Abstract
The free radical scavenging activity of Aspalathus linearis (Rooibos tea) and its effect on reactive oxygen species (ROS), catalase (CAT), and superoxide dismutase (SOD) were investigated in two in vitro disease models of cancer and diabetes. Although the antioxidant activity of this tea has been reported in several studies, its effects in disease models of ROS-induced oxidative stress have not been systematically evaluated to date. The oxygen radical absorbance capacity (ORAC) assay was used in this study to quantify the antioxidant capacity of the extract, whereas the ROS scavenging ability in hyperglycemia-induced human umbilical vein endothelial cells (HUVECs) and HeLa cells were investigated. The CAT and SOD assays were also carried out in the two disease models in order to evaluate the effect of the extract in the stimulation of these two enzyme activities. The extract was observed to have reduced ROS in a dose-dependent manner in both HUVECs and HeLa cells. The stimulation of the CAT and SOD enzyme activities were observed to be dose-dependent as well. The high ORAC value of the extract indicated the presence of antioxidant compounds which could directly quench ROS, whereby this mechanism of action could be hypothesized to have been further complemented through the stimulation of CAT and SOD. Overall, the Aspalathus linearis extract was observed to have increased the CAT and SOD activities in two in vitro disease models of cancer and hyperglycemia. Given the correlation between the ORAC values, the increases in CAT and SOD activities and the reduction in ROS in a dose-dependent manner, it could be hypothesized that the extract had a significant therapeutic potential for either the prevention of the onset of the two diseases or their progression because ROS has been identified as their root causes.
Keywords: antioxidant, reactive oxygen species, Rooibos tea, superoxide dismutase, catalase
Graphical abstract

1. Introduction
Elevation of reactive oxygen species (ROS) such as H2O2, organic peroxides, and also singlet oxygen has been accepted by many studies to be the main cause of a wide range of disease conditions such as diabetes, cardiovascular diseases, and cancer.1 In such cases of excessive production of ROS, endogenous protective mechanisms have been identified to be insufficient to limit or quench ROS and their subsequent damage inflicted especially upon DNA and proteins.2 As additional mechanisms of dietary antioxidants may be of great importance, many artificial and natural agents possessing antioxidant and radical scavenging properties have been proposed to prevent and to treat oxidative damage induced by ROS-developed pathological states. Along with a wide scale of artificially prepared antioxidants, numerous naturally occurring plants and fruits, containing compounds with antioxidant and radical scavenging properties have been studied for the purpose of preventing oxidative stress of different etiologies.3,4 This is due to several advantages they possess, such as low or no toxicity, wide availability, and a diverse range of medicinal properties which are able to promote dismutation and trapping of most or all types of ROS.5,6 Most popular among them are different kinds of herbal teas widely used as nonalcoholic beverages.6
For centuries, herbal teas have been used in traditional medicinal systems for the treatment of infections, ailments, and diseases. These teas are very often an infusion of dried plant parts steeped in boiling water. Despite their widespread and established usage in the East, herbal teas have been gaining popularity in Western countries in recent years as well.7,8 Hundreds of different herbal teas are sold in health food stores which are available as pure or blended samples. Moreover, herbal teas have become a rapidly popular beverage because of their fragrance and associated therapeutic applications.6,9 For instance, tea from Camellia sinensis is the most widely consumed beverage in the world second only to water, owing mostly due to its identification as an important dietary source of natural phenolic antioxidants.
Aspalathus linearis (Rooibos tea) is well known for its rich content of different compounds with antioxidant properties and has recently gained much attention because of its potential use for clinical purposes.9,10 Thus, it seems to be of interest to elucidate whether it is able to initiate its antioxidant properties in disease models of diabetes and cancer, an aspect which has not been systematically studied to date. An identification of its efficacy against these diseases would be of value in its promotion as a supplementary therapy. Thus, this study engaged both chemical and in vitro assays for forming conclusions of its bioactivity against free radicals produced during these disease conditions. The effect of the Aspalathus linearis extract against ROS and its influence on the enzymatic activity of catalase (CAT) and superoxide dismutase (SOD) in hyperglycemia-induced human umbilical vein endothelial cells (HUVECs) and HeLa cells were explored in this study.
