Abstract
It has been suspected that in comparison with glucose or fatty acids, the levels of amino acids may readily change with different forms of exercise. In the present study, we measured the concentrations of amino acids, glucose, triglycerides, total protein and total cholesterol in the blood and/or cerebrospinal fluid (CSF) of rats subjected to forced running exercise on a treadmill, and voluntary running exercise using a wheel, with a constant running distance of 440 m. Rats that performed no running and rats subjected to immobilization stress were used as controls. We observed a few significant changes in the levels of plasma glucose, triglycerides, total protein and total cholesterol in all groups. Whereas, plasma amino acid levels were significantly changed by exercise and stress, especially during the light period. The plasma levels of many amino acids were specifically increased by forced running; some were decreased by immobilization stress. Few amino acids showed similar changes in their levels as a result of voluntary running. In addition, there was a significant difference in the degree of amino acid imbalance between blood and CSF. These results provide the first information on changes in levels of amino acids in plasma and CSF resulting from forced and voluntary exercises.
Keywords: behavior, exercise physiology, rat
Exercise has many beneficial effects on the bodies of animals. In humans, many supplements are now available to replenish energy that is consumed and to relieve fatigue resulting from exercise. In particular, it is well known that intake of amino acids before exercise is beneficial. It has been reported that as the branched-chain amino acids (BCAA) are particularly metabolized in skeletal muscle, intake of BCAA before exercise prevents the muscle obstacle after the exercise [13, 16].
In blood and CSF, individual amino acids are usually each maintained at a constant threshold level. Therefore, even if BCAA are taken orally under normal conditions, their levels in plasma do not change [13]. If an imbalance of amino acids occurs, the body works promptly to restore the balance. It has become clear that the plasma levels of amino acids show characteristic changes in various diseases, such as diabetes [3], cancer [8] and liver disease [4]. In addition, the plasma levels of amino acids change as a result of exercise [2, 5, 7, 12]. In 2009, Jin et al. examined changes in the concentrations of amino acids in the liver, muscle, blood and cerebral cortex in weighted rats subjected to forced swimming [6]. They found that the levels of most amino acids did not decrease or change, but that valine, leucine, isoleucine and phenylalanine in blood and skeletal muscle, valine and lysine in the liver, and isoleucine in the cerebral cortex showed marked increases [6].
Exercise can be classified as either forced exercise or voluntary exercise. In rodents, it has been reported that forced exercise, such as treadmill running and swimming, and voluntary exercise, such as wheel running, have different effects in terms of weight gain, food consumption and survival [11]. Although many studies have compared amino acid levels between sedentary controls and individuals subjected to exercise, hardly any information is available about amino acid imbalance resulting from different types of exercise. We hypothesized that the imbalance among different types of amino acid might vary according to the type of exercise, particularly if forced exercise was compared with voluntary exercise.
High-impact exercises, such as treadmill running and swimming, and long-term exercise, have been used in studies examining the resulting changes in amino acid levels [2, 7]. Because the levels of amino acids in plasma reflect the process of metabolism in muscle and liver, any change resulting from high-impact or long-term exercise might not reflect any differences that are specific to the type of exercise undertaken. Therefore, we have measured plasma amino acid levels after short-term exercise and examined the initial changes. Furthermore, very few studies have examined changes in the levels of amino acids in cerebrospinal fluid (CSF) after exercise. As it would be of considerable interest to determine whether plasma amino acid imbalance influences the levels of amino acids in CSF, we measured amino acid concentrations in both CSF and blood.
Against this background, in the present study using rats, we compared the levels of amino acids between forced exercise (treadmill running), voluntary exercise (wheel running) and sedentary controls. Furthermore, as treadmill running is associated with stress, a group of rats subjected to immobilization was also added as a stress control. As the distance of running is the same between forced exercise and voluntary exercise, it is thought that the major difference between forced and voluntary exercises is participation of mental stress by forced exercise. That is, it may be important to compare the results of these two exercise groups with rat subjected stress only. Therefore, a group of rats subjected to immobilization was also added as a stress control. In addition, we carried out similar experiments during both the dark period (activity phase) and light period (resting phase) in order to examine whether the resulting changes in amino acid levels would differ.
MATERIALS AND METHODS
Animals: Adult male Wistar rats (Charles River, Yokohama, Japan) were housed under controlled temperature (23 ± 1°C) and 12 hr:12 hr light:dark conditions (lights on at 07:00 hr) with food and water available ad libitum during the entire experimental period. All of the procedures were performed in accordance with the guidelines for animal care stipulated by the Japanese Physiological Society and were approved by the ethics committee of Miyazaki University. All efforts were made to minimize animal pain and suffering and the number of animals used.
