Abstract
Chlamydia trachomatis is the most important infectious cause of infertility in women with important implications in public health and for which a vaccine is urgently needed. Recent immunoproteomic vaccine studies found that four polymorphic membrane proteins (PmpE, PmpF, PmpG and PmpH) are immunodominant, recognized by various MHC class II haplotypes and protective in mouse models. In the present study, we aimed to evaluate genetic and protein features of Pmps (focusing on the N-terminal 600 amino acids where MHC class II epitopes were mapped) in order to understand antigen variation that may emerge following vaccine induced immune selection. We used several bioinformatics platforms to study: i) Pmps’ phylogeny and genetic polymorphism; ii) the location and distribution of protein features (GGA(I, L)/FxxN motifs and cysteine residues) that may impact pathogen-host interactions and protein conformation; and iii) the existence of phase variation mechanisms that may impact Pmps’ expression. We used a well-characterized collection of 53 fully-sequenced strains that represent the C. trachomatis serovars associated with the three disease groups: ocular (N=8), epithelial-genital (N=25) and lymphogranuloma venereum (LGV) (N=20). We observed that PmpF and PmpE are highly polymorphic between LGV and epithelial-genital strains, and also within populations of the latter. We also found heterogeneous representation among strains for GGA(I, L)/FxxN motifs and cysteine residues, suggesting possible alterations in adhesion properties, tissue specificity and immunogenicity. PmpG and, to a lesser extent, PmpH revealed low polymorphism and high conservation of protein features among the genital strains (including the LGV group). Uniquely among the four Pmps, pmpG has regulatory sequences suggestive of phase variation. In aggregate, the results suggest that PmpG may be the lead vaccine candidate because of sequence conservation but may need to be paired with another protective antigen (like PmpH) in order to prevent immune selection of phase variants.
Introduction
Chlamydia trachomatis is an obligate intracellular human bacterial pathogen, comprised of 15 to 18 major serovars. Serovars A-C are responsible for ocular infections that result in trachoma [1]. Serovars D-K cause sexually transmitted diseases such as cervicitis and pelvic inflammatory disease (PID), and globally are an important infectious cause of infertility and ectopic pregnancy in women [2]. Serovars L1-L3 also enter through the ano-urogenital tract, but may dissiminate via infection of macrophages to regional draining lymph nodes, causing lymphogranuloma venereum (LGV) [3]. C. trachomatis is the major bacterial cause of sexually transmitted infections (STIs), accounting for ~106 million of the 500 million new cases of STIs that occur each year worldwide [4]. In Europe, almost half of the estimated 47 million STI cases are due to C. trachomatis [4], while nearly 1.4 million infections are reported each year in the United States [5]. Although the estimated global economic burden is uncalculated, over $516 million are spent annually in direct medical costs on genital chlamydial infections in the United States, making C. trachomatis the most costly infection among the nonviral STIs [6–7].
Despite screening and treatment public health programs to control Chlamydia, the incidence of C. trachomatis infection has increased [4, 8–10]. Thus, there is an urgent need for an efficacious vaccine that prevents acquisition and transmission of infection and the development of pelvic inflammatory disease sequelae. Cumulative studies in animal models and human infections [8, 11–23] have shown that systemic and mucosal CD4 T cell-mediated immunity is necessary for protection against C. trachomatis infection. Among antigen candidates that have been studied [11, 15, 24], members of the polymorphic membrane protein family (PmpA-I) have shown promise as vaccine components as they are dominant antigenic targets for cellular immune responses [25–29]. Four of the nine Pmps (PmpE, PmpF, PmpG and PmpH) have been identified via immunoproteomics as dominant T-cell antigens with multiple MHC class II binding peptides for both C. trachomatis and C. muridarum and observed to be protective in the murine genital tract infection model [25, 28–30]. The fact that each Pmp generates different peptides recognized by different MHC class II haplotypes confers on them the capability of immunizing outbred populations [25]. In the murine model, a PmpG epitope was found to persist on splenic antigen presenting cells for at least 6 months [26]. Tetramer staining also demonstrated PmpG as one of the quantitatively dominant antigens recognized by murine CD4 T cells [17].
Pmps are Chlamydia-specific outer membrane proteins whose precise functions remain unknown, but which have been implicated in pathogenesis and host cell adherence. As typical type V autotransporters [31–32], Pmps are capable of translocating to the bacterial surface their N-terminal Sec-dependent leader sequence (passenger domain), containing multiple short repetitive motifs (GGA(I, L, V) and FxxN) [33]. The proteins may also undergo complex infection-dependent post-translational proteolytic processing [34–37]. These proteins mediate in vitro chlamydial attachment to human epithelial and endothelial cells [34, 38]. Previous bioinformatics analyses suggested that six of the 9 pmps are under positive selection either driving bacterial adaptation to specific niches (ocular conjunctiva, epithelial-genitalia and lymph nodes), or during a strains’ diversification within a particular niche [39]. Numerous mutations within bioinformatically predicted HLA class I and II T-cell epitopes for the N-terminal domain for one Pmp (PmpF) have already been identified [40]. Pmps were also found in tissue culture to be variably expressed at the chlamydial cell surface, where each protein seemed subjected to an independent high-frequency on/off switching at the inclusion level [41].
Therefore we aimed to bioinformatically assess the allelic and phase variation of these antigens, in particular for the four most promising vaccine candidates PmpE, PmpF, PmpG and PmpH, in order to understand if putative antigenic escape variants may emerge following vaccination. We performed a detailed bioinformatic analysis using a well-characterized collection of 53 strains whose genomes have been fully sequenced [42–43] and which represent C. trachomatis serovars associated with the three canonical disease groups: ocular, epithelial-genital and LGV. By encompassing strains from temporally and geographically diverse sources around the world, this collection should capture C. trachomatis genetic diversity. Overall, we analyzed 25 strains from epithelial-genital serovars, 20 strains from LGV serovars, and eight strains from ocular serovars.
Materials and Methods
Genetic and phylogenetic analyses
In order to assess the genetic variability of PmpE, PmpF, PmpG and PmpH within C. trachomatis, 53 genome sequences representative of distinct or same-serovar strains were retrieved from GenBank and aligned using the progressiveMauve algorithm of Mauve 2.3.1 [44] with the default parameters and an initial match seed weight appropriate for 1MB genomes. Basically, Progressive Mauve performs a recursive anchor search and a full gapped anchored alignment of the genome sequences using a modified MUSCLE algorithm. For all pmps, individual alignments were extracted from the whole-genome alignment, and visually inspected with MEGA6 software (http://www.megasoftware.net) for further correction.