2. Materials and methods
Fresh samples of Aspalathus linearis were kindly provided by Rooibos World Co. (Nagoya, Japan). A voucher specimen of the sample was deposited in the herbarium of the Temasek School of Applied Sciences (no. 5242). Dubelcco's modified eagle's medium (DMEM), glucose, and antibiotics were purchased from GIBCO (Los Angeles, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Utah, USA). HeLa and HUVECs were purchased from American Type Culture Collection (ATCC, Virginia, USA). Tissue culture treated T75 flasks, 48-well plates, and 6-well plates were purchased from CellStar (USA). SOD assay kit was purchased from Cayman chemicals (Ann Arbor, MI, USA). 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetateacetyl ester (CM-H2DCFDA) was purchased from Invitrogen (Molecular Probe, New York, USA). All other reagents were purchased from Sigma Chemicals, Singapore unless otherwise stated.
2.1. Preparation of tea extracts
One gram of Aspalathus linearis leaves were extracted with 50 mL boiling water. Infusions were allowed to steep for 1 hour with continuous swirling. The remnants were filtered and the extraction was repeated. The collected extracts were combined and freeze-dried overnight. The yield of the dried extract as a percentage weight of the starting fresh plant material was calculated as 20.3%. The extracts were powdered and stored at −20°C until the various analyses were carried out.
2.2. Determination of the total phenolics content and oxygen radical absorbance capacity (ORAC) assay
The method, as described by Singleton and Rossi11 was used for determining the total phenolic content of Aspalathus linearis with modifications as suggested by Huang et al12 using the Folin–Ciocalteu's phenol reagent. The value was determined using a standard curve prepared from gallic acid and expressed as mg gallic acid equivalents/g fresh weight of sample (GAE/g). The ORAC assay was carried out as described by Huang et al13 in 96-well microplate format using a Tecan i-control multi well reader using Infinite 2000 software. Fluorescein disodium was used for the kinetic monitoring of free radical quenching and AAPH was used as the free radical source. The excitation and emission wavelengths were 485 nm and 528538 nm, respectively. The following components were added to a single well: (1) blank (phosphate buffered saline)/trolox standard/sample 20 μL; (2) fluorescein working solution 160 μL; and (3) AAPH 20 μL. The reaction kinetics were monitored for 2 hours at 37°C, following which the area under the curve was used to calculate the ORAC value compared with those of the trolox standards. Results were expressed as μmol Trolox Equivalents per gram fresh weight (μmol TE/g) of sample.
2.3. Establishment of diabetes and cancer cell models
HUVECs were cultured according to the method by Nishikawa et al14 whereas HeLa cells were cultured in 2% gelatine-coated 60 mm petri dishes and grown in DMEM low glucose media supplemented with 20% FBS and 1% antibiotics. HUVECs as well as HeLa cells were seeded at equal density (12,500 cells per well) in gelatine coated 48-well plates in its respective media. Both types of cells were treated with the Aspalathus linearis extracts at concentrations of 0.1 mg/mL, 0.2 mg/mL, 0.3 mg/mL, 0.4 mg/mL, and 0.5 mg/mL for 5 days based on previous studies on toxicity.15 For HeLa cells, 0.6 mg/mL and 0.7 mg/mL concentrations were also evaluated based on biochemical evidence from previous studies.16 For the HUVECs, two control groups of the cells were formed: (1) low glucose (LG) media (with a glucose concentration of 5.6 mM); and (2) high glucose (HG) media (with a glucose concentration of 35 mM). Only the HG HUVECs were incubated with the Aspalathus linearis extracts. It has to be noted that as a comparatively better experimental approach, both cells were exposed to the oxidative stress conditions prior to incubation with Aspalathus linearis in order to mimic the onset of the disease conditions as highlighted in the study by Nishikawa et al.14 As for the HeLa cells, exposure to oxidative stress triggering conditions such as H2O2 were not required due to their existing production of significant amounts of ROS. This production of ROS was further ascertained when a measurement was carried out using a fluorescent probe as described in the next paragraph. Several previous studies have also supported this condition by contrast with other mammalian cells for the study of ROS production.17–19
CM-H2DCFDA was used to detect the concentration of intracellular ROS. Both HUVECs and HeLa cells were exposed to 20 μL of 20 μM CM-H2DCFDA dissolved in phosphate buffered saline (PBS) for 45 minutes. Excess CM-H2DCFDA was removed by washing the cells twice using PBS. The intensity of viable cells was analyzed by the Tecan i-control multi well reader at 37°C using Infinite 2000 software at excitation and emission wavelengths of 492–495 nm and 517–527 nm, respectively. Five replicates were used per concentration of extract for the quantification of the fluorescence intensity.
