Abstract
This parallel-group, randomized controlled pilot study examined daily meditation in a diverse sample of older adults with postherpetic neuralgia. Block randomization was used to allocate participants to a treatment group (n = 13) or control group (n = 14). In addition to usual care, the treatment group practiced daily meditation for six weeks. All participants completed questionnaires at enrollment in the study, two weeks later, and six weeks after that, at the study’s end. Participants recorded daily pain and fatigue levels in a diary, and treatment participants also noted meditation practice. Results at the .10 level indicated improvement in neuropathic, affective, and total pain scores for the treatment group, whereas affective pain worsened for the control group. Participants were able to adhere to the daily diary and meditation requirements in this feasibility pilot study.
Introduction
Postherpetic neuralgia (PHN) is a severe, life-altering condition that afflicts approximately 20% to 30% of individuals who have had herpes zoster, more commonly known as shingles.1 Of the 1 million new cases of shingles in the United States each year, nearly 50% occur in persons 60 years of age or older. By the age of 85, about 50% of adults have had or will have shingles,2 with 60% being women.3 As the U.S. population ages, the incidence of shingles will increase, along with a concomitant increase in PHN. Older adults tend to have more severe cases of shingles and develop PHN more often than do younger adults.4
Although an acute episode of shingles typically lasts 3 to 4 weeks, PHN can last for months or even years.1 The primary cause of morbidity in PHN is pain, and the condition is reported to be one of the most intractable neuropathic pain disorders.5 Individuals describe this pain as “stabbing,” “burning,” “aching,” and “itching,” resulting in fatigue, interrupted sleep, depression, and anxiety.6 The ability to perform activities of daily living can be affected, resulting in a loss of independence and decreased quality of life, especially in an older, more vulnerable population.7–10
The usual mode of treatment for PHN is pharmacotherapy,11,12 with tricyclic antidepressants and anticonvulsants recommended by the International Association for the Study of Pain as first-line therapies.13 Second-line therapies include tramadol and opioid analgesics, with topical capsaicin noted as a possible third-line therapy.13 Given alone, these medications have well-documented side effects14; given in combination to manage the pain of PHN, the probability of adverse drug interactions increases.15–17 Drug-related side effects are more commonly seen in older individuals, who may have comorbid illnesses and may already be taking several medications.18,19 Even with a variety of medications, the pain of PHN might not be well controlled.20,21
Management of chronic pain using complementary and alternative medicine (CAM) therapies has been examined for a variety of conditions.22–25 Some of the most commonly used CAM therapies are relaxation techniques, with meditation comprising three fourths of these.26 Although little research specifically focused on meditation in PHN has been reported in the literature, studies have examined its use in a variety of other chronic conditions. A reduction in the use of pain medications and an improvement in mental health were seen in women with fibromyalgia who received a mindfulness intervention that included meditation.27 A significant decrease in depressive symptoms was found compared with a waitlist control group. Home meditation practice has been associated with improvement in several outcome measures in chronic pain conditions28 and has been found to be useful for older adults with pain.29 Because meditation is typically just one component of mindfulness-based stress reduction (MBSR) programs, investigators have recommended that individual program components (e.g., meditation) be evaluated separately27,30–34 and in samples that are homogeneous regarding the pain condition.22,28
Method
This pilot study was undertaken to determine whether daily mindfulness meditation would decrease the pain associated with PHN and increase mental health and quality of life in a diverse sample of community-dwelling adults age 50 and older. Examining only the meditation component of what is typically an eight-week MBSR program, a parallel-group, randomized controlled design was used to (1) compare initial estimates of treatment effects in study participants who received usual care plus meditation (treatment group) with those who received usual care alone (control group); (2) assess the feasibility of recruitment of a diverse sample and adherence to a six-week meditation program; and (3) examine the acceptability and experience of practicing meditation from the perspective of study participants. A crossover option (i.e., delayed treatment) for participants initially randomly assigned to the control group was added after enrollment in the study commenced. Given this pilot study’s primary focus on the acceptability of the intervention and its feasibility for an older population, study sample size was partially determined by resource and enrollment constraints, and this study was not intended to have sufficient statistical power to detect medium or small effect sizes either within or between groups. The achieved sample size of 27 was sufficient to determine feasibility and acceptability proportions within a rough margin of error (at most 18.9% for 95% confidence intervals).
Recruitment
After study approval by the institution’s human research review committee, participants were primarily recruited through an article in the local newspaper. Inclusion criteria were 50 years of age or older, able to read and write English, and self-reported persistent pain after the shingles rash had resolved. Exclusion criteria were consistent use of meditation in the previous year; medical instability from severe heart disease, lung disease, or diabetes mellitus; multiple recent falls; pain caused by an acute injury in the previous month; unable to stand independently; and underlying serious illness, such as unexplained weight loss, fever, or pain from cancer.
