Abstract
The extract from roasted chicory (Cichorium intybus L.; 菊苣 jú jù) root (chicory root extract), which contains inulin-type fructans, has favorable effects including antihyperglycemic and antidyslipidemic effects and the improvement of bowel movement. In this study, we examined the effects of chicory root extract on blood glucose, lipid metabolism, and fecal properties in 47 healthy adult participants in a randomized, double-blind, placebo-controlled study. The participants were divided into a test group that drank chicory root extract and a placebo group that drank nonchicory root extract (ingesting 300 mL daily for 4 weeks). We performed hematological examinations and body composition measurements, and administered a visual analog scale (VAS) questionnaire for fecal properties at the baseline (Week 0) and after the intervention (Week 4) for the two groups. Although no significant differences in fasting plasma glucose or insulin were observed, hemoglobin A1c was found to decrease by ingesting chicory root extract. No intergroup differences in the levels of lipid metabolism parameters were observed. However, the level of adiponectin was significantly improved in the chicory root extract group when the baseline and postintervention values were compared. In addition, chicory root extract tends to improve the VAS score for fecal properties. These results suggest that chicory root extract could delay or prevent the early onset of diabetes mellitus and improve bowel movements.
Keywords: fecal property, glucose metabolism, lipid metabolism, roasted chicory root extract, Hemoglobin A1c
Graphical abstract
1. Introduction
Lifestyle-related diseases such as obesity, dyslipidemia, type 2 diabetes, and hypertension are widespread and are becoming more prevalent globally. In 2004, the U.S. National Heart, Lung, and Blood Institute, in collaboration with the American Heart Association, convened a conference to define metabolic syndrome.1 Metabolic syndrome is a complex condition, consisting of interrelated risk factors for cardiovascular disease and diabetes. These risk factors include dysglycemia, increased blood pressure, elevated triglyceride (TG) levels, reduced high-density lipoprotein-cholesterol (HDL-Cho) levels, and obesity.2 A previous study showed that functional foods can be protective against the occurrence of several diseases.3 It is thus important to investigate the utility of functional foods and their bioactive components to improve and prevent lifestyle-related diseases.
chicory (Cichorium intybus L.; 菊苣 jú jù) is a major crop in northwestern Europe, and its roots are a rich source of the dietary fiber inulin.4 Chicory roots are used in the preparation of a bitter drink as a substitute for coffee in France and Japan, for example. People of all ages can drink the extract from chicory root, because it has noncaffeine ingredients. Chicory inulin also has the potential to replace dietary fat, resulting in decreased calorie intake. When inulin is mixed with water or any other aqueous liquid, it forms a colloidal suspension providing a white, creamy emulsion.5
Inulin, one of the fructooligosaccharides, is a linear fructose polymer with β(2→1) glycosidic linkages. Inulin is nondigestible, fully soluble,6 and widely found in nature in a variety of plants and in some bacteria and fungi. Some of the plants containing inulin are leeks, onions, garlic, asparagus, Jerusalem artichokes, yacón, and chicory.7
Inulin has various pharmacological effects. For example, the addition of inulin to a moderately high-carbohydrate low-fat diet has a beneficial effect on plasma lipids by decreasing hepatic lipogenesis and plasma triacylglycerol concentrations.8 In a clinical study of women with type 2 diabetes, inulin supplementation (10 g/d) for 2 months improved fasting plasma glucose (FPG), insulin, and hemoglobin A1c (HbA1c) levels, and decreased malondialdehyde levels compared with maltodextrin supplementation.9 Another study reported that ingestion of inulin (12 g/d) for 2 weeks was well tolerated by adult participants and led to a significant improvement of bowel movements with a minimum effect on fecal microflora, however, a slight increase in Bifidobacterium and Lactobacillus was noted in participants who had average fecal levels of Bifidobacterium and low fecal frequency.10
These findings suggest that chicory root, which is rich in inulin, may help counteract metabolic syndrome; a cluster of conditions characterized by hyperglycemia and hyperlipidemia. However, very few clinical studies have assessed the effects of chicory root. Here we evaluated whether the ingestion of the extract from roasted chicory root can improve hyperglycemia, dyslipidemia, and bowel movements in an adult population.
