Abstract
Functional constipation is a common clinical complaint of patients with unsatisfactory treatment outcome. We designed this study to evaluate the efficiency of a traditional herbal preparation (Lax-Asab) in treating chronic constipation. In this double-blind, randomized, placebo-controlled clinical trial, participants with chronic constipation (n = 48) were randomly selected to receive either the Lax-Asab powder (n = 24) or placebo (n = 24) on alternative days for 4 weeks. The Lax-Asab powder contains equal amounts of Cassia angustifolia Vahl. (狹葉番瀉葉 xiá yè fān xiè yè), Mentha piperita L. (胡椒薄荷 hú jiāo bò hé), Zingiber officinale Rosc. (生薑 shēng jiāng), Glycyrrhiza glabra L. (甘草 gān cǎo). A total of 40 patients completed the study. We determined the severity of constipation based on defecation frequency (per week) and defecation difficulties. Of the total of 48 patients who participated, 40 completed the trial [24 men (60%), mean age, 21.0 ± 4.2 years; 16 women (40%), mean age, 20.1 ± 4.3 years]. The mean of weekly defecation frequency increased in both groups; from 1.8 ± 0.41 to 4.8 ± 1.12 times in patients who received Lax-Asab and from 1.7 ± 0.44 to 2.2 ± 0.61 times in patients who received placebo. A time–treatment interaction showed that this increase was significantly higher in the intervention group. Defecation difficulties improved significantly more in patients who received Lax-Asab than patients who received placebo. There was no statistically significant difference between the two groups with regard to the side effects observed. This study confirms the efficacy and tolerability of an Iranian herbal preparation, Lax-Asab, in treating patients with chronic functional constipation.
Keywords: Cassia angustifolia, constipation, herbal medicine, Lax-Asab, treatment
Graphical abstract
1. Introduction
Constipation is a common complaint encountered by physicians, and women are two times as likely to report constipation than men.1 Constipation is commonly multifactorial and can result from metabolic, endocrine, or neurological disorders, or from the adverse effect of medications.2 In functional defecation disorders,3 patients perceived constipation as difficulties in defecating or the presence of hard stools despite a normal evacuation rate.2 The prevalence rate (usually on the higher side) of constipation varies considerably due to different methods applied and differences in study populations. The prevalence of constipation in the Iranian population is reported to be 1.4–37%, and the prevalence of functional constipation is reported to be 2.4–11.2%.4
Chronic constipation impairs health-related quality of life, and thus, appropriate management is very important.3,5 A recent population-based study estimated the economic burden of functional bowel disorders (including functional constipation) in Iran and reported a heavy financial burden on the Iranian national health system.6
Constipation, especially when chronic and severe, can result in complications such as fecal impactions, hemorrhoid and rectal bleeding, colorectal obstruction, colorectal ulcers, rectal prolapse, anal fissures, and pelvic wall insufficiency,7 thereby increasing the burden of the disorder on the patient's health. Thus, constipation is considered a common clinical problem as well. A long-lasting management of chronic constipation usually begins with lifestyle modifications and increased intake of dietary fibers and fluids alone or in combination with an osmotic laxative. Polyethylene glycol, sodium picosulfate, bisacodyl, prucalopride, lubiprostone, and linaclotide are considered to be more effective than placebo in treating chronic functional constipation.8 Although there are also other preparations used for treating chronic functional constipation, only limited evidence is available to support their efficacy. Thus, their use may be restricted to patients who fail to respond to conventional treatments. Many patients are still treated empirically as there is a lack of an evidence-based algorithm so far.9 The high costs and widespread complications associated with chemical drugs force patients to consume alternative medication, which sometimes have higher efficacy and lower side effects and cost. In Western European countries and the United States, approximately 8–50% of individuals use alternative medication; in fact, this rate is even higher in developing countries.10 Patients consider herbal medicines appropriate because they are easy to obtain, affordable, and have fewer side effects.10 We designed this study to evaluate a herbal preparation (Lax-Asab), which is traditionally used for the treatment of chronic constipation in some regions of Iran and is well-known for higher efficacy, lower side effects, and lower cost in comparison with other currently available chemical and herbal remedies.