The principal investigator (PI) screened all potential participants by telephone, followed by an individual face-to-face meeting of qualified participants in a private room at the university’s integrative medicine center. After giving written consent, participants completed four questionnaires and were shown how to complete a daily diary of their pain and fatigue levels. A return appointment was made for two weeks later, at which time participants completed the questionnaires a second time. These two testing times before randomization gave participants time to become familiar with the daily diary and was one way to address assessment reactivity, a factor to consider in studies examining the effectiveness of brief interventions.35, 36
Group assignment was made by a statistician so that randomization occurred in a 1:1 ratio in blocks of four. Participants were blinded to group assignment until after completion of the second set of questionnaires, at which time the treatment participants received training on the meditation. The PI was blinded to group assignment until after completion of the enrollment questionnaires. Eight weeks after enrollment in the study (i.e., six weeks after randomization to groups), all study participants returned to the integrative medicine center for a third completion of the questionnaires. Participants in the treatment group were then interviewed by the PI about their experiences with meditation and any challenges encountered, and participation in the study ended for them. Participants in the control group were invited to continue in the study in a delayed treatment group; data from this crossover, delayed treatment group are not included here. All participants were equally compensated for their time through the initial eight-week period, but those continuing as the delayed treatment group did not receive additional compensation. Figure 1 shows the flow of participants through the study, following the CONSORT guidelines.37,38
Fig. 1.
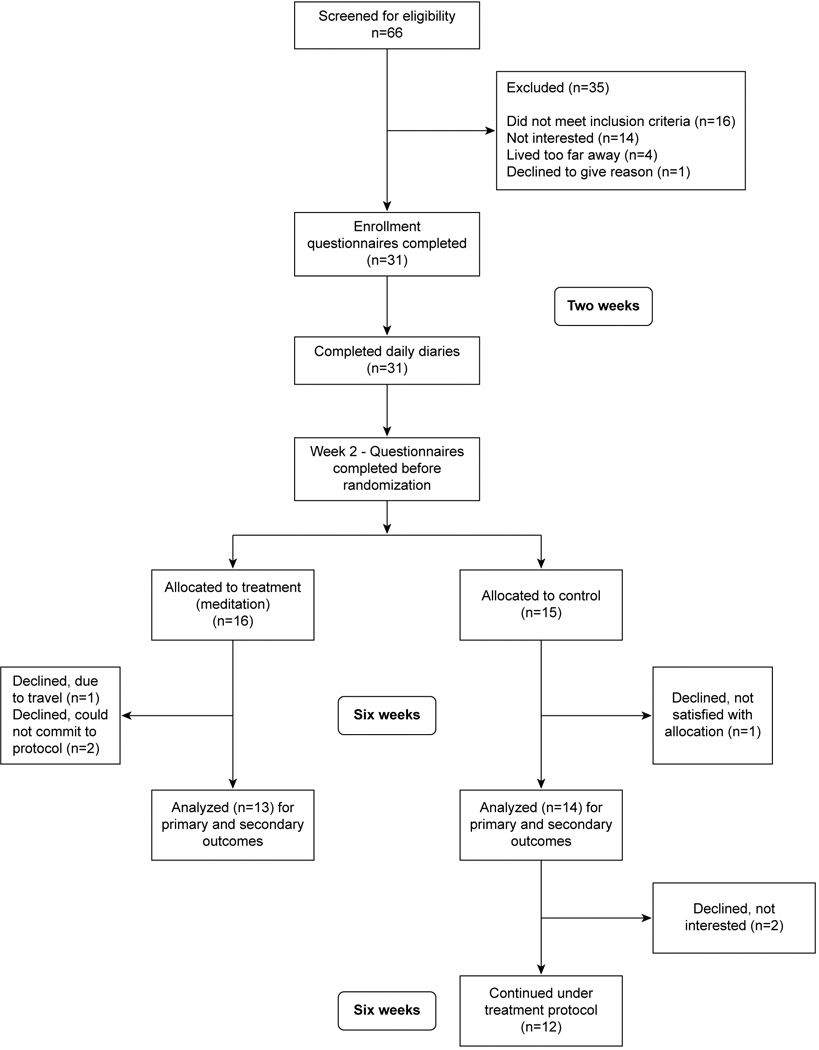
Participant flow through the study.
