Abstract
Background
With apparent declines in malaria worldwide during the last decade and more widespread use of malaria rapid diagnostic tests, healthcare workers in low-resource areas face a growing proportion of febrile patients without malaria. We sought to describe current knowledge and identify information gaps of the etiology severe febrile illness in low-and middle-income countries.
Methods and Findings
We conducted a systematic review of studies conducted in low-and-middle income countries 1980–2013 that prospectively assessed consecutive febrile patients admitted to hospital using rigorous laboratory-based case definitions. We found 45 eligible studies describing 54,578 patients; 9,771 (17.9%) had a positive result for ≥1 pathogen meeting diagnostic criteria. There were no eligible studies identified from Southern and Middle Africa, Eastern Asia, Oceania, Latin American and Caribbean regions, and the European region. The median (range) number of diagnostic tests meeting our confirmed laboratory case definitions was 2 (1 to 11) per study. Of diagnostic tests, 5,052 (10.3%) of 49,143 had confirmed bacterial or fungal bloodstream infection; 709 (3.8%) of 18,142 had bacterial zoonosis; 3,488 (28.5%) of 12,245 had malaria; and 1,804 (17.4%) of 10,389 had a viral infection.
Conclusions
We demonstrate a wide range of pathogens associated with severe febrile illness and highlight the substantial information gaps regarding the geographic distribution and role of common pathogens. High quality severe febrile illness etiology research that is comprehensive with respect to pathogens and geographically representative is needed.
Introduction
Fever is a common reason for seeking healthcare in low- and middle-income countries (LMICs) [1]. Among patients with febrile illness requiring admission case fatality ratios are high, sometimes exceeding 20% [2–6]. Fever etiology research [4,7,8] and the more widespread use of malaria diagnostic tests following changes to malaria treatment guidelines [9,10] have highlighted the problem of malaria over-diagnosis among patients with severe febrile illness. Apparent declines in malaria illnesses and deaths associated with malaria control efforts mean that the proportion of febrile patients with malaria has declined over the past decade [11,12].
While the global burden of disease due to diarrhea and pneumonia has been estimated at the syndrome level [13–15], such an approach has not been taken for fever without localizing features. Instead, illness and death due to some febrile diseases (e.g., dengue, malaria) are estimated [11,16], while others have been neglected (e.g., leptospirosis, Q fever). Comprehensive, standardized, and high quality, multi-center etiology research is being undertaken to understand the causes of severe childhood diarrhea and pneumonia [13,14] but such an approach has not been taken for fever. The many causes of fever are difficult to distinguish clinically [4,7,8] and laboratory services may be limited or absent in low-resource areas [17]. Consequently, clinicians frequently lack information about the local epidemiology of causes of severe febrile illness needed to adapt international management guidelines. Similarly disease control programs lack data to set priorities for prevention.
A robust contemporary picture of treatable and preventable infectious causes of severe febrile illness is urgently needed to improve patient outcomes and to inform disease control efforts in LMICs. Systematic reviews of studies of community-acquired bloodstream infections in Africa [18] and Asia [19] have demonstrated the importance invasive infections among febrile inpatients. A study mapping studies of the aetiology of non-malarial febrile illness in South East Asia [20] highlighted the diversity and geographical variation in a range of causes of fever. It also revealed the substantial information gaps that remain for a range of relevant pathogens.
To describe epidemiologic patterns and to identify data gaps in our understanding of severe febrile illness in low resource areas, we sought to systematically review prospective hospital-based studies of the etiology of febrile illness in LMICs.
Methods
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21,22].
Geographic and human development classification of countries
Countries were categorized into areas and regions according to the United Nations Population Division classification (Table 1) [23]. From each region, low- and middle-income countries were selected according to the 2012 Human Development index (HDI) [24].
Table 1. Etiology of severe febrile illness in low- and middle-income countries systematic review search terms.
| Geographic terms | Etiology terms | |||
|---|---|---|---|---|
| Area | Region | Country | Group | Pathogen (disease) |
| Africa | Eastern Africa | Burundi | Bacterial | (‘blood stream infections’/ ‘blood stream pathogens’/bacteremia/ bacteremia/septicemia/septicaemia fever/sepsis/’septic shock’) |
| Comoros | Bacterial zoonoses | ‘Anaplasma phagocytophilum’/ (anaplasmosis) | ||
| Djibouti | ‘Bartonella bacilliformis’/ (‘Carrión's disease’)/’Bartonella henselae’/ (‘cat scratch disease’)/’Bartonella Quintana’/ (‘trench fever’) | |||
| Eritrea | Borrelia /(borreliosis) | |||
| Ethiopia | Brucella/(brucellosis) | |||
| Kenya | Coxiella/ (‘acute Q fever’) | |||
| Madagascar | Ehrlichia/(ehrlichiosis) | |||
| Malawi | Leptospira/ (leptospirosis) | |||
| Mozambique | ‘Neorickettsia sennetsu’ | |||
| Rwanda | ‘Orientia tsutsugamushi’/(‘scrub typhus’) | |||
| Somalia | Rickettsia/ (murine typhus/’Spotted fever group rickett*/’Typhus group rickett*‘ | |||
| Seychelles | ||||
| Tanzania/’United Republic of Tanzania’ | Fungal | ‘Coccidioides immitis’/ (fungemia/mycoses/coccidiodomycosis) | ||
| Uganda | ‘Cryptococcus neoformans’ (fungemia/mycoses/cryptococcosis) | |||
| Zambia | Histoplasma/ (fungemia/mycoses/ histoplasmosis) | |||
| Zimbabwe | Candida/(fungemia/mycoses/ candidiasis/candidemia) | |||
| Middle Africa | Angola | ‘Blastomyces dermatitidis’/ (fungemia/mycoses/blastomycoses) | ||
| Cameroon | ||||
| ‘Central African Republic’ | Viral | Dengue/(‘dengue fever’ ‘dengue hemorrhagic fever’/’DF’/’DHF’) | ||
| Chad | ‘Chikungunya virus’/ (chikungunya) | |||
| Congo | ‘Yellow fever virus’/ (‘yellow fever’) | |||
| Congo/’Democratic Republic of the Congo’ | ‘West Nile virus’/ (‘West Nile’) | |||
| ‘Equatorial Guinea’ | Influenza/(‘human influenza’) | |||
| Gabon | ‘Measles virus’/(measles) | |||
| ‘Sao Tome and Principe’ | ||||
| Northern Africa | Egypt | Blood parasite | ‘Plasmodium falciparum’/’Plasmodium malariae’/ ‘Plasmodium vivax’/ (malaria) | |
| Morocco | ‘Babesia microti’/(babesiosis) | |||
| ‘South Sudan’ | ‘ Trypanosoma brucei rhodesiense’/’Trypanosoma brucei gambiense’ /’Trypanosoma cruzi’ /(‘African trypanosomiasis’) | |||
| Sudan | ‘Leishmaniasis donovani’ /(‘Visceral leishmaniasis’) | |||
| Tunisia | ||||
| Southern Africa | Botswana | |||
| Lesotho | ||||
| Namibia | ||||
| ‘South Africa’ | ||||
| Swaziland | ||||
| Western Africa | Benin | |||
| ‘Burkina Faso’ | ||||
| ‘Cape Verde’ | ||||
| ‘Cote d'Ivoire’ /’Ivory Coast’ | ||||
| Gambia | ||||
| Ghana | ||||
| Guinea | ||||
| ‘Guinea-Bissau’ | ||||
| Liberia | ||||
| Mali | ||||
| Mauritania | ||||
| Niger | ||||
| Nigeria | ||||
| Senegal | ||||
| ‘Sierra Leone’ | ||||
| Togo | ||||
| Latin America and the Caribbean | The Caribbean | ‘Dominican Republic’ | ||
| Haiti | ||||
| Central America | Belize | |||
| El Salvador | ||||
| Guatemala | ||||
| Honduras | ||||
| Nicaragua | ||||
| Panama | ||||
| South America | Bolivia | |||
| Guyana | ||||
| Paraguay | ||||
| Suriname | ||||
| Asia | South-Central Asia | Afghanistan | ||
| Bangladesh | ||||
| Bhutan | ||||
| India | ||||
| Kyrgyzstan | ||||
| Maldives | ||||
| Nepal | ||||
| Pakistan | ||||
| Tajikistan | ||||
| Turkmenistan | ||||
| Uzbekistan | ||||
| Eastern Asia | China | |||
| Mongolia | ||||
| South-Eastern Asia | Cambodia | |||
| Indonesia | ||||
| ‘Lao People's Democratic Republic’ | ||||
| Myanmar/Burma | ||||
| Philippines | ||||
| Thailand | ||||
| Timor-Leste | ||||
| Viet Nam | ||||
| Western Asia | Iraq | |||
| Jordan | ||||
| ‘State of Palestine’/ ‘Occupied Palestinian Territory’/ Palestine | ||||
| ‘Syrian Arab Republic’/ Syria | ||||
| Yemen | ||||
| Oceania | Melanesia | Fiji | ||
| ‘Papua New Guinea’ | ||||
| ‘Solomon Islands’ | ||||
| Vanuatu | ||||
| Micronesia | Kiribati | |||
| ‘Micronesia’/ ‘Federated States of Micronesia’ | ||||
| Polynesia | Samoa | |||
| Tonga | ||||
| Europe | Eastern Europe | |||
* Truncated term used.
Pathogens, diseases, and case definitions
Three investigators (NP, DRM, JAC) developed a list of pathogens and diseases associated with febrile illness in low- and middle-income countries (Table 1). Case definitions based on laboratory confirmation were used for each pathogen (Table 2).
