Abstract
Euphorbiaceae (Croton macrostachyus H.; 巴豆 bā dòu) is used in Ethiopian folklore medicine for the treatment of malaria, gonorrhea, diabetes, wounds, fungal infections, and helminths. No scientific investigations have been performed to substantiate these claims. This study aimed to investigate the in vivo antiplasmodial activity of 80% methanol extract of the fruit and the root of Croton macrostachyus H. in a rodent model of malaria. The rodent malaria parasite Plasmodium berghei was used to inoculate healthy 8-week-old male Swiss albino mice weighing 23–27 g. Each of the hydroalcoholic crude extracts (200 mg/kg, 400 mg/kg, and 600 mg/kg) were administered to different groups of mice. The parameters of parasitemia, survival time, body weight, temperature, and packed cell volume were determined using Peter's test and Rane's test. Both extracts significantly inhibited parasitemia and increased survival time in infected mice. Maximum suppression and prolongation were obtained at the highest doses used in the study. The crude extracts prevented loss of weight and temperature, but did not affect the packed cell volume. This study suggests that the root and fruit extracts of the plant both have promising antiplasmodial activity against Plasmodium berghei in a dose-dependent manner, which supports the folkloric use of the plant for treating malaria.
Keywords: antimalarial activity, Croton macrostachyus, fruit, Plasmodium berghei, root
Graphical abstract
1. Introduction
Malaria is generally a major public health problem throughout the world, particularly in developing countries. It causes an estimated 0.7–1 million deaths per year.1,2 Approximately one-half of the world's population is at risk of contracting malaria.1,2 Most cases (78%) occur in the African region, followed by Southeast Asia (15%) and Eastern Mediterranean regions (5%).3 Malaria is preventable and curable; however, it remains one of the greatest global public health problems, especially in sub-Saharan Africa.4
Because of increasing resistance to available antimalarial agents, there is broad consensus on the need to develop new antimalarial drugs. Antimalarial drug development can follow several strategies, which range from minor modifications of existing agents to the design of novel agents that act against new targets.5
Natural products have been important sources of different drugs currently available to treat severe Plasmodium falciparum malaria. Quinine and derivatives of artemisinin are the two most important products of plants useful in clinical practice. In the case of artemisinin, relatively simple chemical modifications of the natural parent compound have led to a series of highly potent antimalarials.6,7 The development of these two important drugs from natural sources and the utilization of many plants traditionally in various parts of the world trigger the conduction of in vitro and in vivo studies because natural products can be a source of new antimalarial drugs.
Croton macrostachyus H. (巴豆 bā dòu) is a deciduous tree that is 3–25 m high with large green leaves. It is commonly found on forest edges along rivers, around lakes, woodlands, wooded grasslands, and along road sides. It is native to Eritrea, Ethiopia, Kenya, Nigeria, Tanzania, and Uganda.8,9 The Shinasha, Agew-awi, and Amhara people of Ethiopia use it to treat malaria.10
Up to 80% of the Ethiopian population uses traditional medicine because of the cultural acceptability of healers, the relatively low cost of traditional medicine, and difficult access to modern health facilities.11 Works that have evaluated the safety and efficacy of Ethiopian traditional medicinal plants are not extensive.12 Ethiopia is rich in flora that could be sources of different, new antimalarial agents. Therefore, further screening of plants cannot be overemphasized. This study therefore aimed to enrich the screened Ethiopian medicinal plants and to evaluate the claimed antimalarial activity of Euphorbiaceae (Croton macrostachyus H.; 巴豆 bā dòu) by traditional medicine practitioners.
2. Materials and methods
2.1. Plant materials
The fresh fruit and root of the plant were collected in April 2013 from the Shindi district of Amhara regional state, which is 420 km southwest of Bahir Dar, Ethiopia. The fresh fruits and root were wrapped in plastic sheets during transportation. A taxonomist from Addis Ababa University (Addis Ababa, Ethiopia) identified the plant as Croton macrostachyus. A specimen of the plant was deposited (voucher no. LB001) at the National Herbarium in the College of Natural Sciences at Addis Ababa University for future reference.
2.2. Animals and the parasite
Male Swiss albino mice (age, 8 weeks; weight, 23–27 g) were used; they were bred and maintained at the Ethiopian Health Nutrition and Research Institute (Addis Ababa, Ethiopia). They were housed in the animal house of the Amhara Regional State Animal Health Institute (Bahir Dar, Ethiopia) at a temperature of 22 ± 3 °C, relative humidity of 40–50% and 12-hour light/12-hour dark cycle. The animals were acclimatized for 2 weeks to the experimental environment and provided with commercial food and water ad libitum. A chloroquine-sensitive strain of Plasmodium berghei (ANKA) was obtained from the School of Pharmacy in the Department of Pharmacology at Gondar University (Gondar, Ethiopia). The parasites were maintained by serial passage of blood from infected mice to uninfected mice on a weekly basis.