2. Material and methods
2.1. Test meal preparation
The composition of the extract from roasted chicory root (chicory root extract) used in this study is presented in Table 1. Chicory was harvested from Tenshin Farm, Furano, Hokkaido, Japan. The test meal was prepared by drying, roasting, and crushing the chicory root using a conventional method. The production and packing were carried out at Tenshin Farm in a quality-controlled manufacturing plant in compliance with the Food Sanitation Act (the Ministry of Health, Labor, and Welfare of Japan).
Table 1.
Composition of chicory root extract compared with placebo per 100 mL.
| Component | Chicory | Placebo |
|---|---|---|
| Calories (kcal) | 8 | 4 |
| Water (g) | 97.8 | 99.1 |
| Proteins (g) | 0.1 | 0.1 |
| Lipids (g) | 0.1 | 0.1 |
| Carbohydrates (g) | 1.9 | 0.9 |
| Ash (g) | 0.2 | 0.1 |
| Sodium (mg) | 1.6 | – |
| Inulin (g)a | 0.25 | – |
| Total fructan (g) | 0.6 | – |
| Polyphenol (g) | 0.09 | 0.03 |
The inulin content was calculated as total fructan – fructooligosaccharide.
The participants were instructed to ingest 300 mL/d of chicory root extract (containing 0.25 g inulin/100 mL) or a placebo drink (barley tea containing 10% coffee to imitate the bitter taste of the chicory root extract drink) in two or three parts per day. The reason for choosing barley tea as the placebo is that barley tea almost does not affect human health.11 The participants themselves prepared these drinks every day as follows: 10 g of chicory root or placebo leaves were put in a pot, and 350 mL of hot water was poured over the leaves. It was revealed that chicory inulin has various degrees of polymerization.7
2.2. Study participants
Forty-seven volunteers (8 males and 39 females; age, 33–70 years) were enrolled in this study. None of the participants had a recent history of gastrointestinal disorders, pregnancy, significant disease, surgery, severe allergic reaction to food, or current use of any medication including antihypertensive medication. The age, body weight, height, body mass index (BMI), and body fat percentage of the participants are presented in Table 2.
Table 2.
Characteristics of the participants in the placebo and the chicory root extract intake groups.
| Characteristic | Chicory | Placebo |
|---|---|---|
| No. | n = 24 | n = 23 |
| No. of males (male %) | 5 (20.83%) | 3 (13.04%) |
| Age (y) | 52.92 ± 11.60 | 54.30 ± 10.15 |
| Height (cm) | 159.69 ± 5.86 | 158.62 ± 6.61 |
| Body weight (kg) | 55.26 ± 9.06 | 55.93 ± 10.47 |
| BMI (kg/m2) | 21.61 ± 2.76 | 22.20 ± 3.48 |
| Body fat percentage (%) | 28.30 ± 6.32 | 28.68 ± 8.53 |
Values shown are mean ± standard deviation. Statistical analysis was performed by analysis of variance for age, height, body weight, and body mass index, and by Chi-square test for sex.
BMI = body mass index.
The clinical intervention was carried out as a randomized, double-blind, placebo-controlled trial. At randomization, the 47 eligible participants were randomly and blindly assigned to one of the two groups: the test group that drank chicory root extract drink, and the placebo group that drank the placebo drink.
Hematological examinations and body composition (body weight, BMI, and body fat rate) measurements were performed, and a visual analog scale (VAS) questionnaire for fecal properties was administered at the baseline (Week 0) and after the intervention (Week 4) for the two groups. The blood samples were sent to Sapporo Clinical Laboratory Inc. (Sapporo, Japan) for further analysis. The adiponectin level was measured using the Human Total Adiponectin/Acrp30 Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA). The body composition and blood pressure of participants were measured using a body composition analyzer (InBody device; Biospace Co., Tokyo, Japan) and an automatic blood pressure monitor (OMRON HEM-780; OMRON Corp., Kyoto, Japan), respectively.
All participants provided written informed consent before undergoing any study-related tests, and the protocol was approved by the Ethical Committee of Hokkaido Information University (Hokkaido, Japan). The study protocol conformed to the Declaration of Helsinki.
