Abstract
There are a great number of reports with assertions that oxidative stress is produced by organophosphorus compound (OPC) poisoning and is a cofactor of mortality and morbidity in OPC toxicity. In addition, antioxidants have been suggested as adjuncts to standard therapy. However, there is no substantial evidence for the benefit of the use of antioxidants in survival after acute intoxication of OPCs. The present study was conducted to assess the effectiveness of three non-enzymatic antioxidants (NEAOs), N-acetylcysteine (NAC), glutathione (GSH), and ascorbic acid (AA), in acute intoxication of adult male Wister rats with paraoxon. The efficacy of the antioxidants was estimated as both a pretreatment and a concurrent application along with the standard oxime, pralidoxime (2-PAM). Relative risk of death after 48 hours of application was estimated by Cox regression analysis. The results revealed no benefit of either tested NEAO to the improvement in survival of experimental rats. The application of these antioxidants was found to be deleterious when administered along with pralidoxime compared to the treatment with pralidoxime alone. It has been concluded that the tested non-enzymatic antioxidants are not useful in acute toxicity for improving survival rates. However, the individual toxic dynamics of diversified OPCs should not be overlooked and further studies with different OPCs are suggested.
1. Introduction
There are various groups of organophosphorus compounds which are structurally and toxicologically different. Organophosphorus compounds account for hundreds of thousands of deaths worldwide every year and for even a greater number of casualties [1]. The standard medical treatment of organophosphorus poisoning is atropine + oxime + benzodiazepines, for example, diazepam [2]. In recent years, oxidative stress has been described as one of the co-lethal factors in organophosphorus-induced poisoning [3–11]. A number of clinical studies also suggested the beneficial role of antioxidants in acute OPC poisoning [12–14]. However, a conflicting review was reported by Nurulain et al. [15]. It is noteworthy that among the large number of published articles on the topic, depicting the beneficial role of the use of antioxidants, the mortality improvement studies with acute poisoning are scarcely reported. The assumption and conclusion for oxidative stress and use of antioxidants in OPC poisoning are mainly based on cell/organ level of investigations. Secondly, studies were largely carried out on moderately or highly toxic compounds with sublethal doses or LD50 dose.
The objective of the present study was to assess the use of three common NEAOs, NAC, GSH, and AA as adjuncts to oxime and without oxime in acute poisoning with survival outcome. Paraoxon is an extremely toxic OPC and one of the most potent acetylcholinesterase inhibiting insecticide available. It is around 70% as potent as the nerve agent sarin. It is an active metabolite of the insecticide parathion. The purpose of the study was to quantify the protection incurred by NEAO in case of acute intoxication by an extremely toxic OPC, paraoxon.
2. Materials and Methods
2.1. Experimental Animals
During the entire experiment, the “Guiding Principles in the Care of and Use of Laboratory Animals” have been observed. Animals were handled, ethically treated, and humanly killed as per the rules and instructions of the Ethical Committee. All studies were performed with the approval of the Institutional Ethical Committee (A29/12). The original stock of Wistar rats was purchased from Harlan Laboratories (Harlan Laboratories, Oxon, England). The animals used in the present studies were bred at our own Animal Facility from the original stock. Adult male rats (average weight ± SD: 259 ± 13 g; 95% confidence interval: 258 g–260 g) were housed in polypropylene cages (43 × 22.5 × 20.5 cm3; six rats/cage) in climate- and access-controlled rooms (23 ± 1°C; 50 ± 4% humidity). The day/night cycle was 12 h/12 h. Food and water were available ad libitum. The food was purchased from Emirates Feed Factory (Abu Dhabi, UAE) which is a standard maintenance diet for rats.
2.2. Chemicals
Paraoxon (POX) stock solution (100 mM) was prepared in dry acetone. Working solution for i.p. application was prepared ex tempore by diluting stock solution with saline. The other solutions were prepared before experiment. All the chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steinheim, Germany).
