Abstract
The Mediterranean diet has been proven to be highly effective in the prevention of cardiovascular diseases. Paraoxonase 1 (PON1) has been implicated in the development of those conditions, especially atherosclerosis. The present work describes a systematic review of current evidence supporting the influence of Mediterranean diet and its constituents on this enzyme. Despite the differential response of some genetic polymorphisms, the Mediterranean diet has been shown to exert a protective action on this enzyme. Extra virgin olive oil, the main source of fat, has been particularly effective in increasing PON1 activity, an action that could be due to low saturated fatty acid intake, oleic acid enrichment of phospholipids present in high-density lipoproteins that favor the activity, and increasing hepatic PON1 mRNA and protein expressions induced by minor components present in this oil. Other Mediterranean diet constituents, such as nuts, fruits and vegetables, have been effective in modulating the activity of the enzyme, pomegranate and its compounds being the best characterized items. Ongoing research on compounds isolated from all these natural products, mainly phenolic compounds and carotenoids, indicates that some of them are particularly effective, and this may enhance the use of nutraceuticals and functional foods capable of potentiating PON1 activity.
Keywords: paraoxonase 1, PON1, Mediterranean diet, olive oil, nuts, fruits, nutraceuticals
1. Introduction
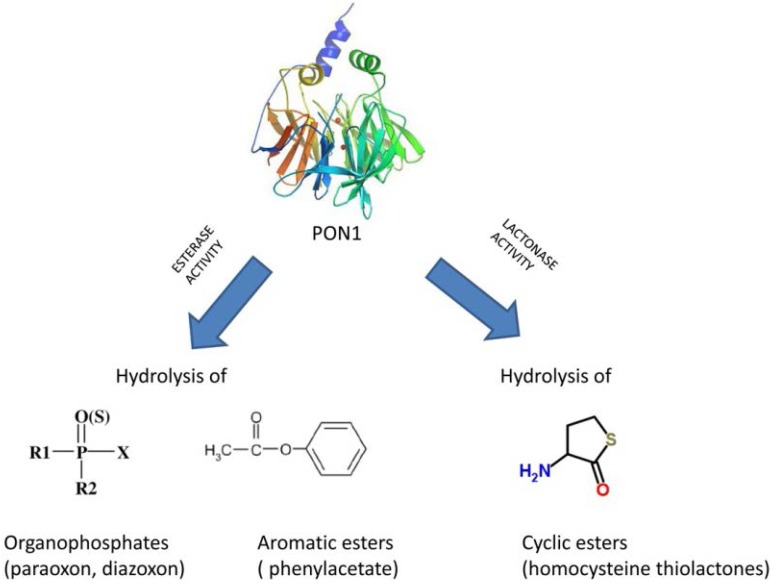
Human serum paraoxonase 1 (PON1), primarily synthesized by the liver, is mainly associated with serum high-density lipoproteins (HDL) [1] and, in vitro, displays a wide range of esterase activities (Figure 1). It has been reported to play a crucial role in the antioxidant activity of HDL by protecting low-density lipoproteins (LDL) against lipid peroxidation and, thus, attenuates the development of atherosclerosis [2]. In fact, PON1-deficiency resulted in increased oxidative stress not only in serum, but also in macrophages, a phenomenon that can contribute to the accelerated atherosclerosis shown in Pon1-deficient mice [3]. PON1 also exerts a protective effect against oxidative damage of cells and modulates the susceptibility of HDL and LDL to atherogenic changes such as homocysteinylation [4]. In addition, it modulates the anti-inflammatory role of HDL [5]. Moreover, low PON1 activity has been found in numerous pathological conditions associated with atherosclerosis, including type 1 and 2 diabetes, hypercholesterolemia and metabolic syndrome, as well as in elderly populations [6,7,8]. All these conditions display a pro-inflammatory baseline state that could be due to low PON1, as its activity is inversely related to cardiovascular risk [2,9].
Figure 1.
Paraoxonase 1 activities in vitro.
The interindividual variation in PON1 activity is partly determined by the existence of genetic polymorphisms since human PON1 shows two major polymorphic isoforms, PON1-Q192R and PON1-L55M. The PON1-192 polymorphism is associated with diminished PON1 concentrations and an increased risk for coronary heart disease in RR-allele subjects [10]. PON1 L55M genotype (MM) frequency was significantly higher in those patients than in controls [11]. PON1 activity can be modulated by environmental conditions as well (Figure 2).
Figure 2.
Effect of dietary components on regulation of paraoxonase 1 activity and its interaction with genetic factors. Adapted from [12] and [13], reproduced with permission from Elsevier.
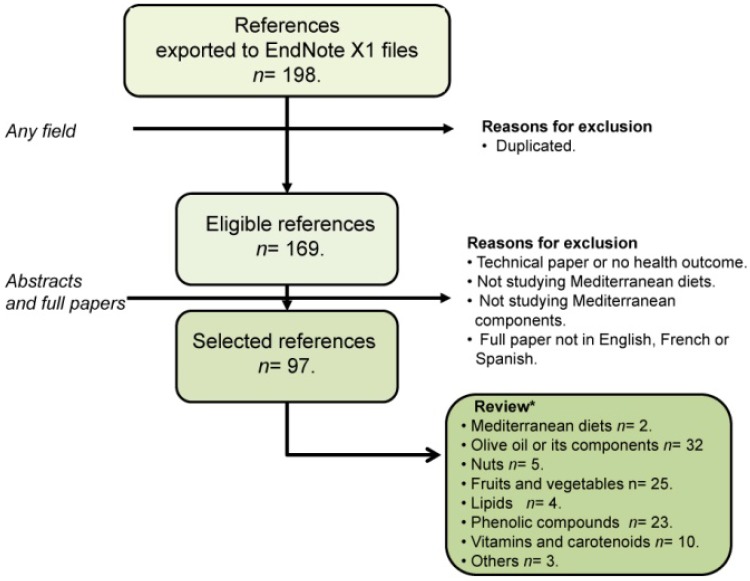
A 2012 review [14] highlighted some of the more recent studies in the field of nutrigenetics at that time, which explored interactions among diet, genetic variation in antioxidant enzymes (including PON) and oxidative stress. It is, therefore, of interest to identify modulators of PON1 activity and concentration, and diet may be one of these factors [15]. The traditional Mediterranean diet used to be the food consumption pattern in countries like Spain, Italy, Greece and southern France during the decade of the sixties, and it has been associated with a lower incidence of coronary heart disease [16,17]. The Mediterranean diet is largely vegetarian in nature and includes the consumption of large quantities of olive oil as the main source of calories [18]. In the present paper, we will focus on the effect of the Mediterranean diet, its typical foods or chemical components and the effect of their intake on PON1 activity. To compile advances in the field, the present report has adhered to systematic review guidelines [19]. As displayed in Figure 3, a search in PubMed [20] using the keywords (PON1 and Mediterranean diet, nut and paraoxonase, olive oil and paraoxonase, food and paraoxonase as Mesh, resveratrol and paraoxonase, rutin and paraoxonase, carotenoids and paraoxonase, catechins and paraoxonase) identified 198 hits from November 1945 to March 11th, 2015. The search was refined by eliminating duplicate documents. The 169 papers obtained were critically reviewed to verify whether they analyzed PON1 and Mediterranean diet or its components. Documents that failed to meet this criterion were excluded. Thus, this review covers the works related to the effects of Mediterranean diet and PON1 in 97 papers.
Figure 3.
Flow chart displaying the stages used to select the references considered. EndNote X1 (Bld 2566, Thomson Reuters: New York, NY, USA, 2007). * Some references may appear in more than one section of the review.
2. PON1 and Mediterranean Diet
Two studies analyzed the effect of the Mediterranean diet as a whole on PON1 activity. In the first, paraoxonase activity was assayed in Greek migrants, who maintain a traditional Mediterranean diet, and compared with that of Anglo-Celtic individuals in Australia. Interestingly, paraoxonase activity correlated directly with circulating carotenoid concentrations in Greeks, and inversely with homocysteine and C-reactive protein in Anglo-Celtics. Such associations were seen only among subjects with the high activity phenotype (defined by the ratio of paraoxonase:arylesterase activity). The data suggest that dietary modulation of atherosclerotic risk may vary according to PON1 phenotype [21]. In the second study, the effect of two different fatty meals was addressed. A Mediterranean-like meal (61% monounsaturated fat) was compared to a Western-like meal (57% saturated fat), and only the former resulted in a significant increase in both PON1 activity and carotenoid concentrations [22]. These studies provide some evidence that the Mediterranean diet can alter PON activity.
3. PON1 and Olive Oil or Its Components
The importance of dietary lipids in paraoxonase activity was recently reviewed by Ferretti and Bacchetti [4] who, in line with other authors [23,24,25], propose that the amount and the composition of dietary lipids are key factors in the modulation of PON1. The effect of dietary lipids is also modulated by PON1 polymorphisms [26] and the molecular mechanisms involved include regulation of hepatic PON1 synthesis or secretion and/or modification of PON1 interactions with HDL [24]. Furthermore, changes in PON1 activity could also be related to dietary intake of oxidized lipids that behave as PON1 inhibitors [27].