2.3. Single-nucleotide polymorphisms of the β2-adrenalin receptor
The β2-adrenergic-modulated cardiovascular function is mediated by relaxing vascular smooth muscle, resulting in lowered blood pressure and a more rapid return of blood to the heart through the β2-adrenergic receptors.12 It was reported that the Arg16Gly (+46 A<G; rs1042713) polymorphism in the β2-adrenergic receptor gene (ADRβ2) has an association with blood pressure phenotypes.13
In this study, genomic DNA was extracted from buccal epithelial cells using the BuccalAmp DNA extraction kit (EPICENTER Biotechnologies, Madison, WI, USA). Forty-four participants (placebo, 21; test, 23) voluntarily agreed to undergo genotyping. In brief, after rinsing out the participant's mouth two times with water, buccal tissue was collected by rolling a collection swab firmly on the inside of the cheek. DNA was extracted according to the manufacturer's protocol, and the region harboring a single-nucleotide polymorphism of interest was amplified by polymerase chain reaction (PCR) using specific primers for each set. Oligonucleotide sequences for the detection of the ADRβ2 gene with Arg16Gly polymorphism were 5′-CACATAACGGGCAGAACGCAC-3′ (forward), 5′-CATGACCAGATCAGCACAGGC-3′ (reverse). The PCR product was directly sequenced with an ABI310 DNA sequencer (Applied Biosystems, Darmstadt, Germany).
2.4. Statistical analysis
The mean and standard deviation of age and other parameters were calculated for each group. The changes in the values of various parameters were analyzed using the Mann–Whitney U test. The paired t test was performed at the baseline and at 4 weeks after the start of the study in each group. Statistical analyses were performed using IBM SPSS Statistic 19 (IBM, Armonk, NY, USA). The p values <0.05 were considered to be statistically significant. The tendency values were defined as those that had p values <0.10.
3. Results
3.1. Effects of chicory root extract on glucose metabolism
There were no significant differences in age, body weight, height, or BMI between the control and chicory (菊苣 jú jù) root groups (Table 2). First, to determine the effect of chicory root extract on blood glucose metabolism, the levels of FPG, HbA1c, and fasting insulin were measured. The participants who ingested the chicory root extract drink for 4 weeks had a significant decrease in the rate of change of HbA1c compared with the placebo group (change in the range of HbA1c from baseline to 4 weeks: placebo, −0.03% ± 0.02%; chicory, −0.09% ± 0.02%; p = 0.018; Fig. 1C). No significant differences in the FPG or insulin levels before and after the intervention were observed (Fig. 1A and B).
Fig. 1.
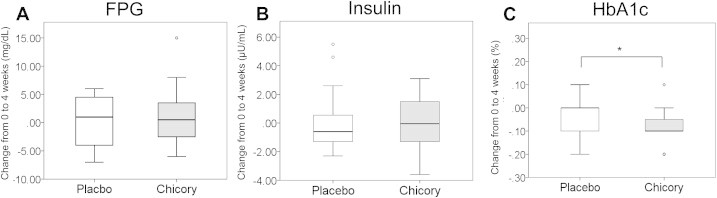
Changes in the level of glucose metabolism parameters from the baseline to the end of intervention in the chicory group (n = 24) and the placebo group (n = 23). (A) Fasting plasma glucose (FPG). (B) Insulin. (C) Hemoglobin A1c (HbA1c). Values are means ± standard error. *p < 0.05.
3.2. Effects of chicory root extract on lipid metabolism
The effect of chicory root extract on lipid metabolism was also examined. No significant between-group differences in the levels of total cholesterol (T-Cho), low-density lipoprotein-cholesterol (LDL-Cho), High-density lipoprotein-cholesterol (HDLCho), Triglyceride or serum adiponectin were observed (Fig. 2).
Fig. 2.
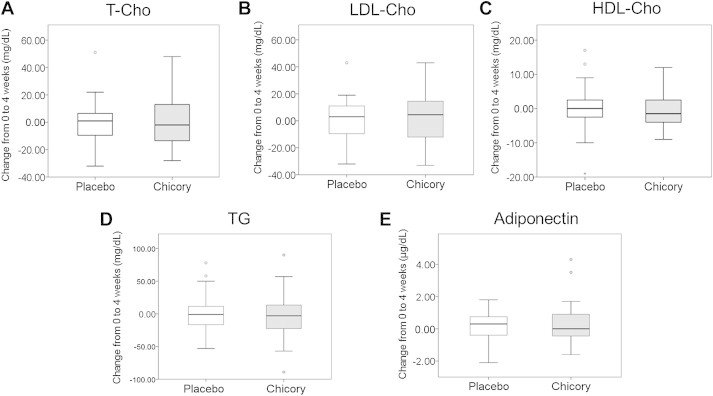
Changes in the level of lipid metabolism parameters from the baseline to the end of the intervention. (A) Total cholesterol (T-Cho). (B) Low-density lipoprotein-cholesterol (LDL-Cho). (C) High-density lipoprotein-cholesterol (HDL-Cho). (D) Triglyceride (TG). (E) Adiponectin. Values are means ± standard error.