2.4. Evaluation of the CAT and SOD activity
The CAT and SOD enzyme assays were performed in both HUVECs and HeLa cells according to the protocol provided in the assay kit by Cayman Chemicals. The results were analyzed by Tecan i-control multi well reader using Infinite 2000 software at 37°C.
2.5. Statistical analysis
Statistical analyses, which included one-way ANOVA and Tukey Test, were carried out using SPSS version 10.0 for Windows (SPSS Inc., IBM, Chicago, IL, USA). Results are expressed as the mean ± SEM (standard error mean) of five or more independent analyses. Values of p < 0.05 were considered to be significant.
3. Results
The extract had an ORAC value of 2705 ± 112 μM TE/g. In comparing with existing antioxidants such as vitamins C (2000 μM TE/g) and E (1900 μM TE/g),13 this value could be deemed as significantly high. The total phenolics content of the extract was evaluated as 54.6 ± 4.1 GAE/g. In considering the ratio between the antioxidant capacity and the total phenolics content, assuming the antioxidant activity to be coming from only the phenolic compounds of the extract, the measured ORAC value could be considered as significantly high as compared with teas of similar origin.9,10
For the HUVECs, Aspalathus linearis extracts at 0.5 mg/mL showed the highest decrease in the fluorescence intensity (Fig. 1A). All concentrations of the extract had statistically significant reductions (p < 0.05) in the fluorescence intensities as compared with the HG control. As the fluorescence intensity represents the presence of ROS, it could be concluded that the extract was able to successfully reduce the ROS levels in all the hyperglycaemic HUVECs. It was also noted that the extract concentration of 0.5 mg/mL did not have a statistically significant difference (p < 0.05) as compared with the LG control indicating the ability of the extract to reduce the ROS to almost nondiseased physiological concentrations, although whether this is reproducible in actual physiological conditions requires further exploration. Similar trends were observed in the extract-treated HeLa cells (Fig. 1B). All concentrations of the extracts had statistically significantly (p < 0.05) decreased the fluorescence intensity in the treated HeLa cells compared with the untreated control.
Fig. 1.
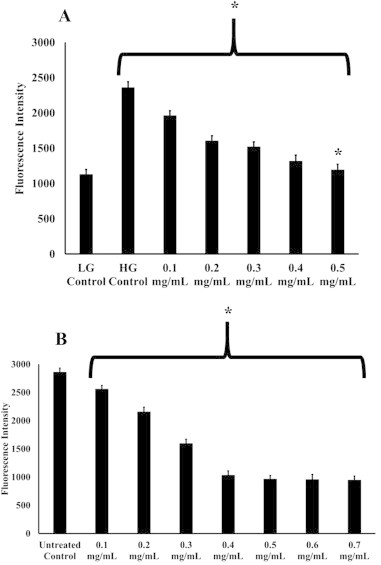
(A) Fluorescence intensity of the HUVECs incubated in 30 mM glucose after treatment with the various concentrations of rooibos tea extracts (mg/mL), 30 mM glucose without treatment (HG control) and 5.6 mM glucose without treatment (LG control). (B) Fluorescence intensity of the HeLa cells treated with the various rooibos tea extracts and untreated with the rooibos tea extracts (untreated control). *p < 0.05 versus the high glucose (HG) control or HeLa cells without any treatment. **p > 0.05 versus the low glucose (LG). HUVECs = human umbilical vein endothelial cells.
The CAT and SOD enzyme activities followed comparative trends to the ROS levels (Fig. 2) indicative of the extract's ability to quench the ROS via the antioxidant compounds as well as through the stimulation of the enzyme activity. Both CAT and SOD activities of the extract-treated HUVECs were statistically significantly higher than the HG control (p < 0.05). The trend in the HeLa cells was somewhat different to the HUVECs. Despite the constant ROS concentration of the HeLa cells >0.4 mg/mL of extract, the two enzyme activities were seen to be increasing proportionately with the concentrations of the extracts. Nevertheless, all the CAT and SOD activities of the extract-treated HeLa cells were statistically significantly higher than the untreated control (p < 0.05). Whether the continuous increases in the CAT and SOD activities in the HeLa cells along with the increasing concentrations of extracts results in a physiological benefit in terms of the oxidative stress levels requires further exploration.