Treatment
Participants in the treatment group had an individual, one-hour session with one of the investigators, a certified MBSR instructor with more than 15 years’ experience. The session focused on what meditation is, what meditation is not, pain and the stress response, the benefits of meditation, being in the moment, and the specific practice of meditation for this study. To enhance treatment fidelity,39 the meditation instructor stayed with an agreed upon script within a one-hour session. She taught only one of the ways to practice mindfulness meditation (i.e., focus on breathing while seated comfortably), using compact discs (CDs) developed for the study. She provided all teaching sessions in a private room at the integrative medicine center, and the PI was present to monitor the sessions for adherence to script and time. All participants received training regarding the CDs by the PI, who also explained how to document the meditation practice in the daily diary. The PI telephoned all participants (treatment and control) weekly and reminded them verbally or through a phone message to mail in their daily diaries each week.
Specifics of the daily meditation
Prior to enrollment of participants, the PI and meditation instructor had considered the age and possible pain level of the target population. Because the meditation literature reports low compliance for meditation interventions (typically, 20 minutes long), the PI and instructor developed a set of six CDs that allowed a gradual increase in meditation time over a six-week period, as depicted in Table 1. Participants were instructed to use the next CD at the end of a week. If the participant did not have time to do the required meditation on any given day, he or she was to listen to a shorter CD, so that at least some time was spent in meditation each day.
Table 1.
Six-week meditation schedule.
| CD Number | Time | Frequency |
|---|---|---|
| 1 | 3 min, 34 sec | 2 times per day |
| 2 | 5 min, 18 sec | 2 times per day |
| 3 | 7 min, 41 sec | 2 times per day |
| 4 | 10 min, 24 sec | 2 times per day |
| 5 | 13 min, 52 sec | 1 time per day |
| 6 | 15 min, 43 sec | 1 time per day |
Measures
Primary outcome measure—pain
The Short-Form McGill Pain Questionnaire (SF-MPQ-2) was used to describe pain intensity. This 22-item questionnaire describes different qualities of pain and related symptoms using an 11-point scale (0 = none to 10 = worst possible).40 The SF-MFQ-2 was designed to provide composite and subscale measures of the major sensory and affective descriptors (e.g., fearful, tiring-exhausting, sickening, etc.) for both neuropathic and non-neuropathic pain.40 Cronbach’s alpha has been reported as excellent for the composite score (.91–.96)41 and acceptable to excellent (.73–.92) for the four subscales (continuous, intermittent, neuropathic, and affective descriptors of pain) in studies that included a clinical trial for treatment of diabetic peripheral neuropathy40 and among U.S. veterans with a variety of chronic pain diagnoses.41 The total and subscale scores of the SF-MPQ-2 have been found to be responsive to change, and such changes were shown to be meaningful to patients.40
Other measures
Quality of life
The RAND 36-Item Health Survey 1.0 is a generic, multidimensional health questionnaire that yields an eight-scale profile of physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions.42 It has been useful in estimating disease burden in a variety of acute and chronic conditions. In more than 25 published studies, reliability statistics have exceeded the minimum standard of .70 recommended for group comparison measurements.43 All questions are scored on a scale from 0 to 100, with 100 representing the highest level of functioning possible. Questions for a subscale are averaged to obtain the score for that subscale (0–100 range). For example, higher scores on the bodily pain measure represent less severe and less debilitating pain.44
Depression
The Center for Epidemiologic Studies Depression Scale is a 20-item scale that measures the major components of depressive symptomatology in the general population (i.e., nonpsychiatric). It has been found to have high internal consistency, with Cronbach’s alphas ranging from .85 to .90 across studies and adequate test–retest reliability.45 Responses have a possible range of 0 to 60, with a score of 16 points or more considered “depressed.”
Anxiety
The State-Trait Anxiety Inventory (STAI) consists of two 20-item scales (a State scale and a Trait scale). Internal consistency reliability estimates for the State and Trait scale scores range from .90 to .94 and from .89 to .96, respectively, in groups of adults,46,47 and the STAI Trait scale has excellent test–retest reliability (with an average r = .88) at multiple time intervals.48
Daily pain level
Participants rated pain and fatigue levels in a daily diary and mailed the diaries to the PI at the end of each week. An 11-point scale was used (0 = No Pain or No Fatigue to 10 = Worst Pain Imaginable or Worst Fatigue Imaginable, respectively). Participants in the treatment group also provided information related to the practice of meditation (i.e., CD used that day, number of times).