2.3. Extraction
The plant materials (i.e., fruit and root) were air dried at room temperature under shade and reduced to appropriate size by grinding with an electric mill. A total of 500 g of the dried root and fruit were each extracted by maceration (100 g of dried fruit and root in 500 mL of 80% methanol) for 72 hours. The extraction process was facilitated by an orbital shaker at 120 rpm. The mixture was first filtered using gauze and further filtered by Whatman filter paper No. 1 (Whatman, Beaconsfield, England). The residue was re-macerated for another 72 hours twice. The filtered extract was then dried by a rotary evaporator (Buchi Rota Vapor, Switzerland) at a temperature of 40 °C. After drying, 54 g (10.8%) and 50 g (10%) of the dry extract of the fruit and root, respectively, were harvested. The dried extracts were maintained at –20 °C until use.
2.4. Acute toxicity testing
Six female Swiss albino mice were randomly divided into two groups of three mice per cage. After being fasted for 2 hours,13,14 the mice in the first group were orally administered 2 g/kg of the fruit extract dissolved in 2% Tween 80, and observed for any signs of toxicity. The mice in the second group were administered 2 g/kg of the root extract dissolved in distilled water; they were observed for gross changes such as loss of appetite, hair erection, lacrimation, tremors, convulsions, salivation, diarrhea, mortality, and other signs of overt toxicity.13
2.5. Phytochemical screening
Following standard procedures, preliminary screening tests were performed on the crude extracts to detect different secondary metabolites.15–19
2.6. In vivo antimalarial tests
2.6.1. Parasite inoculation
The donors were four albino mice that were previously infected with P. berghei and had parasitemia levels of 20%, 24%, 25%, and 25%. The parasitemia of the donor mice was first determined. The donor mice were then sacrificed by decapitation and their blood was collected into a Petri dish treated with 0.5% trisodium citrate. The blood was then diluted with physiological saline (0.9%). The dilution was based on the parasitemia of the donor mice and the red blood cell count of the normal mice20 so that 1 mL blood contained 5 × 107 infected erythrocytes. Each mouse was administered intraperitoneally with 0.2 mL of this diluted blood, which contained 1 × 107 P. berghei-infected erythrocytes.
2.6.2. Grouping and dosing of animals
Fifty mice for each of the extracts were infected and were randomly divided into 20 groups of five mice per group. The mice in groups 1–10 (G1–10) were used to evaluate the antimalarial effects of the fruit using Peter's test and Rane's test. Three fruit extract doses were used: 200 mg/kg (F200), 400 mg/kg (F400), and 600 mg/kg (F600). The negative and positive controls were orally administered 2% Tween 80 (1 mL/100 g of mouse body weight) and chloroquine 15 mg/kg (CQ15), respectively. The mice in groups 11–20 (G11–20) were used for the root extract. Three root extract doses were used: 200 mg/kg (R200), 400 mg/kg (R400), and 600 mg/kg (R600). The negative and positive controls were orally administered distilled water (DW) (2 mL/100 g of mouse body weight) and CQ15, respectively.13 The doses were selected based on the results of the acute toxicity study. A standard gavage was used for the oral administration of the extracts, the vehicles, and the standard drug. The duration of the administration depended on the type of test.
2.6.3. The 4-day suppressive test
This test was used to evaluate the schizontocidal activity of the extracts against P. berghei infection in mice. The method used was described by Peter et al.21 The infected mice were randomly divided into their respective groups, as described under the “Grouping and dosing of animals” section (Amhara State Animal Health Institute). Treatment was started 3 hours after the mice had been inoculated with the parasite on Day 0. Treatment was then continued daily for 4 days from Day 0 to Day 3. After giving the treatment for 4 days, a thin blood film was obtained on Day 4 to determine parasitemia and percentage inhibition. In addition, each mouse was attended daily for the determination of survival time after treatment. Rectal temperature, packed cell volume (PCV), and body weight were also recorded.
2.6.4. Rane's test
The curative potential of the crude extract and the most active fraction were evaluated using the method described by Ryley and Peters.22 On Day 0, the standard inocula of 1 · 107 infected erythrocytes was inoculated in mice intraperitoneally. Seventy-two hours later, the mice were randomly divided into their respective groups and dosed accordingly once daily for 4 days. A Geimsa-stained thin blood film was prepared from the tail blood of each mouse daily for 5 days to monitor the parasitemia level. The survival time for each group was determined by finding the average survival time (days) of mice, starting from their infection in each group for 30 days (D0–D29). Rectal temperature, PCV, and body weight were also recorded.