Fig. 2.
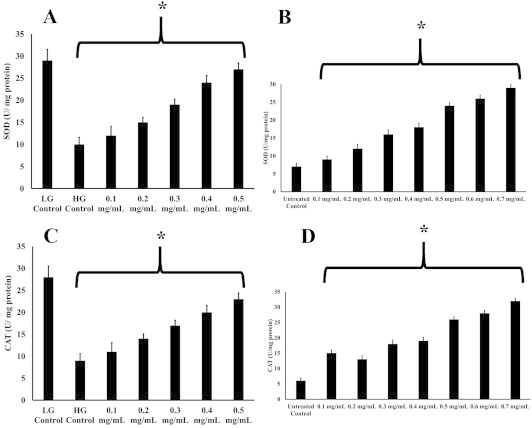
(A) SOD activity and (C) CAT activity of the HUVECs incubated in 30 mM glucose after treatment with the various concentrations of rooibos tea extracts (mg/mL), 30 mM glucose without treatment (HG control) and 5.6 mM glucose without treatment (LG control); **p > 0.05 versus the high glucose (HG) control. (B) SOD activity and (D) CAT activity of the HeLa cells treated with the various rooibos tea extracts and untreated with the rooibos tea extracts (untreated control). *p < 0.05 versus the high glucose (HG) control or HeLa cells without any treatment. CAT = catalase; HG = high glucose; HUVEC = human umbilical vein endothelial cells LG = low glucose; SOD = superoxide dismutase.
4. Discussion
The ORAC assay quantifies the peroxyl radical scavenging capacity by measuring the fluorescein oxidation. Thus, this assay was used as an initial screening step to estimate the radical scavenging ability of the Aspalathus linearis extract. However, because such chemical assays are unable to characterize the behavior of potential antioxidants under actual physiological conditions, it has been recommended to utilize at least two different types of assays (essentially of chemical and in vitro origin) for the investigation of antioxidant activities of samples.12,13 In addition, in order to complement the ORAC results as well as to evaluate the potency of the extract in two disease conditions pertaining to ROS, its effectiveness was determined in both hyperglycemic HUVECs and HeLa cells using CM-H2DCFDA. Fluorescent probes have been successfully used in in vitro studies for the identification of the presence of ROS.14 The oxidation of the probe in the presence of free radicals results in the increase of the fluorescence intensity. This, in turn, translates into a signal which can be easily measured using devices such as microplate readers. Through similar methods as used in this study, an increase in ROS production has been observed in many studies pertaining to cancer and diabetes hence justifying the usage of CM-H2DCFDA as a fluorescent probe to measure the presence of free radicals.20–22
Differing from the HUVECs, the HeLa cells were exposed to extract concentrations >0.5 mg/mL in this study. This was to demonstrate that the ROS concentrations did not decrease any further beyond the concentration of 0.4 mg/mL. Whether the ROS concentration in the HeLa cells >0.4 mg/mL were reflective of a nondiseased physiological status in this instance is nevertheless perplexing. However, it was noted that the fluorescence intensity of the HeLa cells of ≥0.4 mg/mL were comparable with the fluorescence intensity of the HUVECs' LG control. This group could be considered as a representation of typical physiological ROS concentrations existing in nondiseased conditions. The balance between oxidation and antioxidation (REDOX status) is known to be an important factor in maintaining a physiologically healthy biological milieu. An imbalance in the REDOX status observed in many of the chronic diseases further confirms the importance of maintaining the physiological REDOX balance.21,23 In addition, as already well established, hyperglycemia leads to the excessive production of ROS, which in turn damages the tertiary structure of the antioxidant enzymes.24–27 It is likely that the radical scavenging activity of the herb reduced the denaturation of the antioxidant enzymes caused by the free radicals. This protective phenomenon against damage towards antioxidant enzymes has also been observed in a few other plant extracts.28,29
ROS encompass a variety of diverse chemical species including superoxide anions, hydroxyl radicals, and hydrogen peroxide. Some of these species, such as superoxide or hydroxyl radicals, are extremely unstable, whereas others, such as hydrogen peroxide, are freely diffusible and relatively long-lived. These various radical species can either be generated exogenously or produced intracellularly from several different sources. ROS are generally regarded as a final common pathway for cell death and are elicited by all manner of tissue insults, such as diabetes-induced complications, ischemia associated with myocardial infarction or stroke, inflammatory stimuli such as endotoxin, and many anticancer drugs. ROS are also generated in the mitochondria as