From this perspective, there is a renewed interest in virgin olive oil as the main source of fat in the Mediterranean diet, especially first-press extra-virgin olive oil (EVOO), which retains minor compounds that may have antiatherosclerotic properties [28]. Human consumption of virgin olive oil lowers major atherosclerotic risk factors [29]. One proposed mechanism of action has been the increase in HDL levels [30]. However, the functionality of HDL may be as relevant to cardiovascular risk assessment as plasma HDL concentrations [31,32]. The anti-inflammatory activity of HDL has been attributed to apolipoprotein A1 (APOA1) and lipids, including sphingosine-1-phosphate and sphingosylphosphorylcholine [33,34]. However, there is strong evidence that PON1 also participates in this activity, acting alone or in combination with other HDL-associated enzymes to modulate the antioxidant and anti-inflammatory role of HDL [5,35]. Several studies have investigated whether EVOO consumption could improve the atheroprotective activity of HDL. In subjects consuming this oil for 12 weeks, the anti-inflammatory activity of both isolated HDL and PON1 was increased, the anti-inflammatory activity of HDL being modulated by PON1 [9]. Cherki et al., also observed an increase in paraoxonase activity after the consumption of 25 mL/day of either argan oil or EVOO for two weeks, associated with an improvement in antioxidant status [36]. In a postprandial study, it was found that a single meal rich in olive oil raised PON1 arylesterase activity in the serum chylomicron fraction by 25% compared with a meal rich in cream [37], possibly due to the formation of a greater number of chylomicrons and delayed clearance of chylomicron remnants after ingestion of olive oil. A similar finding was observed when thermally stressed olive oil was given to middle-aged and older diabetic women [38]. These results point to a relationship between virgin olive oil consumption and PON1 levels in humans irrespective of experimental design.
The effect of olive oil has been studied in animal models as well. In this regard, in mice consuming fish, soy or olive oils, a significant decrease in postprandial serum PON1 activity was found only after fish oil supplementation, in accordance with the increase in serum oxidizability. PON1, in turn, attenuated postprandial oxidative stress after consumption of soy or olive oil, an action that was probably related to the effect of PON1 lipase-like activity on chylomicron triacylglycerols [39]. Also in animal models, the addition of cholesterol to chow or virgin olive oil diets significantly decreased APOA1 in Apoe-deficient females and serum paraoxonase activities in males. The latter activity was higher in females than in males. Size of aortic lesions was inversely correlated with circulating paraoxonase activity, particularly in males, and the relationship remained after adjusting for APOA1 and HDL cholesterol levels [40]. These results demonstrate that, together with sex, the nutritional regulation of paraoxonase is an important determinant of atherosclerotic lesions. When the comparison was established using different types of Western diets, no differences in plasma PON1 arylesterase activity or APOA1 concentrations were found in Apoe-deficient mice consuming EVOO from different cultivars (Arbequina, Picual, Cornicabra, or Empeltre) with respect to the diet containing palm oil. This fact suggests that the beneficial effects of EVOO could be due to the complex interactions of HDL particles. In fact, an elevated activity of PON1 was found in small, dense, protein-rich HDL. Despite the variation in arylesterase activity, no differences were found in APOA1, the classical PON regulator, a finding that rules out APOA1 as the inductor of this change in enzyme activity. This demonstrates that, even in a westernized diet scenario, cholesterol-poor, APOA4-enriched lipid particles generated by EVOO intake provide potent protection against oxidative stress, in part through increases in arylesterase activity [41]. The antioxidant activity of these small particles has been reported to be rather complex, with enzymatic or non-enzymatic involvement [42,43,44], or perhaps both. In BalbC mice consuming olive oil, there were increases in HDL phospholipids/protein, HDL-PON1 arylesterase activity and the PON1 contribution to HDL-mediated macrophage cholesterol efflux. Therefore, a phospholipid-stimulatory effect on PON1 was proposed. A mutant PON1 lacking the first 20 amino acids, generated by direct mutagenesis, was unable to display the phospholipid stimulatory effect, indicating that this protein domain is critical for the interaction of PON1 with phospholipids and that this interaction is crucial in the olive oil-induced effect [45].
Oleic acid (18:1) is the major fatty acid present in olive oil (Table 1). Due to this fact, it has been proposed that its intake could determine olive oil-induced changes in PON1 by replacing saturated fat and limiting the negative consequences of the latter on this enzyme [24,25]. In this respect, a population-based study showed that high oleic acid intake was associated with increased HDL cholesterol levels and PON1 activity only in subjects with the QR and the RR genotypes of the PON1-192 polymorphism, respectively [26]. Thus, the beneficial effect of increasing oleic acid intake on HDL and PON1 activity would be more pronounced in subjects carrying the R allele. Likewise, olive oil consumption by healthy subjects increased HDL-PC-18:1 levels and HDL-PON1 activities [45]. However, other minor components of olive oil (Table 1) have also been shown to promote changes in PON1 activity. In this respect, Hussain et al., observed decreased hepatic paraoxonase activity in rats with experimental non-alcoholic fatty liver disease consuming unmodified olive oil [46]. However, in a study of senescence-accelerated mouse-prone 8 (SAMP8) mice fed a diet enriched in 10% olive oil containing either high or low amounts of olive oil phenolics for 4.5 months, the results were higher serum PON1 activity and Pon2 mRNA levels in heart tissue of animals consuming the high-phenolic diet. This effect could be attributable to the olive oil phenolic compound, hydroxytyrosol, which may be responsible for the induction of Nrf2-dependent gene expression and the increase in PON activity [47]. Supplements of EVOO with green tea polyphenols given to Apoe-deficient mice also resulted in an increase in serum paraoxonase 1 [48]. Nonetheless, when male Apoe-deficient mice were fed pure hydroxytyrosol at a dose of 10 mg/kg/day for 10 weeks, changes in serum paraoxonase were not observed. These data support the concept that phenolic-enriched products taken out of the original matrix may be not useful [49]. Something similar might occur with squalene (the main hydrocarbon in the unsaponifiable fraction of olive oil). When administered in a glycerol solution, it did not elicit any change in paraoxonase activity in Apoe-deficient mice of either sex [50]. However, the same dose of squalene, but in a different administration regimen (dissolved in the oil fraction of chow diet), promoted changes in HDL-cholesterol and PON1 and decreased reactive oxygen species in lipoproteins and plasma malondialdehyde levels, not only in Apoe-deficient mice, but also in wild-type and Apoa1-deficient mice [51]. The importance of the lipid matrix has also been observed when studying PON1 using high-phenolic preparations of coconut oil [52]. These results indicate that minor components present in EVOO may participate in the increase in PON1 activity, but show a special dependence on the triglyceride matrix present in olive oil. Overall, these findings support an important role for olive oil in PON1 activity, a circumstance that could partly explain the beneficial effect of the Mediterranean diet on health status.
Table 1.
Composition of virgin olive oils.
| Component | Content (g%) |
|---|---|
| Fatty Acids of Triglycerides | |
| Myristic (14:0) | 0.0–0.05 |
| Palmitic (16:0) | 7.5–20 |
| Palmitoleic (16:1n7) | 0.3–3.5 |
| Margaric (17:0) | 0–0.3 |
| Heptadecenoic (17:1) | 0.0–0.3 |
| Stearic (18:0) | 0.5–5.0 |
| Oleic (18:1n9) | 55–83 |
| Linoleic (18:2n6) | 3.5–21 |
| α-linolenic (18:3n3) | 0.0–0.9 |
| Arachidic (20:0) | 0.0–0.6 |
| Eicosenoic (20:1n9) | 0.0–0.4 |
| Behenic (22:0) | 0.0–0.2 |
| Lignoceric (24:0) | 0.0–0.2 |
| Minor Components | |
| Terpene compounds | 0.1–0.3 |
| Phytosterols | 0.1–0.2 |
| Hydrocarbons | |
| Squalene | 0.1–0.8 |
| Carotenes | 0.05–0.1 |
| Phenolic compounds | 0.05–0.1 |
4. PON1 and Nuts
Several epidemiological studies suggest that consumption of nuts promotes a healthy lipid profile associated with a lower risk of cardiovascular disease. These studies have attributed this quality to the nutrients they contain, mainly monounsaturated and polyunsaturated fatty acids and many other bioactive constituents, such as antioxidants, phytosterols and other phytochemicals [58,59]. Dietary interventions aimed to prove the effects of nuts on PON1 have been carried out in humans and in animal models. The former approach involved subjects at increased cardiovascular risk in whom a walnut paste-enriched meat improved the antioxidant status [60,61] and significantly lowered plasma levels of cell adhesion molecules (sVCAm-1 and sICAM-1) and leukotriene B4, compared to low-fat meat. These changes seemed to be related to PON1 activity, and most of the variability could be explained by PON1 and APOA4 polymorphisms [62,63]. However, providing Brazil nuts to normolipidemic individuals at a dose of 45 g per day for 15 days did not modify PON1 activity [64]. In view of these discrepancies, dose may be an important issue. In this regard, while a 2.5 g/day pistachio intake increased serum paraoxonase activity in rats compared with a standard diet, a larger amount of 5 g/day was ineffective [65]. The influence of sex cannot be disregarded and, in this respect, in Apoe-deficient mice, supplementation (30 g/day) with a mixture of three nuts (50% walnuts, 25% almonds and 25% hazelnuts) for 12 weeks raised PON1 activity and increased hepatic Pon2 expression only in females, when compared to those receiving an isoenergetic diet containing palm oil [66]. Overall, these results suggest that the effect of nuts on PON1 may vary according to the varieties consumed, a circumstance that might be influenced by the balance of saturated/unsaturated fatty acids and other accompanying constituents. More studies are required to establish the dose and the role of sex in the response observed.