2.6.5. PCV measurement
The PCV was measured to predict the effectiveness of the test extract and fractions in preventing hemolysis resulting from increased parasitemia associated with malaria. Heparinized capillary tubes were used to collect blood from the tail of the mouse. The capillary tubes were filled with blood to three-quarters of their height and sealed at the dry end with sealing clay. The tubes were then placed in a microhematocrit centrifuge (Gelmanin-Hawksley, Lancing, England) with the sealed end outwards, and centrifuged for 5 minutes at 11,000 rpm. The tubes were then removed from the centrifuge and the PCV was determined using the standard Micro-Hematocrit Reader (Hawksley and Sons). The PCV is a measure of the proportion of red blood cells (RBC) to plasma and is measured before inoculating the parasite and after treatment by the following relationship:
| PCV = volume of erythrocytes in a given volume of blood/total blood volume |
2.6.6. Parasitemia measurement
Thin smears of blood were produced from the blood obtained from the tail of each mouse on Day 4 and Days 3–7 for Peter's test and Rane's test, respectively. The smears were applied on microscope slides (76 mm · 26 mm; Menzel-Glaser, Braunschweig, Germany), fixed with absolute methanol for 15 minutes, and stained with 10% Geimsa stain at pH 7.2 for 15 minutes. The stained slides were then washed gently using DW and then air dried at room temperature. Two stained slides for each mouse were examined under an Olympus microscope (CHK2-F-GS, Taiwan) with an oil immersion nose piece at 100× magnification power. Three different fields on each slide were examined to count the infected RBC. The average of the results was used to calculate the average parasitemia level. The percent parasitemia was calculated using the following formula23:
The percent parasitemia suppression of the extracts was compared with respect to the controls. Parasitemia suppression was calculated using the following formula24:
2.6.7. Monitoring of body weight and temperature changes
The body weight of each mouse in all groups was measured using a sensitive digital weighing balance (Mettler Toledo, Greifensee, Switzerland) before infection and after the completion of the treatment for Peter's test and Rane's test. The rectal temperature of the mice in all groups was measured by a digital rectal thermometer before infection and after the completion of the treatment for both tests.
2.7. Ethical consideration
The animals were maintained and cared for in accordance with the international guidelines for the use and maintenance of experimental animals.13 The study protocol was approved by the board of ethics of the College of Medicine and Health sciences of Bahir Dar University (Bahir Dar, Ethiopia). A formal letter was written to the concerned bodies of the College of Medicine and Health Sciences postgraduate and research coordinator. Diethyl ether was used for euthanasia.
2.8. Data analysis
Data are expressed as the mean ± the standard error of the mean. The data were analyzed using Windows SPSS Version 16.0. One-way analysis of variance (ANOVA) followed by Tukey's honest significant difference post-hoc test was used to determine statistical significance in the comparisons of parasitemia suppression, weight, PCV, rectal temperature, and survival time among groups. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Phytochemical screening
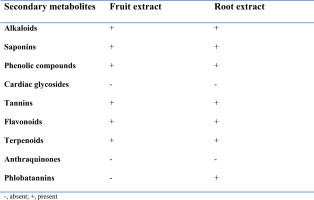
Phytochemical screening of the hydroalcoholic crude extracts of the fruit and root of Croton macrostachyus revealed the presence of alkaloids, phenolic compounds, tannins, terpenoids, saponins, and flavonoids. Anthraquinones and cardiac glycosides were absent in both extracts. Phlobatannins were present only in the root extract (Table 1).
Table 1.
Phytochemical constituents of the root and fruit extracts of Croton macrostachys.
| Secondary metabolites | Fruit extract | Root extract |
|---|---|---|
| Alkaloids | + | + |
| Saponins | + | + |
| Phenolic compounds | + | + |
| Cardiac glycosides | – | – |
| Tannins | + | + |
| Flavonoids | + | + |
| Terpenoids | + | + |
| Anthraquinones | – | – |
| Phlobatannins | – | + |
−, absent; +, present.
3.2. Toxicity studies
The acute toxicity study indicated that both extracts caused no mortality at the dose used (2 g/kg) within the first 24 hours and for the next 14 days. Physical and behavioral observations of the experimental mice also revealed no visible signs of acute toxicity such as lacrimation, loss of appetite, tremors, hair erection, salivation, and diarrhea.
3.3. Effect of the 4-day suppressive test
The results of the study showed that the fruit extract displayed a very good chemosuppressive activity against P. Berghei (Table 2). Percentage inhibition analysis indicated that the extracts produced a dose-dependent decrease in all animals on the 5th day of parasitemia (p < 0.001), compared to the negative controls. The fruit extract F600 and root extract R600 exhibited a significant parasite suppression, compared to the other doses (Table 2). The extract at all doses was also capable of significantly increasing the survival time. The root extract had better suppressive activity and it increased survival time better than the fruit extract.
Table 2.
Parasitemia and survival time measurements in the 4 day suppressive test of the root and fruit extracts of Croton macrostachys.
| Extract administered | Animal group | Parasitemia level | % Suppression | Survival date |
|---|---|---|---|---|
| Fruit extract | CON | 30 ± 0.97 | 0 | 6 ± 0.36 |
| F200 | 18 ± 1.73 | 40a3,b3,c3 | 9 ± 0.33a1,b3,c3 | |
| F400 | 9 ± 0. 31 | 70a3,b1,c3 | 13 ± 0.42a3,b3,c3 | |
| F600 | 4 ± 0.40 | 87a3 | 16 ± 0.60a3,c3 | |
| CQ15 | 0.0 | 100a3 | 30±0a3 | |
| Root extract | CON | 32 ± 1.62 | 0 | 7 ± 0.17 |
| R200 | 17 ± 0.54 | 44a3,c3,d3 | 10 ± 1.14a1,c3,d3 | |
| R400 | 13 ± 0.71 | 75a3,c3,d1 | 14 ± 0.56a3,c,3,d3, | |
| R600 | 3.5 ± 0.52 | 89a3 | 19 ± 1.15a3,c3 | |
| CQ15 | 0.0 | 100a3 | 30.00 ± 0.00a3 |
Data are expressed as means ± SEM; n = 5; a = compared to control, b = to F600 mg/kg, c = to CQ15 mg/kg, d = R600 mg/kg; 1 = p < 0.05, 2 = p < 0.01, 3 = P < 0.001; CON = Control.