For each pmp, MEGA6 was also used to estimate the number of gene variable sites and to compute overall mean distances and matrices of pairwise comparisons at both nucleotide and protein level, based on the number of differences and p-distance value (that calibrates the obtained differences relative to the total number of sites under comparison) among the 53 strains, along with the respective standard error estimates (bootstrap = 1000). Evaluation of variable sites and mean genetic distances was also performed within and between the three disease groups. We also evaluated the impact of the genetic heterogeneity of each group of strains in the overall polymorphism of each pmp, through a sliding-window analysis using the DNA polymorphism tool of the DnaSP software, version 5 [45], with a window size and step size of 15.
Phylogenetic relationships among strains were inferred with MEGA 6, by using the Neighbor-Joining method [46] in conjunction with a bootstrap re-sampling strategy (1000 replicates), as previously described [47]. Evolutionary nucleotide distances were estimated with the Kimura 2-parameter (K2P) model [48] that takes into account transitional and transversional substitution rates, while assuming identical nucleotide frequencies and invariable substitution rates among sites [49]. At the protein level, evolutionary distances were computed based on the number of differences. Because of the different lengths of the sequences, the pairwise-deletion option was chosen to remove all sites containing missing data or alignment gaps from all distance estimations, only when the need arose and not prior to the analysis.
In order to identify specific regions that most contribute to the phylogenetic segregation of taxa within each Pmp, we evaluated the similarity both among the 53 strains and between disease groups. Briefly, SimPlot 3.5.1 software [50] was used to plot a codon-based nucleotide similarity score of each strain against a particular query by estimating pairwise distances with K2P model, without excluding gaps among sequences and considering a transition-transversion substitution rate of 2. The similarity estimations were performed in a sliding window size that ranged from 40 bp for pmpG to 80 bp for pmpF (adjusted according to polymorphism degree), moved across the alignment in a step size of 3 bp. In parallel, SWAAP 1.0.3 software [51] was used to compute the percentage of amino acid (a.a) identity among each pair of sequences throughout all Pmps, over a sliding window that ranged from 20 a.a for both PmpG and PmpH to 40 a.a for PmpF, and a step size of 3 a.a.
These analyses were also performed using gene segments encoding the first ~600 amino acids of each Pmp, as they are part of the surface exposed passenger domain where all MHC class II binding peptides were experimentally mapped [25] (see Results and Discussion section).
Analysis of protein features
Pmps are predicted to have adhesin functions [34, 38, 52]. By using the SeqBuilder module of LaserGene (DNASTAR) we therefore assessed the differential presence among all 53 strains of the repetitive GGA(I, L, V) and FxxN motifs as well as of cysteine residues in each Pmp. Moreover, to shed light on the putative impact of Pmps’ variability on surface probability and immunogenicity, we used the Protean program (DNASTAR) to perform a comparative analysis of the protein sequences. While the surface probability parameter predicts the likelihood of a given region lying on the surface of a protein using the approach of Emini et al. [53], the analysis of immunogenicity uses the approach of Jameson and Wolf [54] that combines several methods for protein structural features (like hydrophilicity, surface probability, flexibility and secondary structure) to predict potential antigenic determinants. For both analyses, the default parameters of each method were used, with surface regions predicted by forming the product of residue specific surface propensities over a range of 5 amino acids and based on a surface decision threshold >1. In order to facilitate the latter analyses, we used one strain representative of the main branches of the respective tree generated with the 53 fully-sequenced genomes, as these strains accurately represent the Pmp genetic backbone of the remainder same-branch taxa. The ocular strain A/Har13, the two epithelial-genital strains D/UW3 and E/150, and the LGV strain L2b/UCH1 were selected as they were among the strains found to be commonly present in the different main branches of all phylogenetic trees. Considering that some Pmps have an additional divergent branch containing genital strains, we opted for including an extra epithelial-genital strain to increase the confidence of the analysis. Thus, the strain G/11074 was also used for the analysis of both PmpH and PmpG, while the strains G/11222 and E/SW2 were used for the analysis of PmpF and PmpE, respectively. After performing preliminary data, we observed that including other strains besides these ones, did not alter the final output. In order to increase the probability of observing relevant disparities among strains for surface probability and immunogenicity, we specially focused on regions falling below a similarity cut-off of 85% (based on SWAAP plots).
Analysis of phase variation
Analysis of phase variation was performed by using different approaches by considering several genetic features known to underlie phase variation mechanisms, such as short sequence repeats (homopolymeric and non-homopolymeric tracts), IS-like sequences, frameshift mutations, small indels, hairpin structures, RNase E cleavage sites, and promoter sequences [55–57]. First, we checked the differential presence of homopolymeric tracts and small indels among all 53 strains by using SeqBuilder (DNASTAR). For each operon, in silico promoter predictions were made by using both the Neural Network Promoter Prediction (NNPP, http://www.fruitfly.org/seq_tools/promoter.html) and the BPROM software (Softberry, http://linux1.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb), to find elements resembling putative σ66, σ28 and/or σ54 promoter sequences that may be differentially present among strains, and thus used as fine-tune transcriptional pmp regulators. Moreover, several putative regulatory elements that are known to affect transcription or translation were also searched throughout each pmp operon and associated regulatory regions using SeqBuilder (DNASTAR). Briefly, the differential existence of putative consensus cleavage sites for RNase E was examined among strains [58–60]. RNaseE is the major endonuclease that generally initiates mRNA degradation in most bacteria [61]. We also searched for the presence of putative Shine-Dalgarno ribosome binding sequences (RBS) [62] and previously described chlamydial RBS [63–66].
