Abstract
Background and Aims
Complete diagnostic autopsies (CDA) remain the gold standard in the determination of cause of death (CoD). However, performing CDAs in developing countries is challenging due to limited facilities and human resources, and poor acceptability. We aimed to develop and test a simplified minimally invasive autopsy (MIA) procedure involving organ-directed sampling with microbiology and pathology analyses implementable by trained technicians in low- income settings.
Methods
A standardized scheme for the MIA has been developed and tested in a series of 30 autopsies performed at the Maputo Central Hospital, Mozambique. The procedure involves the collection of 20 mL of blood and cerebrospinal fluid (CSF) and puncture of liver, lungs, heart, spleen, kidneys, bone marrow and brain in all cases plus uterus in women of childbearing age, using biopsy needles.
Results
The sampling success ranged from 67% for the kidney to 100% for blood, CSF, lung, liver and brain. The amount of tissue obtained in the procedure varied from less than 10 mm2 for the lung, spleen and kidney, to over 35 mm2 for the liver and brain. A CoD was identified in the histological and/or the microbiological analysis in 83% of the MIAs.
Conclusions
A simplified MIA technique allows obtaining adequate material from body fluids and major organs leading to accurate diagnoses. This procedure could improve the determination of CoD in developing countries.
Introduction
Complete diagnostic autopsy (CDA) is considered the gold standard methodology to inform on cause of death (CoD)[1]. Even in the era of high-tech medicine, major clinico-pathological discrepancies are reported in up to a quarter (23.5%) of CDAs[2].
In developing countries non-forensic CDA has always been an infrequent procedure due to several reasons. First of all, most deaths occur outside the health system, thus precluding not only the post-mortem evaluation but frequently even basic medical assistance[3,4]. Secondly, the limited human resources, particularly of trained personnel with technical expertise in this procedure, are a major limitation to undertake CDAs even in tertiary or reference hospitals. Finally, problems related to cultural and/or religious acceptance may negatively influence consent and, consequently, the practice of CDAs in some regions.
To overcome this problem the WHO recommends verbal autopsy (VA), a structured interview to family, friends, and caretakers, as an alternative to CDA in developing countries[5–8]. It has been shown that VA provides mainly a broad syndromic approach, but its performance for specific etiologic diagnosis is poor. Moreover, VA may misclassify a substantial number of deaths, and different researchers using this tool may obtain different results. Recently published estimates on various causes of global cause-specific mortality[9,10] have stirred a profound debate about the validity and adequacy of VA to estimate CoD[5].
Minimally invasive autopsy (MIA) has recently been proposed as an alternative to CDA[11–16]. At present, MIA most often includes the use of imaging techniques, such as MRI or CT scan, coupled with targeted small diagnostic biopsies of key organs obtained by needle puncture[11]. The results obtained so far with this technique seem to be reliable and comparable to the CDA, particularly in stillbirths and neonatal deaths[17–19]. However, these current MIA protocols are neither adequate nor feasible in developing countries due to the need of high-tech imaging studies that, if available, cannot be used for post-mortem studies. Although some studies have performed post mortem biopsies they are often limited to a single organ[12,15,16]. Thus, there is a need for simplified, standardized MIA protocols adequate for low-income settings that can provide accurate information on CoD. We aimed to develop and test a MIA procedure involving organ-directed sampling with pathological and microbiological analyses feasible for trained technicians in low-income settings.
Materials and Methods
Study Design
As part of an ongoing validation study of the MIA compared with the CDA (CaDMIA project) [20] a standard operating procedure (SOP) for the MIA tool was initially developed at the Hospital Clinic of Barcelona (HCB), and later tested and modified at the Department of Pathology of the Maputo Central Hospital (MCH). The study protocol was approved by the National Mozambican Ethics Committee (ref. 342/CNBS/13) and the Ethics Committee of the HCB. For all of the autopsies, a CDA had been requested by their clinicians as part of the medical evaluation and the family had given an informed verbal consent to perform the study.
The MIA procedure
The MIA procedure was performed by a single pathologist with the assistance of a technician. It begins by evaluating the liver, spleen, kidneys and other abdominal organs with a portable ultrasound (US) scan device (Mindray Z6, Mindray Med Int Ltd, Shenzhen, China). In women of childbearing age a suprapubic US scan of the pelvis is also performed. All the lesions identified, abnormal fluids (ascites, pleural effusions), as well as the position of deep organs (particularly the spleen and the kidneys), are recorded. Then, the US gel is removed with cellulose paper and the areas of the corpse to be punctured are cleaned and sterilized initially with tap water and then with 96° alcohol and iodine solution, allowing 5 minutes for each act on the surface of the corpse in order to achieve adequate disinfection for the microbiological analyses[21]. During the cleaning process the skin is carefully inspected in search of any macroscopically evident lesion.