3.3. Effects of chicory root extract on body composition
Changes in the body weight, BMI, and body fat percentage of the participants were analyzed. Although no significant differences in these three parameters were observed between the placebo and chicory groups (Fig. 3), the body fat percentage tended to increase in the placebo group (28.68 ± 1.78% to 29.87 ± 1.71%; p = 0.074), whereas there was no change in the body fat percentage of the chicory group (Table 3).
Fig. 3.
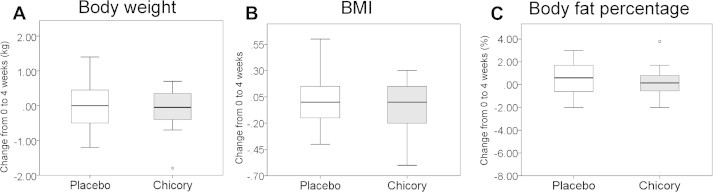
Changes in the level of body components from the baseline to the end of the intervention. (A) Body weight. (B) Body index mass (BMI). (C) Body fat percentage. Values are means ± standard error.
Table 3.
Biochemical data and visual analog scale scores.
| 0 wk | 4 wk | Change value | ||
|---|---|---|---|---|
| FPG (mg/dL) | Placebo | 87.13 ± 1.79 | 87.52 ± 2.13 | 0.39 ± 0.9 |
| Chicory | 85.08 ± 2.17 | 86.45 ± 2.26 | 1.37 ± 1.11 | |
| Insulin (μU/mL) | Placebo | 3.97 ± 0.39 | 4.04 ± 0.51 | 0.07 ± 0.42 |
| Chicory | 4.02 ± 0.38 | 3.91 ± 0.34 | −0.10 ± 0.34 | |
| HbA1c (%) | Placebo | 5.34 ± 0.07 | 5.30 ± 0.07 | −0.03 ± 0.01 |
| Chicory | 5.32 ± 0.06 | 5.22 ± 0.05** | −0.09 ± 0.01*** | |
| T-Cho (mg/dL) | Placebo | 233.43 ± 7.14 | 233.21 ± 7.35 | −0.21 ± 3.64 |
| Chicory | 222.29 ± 6.63 | 222.87 ± 5.98 | 0.58 ± 3.88 | |
| LDL-Cho (mg/dL) | Placebo | 143.26 ± 6.24 | 143.57 ± 6.22 | 0.96 ± 7.11 |
| Chicory | 139.54 ± 5.70 | 142.00 ± 5.5 | 7.79 ± 12.73 | |
| HDL-Cho (mg/dL) | Placebo | 80.83 ± 3.50 | 81.13 ± 3.62 | 0.30 ± 1.53 |
| Chicory | 69.67 ± 3.23 | 69.46 ± 3.99 | −0.20 ± 1.07 | |
| TG (mg/dL) | Placebo | 81.39 ± 7.27 | 82.35 ± 8.81 | 0.95 ± 7.10 |
| Chicory | 101.5 ± 15.27 | 109.29 ± 25.06 | 7.79 ± 12.73 | |
| Adiponectin (μg/mL) | Placebo | 10.92 ± 1.20 | 11.04 ± 1.17 | 0.12 ± 0.20 |
| Chicory | 10.54 ± 1.29 | 10.90 ± 1.33* | 0.36 ± 0.27 | |
| Body weight (kg) | Placebo | 55.93 ± 2.18 | 56.57 ± 2.13 | 0.64 ± 0.60 |
| Chicory | 55.26 ± 1.85 | 55.18 ± 1.81 | −0.08 ± 0.12 | |
| Body fat percentage (%) | Placebo | 28.68 ± 1.78 | 29.87 ± 1.71 | 1.19 ± 0.67 |
| Chicory | 28.3 ± 1.29 | 28.09 ± 1.29 | −0.21 ± 0.44 | |
| BMI | Placebo | 22.2 ± 0.72 | 22.43 ± 0.71 | 0.23 ± 0.20 |
| Chicory | 21.61 ± 0.56 | 21.55 ± 0.56 | −0.05 ± 0.05 | |
| SBP in all participants (mmHg) | Placebo | 120.96 ± 4.72 | 122.00 ± 3.99 | 1.04 ± 2.15 |