5. PON1 and Other Constituents of Mediterranean Diet
Fruits and Vegetables
An important feature of the Mediterranean diet is the high intake of fruits and vegetables. Epidemiological studies have shown a relationship between a reduced risk of cardiovascular disease and fruit consumption [67,68]. Large prospective cohort studies have revealed that a high dietary intake of fruits and vegetables reduces cardiovascular disease risk [67,69]. In a clinical trial in subjects with type 2 diabetes, increased fruit and vegetable consumption augmented the carotenoid concentrations and PON1 associated with the antioxidant properties of HDL [70]. Much of this protective effect has been attributed to the biological activity of the non-nutrient secondary plant metabolites such as flavonoids and diverse phenolic compounds, which, in general, are powerful antioxidants [71]. Intake of antioxidant-rich foods, like fruit and vegetable juices, affect the activity and/or concentration of PON1 [15]. In diabetic patients from two European countries, a flavonoid-rich diet was positively associated with PON1 arylesterase activity [72]. However, in healthy volunteers with adequate vitamin intake, six week diets differing markedly in the amounts of linoleic and oleic acid and vegetables, berries and apples did not differ in their effects on lipid peroxidation or lipoprotein metabolism, and all these diets decreased PON1 activity [73]. Similarly, in nonsmoking women, two diets, one low and the other high in vegetables and having some differences in fatty acid contents, showed that the serum PON1 activity was lower after the high vegetable compared with the low vegetable diet. The reduction of PON1 activity correlated with the reduction in HDL cholesterol. High baseline PON1 activity was related to the presence of the R- and L-alleles of the PON1-192 and PON1-55 genotypes, respectively [74]. These discrepant results evidence a complex picture in which source of fruits and their composition, dietary fat content, special population needs and genetic PON1 isoforms are interacting and, therefore, different outcomes are possible.
Pomegranate has attracted much research interest as a source of some potent phenolic antioxidants (tannins, anthocyanins) whose role in protecting LDL and HDL from lipid oxidation has been clearly demonstrated [75]. This role involves two mechanisms: a direct interaction of pomegranate phenolics with LDL and an indirect effect by which they increase HDL-associated PON1 [76] and augment PON1 synthesis in the liver [77]. The enhancement of PON1 activity by pomegranate was clearly demonstrated in vivo in animal models and in humans, either healthy subjects or patients with diabetes or carotid artery stenosis. First, apolipoprotein E-deficient mice with advanced atherosclerosis that received pomegranate supplementation for two months showed a significant reduction of atherosclerotic lesions. This result may be attributed to the protection of LDL against oxidation and the significant increase in serum paraoxonase activity [78,79]. Using C57BL/6 control mice, Pon1- or Pon2-deficient mice, Rosenblat et al., showed that pomegranate juice consumption also had an antioxidant effect on mouse macrophages that was mediated via stimulation of macrophage Pon2 expression and was independent of PON1 [75]. Secondly, in healthy subjects, serum PON1 arylesterase activity was significantly increased after pomegranate juice consumption for a period of two weeks [80]. Even one week of pomegranate consumption was sufficient to induce serum PON1 activity. This effect was comparable to administration of black currant juice [81]. Thirdly, in diabetic patients, PON1 activity increased significantly by 12% following consumption of pomegranate juice for three months [82]. In another group of patients with type 2 diabetes, administration of 200 mL of pomegranate juice for a shorter period (six weeks) induced similar effects on PON1 activity [83]. In addition, four weeks of Wonderful variety pomegranate juice consumption significantly increased HDL-associated PON1 activity, reversing the dissociation between HDL and PON1 observed in these patients. Association of PON1 with HDL stabilizes the enzyme and stimulates its catalytic activity [84]. Similar results were observed, although to a lesser extent, with administration of an extract of pomegranate peel. Furthermore, in vitro incubation of serum from diabetic patients with pomegranate juice or its purified major phenolic compounds, such as punicalagin, gallic acid and ellagic acid, showed that all of them increased PON1 binding to HDL [85]. Finally, a time-dependent effect of pomegranate consumption on PON1 was observed in patients with carotid artery stenosis. By 12 months, PON1 activity had increased by 83% over the initial level [86].
Much less attention has been devoted to other fruits, whether in preclinical or clinical settings. In an animal model involving Syrian hamsters, consumption of raspberry juice for 12 weeks enhanced PON1 activity if expressed as a ratio to HDL in [87]. Using a different approach, administration of apple juice to a group of male Wistar rats for 28 days protected against the decrease in PON1 activity induced in another group of male Wistar rats that had received no juice when N-nitrosodiethylamine or carbon tetrachloride was given to both groups of animals 24 h before samples were taken. In fact, in the juice-pretreated rats, PON1 activity was actually enhanced despite the administration of the toxicants [88]. In human studies, different groups of patients have been recruited. When the effect of 28-day intake of antioxidant-rich orange and blackcurrant juices (with or without vitamin E supplement) on PON1 activity was explored in patients with peripheral arterial diseases, no significant changes were observed. Despite these results, there was a potential gene-diet interaction since subjects carrying the PON1 L55-allele showed increased PON1 activity following juice consumption without vitamin E [89]. Likewise, although a short intervention period (two weeks) of a low carotenoid diet with tomato or carrot juice failed to affect PON1 activity in healthy volunteers, tomato juice intake reduced lipid peroxidation in subjects carrying the R-allele of the PON1-192 genotype [90]. However, PON1 activity increased in elderly subjects consuming tomato juice, and those who were R-allele carriers were high responders [10]. Administration of lycopene, the carotenoid present in tomato juice, at 224–350 mg/week increased PON1 activity in serum and HDL in moderately overweight middle-aged subjects [91]. In healthy subjects, date consumption decreased basal serum oxidative status and lipid peroxidation, and increased paraoxonase1 activity. Dates are fruits typical of some countries of the Mediterranean basin that possess different concentrations of phenolic compounds (phenolic acids, mainly ferulic acid and coumaric acid derivatives, as well as chlorogenic and caffeic acid derivatives, catechins and a quercetin derivative as well) [92]. These findings suggest differential responses among genotypes and the need to improve identification of chemical compounds provided by the different fruits.
6. PON1 and Chemical Compounds Present in Mediterranean Diet and Their Potential as Nutraceuticals
As pointed out above, often controversial findings regarding the impact of Mediterranean diet constituents on PON1 have raised concern as to the extent of the influence of the complex mixture of chemical compounds on efforts to delineate the most relevant ones and to use them as potential nutraceuticals able to enhance PON1 activity. In this section, the works studying the effect of various compounds present in the Mediterranean diet on PON1 expression/activity are categorized and analyzed.
6.1. Lipids
Several approaches have been used to test the effect of a number of fatty acids on PON1. In this way, to explore the effect of oleic acid, in vitro incubation of serum with di-oleoyl-phosphatidylcholine was carried out, resulting in its incorporation into HDL with a consequent increase in PON1 activity. Furthermore, the contribution of PON1 to HDL-mediated cholesterol efflux from macrophages was greater in di-oleoyl-phosphatidylcholine-enriched HDL in comparison to control HDL [45]. However, administration of a conjugated linoleic acid isomeric mixture (4.5 g/day) for four weeks did not change paraoxonase as compared with safflower oil, but significantly reduced its arylesterase activity in overweight men [93]. Fish oil supplements tested in female patients with rheumatoid arthritis increased serum HDL-C, PON1 levels and arylesterase activity [94]. However, when 2 g/day of purified eicosapentaenoic/docosahexaenoic acids were given to individuals with type 2 diabetes mellitus for six weeks, no significant differences in fasting and postprandial circulating paraoxonase-1 activity were observed [95]. These results seem to indicate that not all fatty acids behave in similar ways regarding PON1, and that oleic acid displays a positive action.
6.2. Phenolic Compounds
Quercetin is a flavonol found in many fruits, vegetables, leaves and grains present in the Mediterranean diet. In this regard, dietary quercetin supplementation (2 mg/g diet) for six weeks induced hepatic Pon1 gene expression with a tendency for greater induction in APOE3 as compared to APOE4 mice, suggesting that PON1 is differentially regulated in response to APOE genotype [96]. However, most dietary quercetin comes from rutin (quercetin-3-O-rutinoside). Rutin is a citrus flavonoid glycoside found in many constituents of the Mediterranean diet (especially the citrus fruits, peaches and apples). Rutin is metabolized by intestinal bacteria [97], releasing quercetin. Al-Rejaie et al., reported that dietary rutin increased the expression of the hepatic Pon1 gene, among other antioxidant genes in hypercholesterolemic male Wistar rats [98].