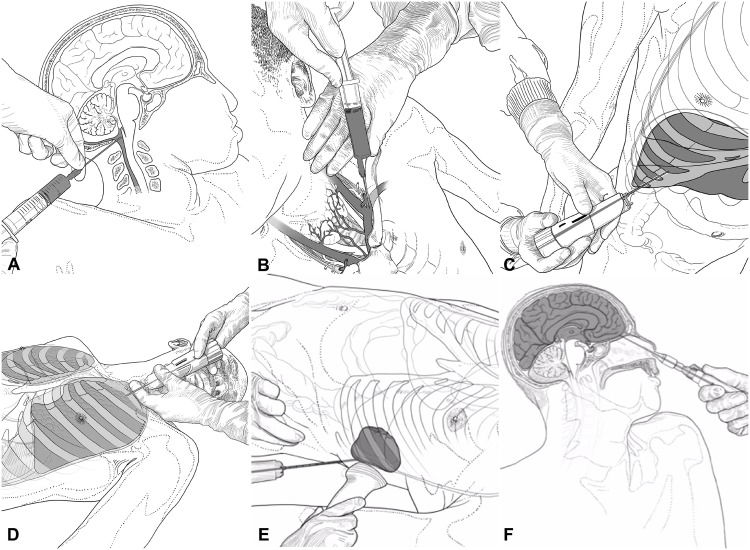
The type and main characteristics of the different needles used in the MIA procedure for each particular biopsy, the sites of puncture and the number of samples to be obtained are summarized in Table 1 and illustrated in Fig 1. The sampling process starts with the collection of CSF by occipital puncture, reaching the cisterna magna (Fig 1A). Up to 5 mL of CSF are collected in a 10 mL sterile tube and two aliquots of 2 mL each are stored in Eppendorf Safe-Lock Tubes at -80°C. Then, 20 mL of blood are extracted by puncture of the subclavian vein through a supraclavicular or infraclavicular approach (Fig 1B). If less than 20 mL of blood are obtained with this approach, intracardiac blood is targeted through a heart puncture at the left 5th intercostal space. Ten mL of blood are collected in an EDTA-containing tube, 10 mL in an aerobic adult or pediatric blood culture bottle (BACTEC), and four large drops of blood are placed onto a Whatman 903 filter (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). If other fluids (pleural effusion or ascites) are detected in the US scan, 20 mL are collected. After the body fluids have been collected, specimens of the major organs are taken.
Table 1. Type and main characteristics of the different needles used in the minimally invasive autopsy procedure for each particular biopsy, puncture sites and number of samples to be obtained.
The organ tissues are presented in the order in which the samples were collected.
| Tissue | Needle | Type | Gauge | Needle length (mm) | Puncture site | Volume/Number of samples for microbiology | Number of samples for histology |
|---|---|---|---|---|---|---|---|
| Cerebrospinal fluid (CSF) | Quincke Spinal # | Manual | 20 | 100 | Occipital puncture | 20 mL | - |
| Blood | Quincke Spinal | Manual | 20 | 100 | Supra/infra-clavicular or left ventricle | 20 mL | - |
| Liver | Unicut * | Manual | 14 | 115 | Anterior right axillar line, 11th-12th intercostal space | 2 cylinders | 4–6 cylinders |
| Lungs | Monopty* | Automatic | 14 | 100 | Right and left clavicular region down to the diaphragm for microbiology samples. Multiple random thoracic punctures for pathology | 2 from left lung, 2 from right lung | 4–6 cylinders from each side |
| Heart | Monopty* | Automatic | 14 | 100 | Left thoracic region 5th intercostal space in a parasternal point | - | 2 cylinders |
| Spleen | Monopty* | Automatic | 14 | 160 | Posterior left axillar line in the 11th-12th intercostal space (locate with US scan) | - | 2 cylinders |
| Kidneys | Monopty* | Automatic | 14 | 160 | Upper abdominal/lumbar area (locate with US scan) | - | 2 cylinders |
| Bone Marrow | T-LokTrephine** | Manual | 8 | 100 | Anterior iliac crest | Half of the cylinder | Half of the cylinder |
| CNS | Biomol*** | Semi Automatic | 16 | 200 | Trans-ethmoidal puncture. Perforation of the cribriform plate with the bone marrow trephine to reach the cranial cavity | 2 cylinders | 4–8 cylinders |
| Uterus | Monopty* | Automatic | 14 | 160 | Central suprapubic region (locate with US scan) | - | 2 cylinders |
| Skin | Biopsy punch ## | Manual | - | 5 | Macroscopically detected lesions | - | 2–3 biopsy punches |
# Becton Dickinson, FranklinLakes, NJ, USA
##KAI Europe GMBH, Solingen, Germany
* BARD Biopsy Systems, Tempe, AZ; USA
**Mana-Tech Ltd, Staffordshire, UK
*** HS Hospital Service S.P.A, Rome, Italy
Fig 1. Procedures for the collection of cerebrospinal fluid (A), peripheral blood (B), liver (C), lung (D), spleen (E), and the central nervous system biopsy (F) (designed by Xabier Sagasta).