Experimental design: Rats were randomly divided into four experimental groups (n=4–13/group), including a sedentary control group (control), forced exercise group (treadmill running), voluntary exercise group (wheel running) and immobilization stress group (immobilization). All treatments including habituation were performed at the same time (11:30–12:30 hr for the light period and 20:30–21:30 hr for the dark period). Forced exercise was performed on a motor treadmill (MK-680, Muromachi-Kikai, Tokyo, Japan). First, the rats were acclimated to the treadmill for five consecutive days at a moderate speed (15–19 m/min) for 20 min. On the day of the experiment, they exercised at a faster speed (22 m/min) for 20 min. To minimize the degree of physical stress apart from the treadmill exercise itself, we did not use electroshock on the treadmill on the experimental day. The rats allowed to run voluntarily were maintained in a cage with free access to a running wheel (MK-750PC; 320 W×190 D×350 H mm, Muromachi-Kikai) for 2 weeks. After habituation, we limited the period of access to the wheel to only 2 hr (11:00–13:00 hr for the light period and 19:00–21:00 hr for the dark period) per day for five consecutive days. The experiment was performed at the same time as habituation, and the sampling was performed when the rats had run approximately 440 m (the same distance as the forced runners). Rats in the immobilization stress group were placed in a cylinder (diameter: 6 cm, length: 20 cm) for 20 min on the experimental day to impose mental stress.
Sample collection and measurement of amino acids: Sampling of blood and CSF was performed immediately after treatment under pentobarbital anesthesia (7.0 mg/100 g body weight, injected intraperitoneally (i.p.)) by cardiac and cisternal puncture, respectively. Blood and CSF samples were centrifuged at 4°C at 14,000 rpm for 4 min. The plasma was mixed with two volumes of 5% (w/w) trichloroacetic acid, centrifuged immediately at 10,000 rpm at 4°C for 20 min to remove precipitated protein and then passed through an Ultrafree-MC filter (Cat. No. UFC5010BK, Millipore, Billerica, MA, U.S.A.), and the concentrations of amino acids were measured with an automatic amino acid analyzer (L-8800A; Hitachi, Tokyo, Japan). We focused on the 20 amino acids that form the components of proteins: valine (Val), leucine (Leu), isoleucine (Ile), alanine (Ala), methionine (Met), proline (Pro), tryptophan (Trp), phenylalanine (Phe), tyrosine (Tyr), threonine (Thr), glutamine (Gln), asparagine (Asn), serine (Ser), glycine (Gly), cysteine (Cys), lysine (Lys), arginine (Arg), histidine (His), aspartic acid (Asp) and glutamic acid (Glu).
Briefly, amino acids were separated by cation-exchange chromatography and detected spectrophotometrically after post-column reaction with ninhydrin reagent. The amino acid concentrations in the CSF samples were measured by liquid chromatography-tandem mass spectrometry (LC/MS/MS) (API 4000, AB SCIEX, Redwood City, CA, U.S.A.). Five µl of each CSF sample was mixed with an equal volume of internal standard solution containing stably labeled amino acids. Subsequently, 10 µl of acetonitrile was added, and the samples were mixed with a vortex mixer. The samples were then centrifuged at 15,000 rpm for 10 min for protein precipitation, and the supernatants were used for subsequent analysis.
To facilitate highly sensitive analysis of amino acids, LC/MS/MS was performed coupled with precolumn derivatization [15]. For the derivatization, 10 µl of supernatant and 30 µl of borate buffer (pH 8.8) were mixed, and 10 µl of the derivatization reagent was added. The samples were then heated at 55°C for 10 min. The reaction mixture was diluted and injected into a reverse-phase high-performance liquid chromatography (HPLC) system (20A series; Shimadzu, Kyoto, Japan). Separation of the derivatized amino acids was performed with an Inertsil C8-3 column (2.1 × 100 mm, 3 µm, GL Sciences, Tokyo, Japan), and the analytes were detected using triple quadrupole tandem mass spectrometry (API3000 LC/MS/MS system; AB/SCIEX) in the selected reaction monitoring mode. The concentrations of plasma glucose, triglycerides, total protein and total cholesterol were measured using a DRI-CHEM 3500V automatic analyzer (FujiFilm, Tokyo, Japan).
Statistical analysis: The data are expressed as the mean ± standard error of the mean (SEM). The statistical significance of differences among the groups was determined by one-way analysis of variance (ANOVA) followed by Tukey’s test.
RESULTS
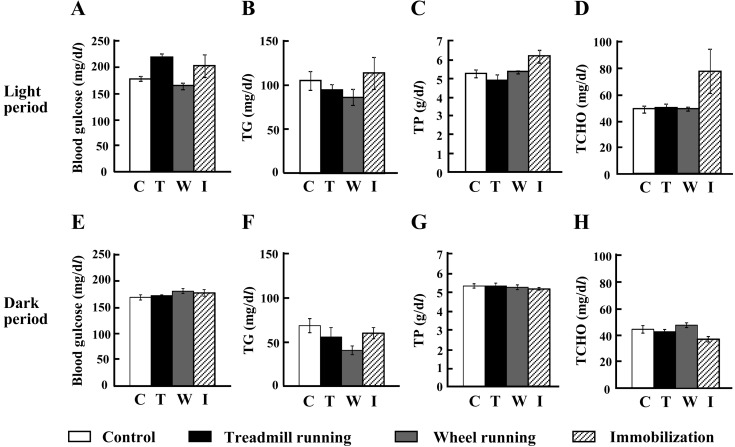
There were no significant differences in blood glucose, triglyceride, total protein and total cholesterol concentrations between the control and experimental groups (n=5–8/group, Fig. 1). No significant changes were observed during both the light and dark periods (Fig. 1).