Results and Discussion
Global polymorphism analysis
In view of Chlamydiae’s reductive evolution, it is remarkable that the pmps vary in number across species and encompass a sizeable chromosome portion (3–5%) [67]. For instance, over 13% of the C. trachomatis specific coding capacity is restricted to this gene family [68]. The evaluation of the mean genetic distances among all 53 strains revealed that pmpF was the most polymorphic pmp gene exhibiting the highest mean nucleotide substitution (214.3 (SE 8.9)) and also the highest p-distance value (0.0691 (SE 0.0029)), which corresponded to a mean of 72.4 (SE 5.0) (7.0%) amino acid substitutions. This degree of polymorphism is followed by pmpH and pmpE with an overall nucleotide variability of 3.6% and 2.4%, respectively. pmpG was observed to be the least variable gene with a polymorphism 6.9-fold lower than that of pmpF. In fact, pmpG exhibited a mean of nucleotide substitutions among all 53 strains of 31.1 (SE 3.7) (1.0%), corresponding to a mean of 13.5 (SE 2.6) (1.3%) amino acid alterations. The high variability shown by some Pmps accords with a recent analysis encompassing ~98% of the C. trachomatis core genome [69] which placed pmpF, pmpE and pmpH within the top 20 most polymorphic chromosomal genes. The set of polymorphic genes includes other important antigens-coding genes, such as CT681/ompA and the paralogously related pmps CT049-CT051 [70–72]. Considering Pmps’ outer membrane localization and putative dual function in adhesion and pathogenesis, the high polymorphism is expected to promote multiple antigenic and/or adherence phenotypes that may influence strains’ pathogenic diversity and tissue specificity.
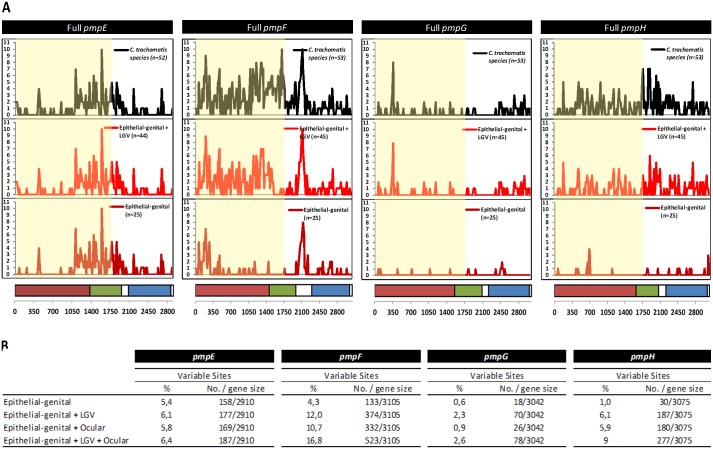
By performing a polymorphism sliding-window analysis throughout each gene (Fig 1), we observed that most of the genetic variability in pmpE is concentrated into the mid-region of the gene (~1050–2100 bp), while a greater nucleotide substitution density is seen within the first two-thirds of pmpF. In contrast, for both pmpG and pmpH, the overall variability appears to be homogeneously distributed throughout each gene, although a polymorphism peak is seen around position 350 for pmpG (corresponding to three amino acids that are specific of LGV strains) We also found distinct scenarios among the four pmps by evaluating the contribution of the different disease groups to the overall species polymorphism (Fig 1). For both pmpF and pmpH, the three disease groups seem to contribute to C. trachomatis diversity, as seen by the successive increase in the overall genetic variability after the independent addition of either LGV or ocular strains to the group of epithelial-genital strains. In fact, the number of variant sites found among epithelial-genital strains augmented ~3-fold for pmpF and ~6-fold for pmpH with addition of LGV strains, and an increase of 1.5-fold was seen after the subsequent inclusion of ocular strains for both genes. For pmpE, the overall species polymorphism is essentially provided by the genetic heterogeneity among the epithelial-genital strains, which harbor almost 85% of the total 187 variant sites found. The dissimilar mutational pattern observed for each pmp may imply differences in how each Pmp fulfills adhesive and antigenic functions in specific niches. Distinct regions were previously identified within pmpE, pmpF and pmpH, where clusters of mutations were found, and were associated with strains’ clustering by cell-tropism or ecological success [73]. In support of this notion, these four pmps were found to be differently targeted by positive selection in each niche, being potentially involved in one or more adaptive processes [39]. For instance, based on bioinformatic predictions that rely essentially on the distribution and exclusive character of nonsilent changes, pmpF is identified to encode specific cell tropism to both the ocular epithelium and mononuclear phagocytes. Besides niche-specific adaptation, some of the pmps were also predicted to evolutionarily impact strains’ niche-specific pathogenicity, as the case of pmpG and pmpH, which appear to contribute to the pathogenic diversity among LGV-causing strains [39]. Both pmpG and pmpH have over-accumulated nonsynonymous substitutions (about 4-fold more frequent than silent mutations) that result in protein variation among LGV strains [74]. Multiple factors may contribute to the genetic diversity of pmps, such as recombinational hotspots involving this large family of paralogous genes since multiple recombination hotspots have been identified throughout the genome [43, 75]. Overall, these data suggest the existence of variant tissue-specific host-interaction motifs that involve different Pmps.
Fig 1. Global polymorphism analysis.
A) Sliding-window analysis of the genetic variability throughout each pmp (window and step size of 15 bp). For each pmp, the black plot represents the polymorphism among all strain collection, while the remainder plots represent the impact of removing strains from the ocular group (red plot) and from the LGV group (magenta plot) on the global polymorphism. The region highlighted in yellow encompasses the 1st 600 amino acids (used in vaccine attempts) where further analyses were performed. The horizontal bars below the plots represent the typical domains of these autotransporter proteins: passenger domain (magenta), middle domain (green), and C-terminal autotransporter domain (blue). B) Impact in both the number and percentage of variable sites found among C. trachomatis strains after the successive addition of LGV and ocular strains to the epithelial-genital group.
Genetic and phylogenetic analyses of Pmps’ N-terminal 600 amino acid domains
Previous immunoproteomic studies using the murine genital infection model [25] revealed that PmpE, PmpF, PmpG and PmpH contain different MHC class II binding peptides in the N-terminal half for both C. muridarum and C. trachomatis serovar D with between one and three different peptides per MHC allele. Therefore, after an initial genetic analysis of the overall polymorphism for the entire genes, the subsequent analyses (genetic, phylogenetic, and study of protein features) were restricted to gene segments encoding the 1st ~600 amino acids of each Pmp that encompass both the surface exposed passenger and middle domains (Fig 1) where all MHC class II-bound peptides were experimentally mapped [25]. The selected gene segments harbor almost 75% of all variant sites estimated for the whole pmpF and pmpE genes and at least 50% of the variant sites for pmpG and pmpH.