Data analysis
Descriptive statistics were used to assess outcome measures for all participants three times: at enrollment, just before randomization to treatment and control groups, and at the end of the study. Pearson’s chi-squared tests were used to compare distributions of categorical variables measured at enrollment between the treatment and control groups, and t tests were used to test for differences in outcomes between the two groups. Analyses of variance were used to examine the significance of main effects and interactions of selected enrollment characteristics and the treatment on different outcomes over time. Pearson’s correlations with cluster-robust standard errors49 were used to examine bivariate associations between selected outcome measures, adjusting for impacts of the nesting of repeated measures within individuals. Due to the small sample size in this pilot study, adjustments for multiple comparisons were not made, and although statistical tests were judged to be significant at the .05 level, the .10 alpha-level was noted as well. Further, calculated p-values should be viewed with some caution due to our use of parametric statistical tests with small samples sizes. Although results at the .10 level were not judged to be statistically significant, effect size measures of Cohen’s d for comparisons of means between groups and dz for comparisons of means within groups were noted for comparisons with p-values less than .10 and compared to the usual cut-offs for “small”, “medium”, and “large” effect sizes of 0.2, 0.5, and 0.8 for d and 0.14, 0.35, and 0.57 for dz.50 Statistical analyses were conducted in SAS 9.251 and Stata 13.1.52
Results
Sample demographics and pain characteristics at entry to study
The mean (SD) age of the participants was 72 (9.6) years, with a range of 55 to 90 years. The participants’ other demographic characteristics are presented in Table 2. Participants rated their pain level at entry to the study, with the majority (85%) reporting moderate or severe pain experienced on a daily basis (67%). Participants were evenly divided in the time since a shingles diagnosis, with almost one third reporting less than a year since diagnosis, one third reporting greater than a year but less than three years, and the rest reporting greater than three years. Three quarters were currently taking at least one PHN-related pain medication. Half the participants indicated they had pain in addition to the pain from PHN. The location of pain from PHN was almost evenly divided among three areas: face, head, or neck (29%); chest and abdomen (33%); and back, extremities, or internal (37%; see Table 3).
Table 2.
Demographic characteristics of study participants.
| Meditation group (n = 13) |
Control group (n = 14) |
Total (N = 27) |
||||
|---|---|---|---|---|---|---|
| N | % | n | % | N | % | |
| Study recruitment | ||||||
| Newspaper article | 10 | 76.9 | 13 | 92.9 | 23 | 85.2 |
| Other | 3 | 23.1 | 1 | 7.1 | 4 | 14.8 |
| Gender | ||||||
| Male | 7 | 53.8 | 5 | 35.7 | 12 | 44.4 |
| Female | 6 | 46.2 | 9 | 64.3 | 15 | 55.6 |
| Ethnicity | ||||||
| American Indian | 1 | 7.7 | 1 | 7.1 | 2 | 7.4 |
| Hispanic | 8 | 61.5 | 3 | 21.4 | 11 | 40.7 |
| White | 4 | 30.8 | 10 | 71.4 | 14 | 51.9 |
| Education | ||||||
| High school graduate or less | 1 | 7.7 | 2 | 14.3 | 3 | 11.1 |
| Some college | 6 | 46.2 | 7 | 50.0 | 13 | 48.1 |
| Bachelor's degree or higher | 6 | 46.2 | 5 | 35.7 | 11 | 40.7 |
| Marital status | ||||||
| Single | 1 | 7.7 | 1 | 7.1 | 2 | 7.4 |
| Married | 9 | 69.2 | 7 | 50.0 | 16 | 59.3 |
| Divorced | 1 | 7.7 | 5 | 35.7 | 6 | 22.2 |
| Widowed | 2 | 15.4 | 1 | 7.1 | 3 | 11.1 |
| Retired | ||||||
| Yes | 10 | 76.9 | 12 | 85.7 | 22 | 81.5 |
| No | 3 | 23.1 | 2 | 14.3 | 5 | 18.5 |
Table 3.
Postherpetic neuralgia pain status at enrollment in the study.
| Meditation (n = 13) |
Control (n = 14) |
Total (N = 27) |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Years since shingles diagnosis | ||||||
| 1 year or less | 4 | 30.8 | 4 | 28.6 | 8 | 29.6 |
| 1–2 years | 5 | 38.5 | 5 | 35.7 | 10 | 37.0 |
| 3 years or more | 4 | 30.8 | 5 | 35.7 | 9 | 33.3 |
| PHN pain medication | ||||||
| None | 3 | 23.1 | 3 | 21.4 | 6 | 22.2 |
| At least one | 10 | 76.9 | 11 | 78.6 | 21 | 77.8 |
| PHN pain level at enrollment | ||||||
| Mild pain | 2 | 15.4 | 2 | 14.3 | 4 | 14.8 |
| Moderate pain | 5 | 38.5 | 5 | 35.7 | 10 | 37.0 |
| Severe or greater pain | 6 | 46.2 | 7 | 50.0 | 13 | 48.1 |
| Days of PHN pain at enrollment | ||||||
| 0–6 Days/week | 5 | 38.5 | 4 | 28.6 | 9 | 33.3 |
| 7 Days/week | 8 | 61.5 | 10 | 71.4 | 18 | 66.7 |
| Other non-PHN pain | ||||||
| No pain | 7 | 53.8 | 6 | 42.9 | 13 | 48.1 |
| Present | 6 | 46.2 | 8 | 57.1 | 14 | 51.9 |
| PHN pain location | ||||||
| Face, head, or neck | 3 | 23.1 | 5 | 35.7 | 8 | 29.6 |
| Front chest & abdomen | 7 | 53.8 | 2 | 14.3 | 9 | 33.3 |
| Back, extremities, or other | 3 | 23.1 | 7 | 50.0 | 10 | 37.0 |
Daily meditation practice
Across the 13 participants in the treatment group, the average rate of reporting whether meditation occurred on a given day or not was 98.2%. Of days in which meditation status was reported, the average rate of meditation was 98.9%. Even if one assumes that every day for which meditation status was not reported represents a day of nonmeditation, the average rate of meditation during the six-week treatment period was 97.1%.