Fig. 1.
Concentrations of blood glucose (mg/dl; A, E), triglycerides (mg/dl; B, F), total protein (g/dl; C, G) and total cholesterol (mg/dl; D, H) were measured after each experimental treatment in the light (n=5–8) and dark (n=5–7) phases. The data represent the mean ± standard error of the mean (SEM). C, control (white bar); T, treadmill running (black bar); W, wheel running (gray bar); I, immobilization (diagonal bar).
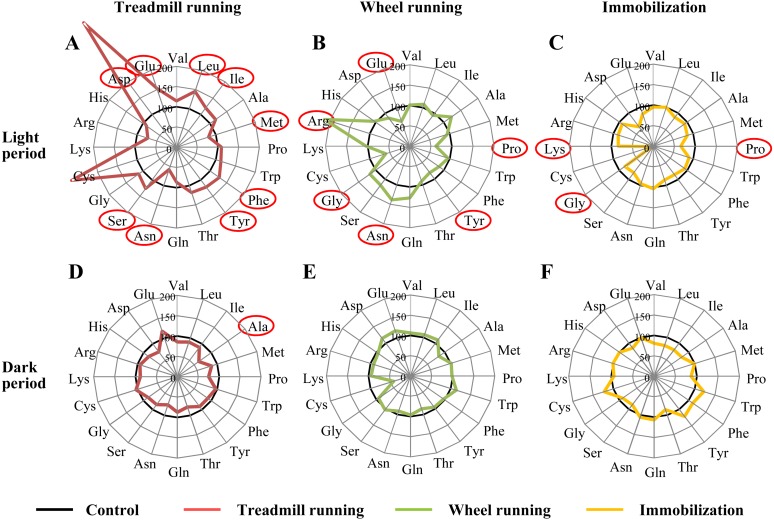
The amino acid profiles and changes in their ratios in each experimental group relative to the control group are shown in Figs. 2 and 3. During the light period (n=8–13/group, Fig. 2A–2C, Table 1), many of the plasma levels of amino acids showed significant changes in the three experimental groups relative to the control group. As a result of treadmill running, the levels of 7 amino acids (Leu, Ile, Phe, Tyr, Ser, Asp and Glu) were significantly elevated, while those of only 2 (Met and Asn) were lower than in the control group (Fig. 2A). Although Cys levels seemed to rise, there was no significant difference for the large variation. In the wheel-running group, the levels of 3 amino acids (Asn, Gly and Arg) were increased, and those of 3 other amino acids (Pro, Tyr and Glu) were decreased relative to the control group (Fig. 2B). In the immobilization stress group, the levels of 3 amino acids (Pro, Gly and Lys) were decreased, and none were increased (Fig. 2C). Furthermore, there were many differences in amino acid levels between the treadmill-running and immobilization groups.
Fig. 2.
Alterations in plasma amino acid concentrations after each experimental treatment in the light (A-C) and dark (D-F) phases. The concentrations of each amino acid are shown as a percentage ratio relative to the concentrations in the control group (n=5–13). A, D: Results for the treadmill-running group (red lines), B, E: wheel-running group (green lines), C, F: immobilization group (yellow lines) and control group (black lines). Amino acids indicated in red showed significant differences vs. the control group (P<0.05).
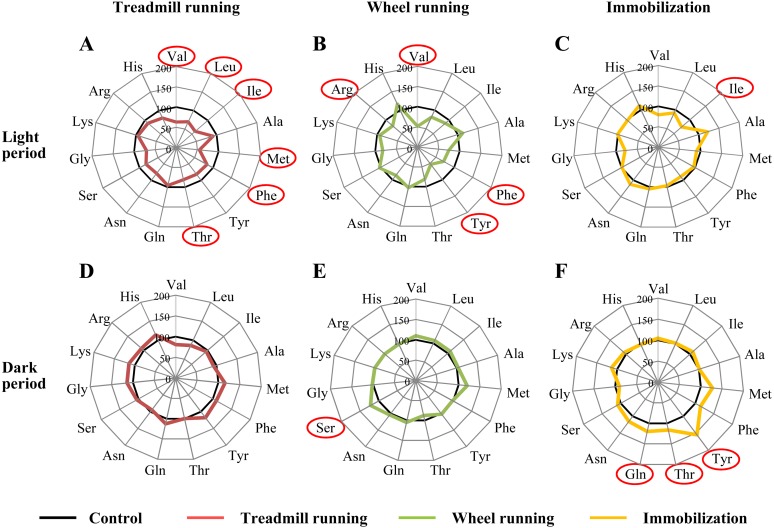
Fig. 3.
Alterations in CSF amino acid concentrations after each experimental treatment in the light (A-C) and dark (D-F) phases. The concentrations of each amino acid are shown as a percentage ratio relative to the concentrations in the sedentary control group (n=4–13). A, D: Results for the treadmill-running group (red lines), B, E: wheel-running group (green lines), C, F: immobilization group (yellow lines) and control group (black lines). Amino acids indicated in red showed significant differences vs. the control group (P<0.05). Pro, Trp, Cys, Asp and Glu were not detectable.