| Chicory | 120.95 ± 4.28 | 120.54 ± 3.89 | 4.63 ± 5.46 | |
| DBP in all participants (mmHg) | Placebo | 75.3 ± 2.23 | 75.35 ± 2.44 | 0.04 ± 1.31 |
| Chicory | 74.61 ± 2.21 | 74.88 ± 2.09 | 3.38 ± 3.28 | |
| SBP in all participants with SNP16A/G allele (mmHg) | Placebo | 114.50 ± 5.79 | 120.60 ± 6.22* | 6.10 ± 2.61 |
| Chicory | 127.00 ± 6.87 | 122.33 ± 6.47 | −4.67 ± 2.69*** | |
| DBP in all participants with SNP16A/G allele (mmHg) | Placebo | 71.50 ± 2.69 | 72.20 ± 2.86 | 0.70 ± 1.97 |
| Chicory | 76.78 ± 3.28 | 77.11 ± 3.57 | 0.33 ± 1.65 | |
| Defecation rhythm (mm) | Placebo | 55.78 ± 6.28 | 54.09 ± 6.37 | −1.70 ± 2.49 |
| Chicory | 54.42 ± 6.64 | 63.54 ± 6.12* | 9.13 ± 3.93 | |
| Defecation straining (mm) | Placebo | 65.43 ± 6.47 | 64.96 ± 5.18 | −0.48 ± 4.59 |
| Chicory | 64.79 ± 5.86 | 65.13 ± 6.11 | 0.33 ± 5.03 | |
| Defecation satisfaction (mm) | Placebo | 69.48 ± 5.73 | 70.91 ± 5.42 | 1.43 ± 4.25 |
| Chicory | 70.38 ± 4.89 | 74.00 ± 4.71 | 3.63 ± 5.44 | |
| Fecal odor (mm) | Placebo | 61.09 ± 5.52 | 62.57 ± 4.99 | 1.48 ± 3.37 |
| Chicory | 73.67 ± 4.12 | 71.75 ± 4.06 | −1.92 ± 3.90 |
Values are mean ± standard error. The comparison was performed by paired t test for the evaluation values at Week 0 and Week 4 in the same group (*p < 0.05, **p < 0.01), and by the Mann–Whitney U test for the change value comparison between the placebo and chicory groups (***p < 0.05).
BMI = body mass index; DBP = diastolic blood pressure; Cho = cholesterol; FPG = fasting plasma glucose; HbA1c = hemoglobin A1c; HDL-Cho = high-density lipoprotein-cholesterol; LDL-Cho = low-density lipoprotein-cholesterol; SBP = systolic blood pressure; T-Cho = Total cholesterol, TG = triglyceride.
3.4. Effects of chicory root extract on blood pressure
The blood pressure of the participants was analyzed. No significant differences in the level of blood pressure were observed between the placebo and chicory groups (Fig. 4A and B). However, we observed that participants with the SNP16A/G allele of ADRβ2 who ingested the chicory root extract drink for 4 weeks showed a significant decrease in systolic blood pressure (SBP) compared with the placebo group (change in SBP from baseline to 4 weeks: placebo group, n = 9: 6.10 ± 2.61 mmHg; chicory group, n = 10: −4.67 ± 2.69 mmHg; p = 0.018; Fig. 4C and D).
Fig. 4.