Anthocyanin is also a flavonoid present in many Mediterranean diet foods (i.e., eggplant, blackberries, plums, grapes, red wine, pomegranates and cherries) [99]. Anthocyanin supplementation (160 mg twice daily for 24 weeks) increased HDL cholesterol, the activity of HDL-PON1 and cholesterol efflux capacity compared with placebo in hypercholesterolemic subjects. Furthermore, HDL-PON1 activity was negatively correlated with HDL lipid hydroperoxides and positively with cholesterol efflux. Based on these findings, anthocyanin may prove to be a cardioprotective nutraceutical [100]. Isoflavones, genistein and daidzein, are also present in some foods of the Mediterranean diet, i.e., fava bean (Vicia faba) and lupine (Lupinus spp.) [101]. Three studies have addressed the influence of dietary isoflavonoids on PON1 activity. In the first, Ustundag et al., studied the protective effect of soy isoflavones (100 mg/kg in diet for eight weeks) on experimental non-alcoholic steatohepatitis and their effects on plasma paraoxonase and arylesterase levels in rats, concluding that soy isoflavones seemed to be effective in preventing liver damage by increasing paraoxonase activity and decreasing lipid peroxidation [102]. In the second study, Mohammadshahi et al. observed that genistein or daidzein reversed the arthritis-induced decreases in paraoxonase and arylesterase activity in rats [103]. In the third study, Schrader et al. screened different flavonoids for their ability to induce PON1 in Huh7 cultured hepatocytes. Genistein was the most potent flavonoid with regard to its PON1-inducing activity, followed by daidzein, luteolin, isorhamnetin and quercetin. Other flavonoids such as naringenin, cyanidin, malvidin and catechin showed little or no PON1-inducing activity. In contrast, when these authors tried to reproduce the genistein effect in vivo by administering this flavonoid to growing rats at a dose of 2 g/kg diet over three weeks, the increases in hepatic Pon1 mRNA and protein levels and in plasma PON1 activity were not reproduced [104]. Catechins deserve special attention since they are present in Mediterranean foods (i.e., peaches, barley grain, wine and vinegar) [105,106,107]. Different experimental models involving toxins or metabolic diseases (i.e., HgCl2 toxicity [108], ethylene glycol-induced renal failure [109], streptozotocin-induced diabetic rats [110], Apoe-deficient mice [111,112] and cystathionine beta synthase-deficient mice [113]); catechins, alone or in combination with other polyphenols (quercetin) or foods (wine); or procyanidins (a group of flavonoids that are oligomeric forms of catechins present in red wine, grapes and apples) [110] have demonstrated up-regulation of PON1 levels or protective activity against LDL oxidation and lipid peroxidation. These studies raised important caveats such as the influence of the chemical nature of flavonoids or isoflavonoids, the experimental approach and selection of the animal model.
Curcumin is a phenolic compound obtained from the root of Curcuma longa and a common spice in the Mediterranean diet. Curcumin supplementation (0.05% in diet) of a high-fat diet significantly elevated plasma HDL cholesterol, APOA1 and paraoxonase activity compared with the control group in hamsters [114]. Moreover, curcumin dose-dependently induced PON1 transactivation in Huh7 cells. However, dietary supplementation with curcumin (500 mg/kg diet) for two weeks in female B6C3F1 mice did not modify the hepatic PON1 mRNA or protein level [115]. In conclusion, curcumin is a potent PON1 inducer in cultured cells in vitro, but its effect in vivo may be influenced by the animal model or dietary fat content.
Resveratrol is a naturally occurring phenolic compound that is present at high levels in wine. In vitro, resveratrol has been shown to enhance PON1 enzyme activity and gene expression in hepatocytes, HepG2, HC04 and A549 cells [116,117,118]. This effect could be mediated by the resveratrol activation of the aryl hydrocarbon receptor as a putative inducer of PON1 [119,120]. More controversial have been the outcomes of resveratrol administration in vivo. Whereas chronic administration of resveratrol significantly decreased serum paraoxonase-1 activity in cystathionine beta synthase-deficient mice [121], in Apoe-deficient mice, serum paraoxonase activity was significantly higher in the resveratrol group [122]. Resveratrol, as other phenolic compounds, is a potent PON1 inducer in cultured cells, but its effect in vivo may be influenced by the animal model or dietary regimen.
6.3. Vitamins and Carotenoids
As was pointed out previously, the Mediterranean dietary pattern is characterized by abundant consumption of fruits and vegetables, which are a good source of all types of vitamins and carotenes. For instance, vitamin E is present in many constituents of this diet (e.g., olive oil, nuts and seeds) and vitamin C in citrus fruits and vegetables. Vitamin E supplementation reversed the decreased serum paraoxonase and arylesterase activities in hypothyroidism induced by propylthiouracil administration to rats [123]. Similarly, vitamin E supplementation for 10 weeks had a protective effect against the decrease in PON1 activity observed in exercise-induced oxidative damage in sedentary dogs [124]. Moreover, the decreased post-exercise PON1 activity was normalized by alpha-tocopherol supplementation in basketball players, [125]. Vitamin C administration restored the decreased serum PON1 activity of hemodialysis patients [126]. The incorporation of vitamin A into the diet of vitamin-A-deficient rats reverted the decreased serum paraoxonase1 induced by vitamin A deficiency [127]. These reports point to a role of vitamins A, E and C in restoring the decreased paraoxonase activity following different oxidative stress-causing conditions.
As was mentioned above, carotenoids are highly present in the Mediterranean diet and may play a role in PON1 activity. Indeed, incubation with β-carotene counteracted the IL-1β-induced decrease in PON1 expression in human endothelial cells. The effect of β-carotene was reversed by a calmodulin-dependent protein kinase kinase inhibitor, a finding that indicates a role of this pathway in PON1 expression [128]. Astaxanthin is a β-carotene-like carotenoid pigment present in crustaceans and salmon, but it is also a metabolite of zeaxanthin that is found in paprika, peppers and leaves of green vegetables, many of them constituents of the Mediterranean diet. Astaxanthin supplementation for 90 days increased PON1 activity in soccer players, an effect that could be due to the protection of free thiol groups against oxidative modification observed during the treatment [129]. Lycopene, a carotenoid present in tomatoes, also displayed a restorative effect upon the decreased PON1 activity observed in rats subjected to experimental diabetes [130]. In moderately overweight middle-aged individuals, McEneny et al. found that lycopene supplementation increased PON1 activity in serum and HDL [91]. However, it has been reported that PON1 polymorphisms may modify the association between serum concentrations of lycopene and oxidative stress parameters [131]. According to these findings, carotenes exert an important effect on PON1, while other potential mechanisms are likewise emerging.
6.4. Coenzyme Q10
CoenzymeQ10 is synthesized in most human tissues, and the enzyme hydroxymethylglutaryl-CoA reductase plays a critical role in its regulation [132]. Rich sources of dietary coenzyme Q10 include mainly meat, poultry, fish and nuts. Fruits, vegetables, eggs, and dairy products are moderate sources of this coenzyme [133,134,135]. Coenzyme Q10 exerts a protective effect on plasma lipoprotein oxidation. Administration of extra virgin olive oil (20 mL) enriched with either 20 or 40 mg of the coenzyme for two weeks induced a dose-dependent increase in paraoxonase-1 activity in healthy subjects [136]. These findings indicate that there is still a potential to improve the quality of the influence of extra virgin olive oil on PON1.
6.5. Taurine
Good sources of dietary taurine are seafood and meat [137]. Administration of taurine to rats with hypothyroidism resulted in a dose-dependent increase in serum paraoxonase and arylesterase activities [138]. This observation prompts the notion that certain amino acids may be particularly active in regulating PON1 expression.
6.6. Trace Elements
Selenium is present in components of the Mediterranean diet such as seafood (mussels), fish (tuna), whole-wheat bread, and seeds (sunflower). Selenium supplementation in male Sprague Dawley rats improved the high fat diet-mediated reduction of serum PON1 enzyme activity by 34% and PON1 protein levels by 21%, with no changes in its hepatic mRNA expression [139]. Thus, this supplementation could be a valuable approach to limiting the adverse effects of high fat diet-induced hypercholesterolemia and may warrant further investigation.
7. Conclusions
One of the key actions of the Mediterranean diet is the prevention of cardiovascular diseases, which can be promoted in part by increased PON1 activity. This hypothesis is supported by the increase in this enzyme when Mediterranean diets have been administered. A dissection of the components of these diets points to EVOO intake as an important factor of PON1 levels. It is noteworthy that some PON1 polymorphisms are crucial determinants of EVOO response. Not only is EVOO consumption replacing intake of noxious saturated fatty acids, but it also contributes to establishing the phospholipid environment of HDL that enhances PON1 activity. In addition, some minor components of EVOO increase hepatic PON1 protein and mRNA expressions. An emerging role of nuts and their components in the activity of this enzyme has also been observed. Likewise, fruits and vegetables are able to modify this activity, the pomegranate and its compounds being well characterized examples. The characterization of isolated compounds from all these and other natural sources, such as phenolic compounds, carotenoids, etc., will lead the way to interesting nutraceuticals and the development of functional foods to enhance the protective role of PON1 (Table 2).
Table 2.
