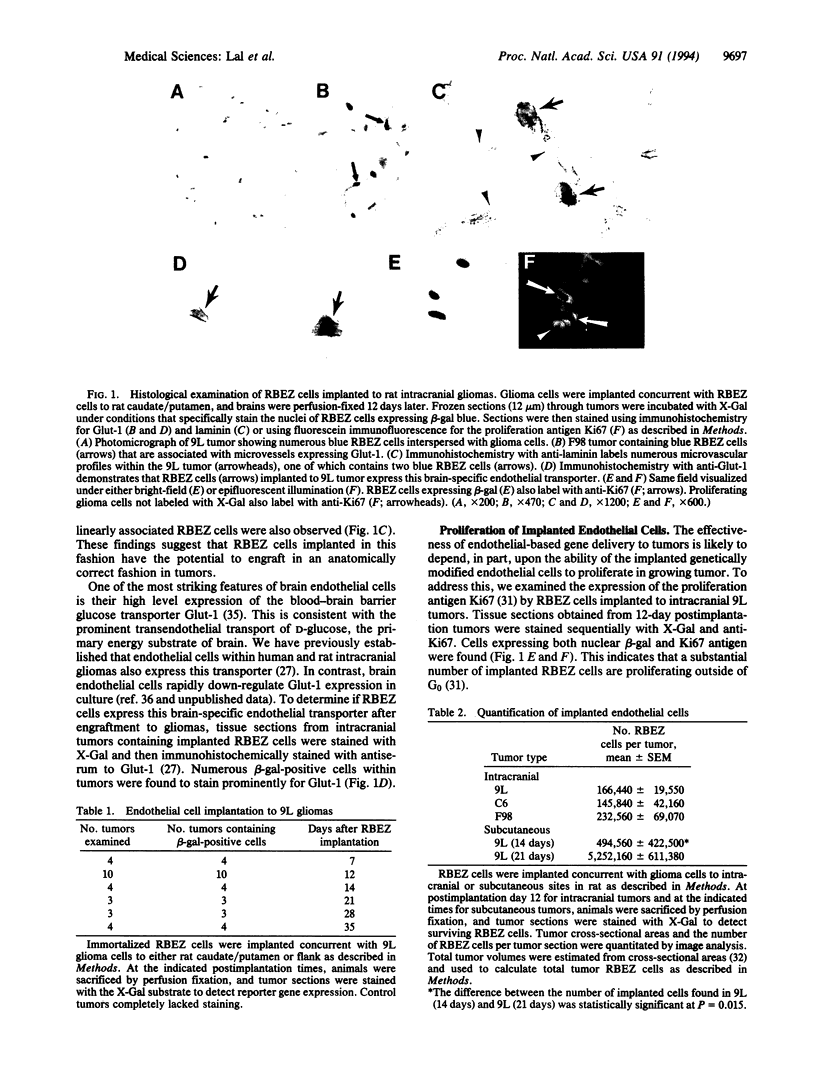
Abstract
The delivery of therapeutic genes to primary brain neoplasms opens new opportunities for treating these frequently fatal tumors. Efficient gene delivery to tissues remains an important obstacle to therapy, and this problem has unique characteristics in brain tumors due to the blood-brain and blood-tumor barriers. The presence of endothelial mitogens and vessel proliferation within solid tumors suggests that genetically modified endothelial cells might efficiently transplant to brain tumors. Rat brain endothelial cells immortalized with the adenovirus E1A gene and further modified to express the beta-galactosidase reporter were examined for their ability to survive implantation to experimental rat gliomas. Rats received 9L, F98, or C6 glioma cells in combination with endothelial cells intracranially to caudate/putamen or subcutaneously to flank. Implanted endothelial cells were identified by beta-galactosidase histochemistry or by polymerase chain reaction in all tumors up to 35 days postimplantation, the latest time examined. Implanted endothelial cells appeared to cooperate in tumor vessel formation and expressed the brain-specific endothelial glucose transporter type 1 as identified by immunohistochemistry. The proliferation of implanted endothelial cells was supported by their increased number within tumors between postimplantation days 14 and 21 (P = 0.015) and by their expression of the proliferation antigen Ki67. These findings establish that genetically modified endothelial cells can be stably engrafted to growing gliomas and suggest that endothelial cell implantation may provide a means of delivering therapeutic genes to brain neoplasms and other solid tumors. In addition, endothelial implantation to brain may be useful for defining mechanisms of brain-specific endothelial differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arosarena O., Guerin C., Brem H., Laterra J. Endothelial differentiation in intracerebral and subcutaneous experimental gliomas. Brain Res. 1994 Mar 21;640(1-2):98–104. doi: 10.1016/0006-8993(94)91861-9. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O. Gene delivery into the brain using virus vectors. Nat Genet. 1993 Mar;3(3):187–189. doi: 10.1038/ng0393-187. [DOI] [PubMed] [Google Scholar]
- Callow A. D. The vascular endothelial cell as a vehicle for gene therapy. J Vasc Surg. 1990 Jun;11(6):793–798. [PubMed] [Google Scholar]
- Chen L. S., Ray J., Fisher L. J., Kawaja M. D., Schinstine M., Kang U. J., Gage F. H. Cellular replacement therapy for neurologic disorders: potential of genetically engineered cells. J Cell Biochem. 1991 Mar;45(3):252–257. doi: 10.1002/jcb.240450305. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Dichek D. A., Neville R. F., Zwiebel J. A., Freeman S. M., Leon M. B., Anderson W. F. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation. 1989 Nov;80(5):1347–1353. doi: 10.1161/01.cir.80.5.1347. [DOI] [PubMed] [Google Scholar]
- Dichek D. A. Retroviral vector-mediated gene transfer into endothelial cells. Mol Biol Med. 1991 Apr;8(2):257–266. [PubMed] [Google Scholar]
- Durieu-Trautmann O., Foignant-Chaverot N., Perdomo J., Gounon P., Strosberg A. D., Couraud P. O. Immortalization of brain capillary endothelial cells with maintenance of structural characteristics of the blood-brain barrier endothelium. In Vitro Cell Dev Biol. 1991 Oct;27A(10):771–778. doi: 10.1007/BF02631242. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Fédérici C., Créminon C., Foignant-Chaverot N., Roux F., Claire M., Strosberg A. D., Couraud P. O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993 Apr;155(1):104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Gerhart D. Z., LeVasseur R. J., Broderius M. A., Drewes L. R. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res. 1989 Apr;22(4):464–472. doi: 10.1002/jnr.490220413. [DOI] [PubMed] [Google Scholar]
- Giordana M. T., Germano I., Giaccone G., Mauro A., Migheli A., Schiffer D. The distribution of laminin in human brain tumors: an immunohistochemical study. Acta Neuropathol. 1985;67(1-2):51–57. doi: 10.1007/BF00688123. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L. The blood-brain barrier. Sci Am. 1986 Sep;255(3):74–83. doi: 10.1038/scientificamerican0986-74. [DOI] [PubMed] [Google Scholar]