Table 1. Alterations in plasma amino acid concentrations during the light period.
| Amino acids (µmol/l) | Control | Treadmill | Wheel running | Immobilization | P value |
|---|---|---|---|---|---|
| Valine | 164.3 ± 4.3 | 188.2 ± 10.6 | 166.4 ± 5.1 | 156.6 ± 3.7# | 0.0084 |
| Leucine | 109.8 ± 4.6 | 152.2 ± 17.3* | 118.8 ± 4.1# | 84.7 ± 6.6# | 0.0003 |
| Isoleucine | 77.5 ± 7.4 | 100.3 ± 5.9* | 72.6 ± 2.6# | 92.3 ± 6.4# | <0.0001 |
| Alanine | 341.0 ± 9.9 | 397.5 ± 28.2 | 417.4 ± 17.6 | 315.6 ± 17.5#$ | 0.0027 |
| Methionine | 60.5 ± 1.8 | 49.2 ± 3.6* | 56.5 ± 2.1 | 51.6 ± 2.6 | 0.0181 |
| Proline | 314.4 ± 16.8 | 341.0 ± 31.8 | 195.4 ± 5.1*# | 206.1 ± 15.2*# | <0.0001 |
| Tryptophan | 72.7 ± 1.7 | 81.9 ± 4.2 | 69.6 ± 2.4 | 65.3 ± 2.8# | 0.0022 |
| Phenylalanine | 59.7 ± 1.4 | 76.3 ± 5.8* | 51.3 ± 1.6# | 55.6 ± 1.4# | <0.0001 |
| Tyrosine | 84.2 ± 1.7 | 102.6 ± 6.3* | 67.8 ± 2.5*# | 72.1 ± 3.1# | <0.0001 |
| Threonine | 247.1 ± 4.6 | 291.7 ± 22.0 | 223.8 ± 10.3 | 213.9 ± 11.3 | 0.0012 |
| Glutamine | 434.5 ± 14.8 | 375.5 ± 12.5 | 551.6 ± 18.9# | 450.0 ± 18.4#$ | <0.0001 |
| Asparagine | 38.5 ± 2.2 | 22.4 ± 1.8* | 53.4 ± 2.5*# | 37.0 ± 1.4#$ | <0.0001 |
| Serine | 203.7 ± 3.1 | 257.6 ± 20.2* | 240.4 ± 11.4 | 162.1 ± 5.1#$ | <0.0001 |
| Glycine | 333.7 ± 8.9 | 375.8 ± 19.3 | 389.9 ± 13.5*# | 279.7 ± 7.6*# | <0.0001 |
| Cysteine | 10.6 ± 1.4 | 28.1 ± 9.3 | 6.3 ± 3.1# | 0.4 ± 0.4# | 0.002 |
| Lysine | 349.7 ± 10.1 | 380.3 ± 21.8 | 362.1 ± 13.3 | 292.2 ± 7.7*#$ | 0.0003 |
| Arginine | 38.5 ± 2.2 | 50.5 ± 9.6 | 147.8 ± 8.8*# | 61.1 ± 10.5$ | <0.0001 |
| Histidine | 57.2 ± 2.0 | 51.1 ± 3.1 | 59.5 ± 1.8 | 52.5 ± 1.4 | 0.0491 |
| Aspartate | 10.4 ± 0.7 | 39.6 ± 8.0* | 8.9 ± 1.0# | 6.1 ± 0.6# | <0.0001 |
| Glutamate | 162.3 ± 5.7 | 248.6 ± 25.5* | 103.9 ± 7.1*# | 133.7 ± 5.2# | <0.0001 |
The data represent the mean ± standard error of the mean (SEM, n=8–13). *; P<0.05 vs Control, #; P<0.05 vs Treadmill running, $; P<0.05 vs Wheel running, as identified by Tukey’s test.
The differences in the levels of individual amino acids between the experimental groups and the control group during the dark period are shown in Fig. 2D–2F and Table 2 (n=5–12/group). In the treadmill-running group, Ala was decreased (Fig. 2D); in the wheel-running group and immobilization stress group, there were no significant changes relative to the control group (Fig. 2E and 2F). There were no common changes in amino acid levels among the groups, but the levels of 3 amino acids (Val, Leu and Ile) were lower in the immobilization stress group than in the wheel-running group.