Analysis of the rectal temperature revealed that 80% methanolic fruit extract of Croton macrostachys significantly prevented the reduction of temperature in a dose-dependent manner (p < 0.01 for F200 and F400; p < 0.001 for F600). The test extract prevented the loss of weight associated with the increase in parasitemia at all dose levels, compared to the negative controls. However, the increase in body weight was not dose-dependent; the highest reduction was caused by F400 (p < 0.001), followed (in decreasing order) by F600 (p < 0.01) and F200 (p < 0.05). There were no detectable differences between the extracts and between the extract and standard in preventing weight loss associated with increasing parasitemia (Table 3).
Table 3.
Temperature, weight and PCV measurements in the 4 day suppressive test of the root and fruit extracts of Croton macrostachys.
| Extract given | Animal group | Temperature |
Weight |
PCV |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D4 | % change | D0 | D4 | % change | D0 | D4 | % change | ||
| Fruit extract | CON | 37.2 ± 0.19 | 36.5 ± 0.19 | −2 | 26.0 ± 0.18 | 24.0 ± 0.43 | −15.5 | 55 ± 0.33 | 53 ± 0.26 | −3.6 |
| F200 | 37.5 ± 0.08 | 37.2 ± 0.07 | −0.8a2 | 26.1 ± 0.12 | 25.3 ± 0.34 | −5.2 a1 | 53 ± 1.34 | 53 ± 1.48 | 0.0 | |
| F400 | 37.1 ± 0.11 | 37.2 ± 0.11 | 0.5a2 | 26.2 ± 0.11 | 25.9 ± 0.15 | −0.6a3 | 54 ± 0.50 | 55 ± 0.70 | 1.8 | |
| F600 | 37.0 ± 0.15 | 37.5 ± 0.15 | 1.3a3 | 26.2 ± 0.17 | 25.7 ± 0.22 | −2.8a2 | 54 ± 0.55 | 56 ± 0.84 | 3.5 | |
| CQ15 | 37.1 ± 0.08 | 37.2 ± 0.08 | 0.2a2 | 26.1 ± 0.09 | 25.5 ± 0.05 | −2.4 a2 | 53 ± 0.55 | 55 ± 0.42 | 3.8 | |
| Root extract | CON | 37.3 ± 0.42 | 32.9 ± 0.21 | −13.3 | 27.9 ± 0.82 | 26.8 ± 1.15 | −4.00 | 53.3 ± 1.46 | 49.7 ± 1.61 | −3.73 |
| R200 | 36.9 ± 0.37 | 36.3 ± 0.47 | −1.6a3 | 27.17 ± 0.64 | 25.15 ± 1.16 | −7.43b3 | 51.7 ± 1.78 | 51.1 ± 1.1 | −1.14 | |
| R400 | 37.5 ± 0.32 | 37.4 ± 0.23 | −0.3a3 | 27.7 ± 1.16 | 26.8 ± 1.08 | −3.82b2 | 52.2 ± 1.25 | 52.2 ± 1.16 | 0.00 | |
| R600 | 37.7 ± 0.49 | 37.4 ± 0.39 | −0.8a3 | 28.1 ± 1.20 | 27.9 ± 1.12 | −0.89b1 | 51.7 ± 1.82 | 53.3 ± 1.90 | 3.23 | |
| CQ15 | 37.4 ± 0.27 | 37.5 ± 0.26 | 0.26a3 | 26.7 ± 0.51 | 27.3 ± 0.83 | 2.36a2 | 53.2 ± 0.48 | 53.9 ± 0.62 | 1.35 | |
Data are expressed as mean ± SEM; n = 5; a = compared to control, b = to CQ 15 mg/kg; 1 = p < 0.05; 2 = p < 0.01, 3 = p < 0.001; D0 = pre-treatment value on day zero, D4 = post-treatment value on day four, CON = control.
The root extract produced similar results. It prevented the reduction in body weight in a dose-dependent manner. All doses also prevented a reduction in temperature. Neither the fruit nor the root extract significantly prevented the reduction of PCV, compared to the negative controls.
3.4. Effect on curative test
The fruit extract in a dose-dependent manner reduced parasitemia by 36%, 64%, and 83% for F200, F400, and F600, respectively (p < 0.001 in all animals), compared to the controls (Table 4). The inhibition by CQ15 was significantly higher than the inhibition by F200 (p < 0.001) and F400 (p < 0.05), but it was not statistically significant with F600. Survival time was not altered by F200, but was significantly increased by F400 (p < 0.001) and F600 (p < 0.001). However, this increase could not match that obtained with chloroquine (Table 4). The root extract produced better chemosuppressive activity than the fruit extract. The suppression was dose-dependent by 43%, 70%, and 88% for R200, R400, and R600, respectively. CQ15 produced a more significant inhibitory effect, compared to R200 (p < 0.001) and R400 (p < 0.05). All three doses increased the survival time significantly [R200 (p < 0.05), R400 (p < 0.001), and R600 (p < 0.001)]. CQ15 produced a more significant increment in survival time, compared to the controls and all extract doses (Table 4).