2.3. Choice of Dosage and Treatment
The acute dosage of paraoxon (≈LD75 and 2x LD75) and safe dose of pralidoxime (1/2 of LD1) against Wister rats were selected according to Nurulain et al. [16]. The NAC was used according to Yurumez et al. [3] who described the effective dose in similar experiments with mice. GSH was used according to Jiang et al. [17] where the dose was found to be beneficial in methyl parathion toxicity. Antunes et al. [18] used 50 mg/kg body weight AA with positive outcome. The average body weight of the animals was ≈250 g. Antioxidants dosage was based on previous studies but their safety profile was checked and found to be non-lethal. The i.p. LD50 for NAC against rats was 1205 mg/kg body weight [19]. The i.p. LD50 for AA and GSH could not be retrieved. However, i.p. LD50 of AA and GSH against mouse was reported in the literature as 10 g/kg body weight [20] and 4.020 g/kg body weight [21] (LookChem.com), respectively. The intravenous (faster systemic circulation than i.p.) LD50 for ascorbic acid against rat was reported as 1.0 g/kg [22]. All the compounds were injected i.p. at different anatomical sites. I.p. injection was opted because it enables the fast systemic circulation compared to intramuscular or oral administration and suitable in quantifying the true effect of the compounds. In concurrent application, all the three injections were delivered within one minute in the following order: POX, 2-PAM, and antioxidant.
2.4. Reference Group
Only Paraoxon Exposure. Animals received i.p. injections of paraoxon, in a dosage of 1 µmol = 272 µg (1.09 mg/kg average body weight; ≈LD75), and 2 µmol = 544 µg (2.18 mg/kg average body weight; 2x LD75), diluted in 500 µL saline solution.
Pralidoxime (2-PAM). 50 µmol/rat = 8.63 mg/rat (=33.5 mg/kg average body weight).
N-Acetylcysteine (NAC). 275 µmol/rat = 45 mg/rat (=225 mg/kg average body weight).
Glutathione Reduced Form (GSH). 490 µmol/rat = 150 mg/rat (750 mg/kg body weight).
Ascorbic Acid (AA). 285 µmol/rat = 50 mg/rat (195 mg/kg body weight).
2.5. Treatment Groups
There were four groups, consisting of 6 rats per compound. Group 1 received only paraoxon; Group 2 received POX + 2-PAM; Group 3 received POX + 2-PAM + NAC; Group 4 received NAC 60 minutes before POX + 2-PAM. The same groupings were applied with GSH and AA but pretreatment was 90 minutes and 30 minutes before POX + 2-PAM, respectively. There was a control group of POX + NAC, POX + GSH, and POX + AA. The animals were monitored for 48 hours and mortality was recorded at 30 minutes, 1, 2, 3, 4, 24, and 48 hours correspondingly. The pretreatment time points for NAC, GSH, and AA are based on their different approximate pharmacokinetic properties.
2.6. Statistical Analysis
Statistical analysis was performed on the mortality data of four cycles. Mortality data were compared and, for each of the seven time points, the respective hazards ratios (relative risks of death) were estimated using the Cox proportional hazards model [23]. Both paraoxon dose and groups (with Group 1, i.e., no pretreatment, as the reference category) were treated as categorical variables. Subsequently, the area under the RR-time curve was determined and pair-wise comparisons (Mann-Whitney U-test) were performed. No Bonferroni's correction for multiple comparisons was applied, and ≤0.05 was considered significant. The SPSS 21.0 (SPSS Inc., Chicago, IL, USA) software package was used for all statistical evaluations.
3. Results
The relative risk of death at the seven time points (30 min, 1, 2, 3, 4, 24, and 48 h), estimated by Cox [23] analysis in simultaneous oxime and antioxidant-treated animals is depicted in Figure 1 and Table 3. Table 1 shows the percentage of mortalities at different time points of observation. RR was compared with untreated animals (Group 1, RR = 1) and adjusted for paraoxon dose (high/low). Statistical comparison was performed on the cumulative relative risk, that is, the area under the RR-time curve. Simultaneous pralidoxime treatment significantly reduced the paraoxon-induced mortality, RR; 0.33 ± 0.03 (P < 0.05) as compared to the no-treatment group (G1; paraoxon only). Simultaneous treatment of NEAO yielded no significant protection. RR was 1.04 ± 0.04, 1.08 ± 0.03, and 0.85 ± 0.28 for NAC, GSH, and AA treatment, respectively. When antioxidants were administered together with PAM, AA treatment group produced statistically significantly higher mortality (RR 1.30 ± 0.12; P < 0.014) than no-treatment group, POX. NAC and GSH applied concurrently with PAM reduced the mortality in comparison with no-treatment group. Pattern of RR for pretreatment with antioxidants was almost the same as mentioned earlier (Figure 2, Tables 2 and 4); that is, only PAM pretreatment provided significant protection (RR 0.34 ± 0.03; P < 0.05). Antioxidant pretreatment without PAM yielded poor protection. RR estimated for NAC was 1.31 ± 0.24; GSH 0.93 ± 0.30; and AA 1.09 ± 0.33. The PAM efficacy was found to be decreased when animals were pretreated with antioxidants. RR values were 0.63 ± 0.15 for NAC, 0.93 ± 0.30 and 1.29 ± 0.0.18 for AA pretreatment, respectively, in comparison with RR 0.47 ± 0.17 for POX + PAM.