Evaluation of the procedure
The evaluation of the procedure was conducted at the MCH, a government-funded quaternary health care facility that serves as the referral center for other hospitals in Southern Mozambique. Mozambique has a total population of around 25, 2 million and the life expectancy at birth in both sexes is 52/53 years[22]. In terms of the number of years of life lost (YLLs) due to premature death in Mozambique, HIV/AIDS, malaria, and lower respiratory infections were the highest ranking causes in 2010 [23].
This study was performed from November 4 to 23, 2013. The initial SOP was applied in a group of 30 adults who died at the MCH. Of all the CDAs requested every day at the Department of Pathology of the HCM (between 5 and 12) two autopsies per day were selected on the basis of most recent time of death. All the deceases had occurred less than 24 hours prior to the procedure A CDA had been requested by the clinicians for all these cases following the standard procedures of the hospital, and the families gave verbal informed consent for the procedure.
The first samples from the liver, right and left lung and CNS biopsies were taken using a sterile new needle, and placed into thioglicolate broth (first sample) and lysis buffer (second sample) for microbiology studies. The subsequent samples were taken using the same needles, and the material obtained was fixed inside a cassette for histology. Only one biopsy of the bone marrow was obtained, and this was divided into two samples, one for microbiology and the other for histological evaluation.
In women of childbearing age a sample from the uterus was taken by central suprapubic puncture. If amniotic fluid was detected, 20 mL was collected in a sterile tube. Finally, if skin lesions were observed a biopsy using a punch was performed at the border of the grossly identified lesion avoiding disfiguration of visible body areas.
The time spent to complete the procedure was recorded for each case.
Histological laboratory procedure
Tissue specimens for histological analysis were fixed in 10% neutral buffered formalin for 4 hours, passed into distilled water, embedded in paraffin, and cut into four-micron sections, which were then stained with H&E as per standard procedures.
Microscopy and analysis of the tissue samples for histological analysis
All samples taken during the MIA procedure were evaluated using an Olympus BX51 light microscope. H&E sections of all the tissues were initially evaluated. When necessary, ancillary histochemical (i.e. Zieh-Neelsen, PAS, Grocott stain, etc.) and immunohistochemical stains were performed in selected blocks to confirm or exclude specific lesions suspected on the H&E stains. Immunohistochemical staining for CD45 (leukocyte common antigen) was performed in all cases in the CNS samples in order to exclude/confirm minimal leukocyte infiltrates.
All the cylinders present in each block were carefully evaluated, recording all the organs present and measuring the area (in mm2) of each organ obtained in the MIA procedure after drawing the perimeter of each tissue cylinder in a Leica dmd 108 microscope.
Determination of the putative cause of death
The histological evaluation of the MIA samples was performed by two pathologists who were blinded to the clinical data or the results of the CDA. The microbiological analyses were supervised by an experienced microbiologist and included conventional blood cultures, CSF, the CNS, liver and lungs, testing for antibodies against hepatitis B and C viruses and HIV and PCR analysis for Plasmodium falciparum and Mycobacterium tuberculosis in all the cases. These routine analyses were complemented by specific molecular assays guided by the results of the histological evaluation and the conventional cultures. A detailed description of the microbiological analyses has been published elsewhere. The results obtained independently in the histological and the microbiological analyses were discussed in a joint session composed by the pathologists, the microbiologist, and an epidemiologist as well as a clinician with expertise in infectious and tropical diseases, and a final putative CoD was assigned to each case based on the combination of all the findings obtained with the MIA samples.
