–1160. [DOI] [PubMed] [Google Scholar]
- 12. Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106: 1367–1373. 10.1038/bjc.2012.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan C, Metz M, Kane S. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91: 187–201. [DOI] [PubMed] [Google Scholar]
- 14. O’Donovan N, Byrne A, O’Connor A, McGee S, Gallagher W, Crown J. Synergistic interaction between trastuzumab and EGFR/HER-2 tyrosine kinase inhibitors in HER-2 positive breast cancer cells. Invest New Drugs. 2011;29: 752–759. 10.1007/s10637-010-9415-5 [DOI] [PubMed] [Google Scholar]
- 15. Warburton C, Dragowska WH, Gelmon K, Chia S, Yan H, Masin D, et al. Treatment of HER-2/neu Overexpressing Breast Cancer Xenograft Models with Trastuzumab (Herceptin) and Gefitinib (ZD1839): Drug Combination Effects on Tumor Growth, HER-2/neu and Epidermal Growth Factor Receptor Expression, and Viable Hypoxic Cell Fraction. Clin Cancer Res. 2004;10: 2512–2524. [DOI] [PubMed] [Google Scholar]
- 16. Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99: 694–705. [DOI] [PubMed] [Google Scholar]
- 17. Gu L, Waliany S, Kane SE. Darpp-32 and its truncated variant t-Darpp have antagonistic effects on breast cancer cell growth and Herceptin resistance. PLoS ONE. 2009;4: e6220 10.1371/journal.pone.0006220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belkhiri A, Dar AA, Peng DF, Razvi MH, Rinehart C, Arteaga CL, et al. Expression of t-DARPP mediates trastuzumab resistance in breast cancer cells. Clin Cancer Res. 2008;14: 4564–4571. 10.1158/1078-0432.CCR-08-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamel S, Bouchard A, Ferrario C, Hassan S, Aguilar-Mahecha A, Buchanan M, et al. Both t-Darpp and DARPP-32 can cause resistance to trastuzumab in breast cancer cells and are frequently expressed in primary breast cancers. Breast Cancer Res Treat. 2010;120: 47–57. 10.1007/s10549-009-0364-7 [DOI] [PubMed] [Google Scholar]
- 20. Hong J, Katsha A, Lu P, Shyr Y, Belkhiri A, El-Rifai W. Regulation of ERBB2 receptor by t-DARPP mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer Res. 2012;72: 4504–4514. 10.1158/0008-5472.CAN-12-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hemmings HC, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310: 503–505. [DOI] [PubMed] [Google Scholar]
- 22. Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402: 669–671. [DOI] [PubMed] [Google Scholar]
- 23. Beckler A, Moskaluk CA, Zaika A, Hampton GM, Powell SM, Frierson HF, et al. Overexpression of the 32-kilodalton dopamine and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein in common adenocarcinomas. Cancer. 2003;98: 1547–1551. [DOI] [PubMed] [Google Scholar]
- 24. Vangamudi B, Peng D-F, Cai Q, El-Rifai W, Zheng W, Belkhiri A. t-DARPP regulates phosphatidylinositol-3-kinase-dependent cell growth in breast cancer. Molec Cancer. 2010;9: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nahta R, Esteva FJ. In vitro effects of trastuzumab and vinorelbine in trastuzumab-resistant breast cancer cells. Cancer Chemo Pharmacol. 2004;53: 186–190. [DOI] [PubMed] [Google Scholar]
- 26. Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70: 440–446. 10.1158/0008-5472.CAN-09-1947 [DOI] [PubMed] [Google Scholar]
- 27. Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: Mechanisms and clinical implications. Crit Rev Oncog. 2012;17: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71: 1871–1882. 10.1158/0008-5472.CAN-10-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers—role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13: R121 10.1186/bcr3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61: 7184–7188. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phosphorylated t-Darpp and total t-Darpp levels were measured by Western analysis in a panel of breast cancer cell lines. BT 1.0C and BT 1.0E are trastuzumab resistant BT474 (BT.HerR) cells that were continuously selected in the presence of trastuzumab. β-actin was used as a protein loading control.
(PDF)
SK.empty and SK.tDp cells were transfected with scrambled or tDp siRNA for 24 hours and subsequently treated with DMSO or trastuzumab for an additional 24 hours. A SRB cell proliferation assay was used to determine relative sensitivity to trastuzumab. Shown are the averages ± SD of three experiments normalized to DMSO-treated controls. **, p<0.01; *, p<0.05.
(PDF)
Colony formation assay showing percent proliferation at various concentrations of AG1478. Colonies, defined as having at least 50 cells, were counted after 14 days treatment.
(PDF)
Western analysis (left) and quantification (right) showing (A) SK.empty and SK.tDp cells and (B) SK-Br-3 and SK.HerR cells treated with 80 μg/ml cycloheximide (CHX) over 72 or 48 hours, respectively. α–tubulin was used as a loading control. EGFR protein expression was normalized to α–tubulin and represented as the fold change relative to the untreated control for each cell line. Error bars represent the SD in three independent experiments.
(PDF)
Each tab in the spreadsheet contains raw data for an individual figure, as labeled. Data for Fig 1A and 1B are calculated IC50 values for the indicated cell lines and drugs, from three replicate experiments. Data for Fig 2B are average (±S.D.) cell proliferation read-outs from each of four experiments, each done in quadruplicate. Data for Fig 3B and 3C are calculated IC50 values and cell proliferation read-outs, respectively, for the indicated transfected cells lines. Data for Fig 4B are calculated IC50 values for the indicated transfected cells (wild type and mutant t-Darpp) from three replicate experiments. Data for Fig 5A and 5B are individual ImageJ readings of phospho-EGFR and tubulin band intensities in the indicated cell lines and EGF incubation times, taken from two to seven gel images.
(XLS)
Combination index analysis showing the interaction of trastuzumab with EGFR-specific kinase inhibitors AG1478 and erlotinib in parental and trastuzumab-resistant BT.HerR cell lines. The combination of either AG1478 or erlotinib with trastuzumab produced high levels of synergy in all cell lines.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Raw data from replicate experiments can be viewed in supplementary document S1 File.