Changes in outcomes over time
Table 4 summarizes the descriptive statistics for outcomes at enrollment, Week 2, and Week 8. Changes in these outcomes over time were examined using t tests at both the p < .05 and the p < .10 levels. Specifically, neuropathic pain showed evidence of improvement between enrollment and study completion for the treatment group that approached significance (p = .069, dz = 0.55), as did the total pain score (p = .057, dz = 0.58). Moreover, there was evidence of a difference in trends that approached significance (p = .058, d = 0.76) for severity of affective pain between the treatment and control groups, with worsening conditions in the control group between enrollment and study completion, and improved measures for the treatment group in the same period. There were also significant (p = .028, dz = 0.45) improvements in health change scores across both groups between time of second testing (Week 2, before randomization) and completion of the study (Week 8), and significant (p = .040, dz = 0.42) decreases in physical role limitation scores across both groups between enrollment and Week-2 testing (indicating possible assessment reactivity issues for this measure). A difference in trends also approached significance (p = .083, d = 0.69) between treatment and control groups from Week-2 testing to completion of the study at Week 8, with increase in physical functioning observed for the treatment group, whereas the control group remained relatively unchanged. Emotional well-being scores showed evidence of improvement (p = .078, dz = 0.53) for the treatment group between Week 2 and Week 8, whereas state anxiety decreased significantly (p = .043, dz = 0.41) across both groups during the same period; depression scores decreased significantly (p = .036, dz = 0.63) for the control group between enrollment and completion of the study at Week 8, but not for the treatment group.
Table 4.
Means and standard deviations of measures at enrollment, immediately before randomization (Week 2), and at completion of study (Week 8).
| Enrollment | Week 2 | Week 8 | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ±SD | |
| Emotional well-beinga | |||
| Meditation | 72.7 ± 20.5 | 66.2 ± 15.6 | 75.4 ± 16.9 |
| Control | 72.1 ± 20.7 | 71.8 ± 20.6 | 74.1 ± 20.5 |
| Health changea | |||
| Meditation | 46.2 ± 17.2 | 40.4 ± 28.0 | 61.5 ± 24.2 |
| Control | 46.4 ± 23.7 | 46.4 ± 23.7 | 51.8 ± 20.7 |
| Bodily paina | |||
| Meditation | 56.3 ± 21.0 | 49.0 ± 27.7 | 54.7 ± 19.1 |
| Control | 53.0 ± 22.6 | 52.9 ± 21.4 | 56.6 ± 20.2 |
| Physical functioninga | |||
| Meditation | 61.2 ± 25.3 | 57.7 ± 30.0 | 68.1 ± 26.1 |
| Control | 66.8 ± 32.5 | 67.1 ± 30.3 | 66.1 ± 33.4 |
| Physical-role limitationsa | |||
| Meditation | 68.8 ± 26.3 | 60.6 ± 32.6 | 54.3 ± 30.2 |
| Control | 76.8 ± 22.0 | 67.4 ± 21.4 | 66.1 ± 23.9 |
| Total CES-D scoreb | |||
| Meditation | 9.2 ± 9.8 | 12.0 ± 12.4 | 9.9 ± 9.1 |
| Control | 10.1 ± 6.2 | 10.4 ± 10.8 | 7.4 ± 5.7 |
| State anxietyc | |||
| Meditation | 28.5 ± 9.5 | 35.2 ± 13.0 | 26.8 ± 9.4 |
| Control | 27.9 ± 6.8 | 28.3 ± 7.4 | 27.0 ± 7.3 |
| Affective paind | |||
| Meditation | 2.7 ± 2.4 | 2.7 ± 2.7 | 1.8 ± 2.0 |
| Control | 1.2 ± 1.6 | 1.3 ± 1.2 | 1.9 ± 2.0 |
| Total pain scored | |||
| Meditation | 3.5 ± 2.2 | 3.2 ± 2.3 | 2.6 ± 1.6 |
| Control | 2.4 ± 1.5 | 2.3 ± 1.2 | 2.1 ± 1.5 |
RAND 36-Item Health Survey 1.0; lower scores indicate worse health.