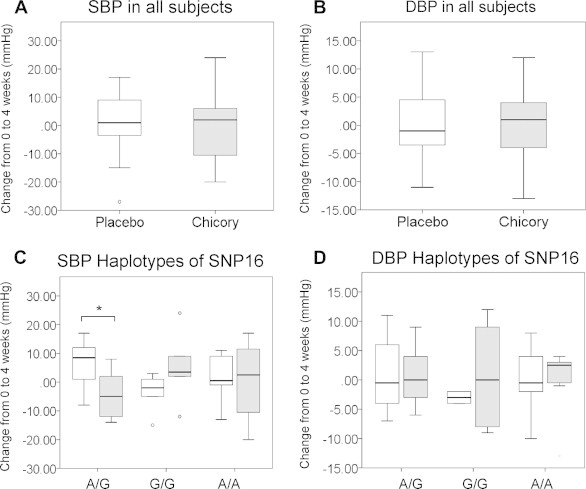
Changes in the systolic blood pressure (SBP) and diastolic blood pressure (DBP) of participants from the baseline to the end of the intervention. (A and B) SBP and DBP values of all participants: chicory group (n = 24), placebo group (n = 23). (C and D) SBP and DBP for participants with SNP16A/G of β2-adrenalin receptor gene (ADRβ2). Chicory group (n = 9), placebo group (n = 10). Values are means ± standard error. *p < 0.05.
3.5. Effects of chicory root extract on fecal properties
For the evaluation of the effects of chicory root extract on fecal properties, the participants completed a VAS questionnaire assessing their defecation rhythm, defecation straining, defecation satisfaction, and fecal odor (0 mm = worst condition and 100 mm = good condition). The defecation rhythm tended to improve after ingesting the chicory root extract drink compared with the placebo drink (change in the VAS score from baseline to 4 weeks: placebo, −1.70 ± 2.49 mm; chicory, 9.13 ± 3.93 mm; p = 0.051).
3.6. Levels of biomarkers of the liver and renal function after the ingestion of chicory root extract
The levels of several biomarkers of liver function and renal function were examined. Minimal changes were observed in the biomarkers of liver function (alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and gamma glutamyl transpeptidase) and in the biomarkers of renal function (blood urea nitrogen and creatinine) after the ingestion of chicory root extract, suggesting that the ingestion of chicory root extract has no or minimal unfavorable effects on the liver and kidneys, even at a dose of 300 mL/d (equivalent to 0.75 g of inulin/d).
4. Discussion
The results of our randomized, double-blind, placebo-controlled, parallel-group trial demonstrated the potential effects of chicory (菊苣 jú jù) root extract on blood glucose, lipid metabolism, and fecal properties. The level of HbA1c was significantly decreased as a result of the 4-week ingestion of chicory root extract. We also observed that the chicory root extract regimen slightly improved the VAS score for fecal property and defecation rhythm. Overall, these results suggest that the chicory root extract improved hyperglycemia and bowel movement.
A previous clinical study showed that ingesting inulin (10 g/d) for 8 weeks decreased FPG and HbA1c levels.9 Other reports suggested that the ingestion of oligosaccharides such as inulin improved glucose metabolism. For example, a carbonated beverage supplemented with indigestible dextrin suppressed the elevation of the postprandial blood glucose level through the inhibition of glucose absorption from the intestine.14 In addition, acarbose, an antidiabetic drug classified as an oligosaccharide, suppressed the elevation of the postprandial blood glucose level by inhibiting the activities of α-amylase and α-glucosidase.15
Moreover, the ingestion of chicory root extract gradually increased the postprandial plasma glucose level because inulin is less susceptible to degradation by α-glucosidase. The aforementioned findings and the results of this study suggest that chicory root extract containing inulin improved HbA1c values by suppressing the postprandial blood glucose level elevation. However, FPG was not improved in our study (Fig. 1A). It was reported that the postprandial serum glucose level plays a major role in the metabolic disequilibrium of patients suffering from mild or moderate hyperglycemia (HbA1c<7.3).16 Further investigation of the effect of chicory root extract on the postprandial blood glucose and insulin levels is needed.
Although no significant between-group differences in the VAS scores of defecation straining, defecation satisfaction, and fecal odor were observed in this study, the defecation rhythm was slightly improved by ingesting the chicory extract drink compared with the placebo drink (Fig. 5). Inulin-type fructans cannot be digested easily by salivary and gastric juices because they are polymers composed mainly of β-(2→1) fructosyl fructose linkages.5 In a patient who received radiotherapy, the ingestion of prebiotic mixtures containing inulin, fructooligosaccharide, and maltodextrin was evaluated for changes in the populations of Lactobacillus and Bifidobacterium.10 Although our clinical test evaluated only fecal properties using a VAS, it is necessary to investigate the intestinal bacterial flora in feces after the ingestion of chicory root extract.
Fig. 5.