Overview of the studies reviewed.
| Characteristics of Studies | Findings | References | |
|---|---|---|---|
| Mediterranean diets | Greek tradition vs. Anglo-Celtics | Paraoxonase activity correlated with carotenoid concentrations | [21] |
| Mediterranean-like meal was compared to a Western-like meal | Increase in PON1 activity and carotenoid concentrations | [22] | |
| Olive oil or its components | Virgin olive oil in humans | Increased PON1 levels | [9,36,37] |
| Olive oil in animal models | Favors PON1 | [39,40,45] | |
| Oleic acid intake in humans | The beneficial effect on PON1 activity was dependent on polymorphisms | [26] | |
| Olive oil and green tea phenolics in animals | Increased PON1 activity | [47,48] | |
| Squalene | Variable effects depending on matrix vehicle | [50,51] | |
| Nuts | Human and animal studies | Effect on PON1 may vary according to different nuts and their constituents | [62,63,64,65,66] |
| Fruits and vegetables | Increased consumption in humans | Augmented PON1 with some fruits and phenotypes | [15,70,71,72,73,74,76,89,90,91,92] |
| Lipids | Human and animal studies | Differential effects depending on different fatty acids | [45,93,94,95] |
| Phenolic compounds | Quercetin in mice | PON1 differentially regulated depending on APOE genotype | [96] |
| Anthocyanin in humans | Increased HDL-PON1 | [100] | |
| Flavonoids and isoflavones | Discrepant results in function of experimental approach | [101,102,103,104,108,109,110,111,112,113] | |
| Curcumin | In vivo effect is influenced by the animal model and dietary fat content | [114,115] | |
| Resveratrol | Animal model and dietary regimen modify the outcome | [116,117,118,119,120,121,122] | |
| Vitamins and carotenoids | Vitamin A, C and E supplementation | Positive action on PON1 | [123,124,125,126,127] |
| β-carotene, astaxanthin, lycopene | Increased PON1 activity | [91,128,129,130,131] | |
| Coenzyme Q10 | Humans consuming olive oil enriched with this compound | Increase in PON1 activity | [136] |
| Taurine | Rats with hypothyroidism | Increase in serum paraoxonase | [138] |
| Trace elements | Selenium supplementation to rats | Increase in serum paraoxonase | [139] |
Acknowledgments
We thank Martha Messman for her assistance in manuscript editing. The work of this group was supported by grants from the Spanish Ministerio de Economía y Competitividad—European Regional Development Fund (2013-41651-R) and the European Social Fund—Gobierno de Aragón (B-69). CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) is an initiative of the Instituto de Salud Carlos III, Spain.
Author Contributions
J.M.L.B., C.G.R. and M.A.N. carried out the search and prepared the draft, and J.O. supervised the work and draft and prepared the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Deakin S., Leviev I., Gomaraschi M., Calabresi L., Franceschini G., James R.W. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 2002;277:4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 2.Durrington P.N., Mackness B., Mackness M.I. Paraoxonase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:473–480. doi: 10.1161/01.ATV.21.4.473. [DOI] [PubMed] [Google Scholar]
- 3.Rozenberg O., Rosenblat M., Coleman R., Shih D.M., Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: Studies in PON1-knockout mice. Free Radic. Biol. Med. 2003;34:774–784. doi: 10.1016/S0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 4.Ferretti G., Bacchetti T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012;22:88–94. doi: 10.1016/j.numecd.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Loued S., Isabelle M., Berrougui H., Khalil A. The anti-inflammatory effect of paraoxonase 1 against oxidized lipids depends on its association with high density lipoproteins. Life Sci. 2012;90:82–88. doi: 10.1016/j.lfs.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Abbott C.A., Mackness M.I., Kumar S., Boulton A.J., Durrington P.N. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995;15:1812–1818. doi: 10.1161/01.ATV.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 7.Seres I., Paragh G., Deschene E., Fulop T., Jr., Khalil A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp. Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Deakin S.P., James R.W. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin. Sci. (Lond.) 2004;107:435–447. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- 9.Loued S., Berrougui H., Componova P., Ikhlef S., Helal O., Khalil A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br. J. Nutr. 2013;110:1272–1284. doi: 10.1017/S0007114513000482. [DOI] [PubMed] [Google Scholar]
- 10.Bub A., Barth S., Watzl B., Briviba K., Herbert B.M., Luhrmann P.M., Neuhauser-Berthold M., Rechkemmer G. Paraoxonase 1 Q192R (PON1–192) polymorphism is associated with reduced lipid peroxidation in R-allele-carrier but not in QQ homozygous elderly subjects on a tomato-rich diet. Eur. J. Nutr. 2002;41:237–243. doi: 10.1007/s00394-002-0389-8. [DOI] [PubMed] [Google Scholar]
- 11.Koubaa N., Nakbi A., Hammami S., Attia N., Mehri S., Ben Hamda K., Ben Farhat M., Miled A., Hammami M. Association of homocysteine thiolactonase activity and PON1 polymorphisms with the severity of acute coronary syndrome. Clin. Biochem. 2009;42:771–776. doi: 10.1016/j.clinbiochem.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Costa L.G., Vitalone A., Cole T.B., Furlong C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Rajkovic M.G., Rumora L., Barisic K. The paraoxonase 1, 2 and 3 in humans. Biochem. Med. (Zagreb) 2011;21:122–130. doi: 10.11613/BM.2011.020. [DOI] [PubMed] [Google Scholar]
- 14.Da Costa L.A., Badawi A., El-Sohemy A. Nutrigenetics and modulation of oxidative stress. Ann. Nutr. Metab. 2012;60(Suppl. 3):27–36. doi: 10.1159/000337311. [DOI] [PubMed] [Google Scholar]
- 15.Durrington P.N., Mackness B., Mackness M.I. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002;22:1248–1250. doi: 10.1161/01.ATV.0000027414.34728.1F. [DOI] [PubMed] [Google Scholar]
- 16.Keys A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995;61(Suppl. 6):1321S–1323S. doi: 10.1093/ajcn/61.6.1321S. [DOI] [PubMed] [Google Scholar]
- 17.Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 18.Wahrburg U., Kratz M., Cullen P. Mediterranean diet, olive oil and health. Eur. J. Lipid Sci. Technol. 2002;104:698–705. doi: 10.1002/1438-9312(200210)104:9/10<698::AID-EJLT698>3.0.CO;2-A. [DOI] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pubmed Website. [(accessed on 11 March 2015)]; Available online: http://www.ncbi.nlm.nih.gov/pubmed/
- 21.Lee C.T., Rowley K., Jenkins A.J., O’Dea K., Itsiopoulos C., Stoney R.M., Su Q., Giles G.G., Best J.D. Paraoxonase activity in Greek migrants and Anglo-Celtic persons in the Melbourne Collaborative Cohort Study: Relationship to dietary markers. Eur. J. Nutr. 2005;44:223–230. doi: 10.1007/s00394-004-0514-y. [DOI] [PubMed] [Google Scholar]
- 22.Blum S., Aviram M., Ben-Amotz A., Levy Y. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann. Nutr. Metab. 2006;50:20–24. doi: 10.1159/000089560. [DOI] [PubMed] [Google Scholar]
- 23.Kudchodkar B.J., Lacko A.G., Dory L., Fungwe T.V. Dietary fat modulates serum paraoxonase 1 activity in rats. J. Nutr. 2000;130:2427–2433. doi: 10.1093/jn/130.10.2427. [DOI] [PubMed] [Google Scholar]
- 24.Thomas-Moya E., Gianotti M., Proenza A.M., Llado I. Paraoxonase 1 response to a high-fat diet: Gender differences in the factors involved. Mol. Med. 2007;13:203–209. doi: 10.2119/2006-00078.Thomas-Moya. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoefel A.L., Hansen F., Rosa P.D., Assis A.M., Silveira S.L., Denardin C.C., Pettenuzzo L., Augusti P.R., Somacal S., Emanuelli T., et al. The effects of hypercaloric diets on glucose homeostasis in the rat: Influence of saturated and monounsaturated dietary lipids. Cell Biochem. Funct. 2011;29:569–576. doi: 10.1002/cbf.1789. [DOI] [PubMed] [Google Scholar]
- 26.Tomas M., Senti M., Elosua R., Vila J., Sala J., Masia R., Marrugat J. Interaction between the Gln-Arg 192 variants of the paraoxonase gene and oleic acid intake as a determinant of high-density lipoprotein cholesterol and paraoxonase activity. Eur. J. Pharmacol. 2001;432:121–128. doi: 10.1016/S0014-2999(01)01482-0. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland W.H., Walker R.J., de Jong S.A., van Rij A.M., Phillips V., Walker H.L. Reduced postprandial serum paraoxonase activity after a meal rich in used cooking fat. Arterioscler. Thromb. Vasc. Biol. 1999;19:1340–1347. doi: 10.1161/01.ATV.19.5.1340. [DOI] [PubMed] [Google Scholar]
- 28.Lou-Bonafonte J.M., Arnal C., Navarro M.A., Osada J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol. Nutr. Food Res. 2012;56:1043–1057. doi: 10.1002/mnfr.201100668. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Jimenez F., Alvarez de Cienfuegos G., Badimon L., Barja G., Battino M., Blanco A., Bonanome A., Colomer R., Corella-Piquer D., Covas I., et al. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Investig. 2005;35:421–424. doi: 10.1111/j.1365-2362.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 30.Mata P., Alvarez-Sala L.A., Rubio M.J., Nuno J., de Oya M. Effects of long-term monounsaturated- vs. polyunsaturated-enriched diets on lipoproteins in healthy men and women. Am. J. Clin. Nutr. 1992;55:846–850. doi: 10.1093/ajcn/55.4.846. [DOI] [PubMed] [Google Scholar]