Table 2. Case definitions for infections sought in systematic review of severe febrile illness in low- and middle-income countries.
| Group | Disease | Confirmed case definition |
|---|---|---|
| Blood and tissue parasites | Babesiosis | Blood film and identification; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens; NAAT |
| Malaria | Blood film and identification; rapid diagnostic test; NAAT | |
| Trypanosomiasis | Blood film and identification | |
| Visceral leishmaniasis | Tissue biopsy or aspirate and identification | |
| Invasive bacterial infections | Bloodstream infection | Blood culture and isolation; urine antigen testing for Streptococcus pneumoniae (adolescents and adults only) or Legionella pneumophila serogroup 1 |
| Invasive fungal infections | Fungemia | Blood culture and isolation |
| Blastomycosis | Fungal culture and isolation; antigen testing | |
| Candidosis | Fungal culture and isolation | |
| Coccidioidomycosis | Fungal culture and isolation; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens; NAAT | |
| Cryptococcosis | Fungal culture and isolation; antigen testing | |
| Histoplasmosis | Fungal culture and isolation; antigen testing of urine or serum; ELISA; NAAT | |
| Bacterial zoonoses | Anaplasmosis | Culture and isolation; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens; NAAT |
| Brucellosis | Culture and isolation; serology with ≥4-fold rise in MAT titer between acute- and convalescent-phase serum specimens | |
| Borreliosis | Culture and isolation; blood film; NAAT | |
| Cat scratch disease | Culture and isolation; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Carrión's disease | Culture and isolation; serology with ≥4-fold in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Ehrlichiosis | Culture and isolation; serology with ≥4-fold in reciprocal titer between acute- and convalescent-phase serum specimens; NAAT | |
| Leptospirosis | Culture and isolation; serology with ≥4-fold in MAT titer between acute- and convalescent-phase serum specimens; NAAT | |
| Q fever | Culture and isolation; serology with ≥4-fold rise in IFA titer between acute- and convalescent-phase serum specimens; NAAT | |
| Scrub typhus | Culture and isolation; serology with ≥4-fold rise in IFA titer between acute- and convalescent-phase serum specimens, NAAT | |
| Spotted fever group rickettsiosis | Culture and isolation; serology with ≥4-fold rise in IFA titer between acute- and convalescent-phase serum specimens; NAAT | |
| Typhus group rickettsiosis | Culture and isolation; serology with four-fold or greater rise in IFA titer between acute- and convalescent-phase serum specimens; NAAT | |
| Trench fever | Culture and isolation; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Viral infections | Dengue fever | Viral culture and isolation; NAAT; NS1; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens; |
| Chikungunya | Viral culture and isolation; NAAT; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Influenza | Viral culture and isolation; NAAT on nasopharyngeal and blood specimens; serology with ≥4-fold rise in HAI titer between acute- and convalescent-phase serum specimens | |
| Japanese B encephalitis | Viral culture and isolation; NAAT; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Measles | Viral culture and isolation; NAAT; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| West Nile virus disease | Viral culture and isolation; NAAT; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens | |
| Yellow fever | Viral culture and isolation; NAAT; serology with ≥4-fold rise in reciprocal titer between acute- and convalescent-phase serum specimens |
NAAT = Nucleic acid amplification test; MAT = microagglutination test; IFA = immunofluorescence assay; HAI = haemagglutination inhibition assay.
Search strategy and selection criteria
We searched three main databases: Ovid Medline, Scopus, and Web of Knowledge. The search included articles in all languages and was limited to articles investigating humans published from the year January 1980 through to July 2013. Search terms were identified and defined with the assistance of an academic liaison librarian (Sarah Gallagher) and are shown in Table 1. The search string combined the geography terms ‘country’ and etiology terms ‘pathogen’ or respective ‘disease’ (Table 1). For blood stream infections and rickettsial infections, only disease terms were searched without pathogen terms. Adjustments to the search strategy were made in accordance with the requirements of each database. Online translation tools were used to evaluate non-English titles, abstracts, and full text articles.
Title and abstract review
One investigator (NP) reviewed titles and abstracts of articles identified by the search strategy. Those that appeared to be prospective studies of consecutive febrile patients enrolled in the emergency department or inpatient service of hospitals in an LMIC during the time period 1980 through 2013 were selected for full-text review. We excluded those that appeared to be: review articles, editorials, behavioural studies, economic impact studies, animal studies, vaccine and drug trials, diagnostic evaluations’ case reports, studies of persons not living in countries of interest such as travellers, or studies of outbreaks or epidemics. References for full-text review were compiled in Endnote version X6 (Thomson Reuters, Philadelphia, PA, USA), after the removal of duplicates, all articles were sought locally and internationally.
Full-text review
Two investigators (DRM, JAC) reviewed full-text articles identified by the title and abstract review. When required, the third investigator (NP) served as tiebreaker, independently reviewing articles to resolve disagreement between the other two investigators. To be eligible for data extraction, full-text articles were confirmed to be prospective studies of consecutive febrile patients enrolled in the emergency department or inpatient service of hospitals in a low- or middle-income country during the time period 1980 through 2013. For the purposes of this review, febrile patients were defined as a person with a history of fever in the past 48 hours; an axillary temperature ≥37.5°C; or a rectal temperature ≥38.0°C. In addition, participants in such studies needed to be evaluated for at least one of the febrile diseases of interest using laboratory-confirmed case definitions (Table 2). We excluded studies of syndromes other than fever; studies of specific subgroups of febrile patients, such as HIV-infected persons; studies of health-care associated infections or studies where such infections could not be distinguished; and studies of outpatients or where outpatients and inpatients could not be distinguished.
Validity assessment
We ensured the validity of the review by adhering to the predefined selection criteria to allow comparison across individual studies. By creating pre-determined case definitions we sought to capture only confirmed cases of infection. However, some variation in microbiological techniques and interpretation of results was unavoidable. We did not exclude studies on the basis of incomplete description of laboratory techniques, blood culture contaminants isolated, or failure to report all pathogens that may have been isolated or identified.
Data extraction
The following data were extracted from each eligible study by one investigator (NP): geographical location of the healthcare facility; healthcare facility rurality; study time dates and duration; study inclusion and exclusion criteria including age range; diagnostic techniques for each infection; number of patients tested for each infection; number tested meeting case definition for each infection; use of additional tests (e.g., HIV serology). When available, we also recorded clinical diagnosis of patients; in-hospital fatality ratio; seasonal variation of pathogens; and pre-admission use of antimicrobials. For the purpose of this review, pediatric studies were defined as those that included patients aged from ≥28 days to <15 years. Studies with mixed populations of adults and children were analyzed as adult studies. Queries regarding data extraction were resolved by return to the original manuscript by three investigators (NP, DRM, JAC).
Statistical analysis
Following data extraction, infections were organized into four groups: blood parasites; bacterial and fungal bloodstream infections; bacterial zoonoses; and viral infections, as shown in Table 2. Data from all individuals in all studies were aggregated to compare prevalence of febrile diseases across studies and regions. Summary statistics were calculated for key variables. Analyses of associations between patient factors or clinical conditions (e.g., HIV infection) and specific febrile diseases were done for studies with data for both the pathogens and factors being assessed. Chi-squared test was used to establish significance of associations and values were expressed as odds ratios (ORs) calculated with STATA software version 13.0 (College Station, TX, USA).
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Search results
The online search completed on 28 September 2013 yielded 135,141 records of which 2,729 articles that appeared to be about febrile illness among humans in LMIC were selected. Of these 863 met criteria for full text review, 823 (95.4%) were available for full-text review of which, 45 (5.5%) were eligible (Fig 1).
Fig 1. PRISMA flow diagram of selection of reports, systematic review of etiology of severe febrile illness in low- and middle-income countries, 1980–2013.
Characteristics of studies and patients
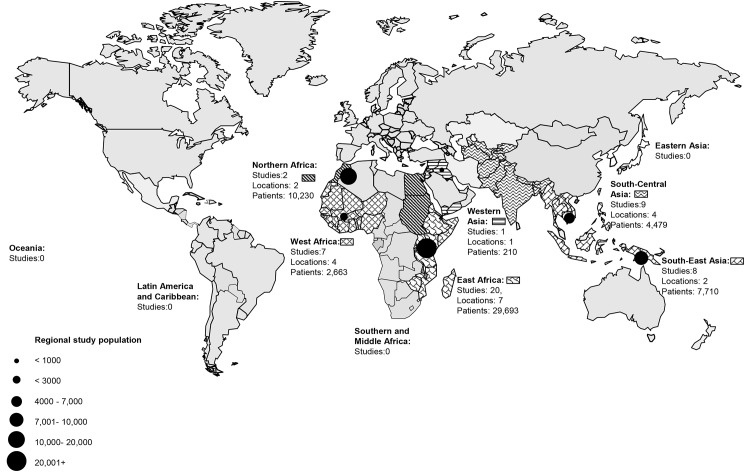
The 45 eligible studies were done in 22 locations and included 54,578 patients tested according to at least one laboratory-based case definition. Of all patients, 29,286 (53.7%) were from Eastern Africa; 10,230 (18.7%) from North Africa; 2,663 (4.9%) from Western Africa; 4,479 (8.2%) from South Central Asia; 7,710; (14.1%) from South East Asia; and 210 (0.4%) from Western Asia. There were no eligible studies identified from Southern and Middle Africa, Eastern Asia, Oceania, Latin American and Caribbean regions, and the European region (Fig 2).
Fig 2. Febrile illness etiology study locations by United Nations population division regions in low- and middle-income countries, 1980–2013.
Infections searched meeting laboratory case definitions
Of the 25 febrile illnesses searched for in this review (Table 2), 16 (64.0%) were investigated according to our predetermined laboratory case definitions by at least one eligible study. Of studies, 22 (48.9%) investigated a single cause of febrile illness according to our selection criteria and laboratory case definitions.[5,6,25–43] The median (range) number of diagnostic tests meeting our confirmed laboratory case definitions was 2 (1 to 11) per study. Of the 45 eligible studies, 8 (17.8%) studies did not meet our inclusion criteria for all of the infectious diseases investigated in the study, with results for those diseases excluded from our analysis (Table 3) [3,25,29,30,36,44–46].