Figure 1.
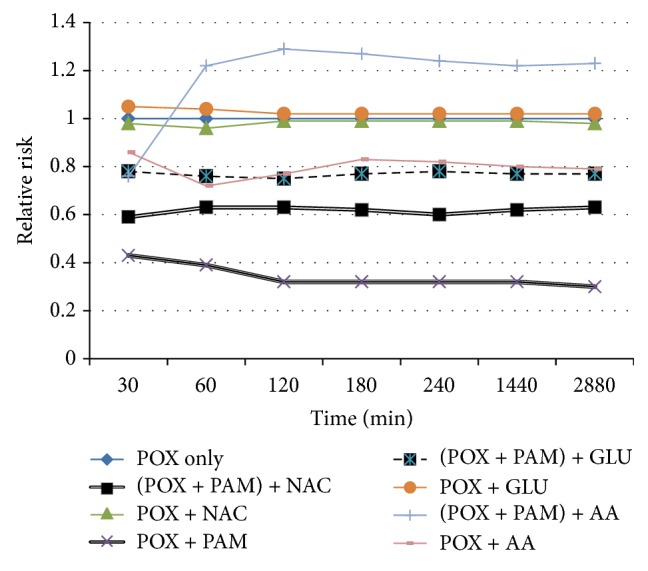
Cumulative relative risk of death overtime after coapplication of compounds. Cumulative relative risk (RR) of death overtime with simultaneous intraperitoneal (i.p.) injection of POX, PAM and NAC, and GSH and AA. The legends on the side are depicting the treatment groups. RR was estimated by Cox [23] analysis, adjusted for POX dose (high/low) for each of the time points examined (30 min and 1, 2, 3, 4, 24, and 48 h). Best protection was conferred by simultaneous PAM treatment only and poor protection was estimated for all non-enzymatic treatment alone. Efficacy of PAM was estimated to decrement when co-administered with antioxidants.
Table 3.
Cox analysis of the cumulative relative risk (RR) of death for co-administration of compounds, including 95% confidence interval (CI), of animals exposed to the paraoxon (POX) and adjusted for POX dose (high/low).
| Groups | RR ± SD | 95% CI | Significance |
|---|---|---|---|
| POX only | 1 | 0-0 | |
| POX + PAM + NAC | 0.66 ± 15 | 0.42–0.91 | P < 0.05 |
| POX + NAC | 1.04 ± 0.04 | 0.97–1.12 | NS |
| POX + PAM | 0.33 ± 0.03 | 0.23–0.43 | P < 0.05 |
| POX + PAM + GSH | 0.81 ± 0.13 | 0.61–1.02 | P < 0.05 |
| POX + GSH | 1.08 ± 0.03 | 1.03–1.14 | NS |
| POX + PAM + AA | 1.30 ± 0.12 | 1.10–1.49 | P < 0.05 |
| POX + AA | 0.85 ± 0.28 | 0.00–3.32 | P < 0.05 |
Table 1.
Mortality [%] after concurrent application of paraoxon, pralidoxime, and NEAO.
| Groups (G) | POX dose μmol/rat | 30 min | 1 hour | 2 hours | 3 hours | 4 hours | 24 hours | 48 hours |
|---|---|---|---|---|---|---|---|---|
| G1: POX only | 1/2 | 79/96 | 83/96 | 88/96 | 88/96 | 88/96 | 88/96 | 88/96 |
| G2: POX + PAM + NAC | 1/2 | 42/78 | 50/78 | 67/89 | 71/89 | 71/89 | 79/89 | 83/94 |
| G3: POX + NAC | 1/2 | 92/100 | 92/100 | 100/100 | 100/100 | 100/100 | 100/100 | 100/100 |
| G4: POX + PAM | 1/2 | 8/75 | 8/79 | 13/79 | 17/79 | 25/79 | 25/83 | 29/83 |
| G5: POX + PAM + GSH | 1/2 | 75/75 | 83/75 | 92/83 | 92/92 | 92/96 | 92/96 | 96/96 |
| G6: POX + GSH | 1/2 | 100/100 | 100/100 | 100/100 | 100/100 | 100/100 | 100/100 | 100/100 |
| G7: POX + PAM + AA | 1/2 | 67/83 | 67/88 | 79/88 | 83/92 | 88/92 | 92/92 | 92/92 |
| G8: POX + AA | 1/2 | 75/100 | 88/100 | 88/100 | 88/100 | 88/100 | 88/100 | 88/100 |
Figure 2.