2. Materials and methods
2.1. Study patients
This double-blind, randomized control clinical trial was carried out at the outpatient clinic of Tabriz University of Medical Sciences, Iran, during 2011–2012. The study protocol was in accordance with the Declaration of Helsinki and was approved by the Regional Ethic Committee on August 20, 2010 (Reference No. 904). The study is also registered as a clinical trial in the Iranian Registry of Clinical Trials (www.irct.ir; Registration No. IRCT201105046388N1). All participants gave written informed consent before participation.
All patients diagnosed by a gastroenterologist to have functional constipation according to the Rome III criteria (for at least 3 months) were included if they agreed to avoid using any laxative medication for 1 week before beginning the study. A thorough physical examination was performed, and patients' medical history and biochemical parameters (including liver, renal, and thyroid function tests) were reviewed and collected before the trial. Any general reasons for constipation mentioned in the medical history were excluded. Imaging or additional evaluations were carried out based on the clinical judgment of the attending gastroenterologist.
Participants with a history of organic gastrointestinal (GI) disorders (including cancer and celiac disease); renal, hepatic, cardiac, pulmonary, neurologic, and hematologic diseases; malignancy; inflammatory bowel disease; drugs or alcohol-use problems; hypersensitivity to herbal preparations; pregnancy; lactation; history of GI bleeding; emergence of intolerable, serious drug side effects; and those noncompliant to the study protocol were excluded from the study. All of the patients were allowed to leave the study at any time and would subsequently receive standard care. Medications that may affect GI motility were not allowed during the study period (e.g., antidepressants, narcotic analgesics, and medications with an anticholinergic effect).
2.2. Study design
The participants were randomized using the RandList analyzer software. Selected patients received either Lax-Asab (Lax-Asab group) or placebo (control or placebo group) for 4 weeks (Fig. 1). The treatment began after a 1-week wash-out period for laxative mediations.
Fig. 1.
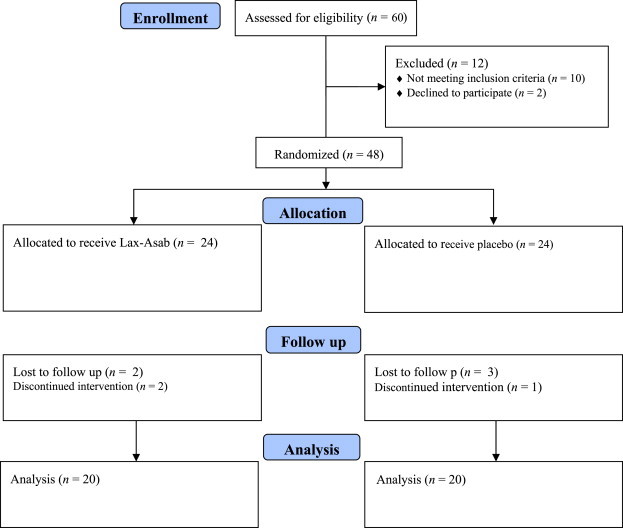
Flow diagram of the study trial.
2.3. Lax-Asab
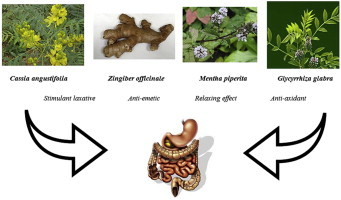
Lax-Asab is a traditional herbal preparation (dried and powdered) that contains equal proportions of Cassia angustifolia Vahl. (狹葉番瀉葉 xiá yè fān xiè yè), Mentha piperita L. (胡椒薄荷 hú jiāo bò hé), Zingiber officinale Rosc. (生薑 shēng jiāng), and Glycyrrhiza glabra L. (甘草 gān cǎo). Because C. angustifolia is the only component with laxative properties (the active component), the mixture of the other three was used as the placebo. Thus, the placebo powder contained the same intergradient as that of Lax-Asab, except C. angustifolia leaves. Lax-Asab and placebo were supplied in identical containers by Saeb-Darou Pharmaceutical Plant Company (Mashhad, Iran). The powdered preparations were identical in appearance, taste, and smell. All study personnel involved in the clinical evaluation and patients were blinded to the study treatments. Patients ingested 1 g of either Lax-Asab or placebo powder, which was provided in identical packages numbered by a study member blinded to clinical evaluations. Patients dissolved the powder in one glass of boiled water and ingested the medication before breakfast on alternative days. Rescue medication was not allowed during the trial. Noncompliance for 3 consecutive days or for a total of 7 days (self-reported by study population) resulted in exclusion.