Table 3. Summary of 45 eligible studies of etiology of severe febrile illness in low- and middle-income countries, 1980–2013.
| First Author (Reference) | Location; study dates | Total no. of patients in study | Hospital type | Age (population type) | Diagnostic tests conducted | N (%) of diseases searched in review investigated in study | Patients (%) with confirmed infection | Patients infected with HIV (proportion of patients tested) | Most common pathogens |
|---|---|---|---|---|---|---|---|---|---|
| Eastern Africa | |||||||||
| Aarsland, S. J. et al[44] | Ethiopia, December 2009—January 2010 | 102 | Urban referral hospital. | 1 month –18 years. Primarily children. | DNA extraction and NAAT from malaria blood smears for S. pneumoniae, Salmonella spp, Rickettsia spp, Borrelia spp, Leptospira spp. (NAAT for Salmonella and S.pneumoniae did not meet case definitions) | 3 (12.0%) | 12 (11.8%) with positive NAAT* | Plasmodium spp, Rickettsia spp, Borrelia spp* | |
| Archibald, L. K., et al[2] | Tanzania; February 1995-April 1995 | 517 | Urban referral hospital | >15 years. | Blood culture. Thick and thin blood smears | 2 (8.0%) | 145 (28.9%) positive blood culture. 49 (9.8%) malaria slide positive | 282 (56.2%) | Mycobacterium tuberculosis, Non-typhoidal Salmonella, S.aureus, |
| Archibald, L. K., et al[56] | Malawi; July 1998—August 1998 | 229 | Urban referral hospital. | 1 month–13 years | Blood culture. Thick and thin blood smears | 2 (8.0%) | 35 (15.3%) positive blood culture. 13 (5.7%) malaria slide positive | 63 (28%) | Non-typhoidal Salmonella, E.coli, Acinetobacter |
| Bell, M., et al[47] | Malawi; March 1998—May 1998 | 238 | Urban referral hospital. | >14 years. Primarily adults | Blood culture (mycobacteria), Thick and thin blood smears | 2 (8.0%) | 67 (28.2%) positive blood culture. 72 (31.2%) malaria slide positive | 173 (75.9%) | Non-typhoidal Salmonella, Mycobacterium tuberculosis, Cryptococcus neoformans |
| Christopher, A., et al[48] | Tanzania; September 2011—Feb 2012 | 317 | Urban referral hospital. | 2–60 months | Blood culture. Thick and thin blood smears | 2 (8.0%) | 21 (6.6%) positive blood culture. 82 (25.9%) malaria slide positive | Plasmodium falciparum, E.coli, Klebsiella spp. | |
| Dougle, M., et al[51] | Kenya; July 1994—October 1994 | 228 | Urban referral, teaching hospital. | > 5 years. Primarily adults | Blood culture. Thick and thin blood smears | 2 (8.0%) | 51 (22.4%) positive blood culture. 25 (11.0%) malaria slide positive | 51 (22.5%) | S enterica serotype Typhi, S.pneumoniae, Non-typhoidal Salmonella |
| Gordon, M. A., et al. [5] | Malawi; December 1997—November 1998 | 9,298 | Urban referral teaching hospital. | Unspecified. Primarily adults | Blood culture | 1 (4.0%) | 449 (16.1%) positive blood culture | Non-typhoidal Salmonella, S.pneumoniae, E.coli | |
| McDonald, L. C., et al[35] | Malawi; August—September 1997 | 128 | Urban referral hospital (Malawi) | > 18 years | Mycobacterial blood culture | 1 (4.0%) | 14 (10.9%) positive blood culture | 101 (78.9%) in Malawi. | Mycobacterium tuberculosis |
| Meremo, A., et al[52] | Tanzania; June 2011—December 2011 | 346 | Urban tertiary referral hospital. | Unspecified. Primarily adults | Blood culture | 1 (4.0%) | 33(9.5%) positive blood culture | 156 (45.0%) | Non-typhoidal Salmonella, S.pneumoniae, E.coli |
| Nadjm, B., et al[53] | Tanzania; July 2006—May 2007 | 3,639 | District, rural hospital | 2 months—13 years. | Blood culture, malaria rapid diagnostic test, thick and thin blood smears | 2 (8.0%) | 341 (9.4%) positive blood culture. 2195 (60.3%) malaria slide positive | 142 (3.9%) | Non-typhoidal Salmonella |
| Petit, P. L. C., et al[54] | Kenya, 1990 | 336 | Study 1 urban and referral | > 8 years. Primarily adults | Blood culture, thick and thin blood smears | 2 (8.0%) | Only study 1–104 (30.9%) positive BSI. 25 (7.4%) malaria slide positive | 12 (3.6%) | Plasmodium spp, Salmonella spp, E.coli |
| Sigaúque, B., et al[40] | Mozambique; May 2001- April 2006 | 18,944 | Rural district hospital | <15 years | Blood culture. Thick and thin blood smears (Blood smears included neonates) | 2 (8.0%) | 1395 (7.4%) true positive blood culture. 9939 (52.5%) with malaria slide positive | Non-typhoidal Salmonella, S.pneumoniae, E.coli, | |
| Ssali, F. N., et al[6] | Uganda; January 2007—April 2007 | 299 | Urban referral, hospital | >15 years. | Blood culture (mycobacterial) | 1 (4.0%) | 71 (23.7%) positive blood culture | 228 (76.3%) | Mycobacterium tuberculosis, S.pneumoniae |
| Strøm, G. E. A[41] | Tanzania; January 2009- June 2009 | 304 | Urban referral hospital. | 1 month- 7 years. | Thick and thin blood smears, malaria rapid diagnostic test, NAAT | 1 (4.0%) | 76 (25.0%) NAAT positive for malaria | Plasmodium falciparum | |
| Lofgren, S. M., et al[34] | Tanzania; August 2007—September 2008 | 628 | Urban referral medical center and Regional hospital. | >13 years. Primarily adults | Histoplasma urine antigen testing | 1 (4.0%) | 7 (1.1%) positive for histoplasmosis | Histoplasma spp | |
| Crump, J. A., et al[4] | Tanzania; September 2007—August 2008 | 870 | Urban referral hospital. | Children (>2 years <13 years) Adults >13 years | Blood culture, thick and thin blood smears. Cryptococcal, S.pneumoniae, H.capsulatum antigen testing. Leptospirosis/ Brucellosis standard microscopic reciprocal test (MAT). Acute and convalescent serological investigation for Q fever and Spotted and typhus fever group rickettsiosis. NAAT for DENG, CHIKV and flavivirus RNA | 11 (44.0%) | Q fever (n = 24; 5.0%) spotted fever rickettsiosis (n = 36; 8.0%) typhus group rickettsiosis (n = 2; 0.4%) chikungunya (n = 55; 7.9%) brucellosis (n = 16; 3.5%) leptospirosis (n = 40; 8.8%) | Chikungunya virus, Leptospira, Rickettsial spp, | |
| Crump, J. A., et al[49] | Tanzania; September 2007—August 2008 | 403 | Urban referral hospital. | >13 years. Primarily adults | Blood culture (mycobacteria), Thick and thin blood smears | 2 (8.0%) | 104 (25.8%) positive blood culture. 8 (2.0%) with malaria slide positive | 161(39%) | S enterica serotype Typhi, S.pneumoniae, E.coli, Mycobacterium tuberculosis |
| Crump, J. A., et al[49] | Tanzania; September 2007—August 2008 | 467 | Urban referral hospital. | >2 years <13 years | Blood culture. Thick and thin blood smears | 2 (8.0%) | 20 (4.3%) positive blood culture. 6 (1.3%) malaria slide positive | 57 (12.2%) | S enterica serotype Typhi, S.pneumoniae, E.coli, Plasmodium spp |
| Western Africa | |||||||||
| Akpede, G. O., et al[55] | Benin; October 1988—October 1989 | 642 | Urban referral hospital. Children's emergency room | 1 month-5 years | Blood culture. Thick and thin blood smears | 2 (8.0%) | 24 (3.7%) positive blood culture. 403 (62.8%) malaria slide positive | Plasmodium spp, S.aureus | |
| Akpede, G. O., et al[60] | Benin; October 1988—October 1989 | 156 | Urban referral hospital. Children's emergency room | 1 month-5 years | Blood culture. Thick and thin blood smears | 2 (8.0%) | 67 (42.9%) positive blood culture. 116 (74.4%) malaria slide positive | Plasmodium spp, S.aureus, Citrobacter spp | |
| Ayoola, O. O., et al[61] | Nigeria; June 1998—November 1998 | 102 | Urban referral hospital. Children's emergency room | 1–12months | Blood culture. Thick and thin blood smears | 2 (8.0%) | 39 (38.2%) positive blood culture. 31 (30.4%) with malaria slide positive | Plasmodium spp, S.aureus, E.coli | |
| Baba, M., et al[45] | Nigeria, July 2008- December 2008 | 310 | Urban, referral, tertiary, teaching hospital | All age groups. Primarily adults | Thick and thin blood smears, Widal test. Plaque reduction neutralization tests for CHIK, YF, DENG, WNV (Did not meet case definitions for Widal and viral tests) | 1 (4.0%) | 49 (15.8%) malaria slide positive | Plasmodium spp | |
| Ki-Zerbo, G. A., et al[57] | Burkina Faso; January 1995—March 1995 | 183 | Teaching hospital | >15 years | Acute and convalescent serological investigation for Spotted and typhus group rickettsiosis and Q fever | 2 (8.0%) | 17 (5.5%) | Rickettsial spp (SFG) Rickettsial spp (TG) Coxiella spp | |
| Lekweiry, K. M., et al[33] | Mauritania; 2009–2010 | 301 | National hospital | 1 month -14 years | Thick and thin blood smears, NAAT for malaria | 1 (4.0%) | 105 malaria positive by NAAT | Plasmodium spp | |