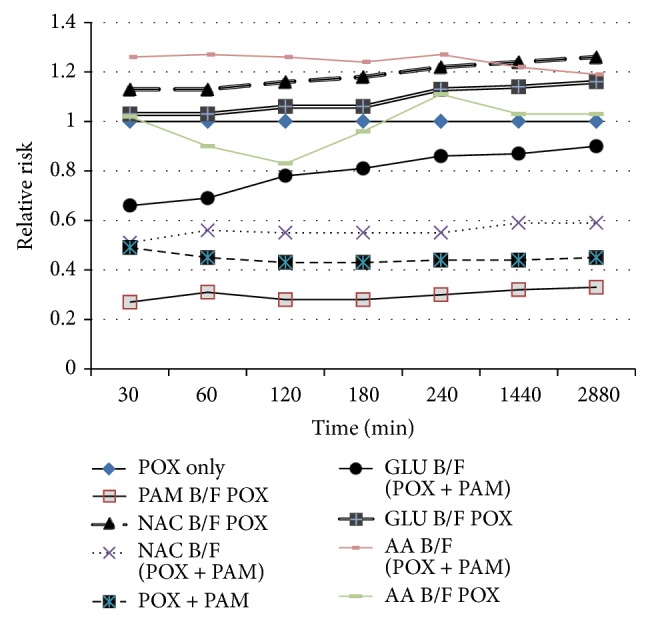
Cumulative relative risk of death over time with pretreatment of compounds. Cumulative relative risk (RR) of death over time with pretreatment, intraperitoneal injections of antioxidants followed by i.p. administration of POX and PAM. The legends on the side are depicting the treatment groups. RR was estimated by Cox [23] analysis, adjusted for POX dose (high/low) for each of the time points examined (30 min, after 30 min, 1, 2, 3, 4, 24, and 48 h). Best protection was conferred by PAM pretreatment only followed by GSH pretreatment. Efficacy of PAM was estimated to decrement when administered with antioxidants. B/F denotes before.
Table 2.
Mortality [%] after pretreatments of NEAO and co-application of paraoxon and pralidoxime.
| Groups (G) | POX DOSE (μmol/rat) | 30 min | 1 hour | 2 hours | 3 hours | 4 hours | 24 hours | 48 hours |
|---|---|---|---|---|---|---|---|---|
| G1: POX only | 1/2 | 79/92 | 83/92 | 88/92 | 88/92 | 88/92 | 88/92 | 88/92 |
| G2: PAM before POX | 1/2 | 4/42 | 17/46 | 17/46 | 17/50 | 17/58 | 17/71 | 17/75 |
| G3: NAC before POX | 1/2 | 94/96 | 94/96 | 100/100 | 100/100 | 100/100 | 100/100 | 100/100 |
| G4: NAC before (POX + PAM) | 1/2 | 17/71 | 33/75 | 38/79 | 42/79 | 42/88 | 50/92 | 50/92 |
| G5: (POX + PAM) | 1/2 | 8/75 | 8/79 | 13/79 | 17/79 | 25/79 | 25/83 | 29/83 |
| G6: GSH before (POX + PAM) | 1/2 | 67/46 | 67/67 | 71/92 | 75/96 | 83/90 | 83/96 | 88/96 |
| G7: GSH before POX | 1/2 | 92/83 | 92/92 | 96/92 | 100/92 | 100/96 | 100/96 | 100/96 |
| G8: AA before (POX + PAM) | 1/2 | 58/83 | 63/83 | 75/88 | 79/92 | 96/92 | 96/92 | 96/96 |
| G9: AA before POX | 1/2 | 58/75 | 67/83 | 75/83 | 75/83 | 75/92 | 83/100 | 83/100 |
Table 4.