Table 2. Alterations in plasma amino acid concentrations during the dark period.
| Amino acids (µmol/l) | Control | Treadmill | Wheel running | Immobilization | P value |
|---|---|---|---|---|---|
| Valine | 149.6 ± 5.3 | 127.9 ± 4.5 | 159.1 ± 15.9 | 121.0 ± 7.2$ | 0.0297 |
| Leucine | 106.4 ± 4.8 | 95.5 ± 5.2 | 114.0 ± 11.1 | 84.5 ± 6.0$ | 0.0412 |
| Isoleucine | 62.8 ± 2.5 | 56.1 ± 2.9 | 69.0 ± 6.1 | 50.0 ± 3.3$ | 0.0131 |
| Alanine | 386.8 ± 24.0 | 245.3 ± 11.6* | 320.4 ± 29.3 | 308.5 ± 12.1 | 0.0035 |
| Methionine | 57.7 ± 2.9 | 49.6 ± 4.2 | 58.6 ± 5.8 | 57.4 ± 3.9 | 0.5722 |
| Proline | 199.5 ± 8.8 | 149.2 ± 5.3 | 195.8 ± 24.7 | 172.8 ± 6.8 | 0.1038 |
| Tryptophan | 61.3 ± 3.1 | 59.2 ± 7.4 | 69.9 ± 6.5 | 74.8 ± 5.3 | 0.1513 |
| Phenylalanine | 54.3 ± 2.0 | 48.9 ± 2.8 | 52.7 ± 5.0 | 60.8 ± 7.2 | 0.4162 |
| Tyrosine | 52.4 ± 2.2 | 47.5 ± 2.7 | 50.0 ± 5.3 | 63.6 ± 6.1 | 0.0877 |
| Threonine | 207.4 ± 7.7 | 162.2 ± 8.8 | 177.3 ± 18.3 | 177.8 ± 8.1 | 0.0535 |
| Glutamine | 526.4 ± 18.6 | 459.3 ± 23.7 | 508.0 ± 45.2 | 562.6 ± 28.5 | 0.2246 |
| Asparagine | 37.9 ± 2.1 | 27.5 ± 1.7 | 34.7 ± 3.9 | 39.8 ± 3.2 | 0.0809 |
| Serine | 220.9 ± 8.0 | 186.9 ± 10.8 | 228.6 ± 23.3 | 190.6 ± 9.5 | 0.1273 |
| Glycine | 329.5 ± 13.8 | 285.6 ± 17.1 | 310.7 ± 30.1 | 299.2 ± 3.9 | 0.4094 |
| Cysteine | 4.6 ± 0.7 | 4.7 ± 0.8 | 1.9 ± 0.3 | 5.6 ± 1.2$ | 0.0267 |
| Lysine | 279.5 ± 8.0 | 248.0 ± 14.7 | 262.6 ± 23.5 | 262.4 ± 8.4 | 0.5038 |
| Arginine | 85.9 ± 15.2 | 81.0 ± 24.3 | 79.5 ± 12.6 | 86.7 ± 4.1 | 0.9821 |
| Histidine | 58.3 ± 1.4 | 49.4 ± 2.0 | 55.7 ± 4.9 | 58.3 ± 3.3 | 0.2798 |
| Aspartate | 6.6 ± 1.7 | 5.0 ± 0.9 | 7.6 ± 1.3 | 5.6 ± 0.8 | 0.6953 |
| Glutamate | 108.1 ± 4.1 | 127.0 ± 6.0 | 127.0 ± 11.5 | 107.6 ± 3.3 | 0.0765 |
The data represent the mean ± standard error of the mean (SEM, n=5–12). *; P<0.05 vs Control, $; P<0.05 vs Wheel running, as identified by Tukey’s test.
In addition to the concentrations of amino acids in plasma, we examined those in CSF and derived the amino acid profiles (Fig. 3). Because the levels of amino acids are lower in CSF than in plasma, some were not detectable in CSF (Pro, Trp, Cys, Asp and Glu). During the light period (n=8–13/group, Fig. 3A–3C, Table 3), the levels of many amino acids were decreased in the treadmill-running group (Val, Leu, Ile, Met, Phe and Thr), but none showed increased concentrations (Fig. 3A). In the wheel-running group, the levels of 4 amino acids (Val, Phe, Tyr and Arg) were lower than in the control group (Fig. 3B), and in the immobilization stress group, the only significant change was a decrease in the concentration of Ile (Fig. 3C). A change in the level of Ile was detected in both the treadmill-running and immobilization stress groups.
Table 3. Alterations in CSF amino acid concentrations during the light period.