The phylogenetic reconstructions based on the first 600 amino acids of each Pmp (S1 Fig), mirror the tree topologies for the whole proteins (S2 Fig) and support the correlation between pmp polymorphism and a strains’ phenotypic association with disease state. For instance, a perfect segregation of strains by full-tropism was observed for PmpH, with strains representative of the three disease groups appearing clustered in distinct separated clades. For all Pmps, the analysis of the matrices of pairwise distances revealed a low heterogeneity within both ocular and LGV strains (Table 1). In contrast, the average evolutionary divergence among the epithelial-genital group varies among Pmps, with PmpE and PmpF exhibiting the highest divergence, 14.5 a.a (SE 2.0) and 14.0 (SE 2.3), respectively, while PmpG and PmpH are much less polymorphic, displaying a polymorphism 7- to 9-fold lower. However, if LGV strains are included in a global group of genital strains, the average amino acid distances increases as epithelial-genital strains are highly dissimilar from LGV strains for some Pmps (Table 1). Overall, these results show that the 1st 600 a.a of PmpG is highly conserved in this 53 strain collection, even among all genital strains (epithelial-genital and LGV), for which the observed mean amino acid distances are about 2-, 3- and 7-fold lower than those seen for PmpH, PmpE and PmpF, respectively. Of note, the segment of the 1st 600 a.a of PmpH is also highly conserved if one considers solely epithelial-genital strains, where no more than 2 a.a differences are seen.
Table 1. Mean amino acid distances within the 1st 600 amino acids of each Pmp.
| PmpE | PmpF | PmpG | PmpH | |||||
|---|---|---|---|---|---|---|---|---|
| No. diff. | SE | No. diff. | SE | No. diff. | SE | No. diff. | SE | |
| Overall mean | 28,0 | 3,4 | 53,2 | 4,4 | 7,7 | 1,8 | 20,9 | 2,7 |
| Within Groups | ||||||||
| Ocular | 1,9 | 0,8 | 1,0 | 0,5 | 0,8 | 0,4 | 0,0 | 0,0 |
| Epithelial-genital | 14,5 | 2,0 | 14,0 | 2,3 | 1,7 | 0,9 | 2,0 | 0,8 |
| LGV | 0,0 | 0,0 | 0,0 | 0,0 | 0,6 | 0,3 | 0,8 | 0,4 |
| Genital (with LGV) | 19,5 | 2,5 | 52,2 | 4,7 | 7,4 | 1,8 | 16,9 | 2,7 |
| Between Groups | ||||||||
| Ocular / Epithelial-genital | 39,2 | 5,3 | 65,5 | 6,9 | 3,4 | 1,4 | 29,9 | 5,0 |
| Ocular / LGV | 47,4 | 6,3 | 111,0 | 8,9 | 11,6 | 3,1 | 45,5 | 6,3 |
| Epithelial-genital / LGV | 19,0 | 3,1 | 84,0 | 7,8 | 12,0 | 3,2 | 31,0 | 5,0 |
Analysis of peptide features
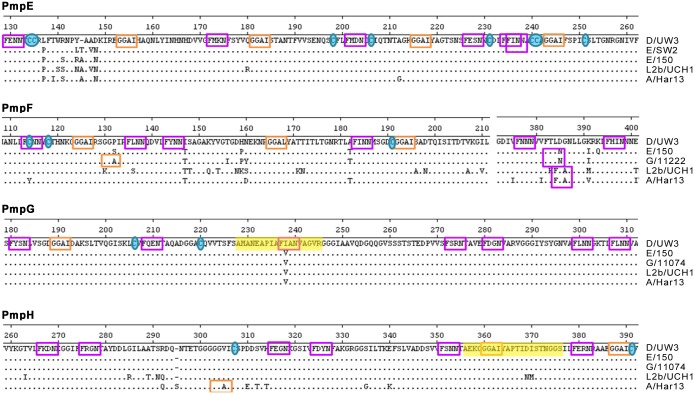
A hallmark of the chlamydial Pmp family is the presence of multiple repeats of the tetrapeptide motifs GGA(I, L, V) and FxxN in their N-terminal half. The repetition of these motifs is seen in very few non-chlamydial proteins, and has been suggested to be directly involved in adherence processes [33, 76]. Thus, considering that Pmps likely have adhesive functions [34, 38] and were found to be variably expressed at the chlamydial surface in vitro [41], we mapped the location and determined the number of the GGA(I, L, V) and FxxN as well as of the cysteine residues within the 1st 600 a.a of each Pmp for all 53 strains (Fig 2). We observed that the FxxN motif occurs on average 12.3-times per Pmp, being ~2-fold more frequent than GGAI motifs, which appear on average 5.8-times per Pmp. The mean incidence of FxxN in the remaining C. trachomatis proteome was shown to be 0.73 per protein, whereas GGAI is predicted to be present as single copy in only 10 other Chlamydia proteins [33]. No GGA(I, L, V) or FxxN motif is found outside the 1st 600 a.a for each Pmp, which is consistent with the exclusive presence of adhesion domains in protein regions that interact with the host. The number of conserved GGAIs varied among the four Pmps, ranging from two for PmpH to eight for PmpE. All Pmps displayed 11 conserved FxxN motifs; one of these fell within the MHC class II (I-Ab)-bound C. trachomatis serovar D-derived peptide (AMANEAPIAFIANVAG) (Fig 3) recently identified in PmpG [25].
Fig 2. Distribution of FxxN motifs (purple), GGA(I, L) motifs (orange), and cysteine residues (black) within the 1st 600 a.a (limited by the vertical dashed line) of each Pmp.
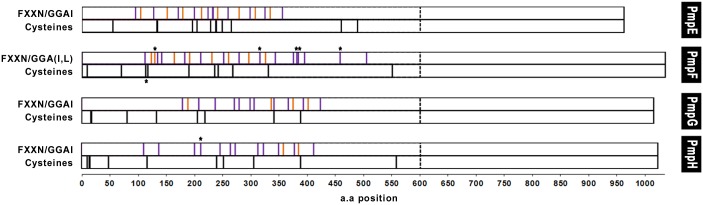
Asterisks indicate motifs nonconserved among all 53 strains.
Fig 3. Pmp alignments showing examples of protein regions containing high concentration of FxxN motifs (purple), GGA(I, L) motifs (orange), and cysteine residues (blue).
MHC class II (I-Ab)-bound C. trachomatis peptides are highlighted in yellow.