Results
Efficiency of the sampling procedure
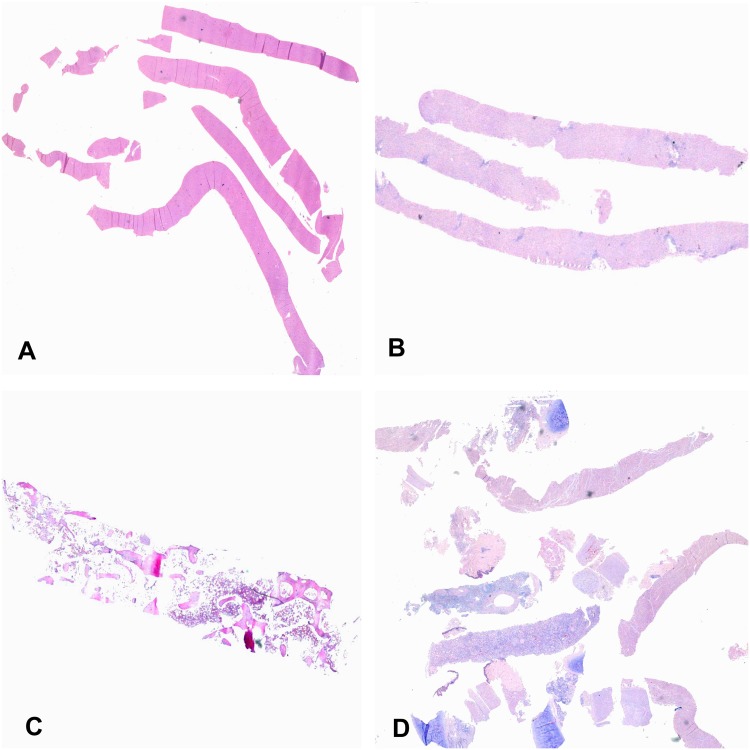
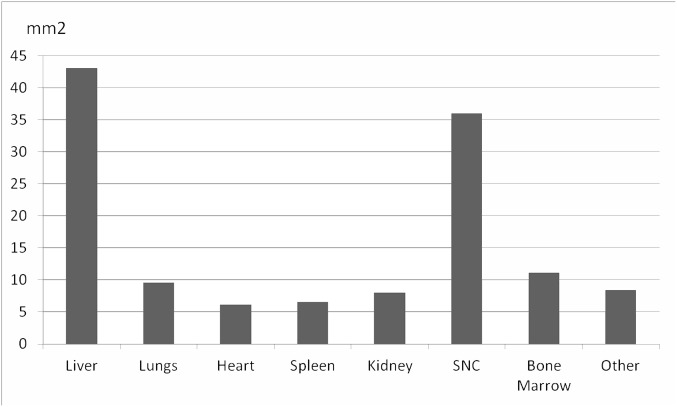
In all the cases, adequate samples of blood and CSF were collected. The percentage of success in terms of adequate samples for histological evaluation was 66.7% (20/30) for the kidney, 70% (21/30) for the spleen, 80% (24/30) for the heart, 96.7% (29/30) for the bone marrow and 100% (30/30) for the liver, lungs and CNS. The skin was sampled only when gross lesions were macroscopically identified (9/30 cases). The uterus was sampled in 11/14 women of childbearing age. In only 54% (6/11) of these cases uterine tissue was successfully obtained for histological evaluation. The number of cylinders obtained from each organ ranged from 0–4 for the kidneys, heart and spleen, 1–4 for the lungs, and 7–9 for the liver and CNS. A representative image of the samples obtained from the CNS, liver, bone marrow and lungs/heart and kidney is shown in Fig 2. Fig 3 shows the median area of tissue sampled in each organ per case. The median area of tissue obtained ranged from 42 mm2 for the liver, to less than 10 mm2 for organs such as the lungs and the heart.
Fig 2. Representative image of the samples obtained from the central nervous system (A), liver (B), bone marrow (C) and lungs/heart and kidney (D) in the minimally invasive autopsy procedure.
Fig 3. Median area of tissue for histological evaluation obtained from each organ in mm2.
A learning curve was observed in terms of time to complete the entire MIA procedure by the pathologist, reducing from 90 minutes for the first procedure performed to 30–45 minutes from the third procedure and thereafter.
Characteristics of the autopsies and diagnostic performance of the MIA
The MIA analysis of 30 cases included 18 women (60%) and 12 males (40%) with a median age of t 35 years (range 17–76). Nineteen out of the 30 (63%) patients were HIV positive.
Table 2 shows the basic demographic data of the patients included in the study, the HIV status, the diagnoses reached in the histological evaluation of the samples obtained through the MIA, the results of the microbiological analyses and the final diagnoses obtained after combining the pathological and the microbiological results. A putative CoD was identified in the histological and/or the microbiological analysis in 83% of the MIAs. A severe disease considered as a possible CoD was identified in the histology samples in 22/30 cases (73%), with 14 cases being infectious diseases, and 8 non- communicable diseases including 4 malignant tumors. Three of the malignant tumors were of viral origin (two liver cell carcinomas, 1 Kaposi’s sarcoma) and one was a large B cell lymphoma negative for the in situ hybridization for Epstein- Barr virus. The MIA tended to yield a CoD in patients 35-year-old or younger more frequently than in those older than 35 years of age, although the difference was not statistically significant (12/17; 70.6% vs. 7/14, 50%; p = 0.288). Fig 4 shows representative examples of CoD identified through MIA sampling.