| Amino acids (µmol/l) | Control | Treadmill | Wheel running | Immobilization | P value |
|---|---|---|---|---|---|
| Valine | 5.7 ± 0.6 | 3.6 ± 0.3* | 3.0 ± 0.3* | 4.6 ± 0.5 | 0.0013 |
| Leucine | 5.3 ± 0.2 | 3.7 ± 0.2* | 4.3 ± 0.3 | 4.9 ± 0.4# | 0.0016 |
| Isoleucine | 2.1 ± 0.1 | 1.3 ± 0.1* | 1.9 ± 0.1# | 1.6 ± 0.2* | 0.0003 |
| Alanine | 56.0 ± 2.9 | 51.3 ± 2.5 | 62.8 ± 2.6 | 68.4 ± 6.0# | 0.0133 |
| Methionine | 5.2 ± 0.4 | 2.9 ± 0.4* | 3.9 ± 0.2 | 4.7 ± 0.8 | 0.0125 |
| Proline | 1.3 ± 0.3 | 0.3 ± 0.1 | ND | 1.0 ± 0.1 | |
| Tryptophan | 1.9 ± 0.3 | 2.1 ± 0.3 | ND | 1.7 ± 0.3 | |
| Phenylalanine | 5.5 ± 0.2 | 4.6 ± 0.2* | 3.9 ± 0.1* | 5.3 ± 0.4$ | 0.0005 |
| Tyrosine | 9.7 ± 0.5 | 7.9 ± 0.5 | 5.1 ± 0.3*# | 8.7 ± 0.8$ | <0.0001 |
| Threonine | 67.2 ± 2.9 | 54.6 ± 1.8* | 54.8 ± 3.6 | 64.9 ± 4.7 | 0.0132 |
| Glutamine | 493.9 ± 18.9 | 473.3 ± 11.4 | 510.1 ± 12.0 | 516.8 ± 28.1 | 0.3826 |
| Asparagine | 5.9 ± 0.3 | 4.7 ± 0.3 | 5.1 ± 0.3 | 6.6 ± 0.5# | 0.0015 |
| Serine | 66.8 ± 4.1 | 55.1 ± 2.1 | 69.3 ± 2.4 | 66.2 ± 4.7 | 0.0349 |
| Glycine | 9.9 ± 1.1 | 7.0 ± 0.3 | 8.1 ± 0.6 | 7.8 ± 1.0 | 0.0875 |
| Cysteine | 1.6 ± 0.2 | 1.5 ± 0.1 | ND | 1.3 ± 0.1 | |
| Lysine | 77.6 ± 2.6 | 73.7 ± 1.8 | 72.9 ± 3.8 | 78.4 ± 3.9 | 0.4982 |
| Arginine | 35.9 ± 1.5 | 32.2 ± 0.5 | 28.3 ± 0.8*# | 35.1 ± 2.2 | 0.008 |
| Histidine | 9.8 ± 0.8 | 7.8 ± 0.3 | 11.4 ± 0.5# | 10.9 ± 0.9# | 0.0032 |
| Aspartate | 1.5 ± 0.3 | 1.9 ± 0.2 | ND | 1.8 ± 1.0 | |
| Glutamate | 6.3 ± 1.4 | 3.3 ± 0.6 | ND | 6.8 ± 2.7 |
The data represent the mean ± standard error of the mean (SEM, n=8–13). *; P<0.05 vs Control, #; P<0.05 vs Treadmill running, $; P<0.05 vs Wheel running, as identified by Tukey’s test.
During the dark period (n=4–12/group, Fig. 3D–3F, Table 4), few changes were evident among the experimental groups when compared with the light period. Treadmill running produced no specific change (Fig. 3D), wheel running resulted in increased levels of Ser (Fig. 3E), and immobilization stress led to increased levels of Tyr, Thr and Gln (Fig. 3F).
Table 4. Alterations in CSF amino acid concentrations during the dark period.
| Amino acids (µmol/l) | Control | Treadmill | Wheel running | Immobilization | P value |
|---|---|---|---|---|---|
| Valine | 2.7 ± 0.1 | 2.2 ± 0.2 | 3.0 ± 0.1# | 2.8 ± 0.2 | 0.0421 |
| Leucine | 4.1 ± 0.2 | 3.5 ± 0.2 | 4.4 ± 0.2# | 4.2 ± 0.4 | 0.0526 |
| Isoleucine | 1.8 ± 0.1 | 1.7 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.2 | 0.3907 |
| Alanine | 57.1 ± 2.2 | 53.1 ± 0.4 | 59.0 ± 1.8 | 57.8 ± 3.7 | 0.4347 |
| Methionine | 2.9 ± 0.3 | 3.3 ± 0.6 | 3.4 ± 0.3 | 3.7 ± 0.2 | 0.4238 |
| Proline | ND | ND | ND | ND | |
| Tryptophan | ND | ND | ND | ND | |
| Phenylalanine | 4.3 ± 0.2 | 4.7 ± 0.2 | 4.2 ± 0.1 | 4.9 ± 0.2 | 0.1255 |
| Tyrosine | 4.3 ± 0.3 | 5.1 ± 0.6 | 4.4 ± 0.3 | 6.7 ± 0.4*$ | 0.0022 |
| Threonine | 52.0 ± 1.5 | 51.8 ± 2.6 | 45.5 ± 1.6 | 60.3 ± 2.9*$ | 0.0011 |
| Glutamine | 455.5 ± 13.3 | 512.8 ± 22.2 | 478.0 ± 15.6 | 545.8 ± 7.8* | 0.0066 |
| Asparagine | 5.0 ± 0.2 | 4.8 ± 0.2 | 5.2 ± 0.4 | 5.8 ± 0.5 | 0.242 |
| Serine | 62.1 ± 1.9 | 64.8 ± 2.8 | 75.7 ± 1.3*# | 67.3 ± 1.4 | 0.0002 |
| Glycine | 7.4 ± 0.5 | 8.4 ± 0.5 | 7.6 ± 0.5 | 6.7 ± 0.6 | 0.4248 |
| Cysteine | ND | ND | ND | ND | |
| Lysine | 63.9 ± 2.4 | 73.0 ± 3.6 | 64.3 ± 1.7 | 72.6 ± 7.3 | 0.1176 |
| Arginine | 28.5 ± 0.9 | 30.4 ± 0.6 | 27.8 ± 1.2 | 30.2 ± 1.6 | 0.3598 |
| Histidine | 11.6 ± 0.6 | 13.2 ± 0.3 | 11.3 ± 0.3 | 11.4 ± 0.5 | 0.1327 |
| Aspartate | ND | ND | ND | ND | |
| Glutamate | ND | ND | ND | ND |
The data represent the mean ± standard error of the mean (SEM, n=4–12). *; P<0.05 vs Control, #; P<0.05 vs Treadmill running, $; P<0.05 vs Wheel running, as identified by Tukey’s test.