The distribution of the two types of peptide motifs is nonrandom, being mainly clustered within a discrete region that spans between ~220 a.a for PmpH to ~280 a.a for PmpF (Fig 2). Most of these motifs appear as doublets of FxxN-x2-25-FxxN and FxxN-x4-20-GGAI, whose differential occurrence, number and spacing vary both among Pmps and among different groups of strains for the same Pmp. They were also found to be in close proximity or adjacent to cysteine residues. The number of conserved cysteines ranges from eight for PmpG to 12 for PmpE. Considering that cysteines are known to play an important role in maintaining structural motifs, such GGAI/FxxN nonrandom distribution and possible cooperative action with the cysteine residues may yield unique structural or functional constraints for interaction with the host cell.
We also observed that the majority of the tetrapeptide motifs occurred in close proximity or fall within regions containing SNPs differentiating specific groups of strains (Fig 3), which may widen adhesion or immunogenic differences within each Pmp, as described for the major chlamydial membrane protein MOMP [77–79]. In support of this, a typical conserved FxxN-X5-GGAI doublet was found to overlap the MHC class II (I-Ab)-bound peptide (AEKGGGAIYAPTIDISTNGGS) identified in PmpH [25], which is conserved solely among ocular and epithelial-genital strains (Fig 3). This epitope is also in very close proximity to another conserved FxxN-X4-GGAI doublet and a conserved cysteine residue. Based on bioinformatic predictions, none of the three MHC class II (I-Ab)-bound C. trachomatis serovar D-derived peptides recently identified in PmpE, PmpG and PmpH [25] fall in regions that impact variability in immunogenicity and surface exposure of the respective proteins (S3 Fig). Although the precise function of the tetrapeptide motifs is yet unknown, it has been shown by using deletion analysis that Pmps’ adhesion capability requires at least the presence of one of these doublets [80]. Also, each Pmp was shown to exhibit in vitro a distinctive adhesion profile depending on the human cell type (epithelial versus endothelial) [34]. The mutational pattern of PmpE, PmpF, PmpG and PmpH was shown to be associated with high efficiency of in vitro attachment to host cells [81]. It has been hypothesized that these short repetitive motifs may be involved in maintaining the Pmp conformation that promotes adhesion, and/or might directly mediate interaction with human receptors [80]. Therefore, the wide variation of these repetitive motifs seen among intra- and inter-Pmps (Figs 2 and 3), is consistent with a role in the formation of niche-specific binding “receptors” for host-interaction.
Analysis of phase variation
Since pmps have been suggested to undergo phase variation-like mechanisms to promote multiple antigenic and/or adherence phenotypes [41], we searched for putative phase variation features on each gene [55–57]. In distinction to pmpE, pmpF and pmpH that reveal no poly(C) or poly(G) stretches, pmpG exhibits an in-frame poly(G) tract of nine residues for all non-LGV strains and eight residues for LGV strains (data not shown). This mirrors that found for C. pneumoniae pmpG family [33, 82–84], suggesting that slipped-strand phase variation [55, 57] may be a common phenotype for PmpG. However, it is not known if the observed poly(G) tract influences pmpG expression in C. trachomatis. Although all annotated genome sequences appear to possess a functional PmpG, we cannot be certain that C. trachomatis strains only harbor in-frame poly(G) tract in pmpG. In fact, it is known that this type of regions may yield biased data in some Next Generation Sequencing. So, it is possible that strains with dissimilar number of “G” (or even a mixture of clones showing different number of “G”) have not been properly annotated. Nevertheless, as only PmpG shows a poly-G tract, which may be involved in phase variation, and because its sequence is highly conserved among all genital strains (including LGV strains), this may be its major mechanism of antigen or niche variation. We also found a non-homopolymeric repetitive motif “PAPAPAPA” in PmpH for some epithelial-genital strains, which is shorter for other epithelial-genital strains and absent for both the LGV and ocular groups (data not shown). A Blast search for this motif was not informative about its putative role, as it seems to be commonly present in diverse proteins, such as regulatory proteins, acid-shock proteins and acetyl-CoA carboxylases, from different species. Hypothetically this genetic feature could be associated with phase variation and would exclusively impact PmpH immunogenicity of epithelial-genital strains. Although this hypothesis is speculative, if PmpH is used as a vaccine component, the use of peptides encompassing all “PA” combinations may be prudent.
It is known that the insertion-excision of mobile elements (like IS-elements) may also impact gene expression [55], and the presence of putative remnants of IS-like elements flanked by direct target repeats has already been described for both pmpB and pmpC [73]. However, no such elements were found in the four pmps under evaluation.
Past studies have shown that expression regulation of C. trachomatis genes is controlled at both transcriptional and translational levels, thus involving multiple complex aspects, like DNA supercoiling, heterogeneity within promoter sequences, cis- and trans-regulatory elements, and mRNA stability. It is known that various chlamydial genes are regulated by two or three different promoters, often with multiple σ factor binding (σ66, σ28 and/or σ54) [85]. We found putative σ66 promoters within both pmpFE and pmpGH regulatory regions by using in silico predictions (S1 and S2 Tables), but no elements resembling a σ28 or σ54 promoter sequence. In distinction to pmpFE, the most promoter predicted for the pmpGH operon contains a transcritptional start site (TSS) that has already been experimentally identified for the L2b/UCH1 strain [86], and it reveals a variable site in the -35 element that distinguishes LGV from epithelial-genital strains. Some variable nucleotide sites are also seen near the -10 elements, the TSSs, or A/T spacer, and several putative consensus RNase E cleavage sites were found throughout both operons and their regulatory regions. The polymorphisms found close or inside Shine-Dalgarno regulatory elements and the nonconservation of RNase E cleavge sites, may suggest heterogeneous expression among strains for both pmpFE and pmpGH operons, supporting previous experimental findings showing Pmp expression differences not only between L2 and E strains, but also within same-serovar strains [87].
Conclusions
Recent vaccine studies in the murine model [29, 88] provided evidence that a vaccine composed of PmpEFGH plus MOMP formulated with a Th1 polarizing adjuvant, was more immunogenic and cleared infection faster than a single antigen vaccine. The suitability of a Pmp-based vaccine will be influenced by antigenic variability displayed during infection of the genital tract. The in silico predictions from the present study suggest that PmpF and PmpE may be less reliable antigens for vaccine purposes due to high sequence polymorphism. This polymorphism, together with putative alterations in structural constraints provided by heterogenity among strains in GGA(I, L)/FxxN motifs and cysteine residues, also suggest a possible role in immunogenicity variability. By contrast, the low polymorphism and high conservation of protein features for PmpG and to a lesser extent for PmpH, suggest that these proteins may be better vaccine candidates. Since phase variation may impact PmpG expression a single-component PmpG subunit vaccine may not provide protection against infection due to phase variation. Based on bioinformatics analysis we suggest that pairing of PmpG with PmpH could be a viable approach, in order to provide a range of epitopes for CD4+ T cell recognition among different MHC genetic backgrounds and to provide cross-protection against multiple antigenic variants of C. trachomatis.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
AN is a recipient of a post-doctoral fellowship (SFRH/BPD/75295/2010) from Fundação para a Ciência e a Tecnologia (FCT).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
AN is a recipient of a post-doctoral fellowship (SFRH/BPD/75295/2010) from Fundação para a Ciência e a Tecnologia (FCT).