| Obaro, S., et al[38] | Nigeria; September 2008—November 2009 | 969 | 7 hospitals | 2 months -5 years | Blood culture | 1 (4.0%) | 111 (11.5%) with positive blood cultures | S enterica serotype Typhi, Non typhoidal Salmonella, S. aureus | |
| North Africa | |||||||||
| Afifi, S., et al[26] | Egypt; 1999–2003 | 10,130 | Public infectious disease hospital | > 4 years. Primarily adults | Blood culture | 1 (4.0%) | 1005 (10.2%) with positive blood culture | Salmonella enterica serotype Typhi, Brucella spp, S.aureus | |
| Hyams, K. C., et al[62] | Sudan; Jan 1984—Feb 1984 | 100 | Urban hospital | > 12 years. Primarily adults | Blood culture, virology test- isolation and acute and convalescent serological investigation for DENV, YF, WNV, CHIK, thick and thing blood smears | 5 (5.0%) | 25 (25%) positive blood culture, 21(21%) virus isolation, 13 (13%) malaria slide positive | Dengue virus, Salmonella enterica serotype Typhi, Plasmodium spp | |
| South Central Asia | |||||||||
| Abbasi et al[25] | Pakistan; September 2007—January 2008 | 112 | Urban teaching hospital | > 13 years. Primarily adults | Thick and thin blood smears. Dengue viral specific immunoglobulin detection (Did not meet dengue case definition) | 1 (4.0%) | 26 (23.2%) malaria slide positive | Plasmodium spp | |
| Akram, D. S[63] | Pakistan; June 1994—September 1994 | 25 | Urban, Pediatric hospital | 1 month- 12 years | Acute and convalescent serology for dengue virus, West Nile virus, JEV | 3 (12.0%) | 10 (4%) serologically confirmed cases | Dengue virus, West Nile virus | |
| Blacksell, S. D., et al[46] | Nepal, Kathmandu; July 2002—June 2004 | 103 | Urban, referral, community general hospital | > 17 years | Blood culture. Serology for scrub typhus, murine typhus, leptospirosis, dengue. Included only for blood culture and paired acute and convalescent sera | 3 (12.0%) | 29 (28.1%) positive blood culture, 14 (13.5%) confirmed serology | Salmonella enterica serotype Typhi, Salmonella enterica Paratyphi A, R.typhi | |
| Chrispal, A., et al[29] | South India; January 2007—January 2008 | 398 | Tertiary care referral hospital | >16 years | Blood culture, thick and thin blood smears, serological testing for scrub typhus, Dengue virus, Leptospira spp, SFG rickettsiosis (did not meet serological case definitions) | 1 (4.0%) | 32 (8.0%) positive blood cultures, 68 malaria slide positive | Salmonella enterica serotype Typhi, Salmonella enterica Paratyphi A, Plasmodium spp | |
| Faruque, L. I[30] | Bangladesh; December 2008—November 2009 | 462 | Six tertiary level, teaching, referral hospital | Unspecified. Primarily adults | Malaria rapid diagnostic test. Serological testing for dengue virus (Did not meet dengue case definition) | 1 (4.0%) | 3 (0.6%) positive for malaria rapid diagnostic test | Plasmodium spp | |
| Kaushik, J. S., et al[32] | India; June 2008—December2008 | 1,680 | Urban tertiary, hospital | 1 month- 12 years | Thick and think blood films for malaria parasites | 1 (4.0%) | 38 (2.3%) malaria slide positive | Plasmodium spp | |
| Murdoch, D. R., et al[36] | Nepal, Kathmandu; Jan 2001—March 2001 and July—August 2001 | 876 | Urban, general hospital | >14 years old. | Blood culture, Urinary antigen testing, serological testing for IgM antibodies dengue virus, Leptospira spp, Scrub typhus and R.typhi (did not meet serological case definition) | 1 (4.0%) | 137 (15.6%) positive blood culture | Salmonella enterica serotype Typhi, Salmonella enterica Paratyphi A | |
| Pattanaik, Sarit S[39] | India; 2008–2009 | 67 | Teaching hospital | >15 years. | Blood culture, NAAT | 1 (4.0%) | No positive results | ||
| Zimmerman, M. D., et al[43] | Nepal, Kathmandu; Jan 2001—March 2001 and July—August 2001 | 756 | Urban, tertiary care hospital | >14 years old | R.typhi NAAT | 1 (4.0%) | 50 (6.6%) positive NAAT | R.typhi | |
| South East Asia | |||||||||
| Archibald, L. K., et al[27] | Thailand, Bangkok; February 1997—April 1997 | 246 | Urban, referral, infectious disease hospital. | >15 years. | Blood culture (mycobacterial) | 1 (4.0%) | 119 (48.4%) positive blood culture | C. neoformans, Mycobacterium tuberculosis, Non-typhoidal Salmonella | |
| Blair, P. J., et al[28] | Cambodia; December 2006—December 2008 | 4,233 | Two referral hospitals | > 2 years | Blood, throat and nasal specimen. rRT- NAAT, virus isolation, HI assay | 1 (4.0%) | 1151 (27.2%) with confirmed influenza | ||
| Chheng, K., et al[3] | Cambodia; October 2009—October 2010 | 1,193 | Urban, referral, government hospital. | < 16 years, neonates excluded | Blood culture. Thick and thin blood smear. Nucleic amplification test, serological testing for JEV, DENV), Acute and convalescent serological testing for R.typhi and Orientia tsutsugamushi, NAAT for Leptospira spp, nasal and throat specimen, rRT-NAAT for influenza (Did not meet case definitions for DENV and JEV) | 6 (24.0%) | 149 (12.5%) positive blood culture, 96 (8.0%) Orientia tsutsugamushi, 27 (2.2%) R.typhi, Influenza 25 (2.1%) 24(2.0%) malaria slide positive, 17 (1.4%) Leptospira spp | Orienta tsutsugamushi, S.aureus R.typhi | |
| McDonald, L. C., et al[35] | Thailand; February 1997—March 1997 and August—September 1997 | 216 | Urban, referral hospital in Thailand. | > 18 years | Mycobacterial blood culture | 1 (4.0%) | 20 (9.3%) positive blood culture | 154 (71.3%) in Thailand | Mycobacterium tuberculosis |
| Cohen, Adam L[58] | Thailand; February 2002—February 2003 | 704 | Four district rural hospitals | > 6 years. Primarily adults | Acute and convalescent serological examination for dengue virus, and Leptospira spp | 2 (8.0%) | 199 (28.3%) with confirmed serology | Dengue virus, Leptospira spp | |
| Kalayanarooj, S., et al[31] | Thailand; April 1994—December 1994 | 172 | One urban children's hospital. One rural provincial hospital | 6 months—14 years | Dengue virus isolation and acute and convalescent serological examination | 1 (4.0%) | 60 (34.9%) with confirmed serology | Dengue virus | |
| Wijedoru, L.P., et al[42] | Cambodia; April 2009—June 2009 | 134 | Children's hospital | > 1 year <16 years | Blood culture | 1 (4.0%) | 5 (3.7%) positive blood culture | Salmonella enterica serotype Typhi | |
| Libraty, D. H., et al[59] | Thailand; 1994–1999 | 812 | One urban children's hospital. One rural provincial hospital | 6 months–14 years | Acute and convalescent serological examination for Leptospira spp and dengue. | 2 (8.0%) | 468 (44.8%) with confirmed serology | Dengue virus, Leptospira spp | |
| Western Asia | |||||||||
| Nimri, L. F., et al[37] | Jordan; 1998–1999 | 210 | Urban pediatric teaching hospital. | 1 month—10 years | Blood culture | 1 (4.0%) | 94 (44.8%) positive blood culture | S.pneumoniae, E.coli, Klebsiella spp | |
*NAAT–Nucleic acid amplification test
*spp.—species
Bacterial and fungal bloodstream infections
Of the 45 eligible studies and 54,578 patients included in this review, blood cultures and antigen testing was conducted in 28 (62.2%) studies among 49,143 (90.0%) patients. All studies described the microbiological techniques used for blood cultures. However, the media used and methods of identification of organisms varied between studies. Minimum acceptable blood culture volumes were reported by 18 (64.3%) of 28 studies using blood cultures and ranged from 1 mL to 3mL in pediatric studies and from 5 mL to 10mL in adult studies. Of 23 studies reporting results of antimicrobial susceptibility testing, all used disc diffusion, or Epsilometer test (E-test) methods [2,3,5,26,27,35–38,46–54]. Organisms thought to be contaminants were reported as being excluded from analysis in 16 (57.1%) of the 28 studies. In six studies providing data from all positive blood cultures, contaminants were isolated from 36 (3.9%) of 920 adult blood cultures [2,50] and 107 (4.2%) of 2,550 pediatric blood cultures [3,49,51,55].
Of patients evaluated with blood culture or antigen testing, 4,852 (10.6%) were reported to have positive result. Table 4 provides a summary of the most common bloodstream isolates according to region and age group in eligible studies.
Table 4. Summary of eligible diagnostic tests and confirmed cases found according to region and age in all eligible studies, 1980–2013.