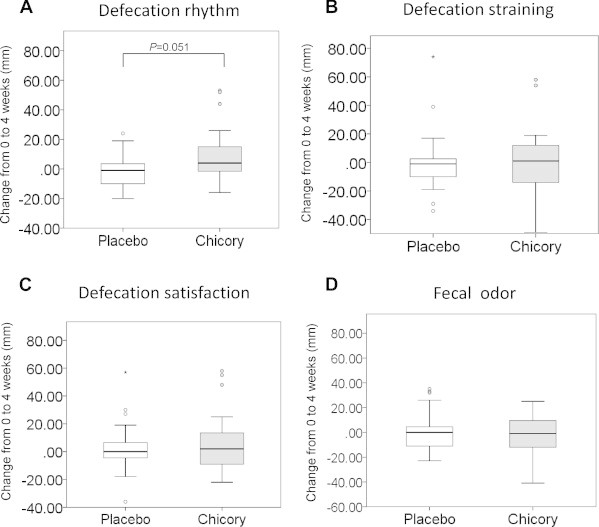
Changes in the visual analog scale scores of the participants from the baseline to the end of the intervention. (A) Defecation rhythm. (B) Defecation straining. (C) Defecation satisfaction. (D) Fecal odor. Values are means ± standard error.
We observed no between-group differences in the levels of lipid metabolism parameters (i.e., T-Cho, LDL-Cho, HDL-Cho, and TG; Fig. 2). Inulin-type fructans have been tested for their capacity to modulate lipid metabolism in humans and in several animal models. In a double-blind, placebo-controlled crossover study with 18 participants, the ingestion of 10 g of inulin/d for 6 weeks decreased the levels of serum TG and T-Cho.8 The expressions of acetyl-coenzyme A carboxylase and fatty acid synthase messenger RNA were also decreased following the ingestion of inulin.5 Our clinical study did not demonstrate any improvement in lipid metabolism, probably because it was designed for a shorter period. However, we found that the level of adiponectin was significantly improved in the chicory root extract group when we compared the baseline and postintervention values (baseline, 10.54 ± 1.29 μg/mL; postintervention, 10.90 ± 1.33 μg/mL; p = 0.041; Table 3). We need to further investigate the increase in adiponectin level following the ingestion of chicory root extract using in vitro or in vivo methods.
Neither the chicory root extract drink nor the placebo drink affected the body composition (body weight, BMI, and body fat percentage). However, after the 4-week ingestion of the placebo drink, a slight increase in body fat percentage was observed (baseline, 28.68 ± 1.78%; postintervention, 29.87 ± 1.71%; p = 0.074), whereas the chicory root extract drink did not induce such a change (baseline, 21.6% ± 0.56%; postintervention, 21.55% ± 0.56%, p = 0.833). Adiponectin, an adipocyte-derived hormone, has an anti-inflammatory function. Although its biological actions have not been fully investigated, adiponectin showed improved insulin resistance in hepatocytes through the activation of adenosine monophosphate-activated kinase.17 In obese patients, the adiponectin level showed an inverse correlation with visceral fat decreases.18 Based on the results of our present study, we propose that the improvement in HbA1c following the 4-week ingestion of chicory root extract was related to the reduction of body fat, thereby increasing the adiponectin level. We need to reconsider the ingestion period and ingestion volume to better understand the effects of chicory root extract on the lowering of lipid levels and the improvement of the body fat percentage.
We also examined the biological action of chicory root extract with respect to the genotypes at SNP16 of ADRβ2. Our findings showed that the ingestion of the chicory root extract decreased the participants' SBP much more effectively among those with an A/G allele at SNP16. Although the reason for this result has not been fully investigated, a study using a hypertensive rat model suggested that mannooligosaccharides were able to suppress an elevation in blood pressure, and this mechanism involved the inhibition of renal tubular sodium reabsorption through the suppression of aldosterone secretion.19 Several studies have indicated that certain genes and their variants can influence the uptake of nutrient components from the diet.20 It is of interest to investigate the molecular mechanisms by which chicory root extract can affect blood pressure through ADRβ2.
5. Conclusion
We have demonstrated the possibility that chicory root extract can modulate hyperglycemia and bowel movement. We used chicory root extract containing less inulin, which is the main bioactive component, compared with the prior studies that used higher concentrations of inulin. Although we need to obtain more detailed data involving other bioactive components such as sesquiterpene, our present findings suggest that the effective use of chicory root extract could be beneficial for lifestyle-related diseases.