References
- 1. Vogel G. Infectious diseases. Tackling neglected diseases could offer more bang for the buck. Science. 2006;311(5761):592–593. doi: 311/5761/592a [pii] 10.1126/science.311.5761.592a . [DOI] [PubMed] [Google Scholar]
- 2. Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349(25):2424–2430. doi: 10.1056/NEJMcp030542 349/25/2424 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3. Schachter J. Chlamydial infections (first of three parts). N Engl J Med. 1978;298(8):428–435. . [DOI] [PubMed] [Google Scholar]
- 4. WHO. Global incidence and prevalence of selected curable sexually transmitted infections– 2008. Geneva, Switzerland: 2012. [Google Scholar]
- 5. CDC. Sexually Transmitted Disease Surveillance 2013. Atlanta, GA: 2014. [Google Scholar]
- 6. Owusu-Edusei K Jr., Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40(3):197–201. doi: 10.1097/OLQ.0b013e318285c6d2 00007435-201303000-00003 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7. Pultorak E, Wong W, Rabins C, Mehta SD. Economic burden of sexually transmitted infections: incidence and direct medical cost of Chlamydia, gonorrhea, and syphilis among Illinois adolescents and young adults, 2005–2006. Sex Transm Dis. 2009;36(10):629–636. doi: 10.1097/OLQ.0b013e3181a96d23 00007435-200910000-00007 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8. Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31(15):1892–1897. doi: S0264-410X(13)00081-9 [pii] 10.1016/j.vaccine.2013.01.024 ; pmcid: PMC4148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottlieb SL, Low N, Newman LM, Bolan G, Kamb M, Broutet N. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine. 2014;32(14):1527–1535. doi: S0264-410X(13)01398-4 [pii] 10.1016/j.vaccine.2013.07.087 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rekart ML, Brunham RC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008;84(2):87–91. doi: sti.2007.027938 [pii] 10.1136/sti.2007.027938 . [DOI] [PubMed] [Google Scholar]
- 11. Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5(2):149–161. doi: nri1551 [pii] 10.1038/nri1551 . [DOI] [PubMed] [Google Scholar]
- 12. Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79(3):986–996. doi: IAI.00881-10 [pii] 10.1128/IAI.00881-10 ; pmcid: PMC3067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins RA, Rank RG, Kelly KA. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun. 2002;70(9):5132–5139. ; pmcid: PMC128225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59(4):1346–1351. ; pmcid: PMC257849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karunakaran KP, Yu H, Foster LJ, Brunham RC. Development of a Chlamydia trachomatis T cell Vaccine. Hum Vaccin. 2010;6(8):676–680. doi: 12299 [pii]. ; pmcid: PMC3056063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li W, Murthy AK, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180(5):3375–3382. doi: 180/5/3375 [pii]. . [DOI] [PubMed] [Google Scholar]
- 17. Li LX, McSorley SJ. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog. 2013;9(10):e1003707 doi: 10.1371/journal.ppat.1003707 PPATHOGENS-D-13-01491 [pii]. ; pmcid: PMC3814678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63(12):4661–4668. ; pmcid: PMC173669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68(12):6979–6987. ; pmcid: PMC97807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175(11):7536–7542. doi: 175/11/7536 [pii]. ; pmcid: PMC3514507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and-independent pathways. J Immunol. 1997;158(7):3344–3352. . [PubMed] [Google Scholar]
- 22. Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63(9):3302–3308. ; pmcid: PMC173455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161(3):1439–1446. . [PubMed] [Google Scholar]
- 24. Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32(14):1563–1571. doi: S0264-410X(13)01111-0 [pii] 10.1016/j.vaccine.2013.08.020 . [DOI] [PubMed] [Google Scholar]
- 25. Karunakaran KP, Yu H, Jiang X, Chan Q, Moon KM, Foster LJ, et al. Outer membrane proteins preferentially load MHC class II peptides: Implications for a Chlamydia trachomatis T cell vaccine. Vaccine. 2015;33(18):2159–2166. doi: S0264-410X(15)00244-3 [pii] 10.1016/j.vaccine.2015.02.055 ; pmcid: PMC4390527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson RM, Yu H, Kerr MS, Slaven JE, Karunakaran KP, Brunham RC. PmpG303-311, a protective vaccine epitope that elicits persistent cellular immune responses in Chlamydia muridarum-immune mice. Infect Immun. 2012;80(6):2204–2211. doi: IAI.06339-11 [pii] 10.1128/IAI.06339-11 ; pmcid: PMC3370596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan C, Hsia RC, Shou H, Haggerty CL, Ness RB, Gaydos CA, et al. Chlamydia trachomatis-infected patients display variable antibody profiles against the nine-member polymorphic membrane protein family. Infect Immun. 2009;77(8):3218–3226. doi: IAI.01566-08 [pii] 10.1128/IAI.01566-08 ; pmcid: PMC2715660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J Immunol. 2009;182(3):1602–1608. doi: 182/3/1602 [pii]. ; pmcid: PMC2637473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun. 2012;80(4):1510–1518. doi: IAI.06338-11 [pii] 10.1128/IAI.06338-11 ; pmcid: PMC3318408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karunakaran KP, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol. 2008;180(4):2459–2465. doi: 180/4/2459 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31. Henderson IR, Lam AC. Polymorphic proteins of Chlamydia spp.—autotransporters beyond the Proteobacteria. Trends Microbiol. 2001;9(12):573–578. doi: S0966-842X(01)02234-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 32. Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68(4):692–744. doi: 68/4/692 [pii] 10.1128/MMBR.68.4.692-744.2004 ; pmcid: PMC539010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grimwood J, Stephens RS. Computational analysis of the polymorphic membrane protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb Comp Genomics. 1999;4(3):187–201. . [DOI] [PubMed] [Google Scholar]