Table 4.
Parasitemia and survival time measurements for Rane's test of the root and fruit extracts of Croton macrostachys.
| Extract administered | Animal group |
Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | % Inhibition | Survival date |
|---|---|---|---|---|---|---|---|---|
| Fruit extract | CON | 9 ± 0.15 | 16 ± 0.13 | 14 ± 0.23 | 31 ± 0.61 | 36 ± 1.10 | 0 | 6 ± 0.31 |
| F200 | 12 ± 0.36 | 28 ± 1.20 | 26 ± 0.32 | 25 ± 0.16 | 23 ± 0.19 | 36a3,b3,c3 | 7 ± 0.31b3,c3 | |
| F400 | 11 ± 0.43 | 19 ± 0.95 | 16 ± 0.85 | 14 ± 0.54 | 13 ± 0.95 | 64a3,c1 | 12 ± 0.47a3,b3,c3 | |
| F600 | 10 ± 0.32 | 14 ± 0.56 | 11 ± 0.47 | 8 ± 0.41 | 6 ± 0.78 | 83a3 | 16 ± 0.42a3,c3 | |
| CQ15 | 11 ± 0.22 | 5 ± 0.09 | 2 ± 0.56 | 0 | 0.00 ± 0.00 | 100a3 | 30±0a3 | |
| Root extract | CON | 21.8 ± 0.58 | 26.6 ± 0.90 | 33.1 ± 0.81 | 40.7 ± 0.67 | 44 ± 1.34 | 0 | 5 ± 0.31 |
| R200 | 20.8 ± 0.83 | 20.8 ± 0.66 | 20.2 ± 0.92 | 19.8 ± 0.50 | 25 ± 0.63 | 43a3,c3,d3 | 9 ± 0.34a1,c3,d2 | |
| R400 | 22.7 ± 0.63 | 21.2 ± 0.51 | 17.4 ± 0.45 | 15.4 ± 0.49 | 13 ± 0.80 | 70a3,c3 | 13 ± 0.76a3,c3,d3 | |
| R600 | 21.8 ± 0.79 | 20.9 ± 0.62 | 15.4 ± 1.02 | 11.5 ± 0.45 | 5 ± 0.65 | 88a3 | 18 ± 0.72a3,c3 | |
| CQ15 | 20.8 ± 0.85 | 15.6 ± 1.02 | 5.5 ± 0.28 | 1.5 ± 0.29 | 0.00 ± 0.00 | 100a3 | 30.0 ± 0.0a3 |
Data are expressed as means ± SEM; n = 5; a = compared to control, b = to F600 mg/kg, c = CQ 15 mg/kg, d = R600 mg/kg; 1 = p < 0.05, 2 = p < 0.01, 3 = P < 0.001; CON = Control.
Rectal temperature analysis indicated that both extracts significantly prevented the reduction of rectal temperature in comparison to the controls F200 (p < 0.01) < F400 (p < 0.001) < F600 (p < 0.001); R200 (p < 0.01) < R400 (p < 0.001) < R600 (p < 0.001). A comparison of the doses did not reveal any apparent change. No detectable changes were observed between the extract doses and CQ15 (Table 5). Similar to the 4-day suppressive test, both extracts failed to show any protective activity against the reduction in the PCV (Table 5).
Table 5.
Rectal temperature, weight and PCV measurements in Rane's test of root and fruit extracts of Croton macrostachys.
| Animal group | T0 | T4 | % Change | W0 | W4 | % Change | PCV0 | PCV4 | % Change |
|---|---|---|---|---|---|---|---|---|---|
| CON | 37.4 ± 0.14 | 36.6 ± 0.05 | −2.2 | 26.2 ± 0.21 | 22.1 ± 0.43 | −18.3 | 54 ± 0.31 | 52 ± 0.21 | −3.9 |
| F200 | 37.3 ± 0.08 | 37.2 ± 0.08a2 | −0.2 | 26.3 ± 0.11 | 24.0 ± 0.16a1 | −9.6 | 53 ± 0.31 | 52 ± 0.40 | −1.9 |
| F400 | 37.4 ± 0.09 | 37.4 ± 0.04a3 | 0.0 | 26.4 ± 0.11 | 26.1 ± 0.05a3 | −0.8 | 52.5 ± 0.43 | 53 ± 0.22 | 0.9 |
| F600 | 37.3 ± 0.09 | 37.4 ± 0.03a3 | 0.2 | 26.5 ± 0.06 | 25.9 ± 0.05a2 | −1.9 | 52 ± 0.31 | 53 ± 0.44 | 1.9 |
| CQ15 | 37.4 ± 0.18 | 37.3 ± 0.13a2 | −0.2 | 26.4 ± 0.04 | 25.8 ± 0.04a2 | −2.3 | 52 ± 0.42 | 53 ± 0.21 | 1.9 |
| CON | 37.8 ± 0.14 | 36.7 ± 0.05 | −2.9 | 26.4 ± 0.21 | 22.4 ± 0.43 | −17.8 | 55 ± 0.31 | 53 ± 0.21 | −3.7 |
| R200 | 37.6 ± 0.08 | 37.0 ± 0.08a2 | −0.2 | 26.8 ± 0.11 | 25.0 ± 0.16a1 | −7.2 | 53 ± 0.31 | 51.5 ± 0.40 | −2.9 |
| R400 | 37.5 ± 0.09 | 37.5 ± 0.04a3 | 0.0 | 26.4 ± 0.11 | 26.1 ± 0.05a3 | −1.1 | 53 ± 0.43 | 53 ± 0.22 | 0.0 |
| R600 | 37.1 ± 0.09 | 37.8 ± 0.03a3 | 0.2 | 26.6 ± 0.06 | 26.4 ± 0.05a3 | −0.8 | 52.5 ± 0.31 | 53 ± 0.44 | 0.9 |
| CQ15 | 37.7 ± 0.18 | 37.1 ± 0.13a2 | −0.2 | 26.7 ± 0.04 | 26.0 ± 0.04a2 | −2.7 | 54 ± 0.42 | 55 ± 0.21 | 1.8 |
N = 5, a = compared to controls, 1 = p < 0.05, 2 = p < 0.01, 3 = p < 0.001.