| Disease | Eastern Africa (n = 29,286) | North Africa (n = 10,230) | Western Africa (n = 2,663) | South Central Asia (n = 4,479) | South East Asia (n = 7,710) | Western Asia | Paediatric (All Regions n = 30,295) | All Regions (n = 54,578) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | Tested | Positive | |||||||||||||||||
| N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | N | (%) region | N | (%) tested | |
| Bacteria and fungal invasive infections (blood culture) | 28,752 | (98.1) | 2,988 | (10.4) | 10,230 | (100.0) | 1,030 | (10.1) | 1,869 | (70.2) | 241 | (12.8) | 1,046 | (23.4) | 166 | (15.9) | 1,573 | (20.4) | 247 | (15.7) | 210 | (100.0) | 94 | (44.8) | 27,001 | (89.1) | 2,282 | (8.5) | 43,680 | (80.0) | 4,766 | (10.9) |
| Gram positive | 748 | (2.6) | 81 | (0.8) | 101 | (5.4) | 9 | (0.9) | 61 | (3.9) | 26 | (12.3) | 693 | (2.6) | 1,026 | (1.9) | ||||||||||||||||
| Streptococcus pneumoniae * | 588 | (2.0) | 4 | (>0.1) | 8 | (0.4) | 2 | (1.2) | 20 | (1.3) | 433 | (1.6) | 622 | (1.4) | ||||||||||||||||||
| Staphylococcus aureus | 160 | (0.6) | 77 | (0.8) | 93 | (4.9) | 7 | (0.7) | 41 | (2.6) | 5 | (2.4) | 260 | (0.9) | 383 | (0.9) | ||||||||||||||||
| Gram negative | 1,457 | (5.0) | 788 | (7.7) | 90 | (4.8) | 151 | (14.4) | 87 | (5.5) | 35 | (16.7) | 1,091 | (4.0) | 2,608 | (6.0) | ||||||||||||||||
| Salmonella enterica | 926 | (3.2) | 513 | (5.0) | 31 | (1.7) | 140 | (13.4) | 44 | (2.8) | 647 | (2.4) | 1,654 | (3.8) | ||||||||||||||||||
| Non-typhoidal Salmonella † | 810 | (2.8) | 0 | - | 8 | (0.4) | 17 | (1.1) | 588 | (2.1) | 835 | (1.9) | ||||||||||||||||||||
| S. enterica serotype Typhimurium | 267 | (0.9) | 267 | (0.6) | ||||||||||||||||||||||||||||
| S. enterica serotype Enteritidis | 121 | (0.4) | 121 | (0.3) | ||||||||||||||||||||||||||||
| Typhoidal Salmonella | 67 | (0.3) | 513 | (5.0) | 22 | (1.2) | 140 | (13.4) | 27 | (1.7) | 59 | (0.2) | 773 | (1.8) | ||||||||||||||||||
| S. enterica serotype Typhi | 63 | (0.2) | 508 | (5.0) | 22 | (1.2) | 75 | (7.2) | 27 | (1.7) | 4 | (1.9) | 59 | (0.2) | 614 | (1.4) | ||||||||||||||||
| S. enterica serotype Paratyphi A | 4 | (>0.1) | 5 | (>0.1) | 65 | (6.2) | 0 | - | 74 | (0.2) | ||||||||||||||||||||||
| Non-Salmonella Enterobacteriaceae | ||||||||||||||||||||||||||||||||
| Escherichia coli | 243 | (0.8) | 19 | (1.0) | 9 | (0.9) | 3 | (0.2) | 9 | (4.3) | 200 | (0.7) | 283 | (0.6) | ||||||||||||||||||
| Klebsiella spp | 39 | (0.1) | 11 | (0.6) | 6 | (0.4) | 8 | (3.8) | 32 | (0.1) | 64 | (0.1) | ||||||||||||||||||||
| Enterobacter spp | 16 | (0.1) | 2 | (0.1) | 1 | (0.1) | 2 | (1.0) | 16 | (0.1) | 20 | (>0.1) | ||||||||||||||||||||
| Citrobacter spp | 5 | (>0.1) | 2 | (0.1) | 4 | (>0.1) | 7 | (>0.1) | ||||||||||||||||||||||||
| Proteus mirabilis | 4 | (>0.1) | 4 | (0.2) | 1 | (>0.1) | 8 | (>0.1) | ||||||||||||||||||||||||
| Shigella spp | 6 | (>0.1) | 1 | (0.5) | 1 | (>0.1) | 7 | (>0.1) | ||||||||||||||||||||||||
| Other Gram negative | ||||||||||||||||||||||||||||||||
| Brucella spp ¶ | 1 | (>0.1) | 275 | (2.7) | 0 | - | 276 | (0.6) | ||||||||||||||||||||||||
| Haemophilus influenzae | 114 | (0.4) | 2 | (0.1) | 9 | (0.6) | 6 | (2.9) | 136 | (0.5) | 131 | (0.3) | ||||||||||||||||||||
| Neisseria meningitidis | 36 | (1.3) | 1 | (0.1) | 4 | (0.3) | 4 | (1.9) | 23 | (0.1) | 45 | (0.1) | ||||||||||||||||||||
| Acinetobacter spp | 17 | (0.6) | 14 | (0.7) | 3 | (0.2) | 17 | (0.1) | 34 | (0.1) | ||||||||||||||||||||||
| Pseudomanas spp | 19 | (0.7) | 4 | (0.2) | 4 | (0.3) | 1 | (0.5) | 14 | (0.1) | 28 | (0.1) | ||||||||||||||||||||
| Burkholderia spp | 1 | (>0.1) | 1 | (0.1) | 14 | (0.9) | 0 | - | 16 | (>0.1) | ||||||||||||||||||||||
| Yeasts ‡ | 50 | (0.2) | 0 | - | 11 | (0.7) | 5 | (>0.1) | ||||||||||||||||||||||||
| Cryptococcus spp | 43 | (0.1) | 10 | (0.6) | 3 | (>0.1) | 53 | (0.1) | ||||||||||||||||||||||||
| Histoplasma spp | 7 | (>0.1) | 1 | (0.1) | 2 | (>0.1) | 8 | (>0.1) | ||||||||||||||||||||||||
| Other | 783 | (2.7) | 161 | (15.6) | 50 | (2.7) | 6 | (0.6) | 99 | (6.3) | 33 | (15.7) | 493 | (1.8) | 1,132 | (2.6) | ||||||||||||||||
| Mycobacteria | 1,815 | (6.2) | 129 | (7.1) | 0 | - | 0 | - | 876 | (19.6) | 0 | - | 462 | (6.0) | 71 | (15.4) | 229 | (0.8) | 0 | - | 3,153 | (5.8) | 200 | (6.3) | ||||||||
| Mycobacterium tuberculosis complex | 127 | (7.0) | 47 | (10.2) | 0 | - | 174 | (5.5) | ||||||||||||||||||||||||
| Mycobacterium avium complex | 2 | (0.1) | 24 | (5.2) | 0 | - | 26 | (0.8) | ||||||||||||||||||||||||
| Bacteria and fungal invasive infections (antigen testing) | 0 | - | ||||||||||||||||||||||||||||||
| Streptococcus pneumoniae * | 403 | (1.4) | 17 | (4.2) | 0 | - | 0 | - | 876 | (19.6) | 51 | (5.8) | 0 | - | 0 | - | 1,279 | (2.3) | 68 | (5.3) | ||||||||||||
| Cryptococcus spp ‡ | 403 | (1.4) | 11 | (2.7) | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 403 | (0.7) | 11 | (2.7) | ||||||||||||||
| Histoplasma spp ‡ | 628 | (2.1) | 7 | (1.1) | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 628 | (1.1) | 7 | (1.1) | ||||||||||||||
| Bacterial zoonoses | ||||||||||||||||||||||||||||||||
| Borrelliosis | 102 | (0.4) | 2 | (2.0) | 0 | - | 0 | - | 0 | - | 0 | 0 | - | 0 | - | 102 | (0.2) | 2 | (2.0) | |||||||||||||
| Brucellosis ¶ | 453 | (1.5) | 16 | 0 | - | 0 | - | 0 | - | 0 | 0 | - | 246 | (0.8) | 5 | (2.0) | 453 | (0.8) | 16 | (3.5) | ||||||||||||
| Leptospirosis | 453 | (1.5) | 40 | (8.8) | 0 | - | 0 | - | 2,339 | (30.3) | 98 | (4.2) | 0 | - | 1,881 | (6.2) | 50 | (2.7) | 2,792 | (5.1) | 138 | (4.9) | ||||||||||
| Rickettsial infections** | 552 | (1.9) | 41 | (7.4) | 0 | - | 183 | (6.9) | 9 | (4.9) | 756 | (16.9) | 50 | (6.6) | 1,193 | (15.5) | 38 | (3.2) | 0 | - | 1,679 | (5.5) | 56 | (3.3) | 2,684 | (4.9) | 138 | (4.9) | ||||
| Spotted fever group | 450 | (1.5) | 36 | (8.0) | 183 | (6.9) | 7 | (3.8) | 0 | - | 243 | (0.8) | 18 | (7.4) | 633 | (1.2) | 43 | (6.8) | ||||||||||||||
| Typhus group | 450 | (1.5) | 2 | (0.4) | 183 | (6.9) | 2 | (1.1) | 756 | (16.9) | 50 | (6.6) | 1,193 | (15.5) | 27 | (2.3) | 1,436 | (4.7) | 27 | (1.9) | 2,582 | (4.7) | 89 | (3.3) | ||||||||
| Unspecified Rickettsia spp | 102 | (0.4) | 3 | (3.0) | 0 | 0 | - | 11 | (0.9) | 0 | - | 11* | (0.9) | 102 | (0.2) | 14 | (13.7) | |||||||||||||||
| Scrub typhus | 0 | - | 0 | - | 0 | - | 103 | (2.3) | 5 | (4.9) | 1,193 | (15.5) | 96 | (8.0) | 0 | - | 1,193 | (3.9) | 96 | (8.0) | 1,296 | (2.4) | 101 | (7.8) | ||||||||
| Q fever | 482 | (1.6) | 24 | (5.0) | 0 | - | 183 | (9.8) | 8 | (4.4) | 0 | - | 0 | 0 | - | 268 | (0.9) | 7 | (2.6) | 586 | (1.1) | 32 | (5.4) | |||||||||
| Blood parasites | ||||||||||||||||||||||||||||||||
| Malaria | 6,789 | (23.2) | 2,659 | (39.1) | 100 | (1.0) | 13 | (13.0) | 1,511 | (56.7) | 657 | (43.5) | 2,652 | (59.2) | 135 | (5.1) | 1,193 | (15.5) | 24 | (2.0) | 0 | - | 9,030 | (29.8) | 926 | (10.3) | 12,245 | (22.4) | 3,488 | (28.5) | ||
| Viral infections | ||||||||||||||||||||||||||||||||
| Influenza | 0 | - | 0 | - | 0 | - | 0 | - | 5,426 | (70.4) | 1,176 | (21.7) | 0 | - | 1,193 | (3.9) | 25 | (2.1) | 5,426 | (9.9) | 1,176 | (21.7) | ||||||||||
| Dengue | 700 | (2.4) | 0 | - | 100 | (1.0) | 21 | (21.0) | 0 | - | 25 | (0.6) | 9 | (36.0) | 1,688 | (21.9) | 542 | (32.1) | 0 | - | 1,341 | (4.4) | 419 | (31.2) | 2,513 | (4.6) | 572 | (22.8) | ||||
| West Nile | 700 | (2.4) | 0 | - | 100 | (1.0) | 0 | - | 0 | - | 25 | (0.6) | 1 | (4.0) | 0 | - | 0 | - | 332 | (1.1) | 0 | - | 825 | (1.5) | 1 | (0.1) | ||||||
| Chikungunya | 700 | (2.4) | 55 | (7.9) | 100 | (1.0) | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 332 | (1.1) | 34 | (10.2) | 800 | (1.5) | 55 | (6.9) | ||||||||
| Yellow fever | 700 | (2.4) | 0 | - | 100 | (1.0) | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 332 | (1.1) | 0 | - | 800 | (1.5) | 0 | - | ||||||||
| Japanese encephalitis | 0 | - | 0 | - | 0 | - | 25 | (0.6) | 0 | - | 0 | - | 0 | - | 0 | - | 25 | (>0.1) | 0 | - | ||||||||||||
* Streptococcus pneumoniae was tested by both blood culture and urine antigen testing, table number of patients tested using each method from each geographical region and positive results from each.