The dietary11 (Food Frequency Questionnaire) and physical activities12 [International Physical Activity Questionnaire, last 7 days (self-administered format)] of patients were controlled weekly according to the standard questionnaire to maintain a constant dietary composition and physical activity during the study. Side effects were recorded each week, beginning with an open question about any complaints and using a checklist asking about cramps, abdominal pain, nausea, vomiting, diarrhea, gastric discomfort, and excess bloating.
The severity of constipation (defined as number of defecation per week) and defecation difficulties (1 = dry stool; 2 = straining force applied during defecation; and 3 = obstruction during defecation) were recorded before and after the trial.
2.4. Statistical analyses
Data were collected using checklists and the efficacy of Lax-Asab, determined by a symptom scale, was compared with that of placebo during and at the end of the study. Data were analyzed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Results are presented as mean ± standard deviation. Chi-square, Fisher exact, and independent t tests, and repeated measurements were used to estimate differences wherever appropriate. A p value < 0.05 was considered statistically significant.
3. Results
Of the 60 chronic constipation patients assessed for eligibility, 48 patients were selected. The patients were randomly enrolled to receive treatment with either Lax-Asab (n = 24) or placebo (n = 24). A flow diagram of the trial is presented in Fig. 1. Of these 48 patients, 40 completed the trial [24 men (60%), mean age, 21.0 ± 4.2 years; 16 women (40%), mean age, 20.1 ± 4.3 years]. The mean weekly defecation time was 1.8 and 1.7 times, respectively, in patients receiving Lax-Asab and placebo (p = 0.729).
Most patients (n = 32, 80%) had previously used at least one medication for constipation in the past 6 months. The primary complaint of patients was incomplete defecation followed by abdominal bloating/distension, abdominal discomfort, and symptoms due to hemorrhoids.
The mean of weekly defecation times increased in both groups (p = 0.001); from 1.8 ± 0.41 to 4.8 ± 1.12 times in patients receiving Lax-Asab and from 1.7 ± 0.44 to 2.2 ± 0.61 times in patients receiving placebo. A time–treatment interaction showed that this increase was significantly higher in the intervention group (p < 0.0001). All the other measured outcomes concerning defecation difficulty were significantly improved in patients receiving Lax-Asab compared with placebo. The results are presented in Table 1.
Table 1.
Outcome of treatment in patients receiving Lax-Asab or placebo.
| Lax-Asab | Placebo | p | OR | 95% confidence interval | |
|---|---|---|---|---|---|
| Hard stool | 2 (10%) | 20 (100%) | <0.001 | 10 | 20.68–37.23 |
| Feeling of obstruction | 1 (5%) | 19 (95%) | <0.001 | 20 | 2.96–135.1 |
| Need for excessive straining | 3 (15%) | 20 (100%) | <0.001 | 6.66 | 2.34–18.92 |
| Sense of incomplete bowel evacuation | 3 (15%) | 20 (100%) | <0.001 | 6.66 | 2.34–18.92 |
OR = odds ratio.
No statistically significant difference between the two groups with regard to side effects was observed. Side effects were reported by three patients who received placebo and by seven who received Lax-Asab [odds ratio = 3.05; 95% confidence interval (0.65–14.13); p = 0.27]. Major side effects reported were watery diarrhea followed by abdominal cramp, nausea, and distension in the intervention group, and abdominal pain followed by distension in the placebo group (Table 2). Other side effects such as allergic reaction or drug-induced hepatitis were not observed/reported.
Table 2.