The extracts exhibited a preventive effect in the weight reduction of P. berghei-infected mice at all dose levels, compared to the controls. There was no significant difference among the extract doses and CQ15 (Table 5).
4. Discussion
An in vivo model was employed for this study because it takes into account a possible prodrug effect and possible involvement of the immune system in the eradication of an infection.20 P. berghei ANKA is used in the prediction of treatment outcomes25; hence it was an appropriate parasite for this study. Several conventional antimalarial agents such as chloroquine, halofantrine, mefloquine—and more recently, artemisinin derivatives—have been identified using the rodent malaria model.26
The 4-day suppressive test, which primarily evaluates the antimalarial activity of candidate agents on early infections, and Rane's test, which evaluates the curative capability of candidate extracts on established infections, are commonly used for antimalarial drug screening. In both methods, the determination of the percent inhibition of parasitemia is the most reliable parameter. A mean group parasitemia level that is ≤ 90% of that the level in mock-treated control animals usually indicates that the test compound is active in standard screening studies.27
The results from the study indicated that, in P. berghei-infected mice, the percentage parasitemia measured in the 4-day test was significantly reduced in both extract-treated groups, compared to mice in the negative control group. This indicates that the plant is endowed with antimalarial activity. In fact, the root extract with 89% and 88% parasitemia suppression and the fruit extract with 87% and 83% parasitemia suppression for Peter's test and Rane's test, respectively, have comparable antimalarial activity to another species of the same genus, Croton zambesicus, which has a maximum parasitemia suppression activity of 80%.28 Alkaloids, phenolic compounds, and terpenoids in these extracts could be responsible for their antimalarial activity. The alkaloids possess antiplasmodial properties. The most famous alkaloid is quinine. Crude extract of Euphorbiaceae (Croton macrostachyus H.; 巴豆 bā dòu) reportedly has antimicrobial activity.29 Furthermore, chloroform and butanol fractions of the plant show a higher growth inhibition activity in bacteria, whereas the aqueous fraction has no growth inhibition effect.30 Drugs with antibacterial activity such as tetracycline and its derivatives have been implicated in malarial treatment. This further reinforces the notion that compounds responsible for antimalarial activity of the plant may be concentrated in nonpolar and semipolar fractions.
Anemia, body weight loss, and body temperature reduction are the general features of malaria-infected mice.31 Therefore, the ideal antimalarial agents from plants are expected to prevent body weight loss in parasite-infected mice. Despite the fact that an increase in weight was not consistent with an increase in the dose, the fruit extract of Croton macrostachys significantly prevented weight loss associated with an increase in the parasitemia level, whereas the root extract prevented weight reduction in a dose-dependent manner. This finding may indicate the presence of appetite-suppressant secondary metabolites in the fruit extract, rather than in the root extract, or some other difference. A decrease in the metabolic rate in infected mice occurs before death and is accompanied by a corresponding decrease in the internal body temperature.32 The rectal temperature ideally decreases as the parasite level escalates. Active compounds should prevent the rapid dropping of the rectal temperature. Both extracts did have a protective effect against temperature reduction in a dose-dependent manner.
The PCV was measured to evaluate the effectiveness of the crude extract and fractions in preventing hemolysis due to a rising parasitemia level. The underlying cause of anemia includes the following mechanisms: the clearance and/or destruction of infected RBC, the clearance of uninfected RBC, and erythropoietic suppression and dyserythropoiesis. Each of these mechanisms has been implicated in human and in mouse malarial anemia.33 Both extracts failed to prevent PCV reduction. The failure of extracts to reverse PCV reduction could probably be associated with the presence of saponins in the extracts, which have strong hemolytic effects.34
In the curative test, blood samples were obtained and smears were prepared daily to evaluate the curative ability of the extract. As indicated in the “Results” section, all doses of the extracts reduced parasitemia after two dosings; however, the standard drug chloroquine started its activity right after the first dose. This delay in activity may indicate the need for a loading dose, or the extract may have a delayed onset of action, or the extracts may need more frequent administrations per day.