Cox analysis of the cumulative relative risk (RR) of death for pretreatment protocol, including 95% confidence interval (CI), of animals exposed to the paraoxon (POX) and adjusted for POX dose (high/low).
| Groups | RR ± SD | 95% CI | Significance |
|---|---|---|---|
| POX only | 1 | 0-0 | — |
| PAM before POX | 0.34 ± 0.19 | 0.04–0.64 | P < 0.05 |
| NAC before POX | 1.31 ± 0.24 | 0.93–1.70 | NS |
| NAC before (POX + PAM) | 0.63 ± 0.15 | 0.39–0.86 | P < 0.05 |
| POX + PAM | 0.47 ± 0.17 | 0.20–0.74 | P < 0.05 |
| GSH before (POX + PAM) | 0.93 ± 0.30 | 0.45–1.41 | NS |
| GSH before POX | 0.84 ± 0.39 | 0.94–1.48 | P < 0.05 |
| AA before (POX + PAM) |
1.29 ± 0.18 | 1.01–1.57 | P < 0.05 |
| AA before POX | 1.09 ± 0.30 | 0.00–4.08 | NS |
4. Discussion
The present study demonstrates the non-effectiveness of the three NEAOs for paraoxon-induced acute toxicity. Non-efficacy was observed in both the pre- and the posttreatment of antioxidants. Recently, it has been documented in the literature that oxidative stress is a co-lethal factor of OPC-induced poising, in addition to AChE inhibition. Moreover, uses of antioxidants have been recommended as adjunct treatment to OPC poisoning. The biochemical estimations of oxidative stress parameters revealed oxidative stress in many OPC-induced subjects, including experimental rats and mice. However, there is no convincing evidence for the use of NEAO in acute OPC-poisoning with survival endpoint. It has also been overlooked that each OPC has unique toxicity profile and the hypothesis/concept may not be generalized for all OPCs. The present study was conducted without atropine to quantify the possible beneficial effect of three antioxidants. Recently, Yurumez et al. [3] determined the beneficial effect of NAC against organophosphate fenthion toxicity (a moderately toxic OPC) in mice and demonstrated that NAC has prophylactic as well as therapeutic activity in OPC poisoning and clearly improves survival rates in mice at a higher dose of NAC. It is not clear whether improved survival rate is due to the antioxidant nature of NAC or some other mechanism is involved. Shadnia et al. [14] used NAC in a clinical trial of an OPC-poisoning case. Type of OPC was not identified in the trial. They found that the group which received NAC needed significantly less atropine than the other one without NAC. The other antioxidants used for OP-induced toxicity are Vitamins C and E, melatonin, and so forth, but only on cellular level and biochemical estimation of oxidative stress parameters. There are also conflicting reports for the oxidative stress produced by OPCs under acute toxic condition. Kose et al. [24] concluded that acute dichlorvos administration did not cause marked oxidative stress and probably does not play a major role in dichlorvos-induced poisoning. Gunay et al. [25] reported no evidence of oxidative stress due to dichlorvos in an acute study on rats. Mostafalou et al. [10] worked on rats hepatocytes treated with a slightly toxic group of OPC, malathion and concluded that the main cause of cell death was mitochondrial dysfunction and reduction of ROS is not sufficient for cell survival. Furthermore, in response to changes in the intracellular environment, mitochondria become producers of excessive reactive oxygen species and release prodeath proteins, resulting in disrupted ATP synthesis and activation of cell death pathways [26]. Our results show that concurrent application of POX + PAM produced better protection than POX + PAM + antioxidant which may be due to the interference of antioxidants with the effect of PAM.
There may be many possible mechanisms to elaborate the failure of NEAO in acute paraoxon poisoning. For instance, mechanistically, loading of NEAO may not have come in systemic circulation or its concentration might be so high that the body cannot compensate it in a short period [13]. Another possibility is that the oxidant produced during acute intoxication by paraoxon is not scavenged by these NEAOs because they may be under the control of enzymatic antioxidants. Furthermore, radical scavengers like ascorbic acid can be pro-oxidant [27]. According to Galley et al. [28] under severe oxidant stress, vitamin C can function as a pro-oxidant by promoting iron-catalyzed reactions. The systemic review and meta-analysis conducted by Bjelakovic et al. [29] found no evidence to support antioxidant supplements for primary or secondary prevention in patients with various diseases caused by oxidative stress. In short, adjunct treatment with NEAOs is not beneficial in acute poisoning of OPC for survival outcome. However based on evidence from the literature, it may be speculated that the use of antioxidants may be beneficial in chronic exposure of OPC which causes different pathophysiological conditions due to oxidative stress.