- 34. Becker E, Hegemann JH. All subtypes of the Pmp adhesin family are implicated in chlamydial virulence and show species-specific function. Microbiologyopen. 2014;3(4):544–556. 10.1002/mbo3.186 ; pmcid: PMC4287181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kiselev AO, Skinner MC, Lampe MF. Analysis of pmpD expression and PmpD post-translational processing during the life cycle of Chlamydia trachomatis serovars A, D, and L2. PLoS One. 2009;4(4):e5191 10.1371/journal.pone.0005191 ; pmcid: PMC2666266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saka HA, Thompson JW, Chen YS, Kumar Y, Dubois LG, Moseley MA, et al. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol Microbiol. 2011;82(5):1185–1203. 10.1111/j.1365-2958.2011.07877.x ; pmcid: PMC3225693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Swanson KA, Taylor LD, Frank SD, Sturdevant GL, Fischer ER, Carlson JH, et al. Chlamydia trachomatis polymorphic membrane protein D is an oligomeric autotransporter with a higher-order structure. Infect Immun. 2009;77(1):508–516. doi: IAI.01173-08 [pii] 10.1128/IAI.01173-08 ; pmcid: PMC2612253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia RC, Tan C, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A. 2006;103(6):1894–1899. doi: 0508983103 [pii] 10.1073/pnas.0508983103 ; pmcid: PMC1413641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borges V, Nunes A, Ferreira R, Borrego MJ, Gomes JP. Directional Evolution of Chlamydia trachomatis towards Niche-Specific Adaptation. J Bacteriol. 2012;194(22):6143–6153. doi: JB.01291-12 [pii] 10.1128/JB.01291-12 ; pmcid: PMC3486361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73(10):6407–6418. doi: 73/10/6407 [pii] 10.1128/IAI.73.10.6407-6418.2005 ; pmcid: PMC1230933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan C, Hsia RC, Shou H, Carrasco JA, Rank RG, Bavoil PM. Variable expression of surface-exposed polymorphic membrane proteins in in vitro-grown Chlamydia trachomatis. Cell Microbiol. 2010;12(2):174–187. doi: CMI1389 [pii] 10.1111/j.1462-5822.2009.01389.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borges V, Pinheiro M, Vieira L, Sampaio DA, Nunes A, Borrego MJ, et al. Complete Genome Sequence of Chlamydia trachomatis Ocular Serovar C Strain TW-3. Genome Announc. 2014;2(1). doi: 2/1/e01204-13 [pii] 10.1128/genomeA.01204-13 ; pmcid: PMC3900901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harris SR, Clarke IN, Seth-Smith HM, Solomon AW, Cutcliffe LT, Marsh P, et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet. 2012;44(4):413–419, S411. doi: ng.2214 [pii] 10.1038/ng.2214 ; pmcid: PMC3378690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5(6):e11147 10.1371/journal.pone.0011147 ; pmcid: PMC2892488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: btp187 [pii] 10.1093/bioinformatics/btp187 . [DOI] [PubMed] [Google Scholar]
- 46. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. . [DOI] [PubMed] [Google Scholar]
- 47. Nunes A, Nogueira PJ, Borrego MJ, Gomes JP. Chlamydia trachomatis diversity viewed as a tissue-specific coevolutionary arms race. Genome Biol. 2008;9(10):R153. doi: gb-2008-9-10-r153 [pii] 10.1186/gb-2008-9-10-r153 ; pmcid: PMC2760880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. . [DOI] [PubMed] [Google Scholar]
- 49. Nei M, Kumar S. Molecular Evolution and Phylogenetics New York: Oxford University Press; 2000. [Google Scholar]
- 50. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73(1):152–160. ; pmcid: PMC103818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pride DT. SWAAP Version 1.0.0—Sliding Windows Alignment Analysis Program: A tool for analyzing patterns of substitutions and similarity in multiple alignments. Distributed by the author. 2000.
- 52. Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out--processing of the Chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol Microbiol. 2004;51(2):319–334. doi: 10.1046/j.1365-2958.2003.03838.x MMI3838 [pii]. . [DOI] [PubMed] [Google Scholar]
- 53. Emini EA, Hughes JV, Perlow DS, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55(3):836–839. ; pmcid: PMC255070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4(1):181–186. . [DOI] [PubMed] [Google Scholar]
- 55. Henderson IR, Owen P, Nataro JP. Molecular switches--the ON and OFF of bacterial phase variation. Mol Microbiol. 1999;33(5):919–932. doi: mmi1555 [pii]. . [DOI] [PubMed] [Google Scholar]
- 56. Moxon R, Bayliss C, Hood D. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu Rev Genet. 2006;40:307–333. 10.1146/annurev.genet.40.110405.090442 . [DOI] [PubMed] [Google Scholar]
- 57. van der Woude MW, Baumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17(3):581–611, table of contents. doi: 10.1128/CMR.17.3.581-611.2004 17/3/581 [pii]. ; pmcid: PMC452554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chelladurai BS, Li H, Nicholson AW. A conserved sequence element in ribonuclease III processing signals is not required for accurate in vitro enzymatic cleavage. Nucleic Acids Res. 1991;19(8):1759–1766. ; pmcid: PMC328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ehretsmann CP, Carpousis AJ, Krisch HM. Specificity of Escherichia coli endoribonuclease RNase E: in vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992;6(1):149–159. . [DOI] [PubMed] [Google Scholar]
- 60. Robertson HD, Dickson E, Dunn JJ. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977;74(3):822–826. ; pmcid: PMC430490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deutscher MP. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34(2):659–666. doi: 34/2/659 [pii] 10.1093/nar/gkj472 ; pmcid: PMC1360286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shine J, Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974;71(4):1342–1346. ; pmcid: PMC388224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allen JE, Stephens RS. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989;171(1):285–291. ; pmcid: PMC209584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Danilition SL, Maclean IW, Peeling R, Winston S, Brunham RC. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990;58(1):189–196. ; pmcid: PMC258428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de la Maza LM, Fielder TJ, Carlson EJ, Markoff BA, Peterson EM. Sequence diversity of the 60-kilodalton protein and of a putative 15-kilodalton protein between the trachoma and lymphogranuloma venereum biovars of Chlamydia trachomatis. Infect Immun. 1991;59(3):1196–1201. ; pmcid: PMC258390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stephens RS, Wagar EA, Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988;170(2):744–750. ; pmcid: PMC210717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nunes A, Gomes JP. Evolution, phylogeny, and molecular epidemiology of Chlamydia. Infect Genet Evol. 2014;23:49–64. doi: S1567-1348(14)00042-2 [pii] 10.1016/j.meegid.2014.01.029 . [DOI] [PubMed] [Google Scholar]