† Non-typhoidal Salmonella and Typhoidal Salmonella were not consistently described to species level, thus total reported number for each group was greater than sum of species. Brucella spp was tested by both blood culture and serological methods, table number of patients tested using each method from each geographical region and positive results from each.
‡ Yeasts (Cryptococcus spp, Histoplasma spp) were tested by both blood culture and antigen testing, table number of patients tested using each method from each geographical region and positive results from each.
** Rickettsia spp were not consistently described to species level, one study from South East Asia testing for typhus group rickettsiosis reported 11 unspecified Rickettsia spp. were identifie
Antimicrobial use before admission was assessed in 14 studies and 13,805 patients, and ranged from 9 (8.7%) of 103 to 111 (47.6%) of 233 [6,26,27,35,36,38,42,46–50,52,56]. Of nine studies evaluating the role of pre-admission antimicrobial exposure on blood culture positivity, five (55.5%) showed that pre-enrolment use of antimicrobials was not associated with a blood culture being positive [36,47,48,50,56]. One (11.1%) study showed a statistically significant increase in blood culture positivity [27], and two (22.2%) study identified fewer positive blood cultures in patients previously treated with antimicrobial drugs than in those who were not [42,49].
Bacterial zoonoses
Out of the 45 eligible studies with 54,578 patients, bacterial zoonoses were investigated in nine (20.0%) and included 14,773 (27.6%) patients. Of the 18,142 tests for seven bacterial zoonotic diseases (borreliosis, brucellosis, leptospirosis, spotted fever and typhus group rickettsiosis, scrub typhus, and Q fever) 702 (3.9% of tests) met the case definition (Table 4).
Of nine studies investigating bacterial zoonoses, six (66.7%) studies met our pre-defined testing criteria for rickettsial infection [3,4,43,44,46,57] and five (55.5%) studies met the pre-defined criteria for leptospirosis [3,4,44,58,59]. In one study from South East Asia investigating febrile children at a tertiary referral urban children’s hospital and a rural provincial hospital, of 14 confirmed leptospirosis cases, 10 (71.4%) were from patients at the rural hospital [59].
Malaria and other blood parasites
Of all studies included in this review, 24 (51.1%) reported the prevalence of malaria parasites identified by thick or thin smear, nucleic acid amplification test (NAAT), or rapid diagnostic test. No study reported the detection of blood parasites other than malaria.
Of all 54,578 patients in this review, 12,245 (22.4%) were enrolled in the 23 studies testing for malaria, of which 3,488 (28.5%) had a positive result according to our laboratory-based case definitions (Table 4) [2,3,25,29,30,32,33,44,45,47–56,60–62].
Of studies testing for malaria, 13 (54.2%) were conducted in Eastern Africa. Of the 12,245 patients tested for malaria, 10,535 (86.0%) were tested using thick or thin malaria blood smears only and one study tested 462 (3.8%) patients for malaria using rapid diagnostic tests only [30]. Three studies used two or more tests to diagnose malaria [41,44,53]. Among the 3,106 Plasmodium spp. that were identified to the species level, 2,928 (94.3%) were Plasmodium falciparum, of which 2,907 (99.3%) were found in the African regions. Plasmodium vivax accounted for 173 (5.6%) of speciated Plasmodium spp. Of the 92 Plasmodium spp. identified among patients in South Central and South East Asia 75 (81.5%) were Plasmodium vivax.
Viral infections
Of the 45 eligible studies with 54,578 patients in this review, viral infections were investigated according to our laboratory case definitions in eight (17.0%) studies including 7,939 (14.4%) patients using 10,389 tests for the six infections; chikungunya, dengue, influenza, Japanese encephalitis, West Nile virus infection, and yellow fever virus (Table 4).
Of viral infections, dengue fever was the most commonly assessed and was investigated using an eligible test in six (12.8%) studies; one (20.0%) each in Eastern Africa [4], North Africa [62], South Central Asia [63] and three (50.0%) studies in South East Asia [3]. In total 2,513 (4.6%) patients were tested for dengue fever using virus isolation, NAAT, or serology according to our case definitions.
HIV co-infection
Of the 15 studies with 9,365 patients that included HIV testing, 1,988 (21.2%) patients were found to be HIV seropositive. There were insufficient data in included studies to investigate the association between HIV and infections other than bacterial and fungal bloodstream infection. In nine studies with adequate data for analysis [2,6,27,35,47,49,52,64],1,667 (59.4%) of 2,805 patients with HIV infection had bloodstream infections versus 1,357 (52.8%) of 2,566 HIV-uninfected patients (OR 1.3, 95% CI = 1.2–1.5, p<0.0001) (Table 5).
Table 5. Causes of bloodstream infection by HIV serostatus in nine fever etiology studies in low- and middle-income countries, 1980–2013.
| Blood culture isolate | Total isolates (% with BSI) | Patients infected with HIV (% with BSI) | Patients not infected with HIV (% with BSI) | OR for those infected with HIV | ||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | OR | P-value | |
| Mycobacterium spp. | 241 | (39.0) | 237 | (38.3) | 4 | (0.6) | 44.5 | p<0.0001 |
| Streptococcus pneumoniae | 82 | (15.1) | 56 | (10.3) | 26 | (4.8) | 2.9 | p<0.0001 |
| Non-typhoidal Salmonella | 60 | (13.8) | 54 | (12.4) | 6 | (1.4) | 16.5 | p<0.0001 |
| Salmonella enterica Typhi | 34 | (10.8) | 2 | (0.6) | 32 | (10.1) | 0.12 | p<0.05 |
| Escherichia coli | 29 | (6.3) | 14 | (3.0) | 15 | (3.2) | NS | |
| Staphylococcus aureus | 25 | (5.6) | 13 | (2.9) | 12 | (2.7) | NS | |
| Total bloodstream infection | 3,024 | - | 1,667 | (55.1) | 1,357 | (44.9) | 1.3 | p<0.001 |
Clinical diagnosis
Five studies, four from Eastern Africa [4,44,47,51,53,56] and one from South Central Asia [46], provided sufficient data regarding both clinical and laboratory confirmed diagnoses for the cause of fever and enabled assessment the accuracy of clinical diagnosis. In Eastern Africa a clinical diagnosis of malaria was recorded in 800 (51.1%) of 1,566 patients of whom 85 (5.4%) had malaria parasites identified through laboratory diagnostic testing. In the study from South Central Asia 52 (50.5%) of 103 patients presenting with fever had a clinical diagnosis of enteric fever. Of the 52 patients with a clinical diagnosis of enteric fever 20 (38.5%) were found to have a positive blood culture for typhoidal Salmonella [46].
Concurrent infections
Seven (15.6%) out of the 45 eligible studies provided information regarding apparent concurrent infections. Of 5,719 patients enrolled in studies reporting such information, 198 (3.5%) were found to have both a positive blood culture for a pathogen and a positive malaria smear [2,48,52,53,55,60,61].
Moreover, one study in South Central Asia showed evidence for mixed infections of S. enterica serotype Typhi with scrub typhus or typhus group rickettsiosis [46].
In-hospital case fatality ratio
Of all 45 studies, 16 (35.6%) including 10,756 patients provided sufficient data regarding in-hospital case fatality ratio [4–6,26,27,32,36,40,41,47,48,53,55,56,61,62]. Of 10,756 patients, 1,307 (12.2%) patients died during their hospital stay. Cause of death information was provided in 12 studies [5,6,26,27,32,40,41,47,48,53,55,56,61].
Seasonal variation
Associations between seasons and the prevalence of febrile illnesses was reported in 11 studies [26,28,30,33,36,40,43,47,55,59]. Of the 47 eligible studies, 18 (38.3%) were conducted for one year or longer. The median (range) study duration was 6 (1 to 60) months.
Data from two Malawian cohorts showed a shift from a predominance of non-typhoidal Salmonella in blood cultures during the wet season to a predominance of S. pneumoniae during the dry season [5,47]. A Mozambique study found no association between season and bloodstream infections [40].
A study from Egypt suggested an association between the onset of the rainy season and a predominance of S. enterica serotype Typhi isolates. In the same study it was shown that brucellosis was reported in all months of the year with peaks in the spring and early summer, coinciding with the parturient seasons of domestic animals [26].
In Nepal S. enterica serotype Typhi and Paratyphi A were the most common bloodstream isolates during both monsoon and winter seasons. However, there was a substantial increase in the proportion of Salmonella Paratyphi A isolates during the monsoon season. In another study done in Nepal, murine typhus was more common during the winter season than the summer [43].
Vector-borne and zoonotic diseases such as dengue and leptospirosis were found to be more common during the rainy seasons in Mauritania and Thailand [33,59]. In Cambodia, influenza virus cases peaked during the rainy season [28].