Side effects reported by the study patients.
| No side effect | Abdominal pain | Nausea | Diarrhea | Distension | Cramp | Any side effect | |
|---|---|---|---|---|---|---|---|
| Placebo | 17 (85.0%) | 2 (10.0%) | 0 (0%) | 0 (0%) | 1 (5.0%) | 0 (0%) | 3 (15.0%) |
| Lax-Asab | 13 (65.0%) | 0 (0%) | 1 (5.0%) | 3 (15.0%) | 1 (5.0%) | 2 (10.0%) | 7 (35.0%) |
4. Discussion
This randomized, double-blind, placebo-controlled study suggested the benefits of Lax-Asab, a traditional herbal remedy containing equal proportions of Cassia angustifolia Vahl. (狹葉番瀉葉 xiá yè fān xiè yè), Mentha piperita L. (胡椒薄荷 hú jiāo bò hé), Zingiber officinale Rosc. (生薑 shēng jiāng), and Glycyrrhiza glabra L. (甘草 gān cǎo), which is used for the treatment of functional constipation. This herbal preparation was well tolerated by our patients. In addition, the formulation increased the frequency of defecation and decreased complaints as early as 2 weeks after beginning the treatment.
There is no single definition of constipation. Patients mostly define constipation by these symptoms: infrequent stools (typically <3 times/wk), hard stools, the need for excessive straining force during defecation, a sense of incomplete bowel evacuation, and excessive time spent in the toilet or unsuccessful defecation. Herbal preparations are commonly used in the symptomatic approach to manage constipation.10 Leaves of senna (C. angustifolia Vahl.) have been used as an alternative medicine and as an aid to treat constipation. They contain anthraquinones that are known to have stimulant laxative properties. However, no regulated manufacturing standards for senna preparations exist yet. Not all uses of senna are approved by the Food and Drug Administration, and senna preparations are often sold as herbal supplements. There are still not enough data to establish a dose–response relationship but it has been reported that a combination of laxative and senna is more efficient in treating constipation.13 Senna leaves are well-known for their laxative effects in Iran but their use is limited due to the side effects, which can be very severe. Common side effects are electrolyte disturbances, potassium deficiency, dehydration, and diarrhea accompanied by cramps. However, in this study, Lax-Asab, an herbal formulation that contains senna leaves along with other herbal ingredients in equal proportions, effectively decreased defecation problems and the patients did not experience any serious side effects.
Ginger (Z. officinale Rosc.) is a well-known flavoring agent in the East and has been traditionally used for GI upsets. Ginger preparations have been reported to be effective against nausea associated with motion sickness and chemotherapy.14 Human studies and clinical trials of peppermint leaf (M. piperita L.) are limited. However, peppermint has a relaxing effect on GI tissue, as well as analgesic and anesthetic effects on the central and peripheral nervous systems in animal models.15 The final ingredient of Lax-Asab, G. glabra L., has various pharmacological properties, including antitumor, antiparasitic, antiulcer, and antioxidative effects.16 Despite the fact that it is has been traditionally used in the preparation, none of these properties seemed efficient at reducing the side effects or increasing the efficacy of the preparation. Further studies may include several study arms to clarify this.
Although we did not use a satisfaction questionnaire, none of participants stopped using Lax-Asab because of bad taste or intolerable side effects. The herbal combination seems to limit the side effects of senna while giving a tolerable taste. However, this is only a preliminary study and further studies for determining the dosage are necessary.
Speculations about complementary and alternative medicines producing only a placebo effect still exist. However, similar to other studies that evaluated the efficacy of herbal preparations in treating functional constipation, a very low placebo effect was observed in this study.17
Safety analysis revealed that there were no statistically significant differences in side effects between the two groups. Adverse GI events included abdominal distension, pain, diarrhea, flatulence, and nausea; their incidence was not different between the two groups. In addition, the side effects were not considered to be related to the study medication.
Although the main significance of these findings could be providing evidence about the efficacy of this preparation for clinicians, so they can use this formulation in their practice with functional constipation patients, these results could be a base for further research to optimize preparations based on senna, involving identifying the exact dosage required and the effects of other ingredients. In addition, although this study did not evaluate the financial benefits of using Lax-Asab, this preparation will obviously cost less, given the fact that patients face a chronic condition lasting for several months, which requires expensive medications otherwise. By contrast, the aforementioned herbal ingredients are low priced and widely available.