- 68. Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–759. . [DOI] [PubMed] [Google Scholar]
- 69. Ferreira R, Antelo M, Nunes A, Borges V, Damiao V, Borrego MJ, et al. In silico scrutiny of genes revealing phylogenetic congruence with clinical prevalence or tropism properties of Chlamydia trachomatis strains. G3 (Bethesda). 2014;5(1):9–19. doi: g3.114.015354 [pii] 10.1534/g3.114.015354 ; pmcid: PMC4291473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc Natl Acad Sci U S A. 2011;108(24):9969–9974. doi: 1101756108 [pii] 10.1073/pnas.1101756108 ; pmcid: PMC3116399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jorgensen I, Valdivia RH. Pmp-like proteins Pls1 and Pls2 are secreted into the lumen of the Chlamydia trachomatis inclusion. Infect Immun. 2008;76(9):3940–3950. doi: 10.1128/IAI.00632-08 IAI.00632-08 [pii]. ; pmcid: PMC2519427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sisko JL, Spaeth K, Kumar Y, Valdivia RH. Multifunctional analysis of Chlamydia-specific genes in a yeast expression system. Mol Microbiol. 2006;60(1):51–66. doi: MMI5074 [pii] 10.1111/j.1365-2958.2006.05074.x . [DOI] [PubMed] [Google Scholar]
- 73. Gomes JP, Nunes A, Bruno WJ, Borrego MJ, Florindo C, Dean D. Polymorphisms in the nine polymorphic membrane proteins of Chlamydia trachomatis across all serovars: evidence for serovar Da recombination and correlation with tissue tropism. J Bacteriol. 2006;188(1):275–286. doi: 188/1/275 [pii] 10.1128/JB.188.1.275-286.2006 ; pmcid: PMC1317584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Borges V, Gomes JP. Deep comparative genomics among Chlamydia trachomatis lymphogranuloma venereum isolates highlights genes potentially involved in pathoadaptation. Infect Genet Evol. 2015;32:74–88. doi: S1567-1348(15)00069-6 [pii] 10.1016/j.meegid.2015.02.026 . [DOI] [PubMed] [Google Scholar]
- 75. Gomes JP, Bruno WJ, Nunes A, Santos N, Florindo C, Borrego MJ, et al. Evolution of Chlamydia trachomatis diversity occurs by widespread interstrain recombination involving hotspots. Genome Res. 2007;17(1):50–60. doi: gr.5674706 [pii] 10.1101/gr.5674706 ; pmcid: PMC1716266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rockey DD, Lenart J, Stephens RS. Genome sequencing and our understanding of chlamydiae. Infect Immun. 2000;68(10):5473–5479. ; pmcid: PMC101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Feher VA, Randall A, Baldi P, Bush RM, de la Maza LM, Amaro RE. A 3-dimensional trimeric beta-barrel model for Chlamydia MOMP contains conserved and novel elements of Gram-negative bacterial porins. PLoS One. 2013;8(7):e68934 doi: 10.1371/journal.pone.0068934 PONE-D-13-07912 [pii]. ; pmcid: PMC3723809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nunes A, Nogueira PJ, Borrego MJ, Gomes JP. Adaptive evolution of the Chlamydia trachomatis dominant antigen reveals distinct evolutionary scenarios for B- and T-cell epitopes: worldwide survey. PLoS One. 2010;5(10). 10.1371/journal.pone.0013171 ; pmcid: PMC2950151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rodriguez-Maranon MJ, Bush RM, Peterson EM, Schirmer T, de la Maza LM. Prediction of the membrane-spanning beta-strands of the major outer membrane protein of Chlamydia. Protein Sci. 2002;11(7):1854–1861. 10.1110/ps.3650102 ; pmcid: PMC2373662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Molleken K, Schmidt E, Hegemann JH. Members of the Pmp protein family of Chlamydia pneumoniae mediate adhesion to human cells via short repetitive peptide motifs. Mol Microbiol. 2010;78(4):1004–1017. 10.1111/j.1365-2958.2010.07386.x ; pmcid: PMC2997323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jeffrey BM, Suchland RJ, Eriksen SG, Sandoz KM, Rockey DD. Genomic and phenotypic characterization of in vitro-generated Chlamydia trachomatis recombinants. BMC Microbiol. 2013;13:142. doi: 1471-2180-13-142 [pii] 10.1186/1471-2180-13-142 ; pmcid: PMC3703283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Grimwood J, Olinger L, Stephens RS. Expression of Chlamydia pneumoniae polymorphic membrane protein family genes. Infect Immun. 2001;69(4):2383–2389. 10.1128/IAI.69.4.2383-2389.2001 ; pmcid: PMC98169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28(6):1397–1406. doi: gkd254 [pii]. ; pmcid: PMC111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shirai M, Hirakawa H, Ouchi K, Tabuchi M, Kishi F, Kimoto M, et al. Comparison of outer membrane protein genes omp and pmp in the whole genome sequences of Chlamydia pneumoniae isolates from Japan and the United States. J Infect Dis. 2000;181 Suppl 3:S524–527. doi: JID991374 [pii] 10.1086/315616 . [DOI] [PubMed] [Google Scholar]
- 85. Tan M. Regulation of gene expression In: Bavoil P, Wyrick PB, editors. Chlamydia: genomics and pathogenesis Horizon Bioscience; 2006. [Google Scholar]
- 86. Albrecht M, Sharma CM, Reinhardt R, Vogel J, Rudel T. Deep sequencing-based discovery of the Chlamydia trachomatis transcriptome. Nucleic Acids Res. 2010;38(3):868–877. doi: gkp1032 [pii] 10.1093/nar/gkp1032 ; pmcid: PMC2817459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nunes A, Gomes JP, Mead S, Florindo C, Correia H, Borrego MJ, et al. Comparative expression profiling of the Chlamydia trachomatis pmp gene family for clinical and reference strains. PLoS One. 2007;2(9):e878 10.1371/journal.pone.0000878 ; pmcid: PMC1963315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yu H, Karunakaran KP, Jiang X, Brunham RC. Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice. Vaccine. 2014;32(36):4672–4680. doi: S0264-410X(14)00791-9 [pii] 10.1016/j.vaccine.2014.06.002 ; pmcid: PMC4148050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.