Discussion
To our knowledge, this is the first systematic review of severe febrile illness etiology for a broad range of pathogens in all LMICs. We show that bacterial and fungal bloodstream infections, bacterial zoonoses, malaria, and viral infections are leading causes of severe febrile illness, and that their relative importance appears to vary by geographic region (Table 4). Our findings confirm that some infectious causes of fever are closely linked to HIV co-infection, that severe febrile illness is associated with high in-hospital case fatality ratios, that some pathogens show seasonal patterns, and that clinical diagnosis is unreliable among febrile patients, especially for pathogens causing systemic infections. Most notably, we demonstrate that there are major gaps in our current understanding of the causes of severe febrile illness in LMICs. Some potentially important pathogens have not been rigorously studied in any country, many studies examined only one or a few pathogens, many countries and some geographic regions had no eligible research on severe febrile illness etiology, and representation of age groups was inconsistent.
Bacterial and fungal bloodstream infections were the most sought febrile disease with a total of 30 (63.8%) out of 47 studies included in this review conducting blood cultures, 22 (47%) of which were conducted in the African continent. Overall, the proportion of severe febrile illness attributed to invasive bacterial and fungal infections was 10.7%, 8.5% among children and 13.9% among adults. Although our ability to examine geographic and age-related patterns of bloodstream infections was limited by incomplete representativeness of studies, some observations are possible. Salmonella enterica was the most common bloodstream isolate. Non-typhoidal Salmonella (NTS) predominated in all African regions, except for Northern Africa where Salmonella Typhi was more common. In Asian regions Salmonella Typhi and Salmonella Paratyphi A predominated. S. pneumoniae was the most common Gram-positive invasive infection in both African and Asian regions. S. pneumoniae was particularly common in paediatric cohorts in both areas accounting for 19.2% of bacteraemia in the African regions and 16.5% in the Asian regions. Fastidious organisms, such as S. pneumoniae, may be less often isolated than those without special growth requirements. This may have affected the relative prevalence of different species in our review.
Plasmodium spp. was the most commonly identified organism among patients with febrile illness overall. As expected, Plasmodium falciparum predominated in the African regions while Plasmodium vivax predominated in Asian regions. Malaria parasite and bacterial bloodstream co-infections were common among patients with positive malaria diagnostic tests especially in areas with year-round intense malaria transmission [2,40,48,51,53,55,60,61]. It is apparent that Plasmodium spp. may act as the prime pathogen; as a co-pathogen, increasing risk for other infections such as NTS bacteremia in some circumstances [65]; or as an bystander in others. In the latter, despite having a positive blood film the patient is suffering from another illness and the parasitemia, that should nonetheless also be treated, is incidental [66].
Studies that used an afebrile control group to calculate the fraction of febrile illness attributable to malaria indicate that incidental parasitemia is likely to be particularly common in malaria-endemic areas [2,47,64]. Such studies could be improved by including routine measurement of malaria parasite density. Furthermore, studies comparing clinical diagnosis with laboratory diagnosis of malaria confirmed that malaria over-diagnosis is common among patients with severe febrile illness. Incidental Plasmodium spp. infection and malaria over-diagnosis increase the risk of the patient not being treated for the actual cause of the current illness [4,8,44,47,51,56,64].
With respect to bacterial zoonoses and viral infections, large geographical areas had no or few studies, and few patients were evaluated for these infections. Those studies that did evaluate for bacterial zoonoses and viral infections varied widely in pathogens sought and diagnostic tests used. Many studies did not collect convalescent serum, precluding conventional standard diagnostic testing and therefore inclusion in our review. Among eligible studies, case fractions were found to be highly variable across regions and age groups and the small number of both studies and participants suggest that prevalence data should be interpreted with caution. Among eligible studies, spotted fever rickettsiosis predominated in African regions, with brucellosis being common in Northern Africa, while typhus group rickettsiosis, scrub typhus, and leptospirosis were particularly common in Asia. Among viral infections, dengue fever was found to be an important cause of febrile illness in Asia and was associated with a high case ratio.
No eligible studies were found from Latin America and the Caribbean, Oceania, some regions of Africa. There were a small number of studies from populous regions of Asia. Furthermore, the majority of LMICs did not have a single eligible study. In addition, we found that there was a limited amount of research in rural settings, despite the majority of countries searched in this review having predominantly rural populations [67]. Future research studies should improve geographic representativeness and include rural study sites.
This systemic review had a number of limitations. We included only studies of severe febrile illness that required admission to hospital emergency or inpatient departments. It is likely that patterns of infection could be quite different in outpatient, primary care, and community settings [68]. We included studies from 1980, just prior to the onset of the global HIV pandemic. We may have missed potentially relevant studies done in earlier years. It is also possible that patterns of infection in the 1980s and 1990s do not reflect the contemporary picture. Because we used conventional standard laboratory-based case definitions, some infections causing the most severe illness resulting in death before acquisition of a convalescent serum sample could not be ascertained. We did not collect data systematically on localised infections among febrile patients. However, such data was rarely reported in the studied included in this review.
Many studies did not enroll all age groups. This meant that age-related differences in severe febrile illness etiology could not be assessed. HIV infection appears to increase risk for a number of infections that may present with severe febrile illness, such as cryptococcal disease, bacteremic disseminated tuberculosis, and NTS bacteremia [2,6,27,35,47,49,52,64]. However, there were insufficient data to assess the role of HIV co-infection for a number of other pathogens evaluated in this study. Several studies found seasonal patterns with some pathogens. However, not all studies ran for a full year and others that did include at least a year of enrollment did not explore seasonality. Ideally, fever etiology research should include all age groups, at least one annual cycle, and routinely assess HIV infection status of participants. Furthermore, while we were restrictive with laboratory case definitions, we were unable to account for variability in some aspects of clinical and laboratory practice at study sites. Finally, data were aggregated by region by combining individual patient results across studies, resulting in a greater influence of larger studies.
We suggest that the current understanding of the etiology of severe febrile illness in LMICs is incomplete. High quality severe febrile illness etiology research that is comprehensive with respect to pathogens and geographically representative could improve patient outcomes by informing patient management guidelines and disease control priorities [69,70]. We recommend that multi-center severe febrile illness research should investigate a broad range of treatable or preventable infections; use standardized and quality assured diagnostic tests with rigorous case definitions; include healthy community controls to allow accurate estimations of attributable case fractions [71,72]; be geographically and demographically representative; have standardized reporting of fever associated with localized infections and should cover at least a full calendar year to incorporate seasonal variation. Such information is an essential component of an effective health system but the gaps in evidence identified by this study are likely to require coordinated resources and expertise to fill in LMICs.
Supporting Information
(XLS)
(DOC)
(DOC)
Acknowledgments
We thank Sarah Gallagher, Academic Liaison Librarian, Health Sciences Library, University of Otago, Dunedin, for assistance with the development of the search strategy and search process for this systematic review.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported in part by United States National Institutes of Health (NIH) grant NIH grant R01TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program; and by the United Kingdom Biotechnology and Biological Sciences Research Council (BBSRC) grant number BB/J010367. Crump also received support from and by United Kingdom BBSRC Zoonoses in Emerging Livestock Systems program (awards BB/L017679, BB/L018926, and BB/L018845). Reyburn was supported by the Bill & Melinda Gates Foundation through the ACT Consortium awarded to the London School of Hygiene and Tropical Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, et al. (2011) The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One 6: e16085 10.1371/journal.pone.0016085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Archibald LK, Den Dulk MO, Pallangyo KJ, Barth Reller L (1998) Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 26: 290–296. [DOI] [PubMed] [Google Scholar]
- 3. Chheng K, Carter MJ, Emary K, Chanpheaktra N, Moore CE, et al. (2013) A Prospective Study of the Causes of Febrile Illness Requiring Hospitalization in Children in Cambodia. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. (2013) Etiology of Severe Non-malaria Febrile Illness in Northern Tanzania: A Prospective Cohort Study. PLoS Negl Trop Dis 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, et al. (2001) Bacteraemia and mortality among adult medical admissions in Malawi—Predominance of non-typhi Salmonellae and streptococcus pneumoniae. J Infect 42: 44–49. [DOI] [PubMed] [Google Scholar]
- 6. Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, et al. (1998) A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol 19: 484–489. [DOI] [PubMed] [Google Scholar]
- 7. D'Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, et al. (2014) Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med 370: 809–817. 10.1056/NEJMoa1214482 [DOI] [PubMed] [Google Scholar]
- 8. Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, et al. (2004) Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ: British Medical Journal 329: 1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Acremont V, Bosman A (2013) WHO Informal Consultation on fever management in peripheral health care settings: a global review of evidence and practice World Health Organization. [Google Scholar]
- 10. World, Health, Organisation, (WHO) (2010) Guidelines for the treatment of malaria, 2nd edition: World Health Organization. [Google Scholar]
- 11.Newman RD (2012) World Malaria Report 2011.
- 12. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet 379: 413–431. [DOI] [PubMed] [Google Scholar]
- 13. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet 382: 209–222. [DOI] [PubMed] [Google Scholar]
- 14. Levine OS, O’Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, et al. (2012) The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis 54: S93–S101. 10.1093/cid/cir1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. (2013) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suaya JA, Shepard DS, Beatty ME (2007) Dengue: burden of disease and costs of illness. Scientific Working Group: Report on Dengue (Vol TDR/SWG/08), Geneva: World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases.
- 17. Archibald LK, Reller LB (2001) Clinical microbiology in developing countries. Emerg Infect Dis 7: 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy EA, Shaw AV, Crump JA (2010) Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10: 417–432. 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deen J, von Seidlein L, Andersen F, Elle N, White NJ, et al. (2012) Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis 12: 480–487. 10.1016/S1473-3099(12)70028-2 [DOI] [PubMed] [Google Scholar]
- 20. Acestor N, Cooksey R, Newton PN, Menard D, Guerin PJ, et al. (2012) Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review—terra incognita impairing treatment policies. PLoS One 7: e44269 10.1371/journal.pone.0044269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151: W65–94. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Population Divison Classification of Countries by major area and region of the world. In: Department of Economic and Social Affairs, editor.
- 24.United Nations Development Project (UNDP) (2012) Human Development Report 2013.