DISCUSSION
To our knowledge, this is the first reported study to have investigated and demonstrated changes in the levels of individual amino acids in both plasma and CSF after different types of short-term exercise.
The concentrations of plasma glucose, total protein, triglycerides and total cholesterol were not significantly changed by these types of exercise, but obvious changes were evident in the amino acid profiles. It is well known that glucose and lipid are used as an energy source for high-intensity or prolonged exercise [9], and the exercise used in this study was mild. However, under such conditions, it was interesting that the plasma levels of amino acids tended to alter rather readily. A greater number of amino acids showed a change in their levels during the light period, and the degree of change differed among the groups. The basal levels of many amino acids were higher during the dark period (active phase) than during the light period (resting phase). Although the reason for the greater change in amino acid levels resulting from exercise during the light period compared with the dark period is unclear, sudden exercise during the light period (resting period) might elicit an abrupt change in metabolism by nervous control, such as from parasympathetic regulation in the resting phase to sympathetic regulation. Generally, it has been shown that physical exercise causes amino acid imbalance by promoting proteolysis relative to protein synthesis in skeletal muscle, which leads to a decrease in plasma BCAA levels [1, 5]. Conversely, in the case of short-term forced exercise, it has been reported that plasma BCAA levels increase [14]. In the present study also, treadmill running led to a significant increase of both Leu and Ile. On the other hand, wheel-running exercise elicited no significant change in BCAA levels, suggesting that the physical or mental impact of forced exercise on the body differs significantly from that of voluntary exercise.
In addition, among the amino acids involved in the urea cycle, Asp and Arg were specifically increased by treadmill running and wheel running, respectively. Ornithine was increased by both forms of exercise (data not shown). Although it is thought that both types of exercise activate the urea cycle, there may be a difference in the strength and speed of the reaction.
Because the concentrations of amino acids in the CSF were very low in comparison to those in the plasma, some amino acids were undetectable. Amino acid metabolism in the brain is essential for the construction and regeneration of cell membranes and organelles and plays an important role in the synthesis of neurotransmitters, neuropeptide proteins and enzymes. Although the absolute levels of amino acids in CSF may differ from those in plasma, it has been generally considered that the amino acid profiles in the two compartments are correlated. However, no such correlation was found in the present study. Plasma amino acids are transferred to brain across the blood-brain barrier (BBB) through the various amino acid transporters. Their transfer varies depending on kinds of amino acids or its transporters. Furthermore, the observation that the concentrations of amino acids in the CSF were very lower than those in the plasma suggests that the mechanism of maintenance of the amino acid concentrations in CSF may be much more strict.
During the light period, the most characteristic change was a decrease of all amino acids in the CSF. The observation that treadmill running during the light period led to an increase of BCAA levels in plasma with a converse decrease of those in CSF was of particular interest. Although the reason for this decrease is unclear, it is known that exercise increases the levels of glutamate and glutamine in the brain [10]. Large neutral amino acids in plasma, including BCAA, are transferred to the brain by counter-transport with glutamine [17]. Especially, transamination of BCAA is a major source of –NH2 for synthesis of glutamate. Therefore, the BCAA in CSF might be used for synthesis of glutamate. Furthermore, when we compared the profiles of plasma amino acids during the light period between the treadmill-running, wheel-running and immobilization stress groups, they all differed from each other. That is, many different kinds of amino acids were increased by treadmill running and wheel running, whereas most were decreased by immobilization. It can be speculated that proteolysis (catabolism) caused by physical exercise in compartments, such as skeletal muscle, would lead to an increased flow of free amino acids to the liver via the bloodstream for use in gluconeogenesis or lipid metabolism. However, in the case of immobilization stress, it can be speculated that amino acids are mobilized from the available pool and that anabolism is promoted in the brain and other areas. In both the treadmill-running and wheel-running groups, on the other hand, the levels of different kinds of amino acids were changed. As a result of treadmill running, Leu, Ile, Phe, Tyr, Ser, Asp and Glu were increased, whereas wheel running increased the levels of Asn, Gly and Arg. These results suggest that even if the running distance is the same, forced running and voluntary running may influence amino acid metabolism in different ways. Treadmill running may induce greater physical fatigue than wheel running, because the change in the amino acid profile induced by the former resembles that induced by long-term exercise.
The amino acid profile shown in the present study might be effective to compare the general change of amino acid, and there may be possibility that amino acid, such as Asp and Cys, having very low basal levels may be misunderstood as large change by variation of each value. Further improvement of profiling of amino acid may be necessary.