Conflicts of interest
The authors state that they have no conflicts of interest to declare.
Acknowledgments
We thank Ms Rina Kawamura, Tomoko Mino, Megumi Shibata, Yoko Suwabe, and Aiko Tanaka for their technical assistance with the data management, and Mr Jungo Hayashi for his management of the clinical trial. This research was supported in part by the Northern Advancement Center for Science and Technology Foundation.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Keane D., Kelly S., Healy N.P., McArdle M.A., Holohan K., Roche H.M. Diet and metabolic syndrome: an overview. Curr Vasc Pharmacol. 2013;11:842–857. doi: 10.2174/15701611113116660173. [DOI] [PubMed] [Google Scholar]
- 4.Roberfroid M.B. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137:2493S–2502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 5.Franck A. Technological functionality of inulin and oligofructose. Br J Nutr. 2002;87:S287–S291. doi: 10.1079/BJNBJN/2002550. [DOI] [PubMed] [Google Scholar]
- 6.Liber A., Szajewska H. Effects of inulin-type fructans on appetite, energy intake, and body weight in children and adults: systematic review of randomized controlled trials. Ann Nutr Metab. 2013;63:42–54. doi: 10.1159/000350312. [DOI] [PubMed] [Google Scholar]
- 7.van Loo J., Coussement P., de Leenheer L., Hoebregs H., Smits G. On the presence of inulin and oligofructose as natural ingredients in the Western diet. Crit Rev Food Sci Nutr. 1995;35:525–552. doi: 10.1080/10408399509527714. [DOI] [PubMed] [Google Scholar]
- 8.Letexier D., Diraison F., Beylot M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr. 2003;77:559–564. doi: 10.1093/ajcn/77.3.559. [DOI] [PubMed] [Google Scholar]
- 9.Pourghassem Gargari B., Dehghan P., Aliasgharzadeh A., Asghari Jafar-Abadi M. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab J. 2013;37:140–148. doi: 10.4093/dmj.2013.37.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Peris P., Velasco C., Lozano M.A., Moreno Y., Paron L., de la Cuerda C. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: a randomised, double-blind, placebo-controlled trial. Nutr Hosp. 2012;27:1908–1915. doi: 10.3305/nh.2012.27.6.5992. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K., Sumi S., Tojo H., Sumi-Inoue Y., I-Chin H., Oi Y. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract. Nutr Res. 2011;31:421–428. doi: 10.1016/j.nutres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler M.G., Elayan H., Milic M., Sun P., Gharaibeh M. Epinephrine and the metabolic syndrome. Curr Hypertens Rep. 2012;14:1–7. doi: 10.1007/s11906-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 13.Pereira A.C., Floriano M.S., Mota G.F., Cunha R.S., Herkenhoff F.L., Mill J.G. Beta2 adrenoceptor functional gene variants, obesity, and blood pressure level interactions in the general population. Hypertension. 2003;42:685–692. doi: 10.1161/01.HYP.0000085648.65419.17. [DOI] [PubMed] [Google Scholar]
- 14.Asakura R., Kametani N., Mitsuda H., Fukuhara I., Takahashi N., Takehara I. Effect of carbonated beverage containing resistant maltodextrin on the postprandial elevation of blood glucose level – a randomized double-blind crossover study. Jpn Pharmacol Ther. 2010;38:621–626. [Google Scholar]
- 15.Rosak C., Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009;5:157–164. doi: 10.2174/157339909788920910. [DOI] [PubMed] [Google Scholar]
- 16.Monnier L., Lapinski H., Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:131–141. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino-Takao I., Fujii S., Ishii A., Han L.K., Okuda H., Kumao T. Effects of mannooligosaccharides from coffee mannan on blood pressure in Dahl salt-sensitive rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:181–184. doi: 10.3177/jnsv.54.181. [DOI] [PubMed] [Google Scholar]
- 20.Sales N.M., Pelegrini P.B., Goersch M.C. Nutrigenomics: definitions and advances of this new science. J Nutr Metab. 2014;2014:202759. doi: 10.1155/2014/202759. [DOI] [PMC free article] [PubMed] [Google Scholar]