- Guerin C., Laterra J., Drewes L. R., Brem H., Goldstein G. W. Vascular expression of glucose transporter in experimental brain neoplasms. Am J Pathol. 1992 Feb;140(2):417–425. [PMC free article] [PubMed] [Google Scholar]
- Jiao S., Wolff J. A. Long-term survival of autologous muscle grafts in rat brain. Neurosci Lett. 1992 Mar 30;137(2):207–210. doi: 10.1016/0304-3940(92)90405-v. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Ko L., Koestner A., Wechsler W. Morphological characterization of nitrosourea-induced glioma cell lines and clones. Acta Neuropathol. 1980;51(1):23–31. doi: 10.1007/BF00688846. [DOI] [PubMed] [Google Scholar]
- Lal B., Cahan M. A., Couraud P. O., Goldstein G. W., Laterra J. Development of endogenous beta-galactosidase and autofluorescence in rat brain microvessels: implications for cell tracking and gene transfer studies. J Histochem Cytochem. 1994 Jul;42(7):953–956. doi: 10.1177/42.7.8014479. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Lim C. S., Chapman G. D., Gammon R. S., Muhlestein J. B., Bauman R. P., Stack R. S., Swain J. L. Direct in vivo gene transfer into the coronary and peripheral vasculatures of the intact dog. Circulation. 1991 Jun;83(6):2007–2011. doi: 10.1161/01.cir.83.6.2007. [DOI] [PubMed] [Google Scholar]
- Maxwell M., Naber S. P., Wolfe H. J., Hedley-Whyte E. T., Galanopoulos T., Neville-Golden J., Antoniades H. N. Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res. 1991 Feb 15;51(4):1345–1351. [PubMed] [Google Scholar]
- Messina L. M., Podrazik R. M., Whitehill T. A., Ekhterae D., Brothers T. E., Wilson J. M., Burkel W. E., Stanley J. C. Adhesion and incorporation of lacZ-transduced endothelial cells into the intact capillary wall in the rat. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12018–12022. doi: 10.1073/pnas.89.24.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Progress toward human gene therapy. Blood. 1990 Jul 15;76(2):271–278. [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Boyce F. M., Stanley J. C., Nabel G. J. Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science. 1989 Jun 16;244(4910):1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Roux F., Durieu-Trautmann O., Chaverot N., Claire M., Mailly P., Bourre J. M., Strosberg A. D., Couraud P. O. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994 Apr;159(1):101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Hall D. E., Porter S., Barbu K., Cannon C., Horner H. C., Janatpour M., Liaw C. W., Manning K., Morales J. A cell culture model of the blood-brain barrier. J Cell Biol. 1991 Dec;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinstine M., Kawaja M. D., Gage F. H. Intracerebral delivery of growth factors: potential application of genetically modified fibroblasts. Prog Growth Factor Res. 1991;3(1):57–66. doi: 10.1016/0955-2235(91)90013-t. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Miwa T., Hoshino T. Embedding and fixation techniques for immunohistochemical staining with anti-DNA polymerase alpha and Ki-67 monoclonal antibodies to analyze the proliferative potential of tumors. Biotech Histochem. 1992 May;67(3):161–164. doi: 10.3109/10520299209110028. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981 May;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Geselowitz D., Chavany C., Fahmy B., Walbridge S., Alger J. R., Neckers L. M. Stability, clearance, and disposition of intraventricularly administered oligodeoxynucleotides: implications for therapeutic application within the central nervous system. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4665–4669. doi: 10.1073/pnas.90.10.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Birinyi L. K., Salomon R. N., Libby P., Callow A. D., Mulligan R. C. Implantation of vascular grafts lined with genetically modified endothelial cells. Science. 1989 Jun 16;244(4910):1344–1346. doi: 10.1126/science.2734614. [DOI] [PubMed] [Google Scholar]
- Yao S. N., Wilson J. M., Nabel E. G., Kurachi S., Hachiya H. L., Kurachi K. Expression of human factor IX in rat capillary endothelial cells: toward somatic gene therapy for hemophilia B. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8101–8105. doi: 10.1073/pnas.88.18.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiebel J. A., Freeman S. M., Kantoff P. W., Cornetta K., Ryan U. S., Anderson W. F. High-level recombinant gene expression in rabbit endothelial cells transduced by retroviral vectors. Science. 1989 Jan 13;243(4888):220–222. doi: 10.1126/science.2911735. [DOI] [PubMed] [Google Scholar]