5. Conclusion
NEAOs like NAC, glutathione, and ascorbic acid have no beneficial role in the survival of rats in acute toxicity with paraoxon. Oxime treatment without the use of antioxidants has been found more effective. The understanding of types of oxidants and mechanism of their action during acute cholinergic crises may help to select suitable and effective antioxidants. Moreover, the different toxic dynamics of diversified OPCs should not be overlooked.
Acknowledgments
The authors are thankful to Professor Georg Petroianu, Florida International University, USA, for generous gift of chemicals for the study and technical support. There are no patents, products in development, or marketed products to declare. This study was supported by grants from College of Medicine & Health Sciences, UAE University, UAE. The funders had no role in study design, data collection and analysis, decision to publish the paper, or preparation of the paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Authors who contributed significantly and read and approved the paper are Syed M. Nurulain, Shreesh Ojha, Kornelia Tekes, Mohammad Shafiullah, Huba Kalasz, and Abdu Adem. Authors who conceived and designed the experiments are Syed M. Nurulain, Kornelia Tekes, and Abdu Adem. Authors who performed the experiments are Syed M. Nurulain and Mohammad Shafiullah. Authors who analyzed the data are Syed M. Nurulain and Shreesh Ojha. Huba Kalasz did the critical review and paper drafting. Authors who contributed reagents/materials/analysis tools are Shreesh Ojha and Abdu Adem. Authors who wrote the paper are Syed M. Nurulain, Shreesh Ojha, and Abdu Adem. Syed M. Nurulain and Shreesh Ojha contributed equally.
References
- 1.Karalliedde L., Senanayake N. Organophosphorus insecticide poisoning. Journal of the International Federation of Clinical Chemistry. 1999;11(2):4–9. [Google Scholar]
- 2.Nurulain S. M. Different approaches to acute organophosphorus poison treatment. Journal of the Pakistan Medical Association. 2012;62(7):712–717. [PubMed] [Google Scholar]
- 3.Yurumez Y., Cemek M., Yavuz Y., Birdane Y. O., Buyukokuroglu M. E. Beneficial effect of N-acetylcysteine against organophosphate toxicity in mice. Biological and Pharmaceutical Bulletin. 2007;30(3):490–494. doi: 10.1248/bpb.30.490. [DOI] [PubMed] [Google Scholar]
- 4.Buyukokuroglu M. E., Cemek M., Yurumez Y., Yavuz Y., Aslan A. Antioxidative role of melatonin in organophosphate toxicity in rats. Cell Biology and Toxicology. 2008;24(2):151–158. doi: 10.1007/s10565-007-9024-z. [DOI] [PubMed] [Google Scholar]
- 5.Yu F., Wang Z., Ju B., Wang Y., Wang J., Bai D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Experimental and Toxicologic Pathology. 2008;59(6):415–423. doi: 10.1016/j.etp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Soltaninejad K., Abdollahi M. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Medical Science Monitor. 2009;15(3):RA75–RA90. [PubMed] [Google Scholar]
- 7.Lukaszewicz-Hussain A. Role of oxidative stress in organophosphate insecticide toxicity—short review. Pesticide Biochemistry and Physiology. 2010;98(2):145–150. doi: 10.1016/j.pestbp.2010.07.006. [DOI] [Google Scholar]
- 8.Abdollahi M., Karami-Mohajeri S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicology and Applied Pharmacology. 2012;258(3):309–314. doi: 10.1016/j.taap.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Ambali S. F., Aliyu M. B. Short-term sensorimotor and cognitive changes induced by acute chlorpyrifos exposure in wistar rats: ameliorative effect of vitamin E. Pharmacologia. 2012;3:31–38. doi: 10.1177/0748233710373086. [DOI] [PubMed] [Google Scholar]
- 10.Mostafalou S., Eghbal M. A., Nili-Ahmadabadi A., Baeeri M., Abdollahi M. Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicology and Industrial Health. 2012;28(9):840–851. doi: 10.1177/0748233711425073. [DOI] [PubMed] [Google Scholar]