- 31.Stock J. Importance of HDL functionality to cardiovascular risk. Atherosclerosis. 2011;218:19–20. doi: 10.1016/j.atherosclerosis.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Lou-Bonafonte J.M., Fito M., Covas M.I., Farras M., Osada J. HDL-related mechanisms of olive oil protection in cardiovascular disease. Curr. Vasc. Pharmacol. 2012;10:392–409. doi: 10.2174/157016112800812827. [DOI] [PubMed] [Google Scholar]
- 33.Baker P.W., Rye K.A., Gamble J.R., Vadas M.A., Barter P.J. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 1999;40:345–353. [PubMed] [Google Scholar]
- 34.Recalde D., Ostos M.A., Badell E., Garcia-Otin A.L., Pidoux J., Castro G., Zakin M.M., Scott-Algara D. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004;24:756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 35.Shih D.M., Gu L., Xia Y.R., Navab M., Li W.F., Hama S., Castellani L.W., Furlong C.E., Costa L.G., Fogelman A.M., et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 36.Cherki M., Derouiche A., Drissi A., El Messal M., Bamou Y., Idrissi-Ouadghiri A., Khalil A., Adlouni A. Consumption of argan oil may have an antiatherogenic effect by improving paraoxonase activities and antioxidant status: Intervention study in healthy men. Nutr. Metab. Cardiovasc. Dis. 2005;15:352–360. doi: 10.1016/j.numecd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Manning P.J., Jong S.A., Ryalls A.R., Sutherland W.H. Paraoxonase 1 activity in chylomicrons and VLDL: The effect of type 2 diabetes and meals rich in saturated fat and oleic acid. Lipids. 2012;47:259–267. doi: 10.1007/s11745-011-3640-3. [DOI] [PubMed] [Google Scholar]
- 38.Wallace A.J., Sutherland W.H., Mann J.I., Williams S.M. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur. J. Clin. Nutr. 2001;55:951–958. doi: 10.1038/sj.ejcn.1601250. [DOI] [PubMed] [Google Scholar]
- 39.Fuhrman B., Volkova N., Aviram M. Postprandial serum triacylglycerols and oxidative stress in mice after consumption of fish oil, soy oil or olive oil: Possible role for paraoxonase-1 triacylglycerol lipase-like activity. Nutrition. 2006;22:922–930. doi: 10.1016/j.nut.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Acin S., Navarro M.A., Carnicer R., Arbones-Mainar J.M., Guzman M.A., Arnal C., Beltran G., Uceda M., Maeda N., Osada J. Dietary cholesterol suppresses the ability of olive oil to delay the development of atherosclerotic lesions in apolipoprotein E knockout mice. Atherosclerosis. 2005;182:17–28. doi: 10.1016/j.atherosclerosis.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 41.Arbones-Mainar J.M., Navarro M.A., Carnicer R., Guillen N., Surra J.C., Acin S., Guzman M.A., Sarria A.J., Arnal C., Aguilera M.P., et al. Accelerated atherosclerosis in apolipoprotein E-deficient mice fed Western diets containing palm oil compared with extra virgin olive oils: A role for small, dense high-density lipoproteins. Atherosclerosis. 2007;194:372–382. doi: 10.1016/j.atherosclerosis.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Graham A., Hassall D.G., Rafique S., Owen J.S. Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis. 1997;135:193–204. doi: 10.1016/S0021-9150(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 43.Valabhji J., McColl A.J., Schachter M., Dhanjil S., Richmond W., Elkeles R.S. High-density lipoprotein composition and paraoxonase activity in Type I diabetes. Clin. Sci. (Lond.) 2001;101:659–670. doi: 10.1042/CS20010111. [DOI] [PubMed] [Google Scholar]
- 44.Kontush A., Chantepie S., Chapman M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 45.Efrat M., Rosenblat M., Mahmood S., Vaya J., Aviram M. Di-oleoyl phosphatidylcholine (PC-18:1) stimulates paraoxonase 1 (PON1) enzymatic and biological activities: In vitro and in vivo studies. Atherosclerosis. 2009;202:461–469. doi: 10.1016/j.atherosclerosis.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Hussein O., Grosovski M., Lasri E., Svalb S., Ravid U., Assy N. Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2007;13:361–368. doi: 10.3748/wjg.v13.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayram B., Ozcelik B., Grimm S., Roeder T., Schrader C., Ernst I.M., Wagner A.E., Grune T., Frank J., Rimbach G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012;15:71–81. doi: 10.1089/rej.2011.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenblat M., Volkova N., Coleman R., Almagor Y., Aviram M. Antiatherogenicity of extra virgin olive oil and its enrichment with green tea polyphenols in the atherosclerotic apolipoprotein-E-deficient mice: Enhanced macrophage cholesterol efflux. J. Nutr. Biochem. 2008;19:514–523. doi: 10.1016/j.jnutbio.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Acin S., Navarro M.A., Arbones-Mainar J.M., Guillen N., Sarria A.J., Carnicer R., Surra J.C., Orman I., Segovia J.C., Torre R., et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. J. Biochem. 2006;140:383–391. doi: 10.1093/jb/mvj166. [DOI] [PubMed] [Google Scholar]
- 50.Guillen N., Acin S., Navarro M.A., Perona J.S., Arbones-Mainar J.M., Arnal C., Sarria A.J., Surra J.C., Carnicer R., Orman I., et al. Squalene in a sex-dependent manner modulates atherosclerotic lesion which correlates with hepatic fat content in apoE-knockout male mice. Atherosclerosis. 2008;197:72–83. doi: 10.1016/j.atherosclerosis.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Gabas-Rivera C., Barranquero C., Martinez-Beamonte R., Navarro M.A., Surra J.C., Osada J. Dietary squalene increases high density lipoprotein-cholesterol and paraoxonase 1 and decreases oxidative stress in mice. PLoS ONE. 2014;9:e104224. doi: 10.1371/journal.pone.0104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arunima S., Rajamohan T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats–A comparative study. Food Funct. 2013;4:1402–1409. doi: 10.1039/c3fo60085h. [DOI] [PubMed] [Google Scholar]
- 53.Boskou D. Olive Oil, Chemistry and Technology. AOCS Press; Champaign, IL, USA: 1996. [Google Scholar]
- 54.Civantos L., Contreras R., Grana R. In: Obtención del Aceite de Oliva Virgen. 2nd ed. Agrícola Española S.A., editor. Editorial Agrícola Española; Madrid, Spain: 1999. [Google Scholar]
- 55.Harwood J., Aparicio R. Handbook of Olive Oil: Analysis and Properties. Kluwer Academic; Dordrecht, The Netherlands: 2000. [Google Scholar]
- 56.Jiménez J., Rondón D., Martínez L., Mataix J. Composición química de los aceites de oliva. In: Mataix J., editor. Aceite de Oliva Virgen: Nuestro patrimonio alimentario. Universidad de Granada, Puleva Food; Granada, Spain: 2001. pp. 115–136. [Google Scholar]
- 57.Montedoro G.F., Taticchi A., Esposto S., Selvaggini R., Urbani S., Servilli M. Antioxidants in virgin olive oil. Olea. 2007;26:5–13. [Google Scholar]
- 58.Sabate J., Wien M. Nuts, blood lipids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2010;19:131–136. [PubMed] [Google Scholar]
- 59.Fito M., Guxens M., Corella D., Saez G., Estruch R., de la Torre R., Frances F., Cabezas C., Lopez-Sabater Mdel C., Marrugat J., et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 60.Canales A., Benedi J., Nus M., Librelotto J., Sanchez-Montero J.M., Sanchez-Muniz F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr. 2007;26:225–232. doi: 10.1080/07315724.2007.10719605. [DOI] [PubMed] [Google Scholar]
- 61.Nus M., Frances F., Librelotto J., Canales A., Corella D., Sanchez-Montero J.M., Sanchez-Muniz F.J. Arylesterase activity and antioxidant status depend on PON1-Q192R and PON1-L55M polymorphisms in subjects with increased risk of cardiovascular disease consuming walnut-enriched meat. J. Nutr. 2007;137:1783–1788. doi: 10.1093/jn/137.7.1783. [DOI] [PubMed] [Google Scholar]
- 62.Canales A., Sanchez-Muniz F.J., Bastida S., Librelotto J., Nus M., Corella D., Guillen M., Benedi J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur. J. Clin. Nutr. 2011;65:703–710. doi: 10.1038/ejcn.2011.20. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Muniz F.J., Canales A., Nus M., Bastida S., Guillen M., Corella D., Olmedilla-Alonso B., Granado-Lorencio F., Benedi J. The antioxidant status response to low-fat and walnut paste-enriched meat differs in volunteers at high cardiovascular Risk carrying different PON-1 polymorphisms. J. Am. Coll. Nutr. 2012;31:194–205. doi: 10.1080/07315724.2012.10720027. [DOI] [PubMed] [Google Scholar]
- 64.Strunz C.C., Oliveira T.V., Vinagre J.C., Lima A., Cozzolino S., Maranhao R.C. Brazil nut ingestion increased plasma selenium but had minimal effects on lipids, apolipoproteins, and high-density lipoprotein function in human subjects. Nutr. Res. 2008;28:151–155. doi: 10.1016/j.nutres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Aksoy N., Aksoy M., Bagci C., Gergerlioglu H.S., Celik H., Herken E., Yaman A., Tarakcioglu M., Soydinc S., Sari I., et al. Pistachio intake increases high density lipoprotein levels and inhibits low-density lipoprotein oxidation in rats. Tohoku J. Exp. Med. 2007;212:43–48. doi: 10.1620/tjem.212.43. [DOI] [PubMed] [Google Scholar]