Table 2. Age, sex, HIV status and pathological and microbiological diagnoses obtained in the 30 minimally invasive autopsies (MIA) performed in the pilot study.
| MIA | Age | Sex | HIV status | Pathological diagnosis | Microbiological diagnosis | Final diagnosis |
|---|---|---|---|---|---|---|
| 1 | 27 | M | - | Pyogenic-granulomatous meningoencephalitis | Rhizopus oryzae | R. oryzaecerebral invasive mucormicosis |
| 2 | 76 | M | - | Cerebral infarction * | - | Cerebral infarction |
| 3 | 32 | M | - | Hepatocellular carcinoma | HBV | Hepatocellular carcinoma (HBV) |
| 4 | 19 | F | - | Non specific | - | Non specific |
| 5 | 25 | F | - | Cardiac hyperthrophy | - | Suggestive of cardiovascular disease |
| 6 | 28 | F | + | Disseminated Kaposi’s sarcoma # | HHV-8 # | Disseminated Kaposi’s sarcoma HHV-8 |
| 7 | 35 | F | + | Pneumocystis pneumonia # | Pneumocystis jiroveci | Pneumocystis pneumonia |
| 8 | 49 | M | - | Cardiac hyperthrophy | - | Suggestive of cardiovascular disease |
| 9 | 35 | F | + | Disseminated necrotizing granulomas | Toxoplasma gondii | Cerebral toxoplasmosis |
| 10 | 27 | F | + | Pyogenic pneumonia | Acinetobacter baumannii | A. baumannii sepsis of pulmonary origin |
| 11 | 33 | M | + | Meningoencephalitis ** | Streptococcus pneumoniae | Septic pneumococcal meningitis |
| 12 | 41 | M | + | Pyogenic meningoencephalitis | Cytomegalovirus | Pyogenic meningoencephalitis |
| 13 | 76 | F | - | Non specific | - | Non specific |
| 14 | 17 | F | + | Pyogenic meningoencephalitis | Mycobacterium tuberculosis | Tuberculous meningitis |
| 15 | 43 | M | + | Pyogenic pneumonia | Pneumocystis jiroveci | Pneumocystis pneumonia |
| 16 | 48 | M | - | Hepatocellular carcinoma | Hepatitis B virus | Hepatocellular carcinoma (HBV) |
| 17 | 53 | F | + | Large B cell lymphoma | - | Large B cell lymphoma |
| 18 | 36 | F | + | Non specific | - | Non specific |
| 19 | 62 | M | - | Cardiac hypertrophy | - | Suggestive of cardiovascular disease |
| 20 | 35 | M | + | Pyogenic pneumonia | - | Pyogenic pneumonia |
| 21 | 29 | F | + | Disseminated necrotizing granulomas # | Mycobacterium tuberculosis | Miliary tuberculosis |
| 22 | 61 | F | - | Meningoencephalitis ** | - | Suggestive of meningoencephalitis |
| 23 | 30 | F | + | Pyogenic meningoencephalitis | Cryptococcus neoformans | Cryptococcal meningitis |
| 24 | 74 | M | - | Non specific | - | Non specific |
| 25 | 57 | F | + | Non specific | - | Non specific |
| 26 | 45 | F | + | Disseminated necrotizing granulomas # | Mycobacterium tuberculosis | Miliary tuberculosis |
| 27 | 27 | F | + | Non specific | Enterococcus faecalis, Mycoplasma hominis | Puerperal sepsis |
| 28 | 29 | F | + | Cryptococcal sepsis # | Cryptococcus neoformans | Cryptococcal sepsis |
| 29 | 30 | M | + | Non specific | Streptococcus dysgalactiae | S. dysgalactiae septicemia |
| 30 | 31 | F | + | Non specific | Toxoplasma gondii | Cerebral toxoplasmosis |
M: male; F: female
* necrosis and hemorrhage in the cerebral parenchyma with negative stains for microorganisms
# etiological agent detected in the pathology sample by immunohistochemistry or special stains
HBV: hepatitis B virus
HHV-8: human herpes virus 8
** minimal perivascular inflammatory infiltrate in CNS parenchyma after immunohistochemical analysis against CD45.
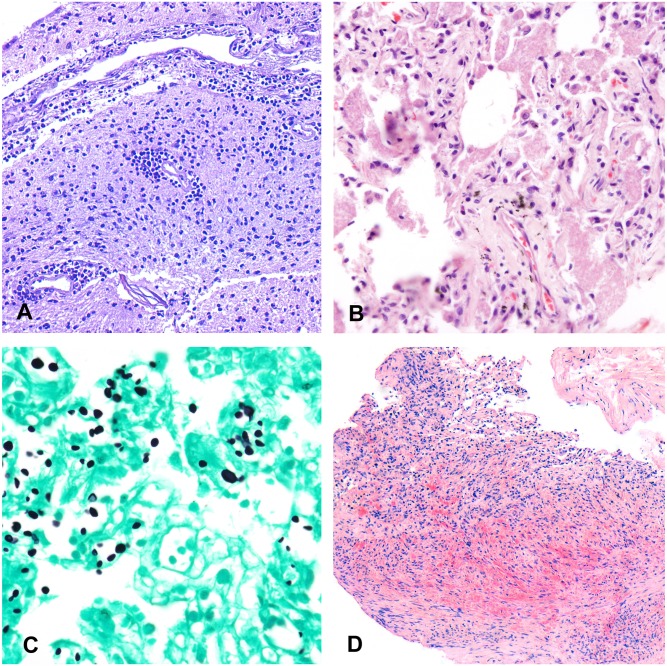
Fig 4. Representative examples of putative causes of death identified with minimally invasive autopsy sampling.