part of the multiple metabolic processes of normal physiology. In susceptible cells, low levels of oxidants can lead to apoptosis, but high levels can cause necrotic death. Therapeutic agents acting via cell death and cell protective pathways are likely to become increasingly prominent in the next few decades. Prevention of cell death will be of importance in the prevention of diabetes, stroke, myocardial infarction, and neurodegenerative conditions, whereas highly selective augmentation of cell death will be sought in anticancer drugs. Enhancement of antioxidant defences through dietary supplementation would seem to provide a more reasonable and practical approach to reduce the level of oxidative stress and there is a wealth of evidence to support the effectiveness of such a strategy in vitro. The development and discovery of novel compounds which behave as mimetics of SOD and CAT, or are able to enhance the activity of these two enzymes has offered an alternative approach with some promise.30,31
Although this study was able to successfully demonstrate the antioxidant activity of Rooibos tea in two in vitro models of diabetes and cancer, caution must be taken when transferring these results to in vivo situations. The disease-protective effects of phenolic compounds may also have adverse prooxidant activities which have been regularly reported in many studies.32,33 Inconsistencies between several studies have been raised over the past few years regarding the association between dietary antioxidants and low risks of diseases. This could be considered as a reflection of the limitation and complexity of human clinical studies as compared with in vitro and in vivo evaluations.33,34
5. Conclusion
From these results it can be concluded that Aspalathus linearis, as a commonly used nonalcoholic beverage, could be an excellent adjuvant supporting the therapy of diabetes and cancer. From the ORAC values and antioxidant enzymes data, it may be surmised that the herbal extract had initiated the antioxidant activities via a twofold pathway: (1) direct scavenging of free radicals; and (2) stimulating the expression and activity of CAT and SOD. As a complete recovery from these disease conditions cannot be expected, nonalcoholic beverages such as Aspalathus linearis can be generally used as supportive therapy in these two diseases as well as any others where free radicals are involved in their pathological progression. For this purpose nevertheless, in vitro and/or in vivo studies, followed by clinical evaluations are essential in order to obtain a more complete picture of the therapeutic efficacies.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by the student TOTE fund, Temasek School of Applied Science, Singapore.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Gutteridge J.M., Halliwell B. 2nd ed. Oxford University Press Inc; New York, NY: 1996. Antioxidants in Nutrition, Health, and Disease. [Google Scholar]
- 2.Pelicano H., Carney D., Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update. 2005;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrios B. Sources of natural phenolic antioxidants. Trends Food Sci Tech. 2006;17:505–512. [Google Scholar]
- 4.Lim Y.Y., Lim T.T., Tee J.J. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. [Google Scholar]
- 5.Chu Y.H., Chang C.L., Hsu H.F. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–566. [Google Scholar]
- 6.Dufresne C.J., Farnworth E.R. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen H.Y., Lin Y.C., Hsieh C.L. Evaluation of antioxidant activity of aqueous extracts of some selected nutraceutical herbs. Food Chem. 2007;104:1418–1424. [Google Scholar]
- 8.Atoui A.K., Mansouri A., Boskou G., Kefalas P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. [Google Scholar]
- 9.von Gadow A., Joubert E., Hansmann C.F. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong, and black tea. Food Chem. 1997;60:73–77. [Google Scholar]
- 10.Bramati L., Minoggio M., Gardana C., Simonetti P., Mauri P., Pietta P. Quantitative characterization of flavonoid compounds in Rooibos tea (Aspalathus linearis) by LC-UV/DAD. J Agric Food Chem. 2002;50:5513–5519. doi: 10.1021/jf025697h. [DOI] [PubMed] [Google Scholar]
- 11.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Eno Vit. 1965;16:1644–1658. [Google Scholar]