- 66.Surra J.C., Barranquero C., Torcal M.P., Orman I., Segovia J.C., Guillen N., Navarro M.A., Arnal C., Osada J. In comparison with palm oil, dietary nut supplementation delays the progression of atherosclerotic lesions in female apoE-deficient mice. Br. J. Nutr. 2013;109:202–209. doi: 10.1017/S000711451200092X. [DOI] [PubMed] [Google Scholar]
- 67.Liu S., Manson J.E., Lee I.M., Cole S.R., Hennekens C.H., Willett W.C., Buring J.E. Fruit and vegetable intake and risk of cardiovascular disease: The Women’s Health Study. Am. J. Clin. Nutr. 2000;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 68.Strandhagen E., Hansson P.O., Bosaeus I., Isaksson B., Eriksson H. High fruit intake may reduce mortality among middle-aged and elderly men. The Study of Men Born in 1913. Eur. J. Clin. Nutr. 2000;54:337–341. doi: 10.1038/sj.ejcn.1600959. [DOI] [PubMed] [Google Scholar]
- 69.Bazzano L.A., He J., Ogden L.G., Loria C.M., Vupputuri S., Myers L., Whelton P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 70.Daniels J.A., Mulligan C., McCance D., Woodside J.V., Patterson C., Young I.S., McEneny J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014;13:16. doi: 10.1186/1475-2840-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Proteggente A.R., Pannala A.S., Paganga G., van Buren L., Wagner E., Wiseman S., van de Put F., Dacombe C., Rice-Evans C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002;36:217–233. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- 72.Lixandru D., Mohora M., Coman A., Stoian I., van Gils C., Aerts P., Manuel Y.K.B. Diet and paraoxonase 1 enzymatic activity in diabetic foot patients from Romania and Belgium: Favorable association of high flavonoid dietary intake with arylesterase activity. Ann. Nutr. Metab. 2010;56:294–301. doi: 10.1159/000298879. [DOI] [PubMed] [Google Scholar]
- 73.Freese R., Alfthan G., Jauhiainen M., Basu S., Erlund I., Salminen I., Aro A., Mutanen M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002;76:950–960. doi: 10.1093/ajcn/76.5.950. [DOI] [PubMed] [Google Scholar]
- 74.Rantala M., Silaste M.L., Tuominen A., Kaikkonen J., Salonen J.T., Alfthan G., Aro A., Kesaniemi Y.A. Dietary modifications and gene polymorphisms alter serum paraoxonase activity in healthy women. J. Nutr. 2002;132:3012–3017. doi: 10.1093/jn/131.10.3012. [DOI] [PubMed] [Google Scholar]
- 75.Rosenblat M., Volkova N., Aviram M. Pomegranate juice (PJ) consumption antioxidative properties on mouse macrophages, but not PJ beneficial effects on macrophage cholesterol and triglyceride metabolism, are mediated via PJ-induced stimulation of macrophage PON2. Atherosclerosis. 2010;212:86–92. doi: 10.1016/j.atherosclerosis.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 76.Aviram M., Rosenblat M. Pomegranate for your cardiovascular health. Rambam Maimonides Med. J. 2013;4:e0013. doi: 10.5041/RMMJ.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khateeb J., Gantman A., Kreitenberg A.J., Aviram M., Fuhrman B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-gamma pathway. Atherosclerosis. 2010;208:119–125. doi: 10.1016/j.atherosclerosis.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan M., Hayek T., Raz A., Coleman R., Dornfeld L., Vaya J., Aviram M. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J. Nutr. 2001;131:2082–2089. doi: 10.1093/jn/131.8.2082. [DOI] [PubMed] [Google Scholar]
- 79.Aviram M., Dornfeld L., Kaplan M., Coleman R., Gaitini D., Nitecki S., Hofman A., Rosenblat M., Volkova N., Presser D., et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 80.Aviram M., Dornfeld L., Rosenblat M., Volkova N., Kaplan M., Coleman R., Hayek T., Presser D., Fuhrman B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 81.Rosenblat M., Volkova N., Attias J., Mahamid R., Aviram M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010;1:99–109. doi: 10.1039/c0fo00011f. [DOI] [PubMed] [Google Scholar]
- 82.Rosenblat M., Hayek T., Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187:363–371. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Parsaeyan N., Mozaffari-Khosravi H., Mozayan M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012;11:11. doi: 10.1186/2251-6581-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rock W., Rosenblat M., Miller-Lotan R., Levy A.P., Elias M., Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008;56:8704–8713. doi: 10.1021/jf801756x. [DOI] [PubMed] [Google Scholar]
- 85.Fuhrman B., Volkova N., Aviram M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition. 2010;26:359–366. doi: 10.1016/j.nut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Aviram M., Rosenblat M., Gaitini D., Nitecki S., Hoffman A., Dornfeld L., Volkova N., Presser D., Attias J., Liker H., et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Suh J.H., Romain C., Gonzalez-Barrio R., Cristol J.P., Teissedre P.L., Crozier A., Rouanet J.M. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011;2:400–405. doi: 10.1039/c1fo10047e. [DOI] [PubMed] [Google Scholar]
- 88.Kujawska M., Ignatowicz E., Ewertowska M., Markowski J., Jodynis-Liebert J. Cloudy apple juice protects against chemical-induced oxidative stress in rat. Eur. J. Nutr. 2011;50:53–60. doi: 10.1007/s00394-010-0114-y. [DOI] [PubMed] [Google Scholar]
- 89.Dalgard C., Christiansen L., Jonung T., Mackness M.I., de Maat M.P., Horder M. No influence of increased intake of orange and blackcurrant juices and dietary amounts of vitamin E on paraoxonase-1 activity in patients with peripheral arterial disease. Eur. J. Nutr. 2007;46:354–363. doi: 10.1007/s00394-007-0675-6. [DOI] [PubMed] [Google Scholar]
- 90.Bub A., Barth S.W., Watzl B., Briviba K., Rechkemmer G. Paraoxonase 1 Q192R (PON1–192) polymorphism is associated with reduced lipid peroxidation in healthy young men on a low-carotenoid diet supplemented with tomato juice. Br. J. Nutr. 2005;93:291–297. doi: 10.1079/BJN20041309. [DOI] [PubMed] [Google Scholar]
- 91.McEneny J., Wade L., Young I.S., Masson L., Duthie G., McGinty A., McMaster C., Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013;24:163–168. doi: 10.1016/j.jnutbio.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 92.Rock W., Rosenblat M., Borochov-Neori H., Volkova N., Judeinstein S., Elias M., Aviram M. Effects of date (Phoenix dactylifera L., Medjool or Hallawi Variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status: A pilot study. J. Agric. Food Chem. 2009;57:8010–8017. doi: 10.1021/jf901559a. [DOI] [PubMed] [Google Scholar]
- 93.Pfeuffer M., Fielitz K., Laue C., Winkler P., Rubin D., Helwig U., Giller K., Kammann J., Schwedhelm E., Boger R.H., et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J. Am. Coll. Nutr. 2011;30:19–28. doi: 10.1080/07315724.2011.10719940. [DOI] [PubMed] [Google Scholar]
- 94.Ghorbanihaghjo A., Kolahi S., Seifirad S., Rashtchizadeh N., Argani H., Hajialilo M., Khabazi A., Alizadeh S., Bahreini E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: A double-blind randomized controlled trial. Arch. Iran Med. 2012;15:549–552. [PubMed] [Google Scholar]
- 95.Stirban A., Nandrean S., Gotting C., Stratmann B., Tschoepe D. Effects of n-3 polyunsaturated fatty acids (PUFAs) on circulating adiponectin and leptin in subjects with type 2 diabetes mellitus. Horm. Metab. Res. 2014;46:490–492. doi: 10.1055/s-0033-1363225. [DOI] [PubMed] [Google Scholar]
- 96.Boesch-Saadatmandi C., Niering J., Minihane A.M., Wiswedel I., Gardeman A., Wolffram S., Rimbach G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis. 2010;211:110–113. doi: 10.1016/j.atherosclerosis.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 97.Bokkenheuser V.D., Shackleton C.H., Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987;248:953–956. doi: 10.1042/bj2480953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Rejaie S.S., Aleisa A.M., Sayed-Ahmed M.M., Al-Shabanah O.A., Abuohashish H.M., Ahmed M.M., Al-Hosaini K.A., Hafez M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013;13:136. doi: 10.1186/1472-6882-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaganath I.B., Crozier A. Dietary Flavonoids and Phenolic Compounds. In: Fraga C.G., editor. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. pp. 1–49. [Google Scholar]
- 100.Zhu Y., Huang X., Zhang Y., Wang Y., Liu Y., Sun R., Xia M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014;99:561–569. doi: 10.1210/jc.2013-2845. [DOI] [PubMed] [Google Scholar]
- 101.Kaufman P.B., Duke J.A., Brielmann H., Boik J., Hoyt J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997;3:7–12. doi: 10.1089/acm.1997.3.7. [DOI] [PubMed] [Google Scholar]
- 102.Ustundag B., Bahcecioglu I.H., Sahin K., Duzgun S., Koca S., Gulcu F., Ozercan I.H. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig. Dis. Sci. 2007;52:2006–2014. doi: 10.1007/s10620-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 103.Mohammadshahi M., Haidari F., Saei A.A., Rashidi B., Mahboob S., Rashidi M.R. Soy protein, genistein, and daidzein improve serum paraoxonase activity and lipid profiles in rheumatoid arthritis in rats. J. Med. Food. 2013;16:147–154. doi: 10.1089/jmf.2012.2509. [DOI] [PubMed] [Google Scholar]