- 25. Abbasi A, Butt N, Sheikh QH, Bhutto AR, Munir SM, et al. (2009) Clinical Features, Diagnostic Techniques and Management of Dual Dengue and Malaria Infection. Jcpsp-Journal of the College of Physicians and Surgeons Pakistan 19: 25–29. [PubMed] [Google Scholar]
- 26. Afifi S, Earhart K, Azab MA, Youssef FG, El Sakka H, et al. (2005) Hospital-based surveillance for acute febrile illness in Egypt: A focus on community-acquired bloodstream infections. Am J Trop Med Hyg 73: 392–399. [PubMed] [Google Scholar]
- 27. Archibald LK, McDonald LC, Rheanpumikankit S, Tansuphaswadikul S, Chaovanich A, et al. (1999) Fever and human immunodeficiency virus infection as sentinels for emerging mycobacterial and fungal bloodstream infections in hospitalized patients ≥15 years old, Bangkok. J Infect Dis 180: 87–92. [DOI] [PubMed] [Google Scholar]
- 28. Blair PJ, Wierzba TF, Touch S, Vonthanak S, Xu X, et al. (2010) Influenza epidemiology and characterization of influenza viruses in patients seeking treatment for acute fever in Cambodia. Epidemiol Infect 138: 199–209. 10.1017/S095026880999063X [DOI] [PubMed] [Google Scholar]
- 29. Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JAJ, et al. (2010) Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in South India. Trop Doct 40: 230–234. 10.1258/td.2010.100132 [DOI] [PubMed] [Google Scholar]
- 30. Faruque LI (2012) Hospital-Based Prevalence of Malaria and Dengue in Febrile Patients in Bangladesh. The American journal of tropical medicine and hygiene 86: 58–64. 10.4269/ajtmh.2012.11-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, et al. (1997) Early clinical and laboratory indicators of acute dengue illness. J Infect Dis 176: 313–321. [DOI] [PubMed] [Google Scholar]
- 32. Kaushik JS, Gomber S, Dewan P (2012) Clinical and Epidemiological Profiles of Severe Malaria in Children from Delhi, India. Journal of Health Population and Nutrition 30: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lekweiry KM, Basco LK, Salem MSOA, Hafid JE, Marin-Jauffre A, et al. (2011) Malaria prevalence and morbidity among children reporting at health facilities in Nouakchott, Mauritania. Trans R Soc Trop Med Hyg 105: 727–733. 10.1016/j.trstmh.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 34. Lofgren SM, Kirsch EJ, Maro VP, Morrissey AB, Msuya LJ, et al. (2012) Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans R Soc Trop Med Hyg 106: 504–507. 10.1016/j.trstmh.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonald LC, Archibald LK, Rheanpumikankit S, Tansuphaswadikul S, Eampokalap B, et al. (1999) Unrecognised Mycobacterium tuberculosis bacteraemia among hospital inpatients in less developed countries. Lancet 354: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 36. Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, et al. (2004) The etiology of febrile illness in adults presenting to Patan Hospital in Kathmandu, Nepal. Am J Trop Med Hyg 70: 670–675. [PubMed] [Google Scholar]
- 37. Nimri LF, Rawashdeh M, Meqdam MM (2001) Bacteremia in children: Etiologic agents, focal sites, and risk factors. J Trop Pediatr 47: 356–360. [DOI] [PubMed] [Google Scholar]
- 38. Obaro S, Lawson L, Essen U, Ibrahim K, Brooks K, et al. (2011) Community Acquired Bacteremia in Young Children from Central Nigeria- A Pilot Study. BMC Infect Dis 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pattanaik SS, Tripathy R, Panda AK, Sahu AN, Das BK (2012) Bacteraemia in adult patients presenting with malaria in India. Acta Trop 123: 136–138. 10.1016/j.actatropica.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 40. Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, et al. (2009) Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28: 108–113. 10.1097/INF.0b013e318187a87d [DOI] [PubMed] [Google Scholar]
- 41. Strøm GEA, Haanshuus CG, Fataki M, Langeland N, Blomberg B (2013) Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malar J 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wijedoru LP, Kumar V, Chanpheaktra N, Chheng K, Smits HL, et al. (2012) Typhoid fever among hospitalized febrile children in Siem Reap, Cambodia. J Trop Pediatr 58: 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zimmerman MD, Murdoch DR, Rozmajzl PJ, Basnyat B, Woods CW, et al. (2008) Murine typhus and febrile illness, Nepal. Emerg Infect Dis 14: 1656–1659. 10.3201/eid1410.080236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aarsland SJ, Castellanos-Gonzalez A, Lockamy KP, Mulu-Droppers R, Mulu M, et al. (2012) Treatable bacterial infections are underrecognized causes of fever in ethiopian children. Am J Trop Med Hyg 87: 128–133. 10.4269/ajtmh.2012.12-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baba M, Logue CH, Oderinde B, Abdulmaleek H, Williams J, et al. (2013) Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. Journal of Infection in Developing Countries 7: 51–59. 10.3855/jidc.2411 [DOI] [PubMed] [Google Scholar]
- 46. Blacksell SD, Sharma NP, Phumratanaprapin W, Jenjaroen K, Peacock SJ, et al. (2007) Serological and blood culture investigations of Nepalese fever patients. Trans R Soc Trop Med Hyg 101: 686–690. [DOI] [PubMed] [Google Scholar]
- 47. Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, et al. (2001) Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis 5: 63–69. [DOI] [PubMed] [Google Scholar]
- 48. Christopher A, Mshana SE, Kidenya BR, Hokororo A, Morona D (2013) Bacteremia and resistant gram-negative pathogens among under-fives in Tanzania. Ital J Pediatr 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang LY, et al. (2011) Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health 16: 830–837. 10.1111/j.1365-3156.2011.02774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, et al. (2011) Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 52: 341–348. 10.1093/cid/ciq103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dougle M, Hendriks E, Sanders E, Dorigo-Zetsma J (1997) Laboratory investigations in the diagnosis of septicaemia and malaria. East Afr Med J 74: 353 [PubMed] [Google Scholar]
- 52. Meremo A, Mshana SE, Kidenya BR, Kabangila R, Peck R, et al. (2012) High prevalence of Non-typhoid salmonella bacteraemia among febrile HIV adult patients admitted at a tertiary Hospital, North-Western Tanzania. Int Arch Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, et al. (2010) WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ 340: c1350 10.1136/bmj.c1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petit PLC, Haarlem JV, Poelman M, Haverkamp MCP, Wamola IA (1995) Bacteraemia in patients presenting with fever. East Afr Med J 72: 116–120. [PubMed] [Google Scholar]
- 55. Akpede GO, Abiodun PO, Sykes RM (1992) Relative contribution of bacteraemia and malaria to acute fever without localizing signs of infection in under-five children. J Trop Pediatr 38: 295–298. [DOI] [PubMed] [Google Scholar]
- 56. Archibald LK, Kazembe PN, Nwanyanwu O, Mwansambo C, Reller LB, et al. (2003) Epidemiology of bloodstream infections in a bacille Calmette-Guérin-vaccinated pediatric population in Malawi. J Infect Dis 188: 202–208. [DOI] [PubMed] [Google Scholar]
- 57. Ki-Zerbo GA, Tall F, Nagalo K, Ledru E, Durand G, et al. (2000) Rickettsiosis and Q fever in pyretic patients hospitalized at the Bobo-Dioulasso Hospital (Burkina Faso). Med Mal Infect 30: 270–274. [Google Scholar]
- 58. Cohen AL (2007) Rapid diagnostic tests for dengue and leptospirosis: antibody detection is insensitive at presentation Rapid diagnostic tests in the tropics. Trop Med Int Health 12: 47–51. [DOI] [PubMed] [Google Scholar]
- 59. Libraty DH, Myint KSA, Murray CK, Gibbons RV, Mammen MP, et al. (2007) A comparative study of leptospirosis and dengue in Thai children. PLoS Negl Trop Dis 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Akpede GO, Abiodun PO, Sykes RM (1993) Acute fevers of unknown origin in young children in the tropics. J Pediatr 122: 79–81. [DOI] [PubMed] [Google Scholar]
- 61. Ayoola OO, Adeyemo AA, Osinusi K (2002) Predictors of bacteraemia among febrile infants in Ibadan, Nigeria. Journal of Health Population and Nutrition 20: 223–229. [PubMed] [Google Scholar]
- 62. Hyams KC, Oldfield EC, McNair Scott R, Bourgeois AL, Gardiner H, et al. (1986) Evaluation of febrile patients in Port Sudan, Sudan: Isolation of dengue virus. Am J Trop Med Hyg 35: 860–865. [DOI] [PubMed] [Google Scholar]
- 63. Akram DS (1998) Dengue Virus Infection among Children with Undifferentiated Fever in Karachi. Indian J Pediatr 65: 735–740. [DOI] [PubMed] [Google Scholar]
- 64. Archibald LK, McDonald LC, Nwanyanwu O, Kazembe P, Dobbie H, et al. (2000) A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: Implications for diagnosis and therapy. J Infect Dis 181: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 65. Scott JAG, Berkley JA, Mwangi I, Ochola L, Uyoga S, et al. (2011) Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. The Lancet 378: 1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koram KA, Molyneux ME (2007) When is "malaria" malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. The American journal of tropical medicine and hygiene 77: 1. [PubMed] [Google Scholar]
- 67.(2011) World Urbanization Prospects, the 2011 Revision, Data on Urban and Rural Populations. United Nations, Department of Social and Economic Social Affairs.
- 68. Crump JA, Youssef FG, Luby SP, Wasfy MO, Rangel JM, et al. (2003) Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis 9: 539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crump JA (2012) Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin Infect Dis 54: 1107–1109. 10.1093/cid/cis024 [DOI] [PubMed] [Google Scholar]
- 70. Crump JA (2014) Time for a comprehensive approach to the syndrome of fever in the tropics. Trans R Soc Trop Med Hyg 108: 61–62. 10.1093/trstmh/trt120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greenwood B (1997) The epidemiology of malaria. Ann Trop Med Parasitol 91: 763–770. [DOI] [PubMed] [Google Scholar]
- 72. Smith T, Schellenberg JA, Hayes R (1994) Attributable fraction estimates and case definitions for malaria in endemic. Stat Med 13: 2345–2358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.