A) Meningoencephalitis (hematoxylin and eosin, 200x); B) Pneumocysttis jiroveci pneumonia (hematoxylin and eosin, 200x); C) Cryptococcus neoformans infecting the lung (PAS metenamine silver stain, 200x); D) Kaposi’s sarcoma involving the lung (hematoxylin and eosin, 100x).
Significant diagnostic information for CoD determination was obtained from the histological samples from the lung, CNS and liver (6 cases each), spleen (3 cases) and skin (1 case). In contrast, the samples from bone marrow, heart, kidney or uterus rendered no information on CoD. For the microbiological samples, the diagnostic information was obtained from the CNS (12 cases), CSF (9), lung (8), liver (7) and blood (3). The microorganism was identified in a single organ in 4 cases, in two organs in 7, in three organs in 1 and in four and five organs in two each.
Meningoencephalitis was the leading CoD (6 cases) among the infectious diseases. The histological samples of the CNS showed clear pyogenic and/or granulomatous reaction in four cases and only a mild perivascular inflammatory infiltrate, enhanced in the immunohistochemical analysis with CD45, in the remaining two cases. In 5/6 of the meningoencephalitis an increase in the leukocyte count was identified in the CSF. The etiologic agent causing the death was identified only by histological techniques in one case (human herpes virus 8 in Kaposi’s sarcoma), by both special histological stains and microbiological techniques in 4 cases, and only by microbiological analyses in 10 cases. In 6 out of these 10 cases a pathological lesion attributable to the microorganism identified was clearly observed, whereas in three cases the final infectious CoD was achieved only by microbiological analyses in the context of non-specific histological changes. In these cases the microorganisms putatively causing the death were identified in multiple organs and fluids (M. hominis and E. faecalis [case 27], S. dysgalactiae [case 29]) or in both the brain and the CSF (T. gondii, case 30).
Discussion
There is a need to find alternative post-mortem examination methods that can be used as tools to advance medical knowledge on CoD and thereby guide effective interventions in low-income countries. This study has developed a standardized protocol of MIA for low-income settings, which has shown to provide adequate samples for both histological and microbiological analysis from most major organs. This simplified technique can be implemented in developing countries and would allow the etiologic diagnosis of a significant number of patients. This standardized protocol of MIA developed achieved the major aims of the study allowing: 1 the collection of as many tissue samples as necessary to make a reliable final diagnosis; and 2) the tissue samples to be adequately preserved in order to maintain high quality material for the analyses. The decision as to the organs to be sampled was taken according to previous experience in autopsy studies[24–27]. During the preparation of the standard operating procedure the observation that the spleen and the kidney were difficult to locate led us to introduce the use of US scan to identify these organs. Once the organ was located, the puncture was done without US guidance. The selection of the type and gauge of the needles to be used was based on clinical experience as well as in the scant information reported in other postmortem studies[4]. The thickest available needles were selected because of the impossibility of clinical complications.
In this study the percentage of success was 100% for the liver, lung and CNS and slightly lower for the other organs. These findings are concordant with other studies [18,28] that, although including a small number of cases, also reported successful sampling of organs such as the lung. The current findings were also similar to those reported in a study carried out almost 45 years ago that included 294 adult cases, in which adequate samples were obtained from the liver in 92% of cases, the heart in 55%, the lung in 46% and the kidney in 34% [28]. The reduced percentage of success for the sampling of the uterus in women of childbearing age, an organ not targeted in previous studies, could be explained by its difficult accessibility behind the urinary bladder. Few previous studies have attempted to collect CNS samples in autopsies[15,29]. In two of the studies performed in children [12,15] a supraorbital puncture was used. In a study from Uganda a trans-nasal approach was used, as in our series, because the supraorbital plate in adults is completely ossified and extremely resistant to perforation, whereas the cribriform plate is softer and easier to pass through with a bone trephine. The percentage of success in CNS sampling in our study (100%) was identical to the success rate obtained in the Malawian study [12] and very similar to that obtained in the Ugandan study [30]. Finally, although the rate of success in sampling attainment was high for many organs, the median area of tissue sampled in each organ was quite different; suggesting that the small size of the samples obtained for several organs such as the lung, heart, spleen and kidney may hinder reaching a diagnosis in focal lesions.