- 12.Huang D., Ou B.X., Hampsch-Woodill M., Flanagan J.A., Deemer E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J Agric Food Chem. 2002;50:1815–1821. doi: 10.1021/jf0113732. [DOI] [PubMed] [Google Scholar]
- 13.Huang D.J., Ou B.X., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multi-channel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa T., Edelstein D., Du X.L. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 15.Joubert E., Winterton P., Britz T.J., Gelderblom W.C.A. Antioxidant and prooxidant activities of aqueous extracts and crude polyphenolic fractions of Rooibos (Aspalathus linearis) J Agric Food Chem. 2005;53:10260–10267. doi: 10.1021/jf051355a. [DOI] [PubMed] [Google Scholar]
- 16.Kunishiro K., Tai A., Yamamoto I. Effects of Rooibos tea extract on antigen-specific antibody production and cytokine generation in vitro and in vivo. Biosci Biotechnol Biochem. 2001;65:2137–2145. doi: 10.1271/bbb.65.2137. [DOI] [PubMed] [Google Scholar]
- 17.Denicourt C., Dowdy S.F. Targeting apoptotic pathways in cancer cells. Science. 2004;305:14111413. doi: 10.1126/science.1102974. [DOI] [PubMed] [Google Scholar]
- 18.Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Rieux-Laucat F., Fischer A., Deist F.L. Cell-death signaling and human disease. Curr Opin Immunol. 2003;15:325–331. doi: 10.1016/s0952-7915(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 20.Yokozawa T., Dong E., Nakagawa T. In vitro and in vivo studies on the radical scavenging activity of tea. J Agric Food Chem. 1998;46:2143–2150. [Google Scholar]
- 21.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 22.Frankel E.N., Finley J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J Agric Food Chem. 2008;56:4901–4908. doi: 10.1021/jf800336p. [DOI] [PubMed] [Google Scholar]
- 23.Moon J.K., Shibamoto T. Antioxidant assays for plant and food components. J Agric Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 24.Hannon-Fletcher M.P.A., O'Kane M.J., Moles K.W., Weatherup C., Barnett C.R., Barnett Y.A. Levels of peripheral blood cell DNA damage in insulin dependent diabetes mellitus human subjects. Mutat Res. 2000;460:53–60. doi: 10.1016/s0921-8777(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 25.Nordwall M., Bojestig M., Arnqvist H.J., Ludvigsson J. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type I diabetes: the Linköping diabetes complications study. Diabetologia. 2004;46:1266–1272. doi: 10.1007/s00125-004-1431-6. [DOI] [PubMed] [Google Scholar]
- 26.Onaran I., Guven G.S., Ozdas S.B., Kanigur G., Vehid S. Metformin does not prevent DNA damage in lymphocytes despite its antioxidant properties against cumene hydroperoxide-induced oxidative stress. Mutat Res. 2006;611:1–8. doi: 10.1016/j.mrgentox.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complication Trial Research Group (DCTT)/Epidemiology of Diabetes Interventions and Complications Research Group Long-term defects of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waisundara V.Y., Hsu A., Huang D.J., Tan B.K.H. Scutellaria baicalensis enhances the antidiabetic activity of Metformin in streptozotocin-induced diabetic Wistar rats. Am J Chinese Med. 2008;36:517–540. doi: 10.1142/S0192415X08005953. [DOI] [PubMed] [Google Scholar]
- 29.Waisundara V.Y., Huang M.Q., Hsu A., Huang D.J., Tan B.K.H. Characterization of the antidiabetic and antioxidant effects of Rehmannia Glutinosa in streptozotocin-induced diabetic Wistar rats. Am J Chinese Med. 2008;36:1083–1104. doi: 10.1142/S0192415X08006594. [DOI] [PubMed] [Google Scholar]
- 30.Finkel T. Oxygen radicals and signalling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 31.Nemoto S., Takeda K., Yu Z.X., Ferrans V.J., Finkel T. A role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20:7311–7318. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Y.L. Challenges in providing credible scientific evidence of health benefits of dietary polyphenols. J Func Food. 2013;5:524–526. [Google Scholar]
- 33.Serafini M. Back to the origin of the “antioxidant hypothesis”: the lost role of the antioxidant network in disease prevention. J Sci Food Agric. 2006;86:1989–1991. [Google Scholar]
- 34.Van Poppel G. Carotenoids and cancer: an update with emphasis on human intervention studies. Eur J Canc. 1993;29A:1335–1344. doi: 10.1016/0959-8049(93)90087-v. [DOI] [PubMed] [Google Scholar]