In the present study, we showed that different types of exercise (forced and voluntary) cause different patterns of change in the levels of amino acids in plasma and CSF. Furthermore, the amino acid profile resulting from treadmill running differed from that induced by immobilization stress. These results suggest that the changes in amino acid levels induced by treadmill running are not solely attributable to the mental stress associated with forced exercise. Rather, they may be due to the differences in interaction between physical and mental fatigue. Moreover, no correlation was found between amino acid levels in plasma and those in CSF. These results suggest that the levels of amino acids in CSF are not always affected by a change in peripheral plasma levels. Further investigations will be necessary to clarify the factors responsible for imbalances in amino acid levels, and how amino acid homeostasis is maintained.
Acknowledgments
ACKNOWLEDGMENT. This study was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan and by JST, CREST.
REFERENCES
- 1.Dohm G. L., Kasperek G. J., Tapscott E. B., Beecher G. R.1980. Effect of exercise on synthesis and degradation of muscle protein. Biochem. J. 188: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohm G. L., Beecher G. R., Warren R. Q., Williams R. T.1981. Influence of exercise on free amino acid concentrations in rat tissues. J. Appl. Physiol. 50: 41–44. [DOI] [PubMed] [Google Scholar]
- 3.Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr1970. Plasma amino acid levels in diabetic ketoacidosis. Diabetes 19: 727–728. doi: 10.2337/diab.19.10.727 [DOI] [PubMed] [Google Scholar]
- 4.Holm E., Sedlaczek O., Grips E.1999. Amino acid metabolism in liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2: 47–53. doi: 10.1097/00075197-199901000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Ji L. L., Miller R. H., Nagle F. J., Lardy H. A., Stratman F. W.1987. Amino acid metabolism during exercise in trained rats: the potential role of carnitine in the metabolic fate of branched-chain amino acids. Metabolism 36: 748–752. doi: 10.1016/0026-0495(87)90111-9 [DOI] [PubMed] [Google Scholar]
- 6.Jin G., Kataoka Y., Tanaka M., Mizuma H., Nozaki S., Tahara T., Mizuno K., Yamato M., Watanabe Y.2009. Changes in plasma and tissue amino acid levels in an animal model of complex fatigue. Nutrition 25: 597–607. doi: 10.1016/j.nut.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 7.Kasperek G. J.1989. Regulation of branched-chain 2-oxo acid dehydrogenase activity during exercise. Am. J. Physiol. 256: E186–E190. [DOI] [PubMed] [Google Scholar]
- 8.Lai H. S., Lee J. C., Lee P. H., Wang S. T., Chen W. J.2005. Plasma free amino acid profile in cancer patients. Semin. Cancer Biol. 15: 267–276. doi: 10.1016/j.semcancer.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Lampman R. M., Schteingart D. E.1991. Effects of exercise training on glucose control, lipid metabolism, and insulin sensitivity in hypertriglyceridemia and non-insulin dependent diabetes mellitus. Med. Sci. Sports Exerc. 23: 703–712. doi: 10.1249/00005768-199106000-00009 [DOI] [PubMed] [Google Scholar]
- 10.Maddock R. J., Casazza G. A., Buonocore M. H., Tanase C.2011. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H-MRS study. Neuroimage 57: 1324–1330. doi: 10.1016/j.neuroimage.2011.05.048 [DOI] [PubMed] [Google Scholar]
- 11.Narath E., Skalicky M., Viidik A.2001. Voluntary and forced exercise influence the survival and body composition of ageing male rats differently. Exp. Gerontol. 36: 1699–1711. doi: 10.1016/S0531-5565(01)00145-0 [DOI] [PubMed] [Google Scholar]
- 12.Okamura K., Matsubara F., Yoshioka Y., Kikuchi N., Kikuchi Y., Kohri H.1987. Exercise-induced changes in branched chain amino acid/aromatic amino acid ratio in the rat brain and plasma. Jpn. J. Pharmacol. 45: 243–248. doi: 10.1254/jjp.45.243 [DOI] [PubMed] [Google Scholar]
- 13.Ra S. G., Miyazaki T., Ishikura K., Nagayama H., Komine S., Nakata Y., Maeda S., Matsuzaki Y., Ohmori H.2013. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 10: 51. doi: 10.1186/1550-2783-10-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahlin K., Katz A., Broberg S.1990. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 259: C834–C841. [DOI] [PubMed] [Google Scholar]
- 15.Shimbo K., Oonuki T., Yahashi A., Hirayama K., Miyano H.2009. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 23: 1483–1492. doi: 10.1002/rcm.4026 [DOI] [PubMed] [Google Scholar]
- 16.Shimomura Y., Inaguma A., Watanabe S., Yamamoto Y., Muramatsu Y., Bajotto G., Sato J., Shimomura N., Kobayashi H., Mawatari K.2010. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 20: 236–244. [DOI] [PubMed] [Google Scholar]
- 17.Smith Q. R.2000. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 130Suppl: 1016S–1022S. [DOI] [PubMed] [Google Scholar]