In this study a severe disease considered as a possible CoD was identified in the histological analysis in more than half of the cases (60%). The leading CoD was an infectious disease. This diagnosis tended to be more frequent among 35-year-old or younger patients than in those older than 35. This is consistent with infectious diseases being more frequent in young compared to older patients at least in developing countries[31]. Over 60% of the patients had uncontrolled or poorly controlled HIV infections. The highly widespread infections occurring in these immunocompromised hosts may have positively impacted the ability to detect the disease and the pathogen in the limited samples obtained in the MIA.
Importantly, significant diagnostic information for CoD determination was obtained from the samples of the lung, the CNS, liver, spleen and skin, all of which are organs frequently involved in infectious diseases, whereas the samples from bone marrow, heart, kidney and uterus rendered little or no information on CoD. If these findings are confirmed in subsequent studies, the MIA procedure could be significantly simplified by eliminating the use of US examination and the sampling of these non-contributive organs.
Interestingly, in this study it was possible to obtain adequate material for microbiological analysis, which allowed confirmation of the pathological results and the etiological characterization of the microorganisms causing the death in a significant number of cases, including fungal, viral and bacterial diseases. In one case (case 12) the pathological exam identified a meningoencephalitis and cytomegalovirus was detected by microbiological methods. Although meningoencephalitis can rarely [32] be associated with cytomegalovirus, in the absence of cytomegalic inclusions in the tissue, the disease cannot be conclusively attributed to this infection. The main limitations of the MIA procedure are related to the cost of the biopsy needles, the requirement of complex and expensive microbiological platforms and qualified microbiologists, and the need for pathologists specifically trained in the evaluation of small samples with limited representation of the lesions. Another possible limitation is that the diagnostic success of the procedure may be influenced by the dissemination of the disease, and could be significantly reduced in focal lesions and in limited infections in immunocompetent hosts. Finally, whereas MIA provided a high number of infectious diseases putatively causing the death, it is likely to be less effective for non-infectious diseases.
In conclusion, the results of this study confirm that MIA is a feasible and reliable tool that allows successful collection of samples from key organs. Further studies comparing the results of MIA with those of CDA are ongoing to test the validity of MIA. This procedure could be a valuable tool to improve the knowledge of CoD in developing countries where infectious diseases are common causes of death.
Acknowledgments
We would like to thank the families of all the patients included in this study. The authors are grateful to all the members of the Department of Pathology of the MCH, whose support made this study possible and also to the staff of the “Centro de Investigação em Saúde de Manhiça” for their logistical support. We specifically thank Mr. Bento Nhancale for his invaluable support to the study.
Data Availability
All relevant data are within the paper.
Funding Statement
The CaDMIA research project (validation of the minimally invasive autopsy tool for cause of death investigation in developing countries) is funded by the Bill & Melinda Gates Foundation (Global Health grant number OPP1067522) and by Spain’s Instituto de Salud Carlos III (FIS, PI12/00757). QB has a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I+D+I 2008-2011, grant number: CP11/00269).
References
- 1. Fligner CL, Murray J, Roberts DJ. Synergism of verbal autopsy and diagnostic pathology autopsy for improved accuracy of mortality data. Popul Health Metr. 2011;9:25 10.1186/1478-7954-9-25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuijpers CC, Fronczek J, van de Goot FR, Niessen HW, van Diest PJ, Jiwa M. The value of autopsies in the era of high-tech medicine: discrepant findings persist. J Clin Pathol. 2014;10–202122. [DOI] [PubMed] [Google Scholar]
- 3. Lishimpi K, Chintu C, Lucas S, Mudenda V, Kaluwaji J, Story A, et al. Necropsies in African children: consent dilemmas for parents and guardians. Arch Dis Child. 2001;84:463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ugiagbe EE, Osifo OD. Postmortem examinations on deceased neonates: a rarely utilized procedure in an African referral center. Pediatr Dev Pathol. 2012;15:1–4. [DOI] [PubMed] [Google Scholar]
- 5. Butler D. Verbal autopsy methods questioned. Nature. 2010;467:1015. [DOI] [PubMed] [Google Scholar]