- 104.Schrader C., Ernst I.M., Sinnecker H., Soukup S.T., Kulling S.E., Rimbach G. Genistein as a potential inducer of the anti-atherogenic enzyme paraoxonase-1: Studies in cultured hepatocytes in vitro and in rat liver in vivo. J. Cell. Mol. Med. 2012;16:2331–2341. doi: 10.1111/j.1582-4934.2012.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carrero-Gálvez M., García-Barroso C., Pérez-Bustamante J.A. Analysis of polyphenolic compounds of different vinegar samples. Z Lebensm Unters Forsch. 1994;199:29–31. doi: 10.1007/BF01192948. [DOI] [Google Scholar]
- 106.Cheng G.W., Crisosto C.H. Browning potential, phenolic composition, and polyphenoloxidase activity of buffer extracts of peach and nectarine skin tissue. J. Am. Soc. Hortic. Sci. 1995;120:835–838. [Google Scholar]
- 107.Quinde-Axtell Z., Baik B.K. Phenolic compounds of barley grain and their implication in food product discoloration. J. Agric. Food Chem. 2006;54:9978–9984. doi: 10.1021/jf060974w. [DOI] [PubMed] [Google Scholar]
- 108.Jaiswal N., Rizvi S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male Wistar rats subjected to oxidative stress. J. Sci. Food Agric. 2014;94:2752–2757. doi: 10.1002/jsfa.6620. [DOI] [PubMed] [Google Scholar]
- 109.Amengual-Cladera E., Nadal-Casellas A., Gomez-Perez Y., Gomila I., Prieto R.M., Proenza A.M., Llado I. Phytotherapy in a rat model of hyperoxaluria: The antioxidant effects of quercetin involve serum paraoxonase 1 activation. Exp. Biol. Med. (Maywood) 2011;236:1133–1138. doi: 10.1258/ebm.2011.011090. [DOI] [PubMed] [Google Scholar]
- 110.Kiyici A., Okudan N., Gokbel H., Belviranli M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J. Med. Food. 2010;13:725–728. doi: 10.1089/jmf.2009.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuhrman B., Aviram M. Preservation of paraoxonase activity by wine flavonoids: Possible role in protection of LDL from lipid peroxidation. Ann. N. Y. Acad. Sci. 2002;957:321–324. doi: 10.1111/j.1749-6632.2002.tb02933.x. [DOI] [PubMed] [Google Scholar]
- 112.Hayek T., Fuhrman B., Vaya J., Rosenblat M., Belinky P., Coleman R., Elis A., Aviram M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler. Thromb. Vasc. Biol. 1997;17:2744–2752. doi: 10.1161/01.ATV.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 113.Hamelet J., Demuth K., Dairou J., Ledru A., Paul J.L., Dupret J.M., Delabar J.M., Rodrigues-Lima F., Janel N. Effects of catechin on homocysteine metabolism in hyperhomocysteinemic mice. Biochem. Biophys. Res. Commun. 2007;355:221–227. doi: 10.1016/j.bbrc.2007.01.142. [DOI] [PubMed] [Google Scholar]
- 114.Jang E.M., Choi M.S., Jung U.J., Kim M.J., Kim H.J., Jeon S.M., Shin S.K., Seong C.N., Lee M.K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 115.Schrader C., Schiborr C., Frank J., Rimbach G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2011;105:167–170. doi: 10.1017/S0007114510004356. [DOI] [PubMed] [Google Scholar]
- 116.Curtin B.F., Seetharam K.I., Dhoieam P., Gordon R.K., Doctor B.P., Nambiar M.P. Resveratrol induces catalytic bioscavenger paraoxonase 1 expression and protects against chemical warfare nerve agent toxicity in human cell lines. J. Cell. Biochem. 2008;103:1524–1535. doi: 10.1002/jcb.21543. [DOI] [PubMed] [Google Scholar]
- 117.Wagner A.E., Boesch-Saadatmandi C., Breckwoldt D., Schrader C., Schmelzer C., Doring F., Hashida K., Hori O., Matsugo S., Rimbach G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes—Role of the redox-regulated transcription factor Nrf2. BMC Complement. Altern. Med. 2011;11:1. doi: 10.1186/1472-6882-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta N., Kandimalla R., Priyanka K., Singh G., Gill K.D., Singh S. Effect of Resveratrol and Nicotine on PON1 Gene Expression: In Vitro Study. Indian J. Clin. Biochem. 2014;29:69–73. doi: 10.1007/s12291-013-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gouedard C., Barouki R., Morel Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler. Thromb. Vasc. Biol. 2004;24:2378–2383. doi: 10.1161/01.ATV.0000146530.24736.ce. [DOI] [PubMed] [Google Scholar]
- 120.Guyot E., Coumoul X., Chasse J.F., Khallouki F., Savouret J.F., Poirot M., Barouki R. Identification of a new stilbene-derived inducer of paraoxonase 1 and ligand of the Aryl hydrocarbon Receptor. Biochem. Pharmacol. 2012;83:627–632. doi: 10.1016/j.bcp.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 121.Noll C., Hamelet J., Ducros V., Belin N., Paul J.L., Delabar J.M., Janel N. Resveratrol supplementation worsen the dysregulation of genes involved in hepatic lipid homeostasis observed in hyperhomocysteinemic mice. Food Chem. Toxicol. 2009;47:230–236. doi: 10.1016/j.fct.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Do G.M., Kwon E.Y., Kim H.J., Jeon S.M., Ha T.Y., Park T., Choi M.S. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 123.Sarandol E., Tas S., Dirican M., Serdar Z. Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: Effect of vitamin E supplementation. Cell Biochem. Funct. 2005;23:1–8. doi: 10.1002/cbf.1119. [DOI] [PubMed] [Google Scholar]
- 124.Motta S., Letellier C., Ropert M., Motta C., Thiebault J.J. Protecting effect of vitamin E supplementation on submaximal exercise-induced oxidative stress in sedentary dogs as assessed by erythrocyte membrane fluidity and paraoxonase-1 activity. Vet. J. 2009;181:288–295. doi: 10.1016/j.tvjl.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Tsakiris S., Karikas G.A., Parthimos T., Tsakiris T., Bakogiannis C., Schulpis K.H. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur. J. Clin. Nutr. 2009;63:215–221. doi: 10.1038/sj.ejcn.1602918. [DOI] [PubMed] [Google Scholar]
- 126.Ferretti G., Bacchetti T., Masciangelo S., Pallotta G. Lipid peroxidation in hemodialysis patients: Effect of vitamin C supplementation. Clin. Biochem. 2008;41:381–386. doi: 10.1016/j.clinbiochem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 127.Gatica L.V., Vega V.A., Zirulnik F., Oliveros L.B., Gimenez M.S. Alterations in the lipid metabolism of rat aorta: Effects of vitamin a deficiency. J. Vasc. Res. 2006;43:602–610. doi: 10.1159/000096247. [DOI] [PubMed] [Google Scholar]
- 128.Yamagata K., Tanaka N., Matsufuji H., Chino M. β-carotene reverses the IL-1 β-mediated reduction in paraoxonase-1 expression via induction of the CaMKKII pathway in human endothelial cells. Microvasc. Res. 2012;84:297–305. doi: 10.1016/j.mvr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Baralic I., Djordjevic B., Dikic N., Kotur-Stevuljevic J., Spasic S., Jelic-Ivanovic Z., Radivojevic N., Andjelkovic M., Pejic S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother. Res. 2013;27:1536–1542. doi: 10.1002/ptr.4898. [DOI] [PubMed] [Google Scholar]
- 130.Yegin S.C., Yur F., Ceylan E. Effect of lycopene application in rats with experimental diabetes using lipoprotein, paraoxonase and cytokines. J. Membr. Biol. 2013;246:621–626. doi: 10.1007/s00232-013-9575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mackinnon E.S., El-Sohemy A., Rao A.V., Rao L.G. Paraoxonase 1 polymorphisms 172T-->A and 584A-->G modify the association between serum concentrations of the antioxidant lycopene and bone turnover markers and oxidative stress parameters in women 25–70 years of age. J. Nutrigenet. Nutrigenomics. 2010;3:1–8. doi: 10.1159/000316636. [DOI] [PubMed] [Google Scholar]
- 132.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 133.Kamei M., Fujita T., Kanbe T., Sasaki K., Oshiba K., Otani S., Matsui-Yuasa I., Morisawa S. The distribution and content of ubiquinone in foods. Int. J. Vitam. Nutr. Res. 1986;56:57–63. [PubMed] [Google Scholar]
- 134.Mattila P., Kumpulainen J. Coenzymes Q9 and Q10: Contents in foods and dietary intake. J. Food Comp. Anal. 2001;14:409–417. doi: 10.1006/jfca.2000.0983. [DOI] [Google Scholar]
- 135.Weber C., Bysted A., sHolmer G. Coenzyme Q10 in the diet—daily intake and relative bioavailability. Mol. Asp. Med. 1997;18:S251–S254. doi: 10.1016/S0098-2997(97)00003-4. [DOI] [PubMed] [Google Scholar]
- 136.Bruge F., Bacchetti T., Principi F., Scarpa E.S., Littarru G.P., Tiano L. Olive oil supplemented with Coenzyme Q(10): Effect on plasma and lipoprotein oxidative status. Biofactors. 2012;38:249–256. doi: 10.1002/biof.1015. [DOI] [PubMed] [Google Scholar]
- 137.Rana S.K., Sanders T.A. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986;56:17–27. doi: 10.1079/BJN19860082. [DOI] [PubMed] [Google Scholar]
- 138.Dirican M., Tas S., Sarandol E. High-dose taurine supplementation increases serum paraoxonase and arylesterase activities in experimental hypothyroidism. Clin. Exp. Pharmacol. Physiol. 2007;34:833–837. doi: 10.1111/j.1440-1681.2007.04615.x. [DOI] [PubMed] [Google Scholar]
- 139.Kaur H.D., Bansal M.P. Studies on HDL associated enzymes under experimental hypercholesterolemia: Possible modulation on selenium supplementation. Lipids Health Dis. 2009;8:55. doi: 10.1186/1476-511X-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]