- 11.Uchendu C., Ambali S. F., Ayo J. O. The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: a review. African Journal of Agricultural Research. 2012;7(18):2720–2728. [Google Scholar]
- 12.Zhang J.-W., Lv G.-C., Zhao Y. The significance of the measurement of serum xanthine oxidase and oxidation markers in patients with acute organophosphorus pesticide poisoning. Journal of International Medical Research. 2010;38(2):458–465. doi: 10.1177/147323001003800209. [DOI] [PubMed] [Google Scholar]
- 13.Hundekari I. A., Suryakar A. N., Rathi D. B. Oxidative stress and antioxidant status in acute organophosphorus pesticides poisoning cases of North Karnataka (India) International Journal of Environmental Health Research. 2011;11(1):39–44. [Google Scholar]
- 14.Shadnia S., Ashrafivand S., Mostafalou S., Abdollahi M. N-acetylcysteine a novel treatment for acute human organophosphate poisoning. International Journal of Pharmacology. 2011;7(6):732–735. doi: 10.3923/ijp.2011.732.735. [DOI] [Google Scholar]
- 15.Nurulain S. M., Szegi P., Tekes K., Naqvi S. N. Antioxidants in organophosphorus compounds poisoning. Arhiv za Higijenu Rada i Toksikologiju. 2013;64(1):169–177. doi: 10.2478/10004-1254-64-2013-2294. [DOI] [PubMed] [Google Scholar]
- 16.Nurulain S. M., Lorke D. E., Hasan M. Y., et al. Efficacy of eight experimental bispyridinium oximes against paraoxon-induced mortality: comparison with the conventional oximes pralidoxime and obidoxime. Neurotoxicity Research. 2009;16(1):60–67. doi: 10.1007/s12640-009-9048-7. [DOI] [PubMed] [Google Scholar]
- 17.Jiang N., Lu L., Wang T., Zhang L., Xin W., Fu F. Reduced glutathione attenuates liver injury induced by methyl parathion in rats. Toxicology Mechanisms and Methods. 2010;20(2):69–74. doi: 10.3109/15376510903575782. [DOI] [PubMed] [Google Scholar]
- 18.Antunes L. M., Darin J. D., Bianchi M. D. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacological Research. 2000;41(4):405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 19.Lewis R. J. Sr., editor. Sax's Dangerous Properties of Industrial Materials. 11th. Hoboken, NJ, USA: Wiley-Interscience, John Wiley & Sons; 2004. [Google Scholar]
- 20.Ash M., Ash I. Handbook of Preservatives. Synapse Information Resources; 2004. [Google Scholar]
- 21. 2015, http://www.lookchem.com/Glutathione/
- 22.Demole V. CVII. On the physiological action of ascorbic acid and some related compounds. Biochemical Journal. 1934;28:770–773. doi: 10.1042/bj0280770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox D. R. Regression models and life tables. Journal of the Royal Statistical Society: Series B. 1972;34:189–220. [Google Scholar]
- 24.Kose A., Gunay N., Kose B., Ocak A. R., Erel O., Demiryurek A. T. Effects of atropine and pralidoxime pretreatment on serum and cardiac oxidative stress parameters in acute dichlorvos toxicity in rats. Pesticide Biochemistry and Physiology. 2010;97(3):249–255. doi: 10.1016/j.pestbp.2010.03.004. [DOI] [Google Scholar]
- 25.Gunay N., Kose A., Tarakcioglu M., Gunay N. E., Demiryurek A. T. Evaluation of cardiac oxidative stress parameters and mortality in a rat model of organophosphate poisoning.in acute dichlorvos toxicity in rats. Pesticide Biochemistry and Physiology. 2010;97(3):249–255. [Google Scholar]
- 26.Kubli D. A., Gustafsson Å. B. Mitochondria and mitophagy: the yin and yang of cell death control. Circulation Research. 2012;111(9):1208–1221. doi: 10.1161/circresaha.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buettner G. R., Jurkiewicz B. A. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiation Research. 1996;145(5):532–541. doi: 10.2307/3579271. [DOI] [PubMed] [Google Scholar]
- 28.Galley H. F., Davies M. J., Webster N. R. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radical Biology and Medicine. 1996;20(1):139–143. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 29.Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews. 2012;14(3) doi: 10.1002/14651858.CD007176.pub2.CD007176 [DOI] [PMC free article] [PubMed] [Google Scholar]