A possible limitation of this study is that although we evaluated the changes in bowel movements (determined based on frequency of defecations), other potentially related variables such as stool consistency, stool weight, and ease of defecation were not evaluated. In other words, measurements were limited to subjective reports, and objective evaluations, which would be time consuming and costly, were not feasible. However, these results would have clinical relevance as subjective complaints have a very important impact on the everyday life of patients, especially in those with functional disorders. Another limitation was the short duration of follow up, which was a result of the preliminary nature of this study and should be improved in further studies that could target cost effectiveness as well.
5. Conclusion
This study provides evidence for the safe and effective use of Lax-Asab in treating chronic functional constipation.
Conflicts of interest
None to declare.
Acknowledgments
This study was supported by the Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.McCrea G.L., Miaskowski C., Stotts N.A., Macera L., Varma M.G. A review of the literature on gender and age differences in the prevalence and characteristics of constipation in North America. J Pain Symptom Manage. 2009;37:737–745. doi: 10.1016/j.jpainsymman.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Lembo A., Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 3.Dennison C., Prasad M., Lloyd A., Bhattacharyya S.K., Dhawan R., Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Iraji N., Keshteli A.H., Sadeghpour S., Daneshpajouhnejad P., Fazel M., Adibi P. Constipation in Iran: SEPAHAN Systematic Review No. 5. Int J Prev Med. 2012;3:S34–S41. [PMC free article] [PubMed] [Google Scholar]
- 5.Irvine E.J., Ferrazzi S., Pare P., Thompson W.G., Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi-Dehkordi B., Vahedi M., Pourhoseingholi M.A. Economic burden attributable to functional bowel disorders in Iran: a cross-sectional population-based study. J Dig Dis. 2011;12:384–392. doi: 10.1111/j.1751-2980.2011.00526.x. [DOI] [PubMed] [Google Scholar]
- 7.Leung L., Riutta T., Kotecha J., Rosser W. Chronic constipation: an evidence-based review. J Am Board Fam Med. 2011;24:436–451. doi: 10.3122/jabfm.2011.04.100272. [DOI] [PubMed] [Google Scholar]
- 8.Portalatin M., Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25:12–19. doi: 10.1055/s-0032-1301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eoff J.C. Optimal treatment of chronic constipation in managed care: review and roundtable discussion. J Manag Care Pharm. 2008;14:1–15. doi: 10.18553/jmcp.2008.14.S8-A.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langmead L., Rampton D.S. Review article: herbal treatment in gastrointestinal and liver disease–benefits and dangers. Aliment Pharmacol Ther. 2001;15:1239–1252. doi: 10.1046/j.1365-2036.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 11.Asghari G., Rezazadeh A., Hosseini-Esfahani F. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br J Nutr. 2012;108:1109–1117. doi: 10.1017/S0007114511006313. [DOI] [PubMed] [Google Scholar]
- 12.Vasheghani-Farahani A., Tahmasbi M., Asheri H., Ashraf H., Nedjat S., Kordi R. The Persian, last 7-day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian J Sports Med. 2001;2:106–116. doi: 10.5812/asjsm.34781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnunen O., Winblad I., Koistinen P., Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47:253–255. doi: 10.1159/000139866. [DOI] [PubMed] [Google Scholar]
- 14.Ryan J.L., Heckler C.E., Roscoe J.A. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479–1489. doi: 10.1007/s00520-011-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 16.Franceschelli S., Pesce M., Vinciguerra I. Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules. 2011;16:5720–5734. doi: 10.3390/molecules16075720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C.W., Bian Z.X., Zhu L.X., Wu J.C., Sung J.J. Efficacy of a Chinese herbal proprietary medicine (hemp seed pill) for functional constipation. Am J Gastroenterol. 2011;106:120–129. doi: 10.1038/ajg.2010.305. [DOI] [PubMed] [Google Scholar]