- 6. Byass P. Usefulness of the Population Health Metrics Research Consortium gold standard verbal autopsy data for general verbal autopsy methods. BMC Med. 2014;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garenne M. Prospects for automated diagnosis of verbal autopsies. BMC Med. 2014;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jha P. Reliable direct measurement of causes of death in low- and middle-income countries. BMC Med. 2014;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–1623. [DOI] [PubMed] [Google Scholar]
- 10. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D,et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. [DOI] [PubMed] [Google Scholar]
- 11. Weustink AC, Hunink MG, van Dijke CF, Renken NS, Krestin GP, Oosterhuis JW. Minimally invasive autopsy: an alternative to conventional autopsy? Radiology. 2009;250:897–904. [DOI] [PubMed] [Google Scholar]
- 12. Milner DA Jr, Dzamalala CP, Liomba NG, Molyneux ME, Taylor TE. Sampling of supraorbital brain tissue after death: improving on the clinical diagnosis of cerebral malaria. J Infect Dis. 2005;191:805–808. [DOI] [PubMed] [Google Scholar]
- 13. Celiloglu OS, Celiloglu C, Kurnaz E, Ozdemir R, Karadag A. Diagnostic contribution of postmortem needle biopsies in neonates. Turk Patoloji Derg. 2013;29:122–126. 10.5146/tjpath.2013.01162 [DOI] [PubMed] [Google Scholar]
- 14. El-Reshaid W, El-Reshaid K, Madda J. Postmortem biopsies: the experience in Kuwait. Med Princ Pract. 2005;14:173–176. [DOI] [PubMed] [Google Scholar]
- 15. Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner GD, Bunthi C, Wonodi CB, Morpeth SC, Molyneux CS, Zaki SR, et al. The role of postmortem studies in pneumonia etiology research. Clin Infect Dis. 2012;54 Suppl 2:S165–S171. 10.1093/cid/cir1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breeze AC, Jessop FA, Whitehead AL, Set PA, Berman L, Hackett GA, et al. Feasibility of percutaneous organ biopsy as part of a minimally invasive perinatal autopsy. Virchows Arch. 2008;452:201–207. [DOI] [PubMed] [Google Scholar]
- 18. Sebire NJ, Weber MA, Thayyil S, Mushtaq I, Taylor A, Chitty LS. Minimally invasive perinatal autopsies using magnetic resonance imaging and endoscopic postmortem examination ("keyhole autopsy"): feasibility and initial experience. J Matern Fetal Neonatal Med. 2012;25:513–518. [DOI] [PubMed] [Google Scholar]
- 19. Thayyil S, Chitty LS, Robertson NJ, Taylor AM, Sebire NJ. Minimally invasive fetal postmortem examination using magnetic resonance imaging and computerised tomography: current evidence and practical issues. Prenat Diagn. 2010;30:713–718. [DOI] [PubMed] [Google Scholar]
- 20. Bassat Q, Ordi J, Vila J, Ismail MR, Carrilho C, Lacerda M, et al. Development of a post-mortem procedure to reduce the uncertainty regarding causes of death in developing countries. Lancet Glob Health. 2013;1:e125–e126. [DOI] [PubMed] [Google Scholar]
- 21. Little JR, Murray PR, Traynor PS, Spitznagel E. A randomized trial of povidone-iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med. 1999;107:119–125. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. World Health Statistics. World Health Organization. 2013. Ref Type: Internet Communication
- 23. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo P, Menendez C, Mayor A, Carrilho C, Ismail MR, Lorenzoni C, et al. Massive Plasmodium falciparum visceral sequestration: a cause of maternal death in Africa. Clin Microbiol Infect. 2013;19:1035–1041. [DOI] [PubMed] [Google Scholar]
- 25. Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67–e74. [DOI] [PubMed] [Google Scholar]
- 26. Menendez C, Romagosa C, Ismail MR, Carrilho C, Saute F, Osman N, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ordi J, Ismail MR, Carrilho C, Romagosa C, Osman N, Machungo F, et al. Clinico-pathological discrepancies in the diagnosis of causes of maternal death in sub-Saharan Africa: retrospective analysis. PLoS Med. 2009;6:e1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cox JA, Lukande RL, Kalungi S, Van Marck E, Van de Vijver K, Kambugu A, et al. Needle autopsy to establish the cause of death in HIV-infected hospitalized adults in Uganda: a comparison to complete autopsy. J Acquir Immune Defic Syndr. 2014;67:169–176. [DOI] [PubMed] [Google Scholar]
- 29. Milner DA Jr, Valim C, Carr RA, Chandak PB, Fosiko NG, Whitten R, et al. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar J. 2013;12:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cox JA, Lukande RL, Kalungi S. Practice of percutaneous needle autopsy; a descriptive study reporting experiences from Uganda. BMC Clin Pathol. 2014;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sepulveda J, Murray C. The state of global health in 2014. Science. 2014;345:1275–1278. [DOI] [PubMed] [Google Scholar]
- 32. Frange P, Boutolleau D, Leruez-Ville M, Touzot F, Cros G, Heritier S, et al. Temporal and spatial compartmentalization of drug-resistant cytomegalovirus (CMV) in a child with CMV meningoencephalitis: implications for sampling in molecular diagnosis. J Clin Microbiol. 2013;51:4266–9. 10.1128/JCM.02411-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.