The active compound has yet to be identified, although the antimalarial activity of Croton macrostachyus could be attributed to a single metabolite or a combination of its secondary metabolites such as alkaloids, flavonoids, terpenoids, and phenolic compounds. These metabolites have been reported in the literature as having different extents of antimalarial activity.35–37 Many species of the genus Croton also reportedly have promising antimalarial activity in different in vitro and in vivo studies.38
In vivo antiplasmodial activity can be classified as “moderate”, “good”, and “very good” if an extract displays a percentage parasitemia suppression ≥ 50% at a dose of 500 mg/kg, 250 mg/kg, and 100 mg/kg body weight per day, respectively.39 Based on this classification, the root and fruit extract have good antiplasmodial activity, even though the root extract somewhat showed better suppressive effect than did the fruit extract.
5. Conclusion
The results obtained from the present study revealed that both extracts prevented a reduction in body weight and in rectal temperature, which are associated with increasing parasitemia, whereas both extracts failed to prevent a significant reduction in the PCV. Survival time of the extract-treated mice was also significantly prolonged. In the study, both extracts produced significant parasitemia suppression in both models. Further studies are required to determine the chemical constituent responsible for the antimalarial activity of Euphorbiaceae (Croton macrostachyus H.; 巴豆 bā dòu). The plant is also an active area of research because it is used traditionally for many disorders. The results of the study can meanwhile be cited as evidence for claims concerning the antimalarial activity of the plant in Ethiopian traditional medicine.
Conflict of interest
None.
Acknowledgments
The financial and material support of Bahir Dar University (Bahir Dar, Ethiopia) is highly acknowledged. It is also my pleasure to thank the Amhara Regional State Animal Health Institute for allowing me to conduct my research and providing me material and human resources. I highly acknowledge Ato Maru Desta and Ato Habitamu Kefiyalew for their contribution to this work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.World Economic Forum . School of Public Health; Cologny/Geneva, Switzerland: 2006. Malaria and Business 2006. Global Health Initiative in Cooperation with Harvard University. [Google Scholar]
- 2.World Health Organization (WHO) Switzerland; Geneva: 2008. World Malaria Report 2008. WHO. [Google Scholar]
- 3.World Health Organization (WHO) Switzerland; Geneva: 2010. World Malaria Report 2010. WHO. [Google Scholar]
- 4.Irungu B.N., Mbabu M.J., Kiboi D.M., Moindi E., Kinyua J., Romano M. In vivo antimalarial and acute toxicity properties of hexane and chloroform extracts from Clausena anisata (Willd.) Benth. AJPT. 2012;1:24–29. [Google Scholar]
- 5.Rosenthal J.P. Antimalarial drug discovery, old and new approaches. J Exp Biol. 2003;206:3735–3744. doi: 10.1242/jeb.00589. [DOI] [PubMed] [Google Scholar]
- 6.Wright W.C. Plant derived antimalarial agents, new leads and challenges. Phytochem Rev. 2005;4:55–61. [Google Scholar]
- 7.Batista R., Júnior S.J.A., Oliveira B.A. Plant-derived antimalarial agents, new leads and efficient phytomedicines, part II. Non-alkaloidal natural products. Molecules. 2009;14:3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapingu C.M., Guillaume D., Mbwambo H.Z., Moshi J.M., Uliso C.F., Mahunnah A.L.R. Diterpenoids from the roots of Croton macrostachys. Phytochem. 2000;54:767–770. doi: 10.1016/s0031-9422(00)00166-7. [DOI] [PubMed] [Google Scholar]
- 9.Orwa C., Mutua A., Kindt R., Jamnadass R., Simons A. 2009. Agroforestry Database: a Tree Reference and Selection Guide Version 4.http://www.worldagroforestry.org/af/treedb Available at. [Last Accessed on 04.02.12] [Google Scholar]
- 10.Gidey M., Tekilehaimanot T., Animut A., Mekonen Y. Medicinal plants of Shinasha, Agew-awi and Amhara peoples in North West Ethiopia. J Ethnopharmacol. 2007;110:516–525. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Kassaye K.D., Amberbir A., Getachew B., Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20:127–134. [Google Scholar]
- 12.Tekalign D., Yalemtsehay M., Abebe A. In vivo antimalarial activity of Clerodendrum myricoids, Dodonea augustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24:25–29. [Google Scholar]
- 13.Organization for Economic Co-operation and Development (OECD) OECD; Paris, France: 2001. Environment, Health and Safety Publications Series on Testing and Assessment, No 24: Guidance Document on Acute Oral Toxicity Testing. [Google Scholar]
- 14.Center for Drug Evaluation and Research (CDER) CDER; Silver Spring, MD: 1996. Guidance for Industry Single Dose Acute Toxicity Testing for Chemicals. [Google Scholar]