Center for Epidemiologic Studies-Depression Scale; lower scores indicate less depression.
State-Trait Anxiety Inventory; lower scores indicate less anxiety.
Short-Form McGill Pain Questionnaire; lower scores indicate less pain.
Relationships between PHN pain and outcome measures across enrollment, Week 2, and Week 8
Table 5 shows that neuropathic, affective, and total pain scores have significant negative relationships with physical functioning, role limitations due to physical health, and emotional well-being, and significant positive correlations between these three measures of pain, depression, and anxiety. Overall, the strongest associations between measures of physical and emotional health and pain were with affective pain.
Table 5.
Correlations among selected pain measures and measures of physical and emotional health (N = 81).
| Neuropathic paina | Affective paina | Total pain scorea | |
|---|---|---|---|
| Pearson’s correlation | Pearson’s correlation | Pearson’s correlation | |
| Health changeb | −0.11 | −0.27 | −0.22† |
| Physical role limitationb | −0.37* | −0.42** | −0.43* |
| Physical functioningb | −0.39* | −0.63*** | −0.51** |
| Emotional well-beingb | −0.27** | −0.46*** | −0.34** |
| Depressionc | 0.48** | 0.55*** | 0.54** |
| State anxietyd | 0.32† | 0.38* | 0.34† |
Short-Form McGill Pain Questionnaire; higher scores indicate more pain.
RAND 36-item Health Survey 1.0; higher scores indicate better health.
Center for Epidemiologic Studies-Depression Scale; higher scores indicate more depression.
State-Trait Anxiety Inventory; higher scores indicate more anxiety.
p < .10.
p < .05.
p < .01.
p < .001.
Discussion
In addressing the first aim of this pilot study to compare initial estimates of treatment effects, we found that affective pain and physical functioning improved in the treatment group compared with the control group. Physical functioning was particularly important for these community-dwelling older adults. Although these changes were not statistically significant, the trend was in the direction supporting the intervention. We also observed correlations showing especially strong relationships between severity of affective pain and measures of physical and emotional health.
Our question about the feasibility of the treatment for a diverse sample was addressed by the response of potential participants to the study, successful enrollment of those meeting study criteria, and high compliance (97.1%) with the study protocol. We believe we would not have seen this rate if the treatment had not been acceptable to the participants in the treatment group. Additional support was indicated by 12 of the 14 control-group participants (85.7%) choosing to continue in the crossover phase for an additional six weeks to receive the treatment.
It is possible that the six-week length of time for practicing meditation may not have been long enough for participants to experience the full benefits of meditation. Due to the high degree of adherence reported by participants to the six-week protocol in the pilot study, we anticipate that they would be able to adhere to an eight-week protocol, which is the standard length of time seen in MBSR programs.53 Relatedly, the high degree of adherence reported by participants for daily diary completion and follow up evaluations further supports the continued use of these tools in larger studies.
Limitations
Participants who reported pain related to other conditions (n = 14) might not have differentiated their PHN pain from their other pain, which could have affected their ratings. Medications taken while enrolled in the study, which were not recorded, may have affected daily ratings and responses to questionnaires. It is not known what effect investigator attention or daily diary ratings may have had on the responses to the questionnaires. Receptivity to the meditation practice could have been affected by these daily assessments, and questionnaire responses by both groups may have been affected by the daily diary recordings.54 Although randomization and holding nontreatment conditions constant for all participants (i.e., three testing times, weekly phone calls) were ways to enhance internal validity in our study, external validity issues were not addressed by our study design. In other words, generalizability of the findings to individuals who are not contacted weekly or keeping daily diaries is likely not possible.
Implications and Recommendations
Geriatric clinical nurse specialists and all nurses who work with older adults should take note of the severity of this painful condition, as confirmed by the reported pain levels in this sample of individuals with postherpetic neuralgia. Thorough pain assessment is especially important in nonverbal or cognitively impaired older adults with a history of shingles.
Study results support several suggestions for future research in this area. Acceptance of and adherence to this intervention was high, indicating that a larger study with sufficient sample size would be feasible and could yield clearer, more conclusive results. Effect size measures noted in this study could also be used to inform power analyses for such studies. Increasing the length of time for the treatment from six weeks to at least eight weeks in a future study is also recommended.
The pilot study showed that mindfulness meditation was acceptable to a diverse sample of older adults, with Hispanics comprising 40% of the study enrollment. This is an important consideration for replicating this intervention and focusing on this group because Hispanic older adults comprise one of the most rapidly growing segments of our aging population.55 As a CAM intervention, meditation offers a relatively inexpensive, nonpharmacologic complementary therapy for managing pain related to PHN. Future studies should specifically include data about type and amount of medication used for pain while enrolled in the study. This would provide additional objective data in evaluating the effects of meditation for pain management. An intervention study, with a larger sample size focusing on an older population living in the community, could contribute to the literature base supporting meditation for pain relief. It could provide specific guidelines for other nurses interested in the use of meditation as a clinical intervention for PHN.
In addition to mindfulness meditation, future studies could include the comparison of a variety of meditative practices (e.g., yogic, mantrum, tai chi) alone or in conjunction with the usual teaching and practice of meditation within a structured 8 to 10 week program. This would help determine which practice and delivery mechanisms might be most acceptable to and cost effective for older adults in pain. Studies could also include homebound older adults in pain or those in long term care facilities.
Results of this pilot study will support a follow up randomized clinical trial examining meditation in adults with postherpetic neuralgia and related conditions. Based on participant acceptability and adherence to all components of this feasibility study, modifications will be made to allow for sufficient time for the actual meditative practice (i.e., having a minimum of 15 minutes daily for 8 weeks before post test measurement of variables). Statistically significant findings in future studies will lend support to the incorporation this nonpharmacologic approach into the plan of care for the management of chronic pain in older adults.
Acknowledgments
The investigators thank the staff at the UNM Center for Life for assistance with scheduling, and Anne Mattarella, MA, for her editorial assistance. This project was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000041.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKendrick MW, Ogan P, Care CC. A 9 year follow up of post herpetic neuralgia and predisposing factors in elderly patients following herpes zoster. J Infect. 2009;59:416–420. doi: 10.1016/j.jinf.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Eng J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 5.Christo PJ, Hobelmann G, Maine DN. Evidence-based herpetic neuralgia in older adults. Drugs Aging. 2007;24:1–19. doi: 10.2165/00002512-200724010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Schmader KE, Harpaz R, Oxman MN. Current treatment and future strategies for herpes zoster and postherpetic neuralgia. Ann Longterm Care Clin Care Aging Clin Geriatr. 2007;15(9) suppl B [Google Scholar]
- 7.Young MK, Wood M, Jean-Noel N. Post herpetic neuralgia in older adults: culture, quality of life, and the use of alternative/complementary therapies. Holist Nurs Pract. 2007;21:124–134. doi: 10.1097/01.HNP.0000269149.80978.15. [DOI] [PubMed] [Google Scholar]
- 8.Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract. 2011;11:397–402. doi: 10.1111/j.1533-2500.2010.00432.x. [DOI] [PubMed] [Google Scholar]
- 9.Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–1736. doi: 10.1503/cmaj.091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmader KE, Sloane R, Pieper C, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RW, McElhaney J. Postherpetic neuralgia in the elderly. Int J Clin Pract. 2009;63:1386–1391. doi: 10.1111/j.1742-1241.2009.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125–132. doi: 10.2147/JPR.S57242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3, suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruckenthal P, Barkin RL. Options for treating postherpetic neuralgia in the medically complicated patient. Ther Clin Risk Manag. 2013;9:329–340. doi: 10.2147/TCRM.S47138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampathkumar L, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–280. doi: 10.4065/84.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zin CS, Nissen LM, Smith MT, et al. An update on the pharmacological management of post-herpetic neuralgia and painful diabetic neuropathy. CNS Drugs. 2008;22:417–442. doi: 10.2165/00023210-200822050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Flossos A, Kostakou C. A review of postherpetic neuralgia. [Cited November 4, 2013];Internet J Pain Symptom Control Palliat Care. 2006 4 Available at: http://web.ebscohost.com/ehost/detail?vid=6&sid=7f65863b-074a-49f2-a23c-ba1914186beb%40sessionmgr13&hid=1&bdata=JnNpdGU9ZWhvc3QtbGl2ZQ%3d%3d#db=ccm&AN=2009274896. [Google Scholar]