- 15.Trease G.E., Evans W.C. 13th ed. Macmillan Publishers; London, England: 1989. A Textbook of Pharmacognosy. Brailliere Tindal. [Google Scholar]
- 16.Jones P., Kinghorn D. Extraction of Plant Secondary Metabolites. In: Sarker D., Latif Z., Gray A., editors. Methods in Biotechnology Natural Products Isolation. Human Press; Totowa, NJ: 2006. pp. 323–351. [Google Scholar]
- 17.Sofowara A. 2nd ed. Spectrum Books; Ibadan, Nigeria: 1993. Medicinal Plants and Traditional Medicines in Africa. [Google Scholar]
- 18.World Health Organization (WHO) WHO; Geneva, Switzerland: 1987. The Promotion and Development of Traditional Medicine, Technical Report Series 622. [PubMed] [Google Scholar]
- 19.Waako P.J., Gumede B., Smith P., Folb P.I. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum and Momordica foetida. J Ethnopharmacol. 2005;99:137–143. doi: 10.1016/j.jep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Hilou A., Nacoulma G., Guiguemde T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus and Boerhaavia erecta in mice. J Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Peter W., Portus H., Robinson L. The four-day suppressive in vivo antimalarial test. Ann Trop Med Parasitol. 1995;69:155–171. [Google Scholar]
- 22.Ryley J.F., Peters W. The antimalarial activity of some quinoline esters. Ann Trop Med Parasitol. 1995;84:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 23.Kalra B.S., Chawla S., Gupta P., Valecha N. Screening of antimalarial drugs. Ind J Pharmacol. 2006;38:5–12. [Google Scholar]
- 24.Dikasso D., Mekonnen E., Debella A. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop J Health Dev. 2006;20:112–118. [Google Scholar]
- 25.Madara A., Ajayi J.A., Salawu O.A., Tijani A.Y. Anti-malarial activity of ethanolic leaf extract of Piliostigma thonningii Schum. (Caesalpiniacea) in mice infected with Plasmodium berghei berghei. Afr J Biotechnol. 2010;9:3475–3480. [Google Scholar]
- 26.Peter I.T., Anatoli V.K. ASM Press; Washington, DC: 1998. The Current Global Malaria Situation. Malaria Parasite Biology, Pathogenesis, and Protection; pp. 11–22. [Google Scholar]
- 27.Okokon J.E., Ofodum K.C., Ajibesin K.K., Danladi B., Gamanil K.S. Pharmacological screening and evaluation of antiplasmodial activity of Croton Zambesicus against Plasmodium berghei in mice. Ind J Pharmacol. 2005;379:243–246. [Google Scholar]
- 28.Bero J., Ganfon H., Jonville M. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J Ethnopharmacol. 2009;122:439–444. doi: 10.1016/j.jep.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Tefera M. Department of Biology, Addis Ababa University; Addis Ababa, Ethiopia: 2006. In vitro Evaluation of Antimicrobial Activities of Albizia gummifera and Croton macrostachyus Against Clinical Isolates of Neisseria gonorrhoeae. MSc Thesis. [Google Scholar]
- 30.Langhorne J., Quin S.J., Sanni L.A. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. In: Perlmann P., Troye-Blomberg M., editors. Malaria Immunology. 2nd ed. Karger Publishers; Stockholm, Sweden: 2002. pp. 204–228. [DOI] [PubMed] [Google Scholar]
- 31.Yen W.J. Possible anti-obesity therapeutics from nature—a review. Phytochem. 2010;71:1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z.G., Sun H.X., Fang W.H. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins on the immune responses to ovalbumin in mice. Vaccine. 2005;23:5196–5203. doi: 10.1016/j.vaccine.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Okokon J.E., Effiong I., Ettebong E. In vivo antimalarial activities of ethanolic crude extracts and fractions of leaf and root of Carpolobia lutea. Pak J Pharm Sci. 2011;24:57–61. [PubMed] [Google Scholar]
- 34.Kaur K., Jain M., Kaur T., Jain R. Antimalarials from nature. Bioorg Med Chem. 2009;17(9):3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira A., Balla J., Jeney V., Balla G., Soares M.P. A central role for free heme in the pathogenesis of severe malaria: the missing link. J Mol Med. 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 36.Okokon J.E., Iyadi K., Effiong C. Effect of subchronic administration of ethanolic leaf extract of Croton zambesicus on haematological parameters of rats. Nig J Physiol Sci. 2004;19:10–13. [Google Scholar]
- 37.Boyom F.F., Kemgne E.M., Tepongning R., Mbacham W.F., Tsamo E., Zollo P.H.A. Antiplasmodial activity of extracts from seven medicinal plants used in malaria treatment in Cameroon. J Ethnopharmacol. 2009;123:483–488. doi: 10.1016/j.jep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Deharo E., Bourdy G., Quenevo C., Munoz V., Ruiz G., Sauvain M. A search for national bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tecana Indians. J Ethnopharmacol. 2001;77:91–98. doi: 10.1016/s0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 39.Munoz V., Sauvain M., Bourdy G. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. J Ethnopharmacol. 2000;69:127–137. doi: 10.1016/s0378-8741(99)00148-8. [DOI] [PubMed] [Google Scholar]