- 19.Johnson RW, Bouhassira D, Kassianos G, et al. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:1–13. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinke T, Edte A, Schmitt S, et al. Impact of herpes zoster and post-herpetic neuralgia on patients’ quality of life: a patient-reported outcomes survey. J Public Health. 2010;18:367–374. doi: 10.1007/s10389-010-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs Aging. 2012;29:863–869. doi: 10.1007/s40266-012-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morone NE, Rollman BL, Moore CG, et al. A mind-body program for older adults with chronic low back pain: results of a pilot study. Pain Med. 2009;10:1395–1407. doi: 10.1111/j.1526-4637.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui R, Cheng A, Chiu M, et al. Integrative approach to the treatment of postherpetic neuralgia: a case series. Altern Med Rev. 1999;4:429–435. [PubMed] [Google Scholar]
- 24.Hui F, Boule E, Vayda E, et al. A randomized controlled trial of a multifaceted integrated complementary-alternative therapy for chronic herpes zoster-related pain. Altern Med Rev. 2012;17:57–68. [PubMed] [Google Scholar]
- 25.Chiesa A, Serretti A. Mindfulness-based treatments for chronic pain: a systematic review of the evidence. J Altern Complement Med. 2011;17:83–93. doi: 10.1089/acm.2009.0546. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 27.Sephton SE, Salmon P, Weissbecker I, et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis Rheum. 2007;57(1):77–85. doi: 10.1002/art.22478. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig S, Greeson JM, Reibel DK, et al. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Karp JF, Shega JW, Morone NE, et al. Advances in understanding the mechanisms and management of persistent pain in older adults. Br J Anaesth. 2008;101:111–120. doi: 10.1093/bja/aen090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 31.Lush E, Salmon P, Floyd A, et al. Mindfulness meditation for symptom reduction in fibromyalgia: psychophysiological correlates. J Clin Psychol Med Settings. 2009;16:200–207. doi: 10.1007/s10880-009-9153-z. (2009). [DOI] [PubMed] [Google Scholar]
- 32.Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann Behav Med. 2006;32(3):227–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- 33.Texeira ME. Meditation as a treatment for chronic pain: an integrative review. Holist Nurs Pract. 2008;22:225–234. doi: 10.1097/01.HNP.0000326006.65310.a7. [DOI] [PubMed] [Google Scholar]
- 34.Goyal M, Singh S, Sibinga EMS, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donovan DD, Bogenschutz MP, Perl H, et al. Study design to examine the potential role of assessment reactivity in the Screening, Motivational Assessment, Referral, and Treatment in Emergency Departments (SMART-ED) protocol. Addict Sci Clin Pract. 2012;7:16. doi: 10.1186/1940-0640-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aaron LA, Turner JA, Mancl L, et al. Electronic diary assessment of pain-related variables: is reactivity a problem? J Pain. 2005;6(2):107–115. doi: 10.1016/j.jpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. J Clin Epidemiol. 2010;63:e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 39.Bellg AJ, Borrelli B, Resnick BR, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 40.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009;144:35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised Short-Form McGill Pain Questionnaire (SF-MPQ-2) J Pain. 2012;13(12):1250–1257. doi: 10.1016/j.jpain.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ware JE, Jr, Sherbourne CD, Davies A. The MOS 36-item short form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–481. [PubMed] [Google Scholar]
- 43.Tsai C, Bayliss MS, Ware JE. SF-36 ® Health Survey Annotated Bibliography: Second Edition. Boston, MA: Health Assessment Lab, New England Medical Center; 1997. (1988–1996). [Google Scholar]
- 44.Ware JE, Kosinski M, Keller SK. SF-36® Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 45.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 46.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 47.Grös DR, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Anxiety Inventory for Cognitive and Somatic Anxiety (STICSA): comparison to the State-Trait Anxiety Inventory (STAI) Psychol Assessment. 2007;19(4):369–381. doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- 48.Barnes LB, Harp D, Jung WS. Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Educ Psychol Meas. 2002;62(4):603–618. [Google Scholar]
- 49.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence: Erlbaum Associates, Inc; (1988). [Google Scholar]
- 51.SAS. Version 9.2. Cary, NC: SAS Institute Inc.; 2010. [computer program]. [Google Scholar]
- 52.Stata. Version 13.1. College Station, TX: StataCorp LP; 2012. [computer program]. [Google Scholar]
- 53.Carmody J, Baer RA. How long does a mindfulness-based stress reduction program need to be? A review of class contact hours and effect sizes for psychological distress. J Clin Psychol. 2009;65(6):627–638. doi: 10.1002/jclp.20555. [DOI] [PubMed] [Google Scholar]
- 54.McCambridge J, Butor-Bhavasr K, Wittonz J, Elbourne D. Can research assessments themselves cause bias in behavior change trials? A systematic review of evidence from Solomon 4-group studies. PLoS ONE. 2011;6(10):e25223. doi: 10.1371/journal.pone.0025223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent GK, Velkoff VA. The next four decades. The older population in the United States: 2010 to 2050. Washington, DC: US Department of Commerce; 2010. [Google Scholar]


