Abstract
Spleen tyrosine kinase (SYK) is an important component of the intracellular signalling pathway for various immunoreceptors, and SYK inhibition has shown promise in preclinical models of autoimmune and glomerular disease. The description of SYK expression in human renal tissue, which would be desirable ahead of clinical studies, is however lacking. Here, we have conducted immunohistochemical analysis for total and phosphorylated SYK in biopsy specimens from over 120 patients with a spectrum of renal pathologies, including thin basement membrane lesion, minimal change disease, membranous nephropathy, IgA nephropathy, lupus nephritis (LN), ANCA associated glomerulonephritis (AAGN), anti-glomerular basement membrane (anti-GBM) disease, and acute tubular necrosis (ATN). We found significant SYK expression in proliferative glomerulonephritis, and that glomerular expression levels correlated with presenting serum creatinine and histological features of disease activity that predict outcome in IgA nephropathy, LN, AAGN and anti-GBM disease. SYK is phosphorylated within pathological lesions, such as areas of extra- and endocapillary proliferation, and appeared to localise both to infiltrating leucocytes and resident renal cells within diseased glomeruli. These data strongly implicate SYK in the pathogenesis of proliferative glomerulonephritides, suggesting that these conditions may respond to SYK inhibitor treatment, and our results may inform the development of future clinical studies in this field.
Introduction
Spleen Tyrosine Kinase (SYK) is a non-receptor tyrosine kinase that is highly expressed in haematopoietic cells, where it has a critical function in classical immunoreceptor signalling. It has a well-characterised role in the intra-cellular signal transduction pathway for the B cell surface receptor (BCR)1 and for activatory Fc receptors (FcR) expressed on a variety of immune effector cells, including myeloid cells2,3 and mast cells4. In addition, a role for SYK in signalling in other cell types and for other receptors, such as integrins and C-type lectins, is increasingly recognised5. The SYK molecule, of molecular weight 72kDA, has a multi-domain structure6, characterised by two N-terminal SH2 domains, which allow it to interact with immunoreceptor-tyrosine based activation motifs (ITAM) on the intracellular domain (or on related adaptor proteins) of the receptors for which it mediates signalling, and a C-terminal kinase domain. Upon receptor engagement, SYK is phosphorylated on multiple tyrosine residues, leading to conformational changes that allow this C-terminal kinase domain to interact with a variety of downstream targets, including PLC-γ, PI3K and MAP kinases, resulting in activation of a variety of cellular responses including proliferation, differentiation, phagocytosis and cytokine production.
Given its important role in generating and effecting adaptive immune responses, SYK has emerged as a potential therapeutic target in autoimmune disease. Both genetic and pharmacological manipulation of SYK activity have shown efficacy in treating in vivo models of autoimmune disease7,8, and several small molecule inhibitors have progressed to clinical investigation9. We have previously reported that SYK inhibition with fostamatinib, a kinase inhibitor with high selectivity for SYK that has progressed to phase III study of non-renal diseases10,11, is effective in treating two distinct rodent models of immune-mediated glomerulonephritis (GN)12,13. We also reported that SYK is expressed in Immunoglobulin A (IgA) nephropathy14. However, a systematic analysis of SYK expression in other glomerular diseases, though desirable ahead of potential clinical studies, is lacking.
Here, we have analysed SYK expression by immunohistochemistry on renal biopsies from patients with a range of glomerulonephritides. We report that SYK is expressed and activated in proliferative GN, and that SYK expression levels appear to correlate with disease activity, suggesting that SYK inhibition may be a rational therapeutic target warranting further clinical investigation in glomerular disease.
Results
Method development and characterisation of SYK staining in normal renal tissue
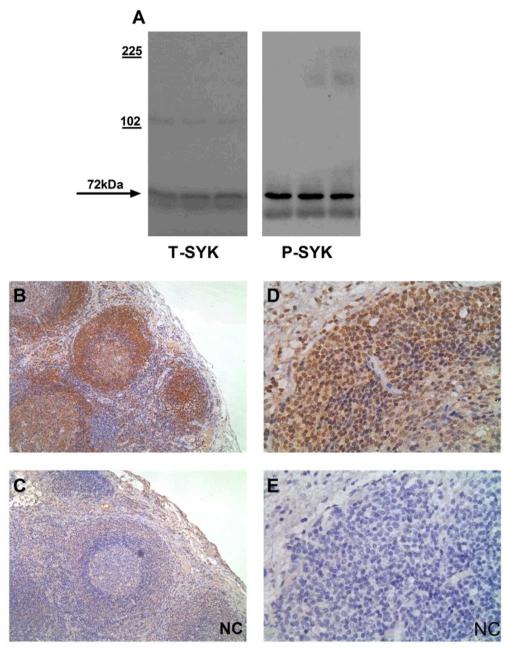
This was an immunohistochemistry (IHC)-based study for which we used commercially available primary antibodies. For total SYK (T-SYK) staining, we used an antibody directed against the N-terminus of SYK that should detect both major splice variants of the protein. For phophorylated SYK (phospho-SYK) staining, we used an antibody directed against phospho-tyrosine 525/526, located within the activation loop of the catalytic domain of SYK, in order to detect functionally activated protein likely to be relevant to disease pathogenesis. We previously reported the use of these antibodies for detection of SYK in human renal mesangial cells and in rat tissue by immunoblotting and IHC, respectively14. For this study, we further confirmed their reactivity and specificity for SYK by Western blot of lysates of human peripheral blood mononuclear cells (Figure 1A). We performed positive control IHC on human lymph node tissue and found positive staining for both T-SYK and phospho-SYK localised to lymphoid follicles, consistent with an important role for SYK in maintaining follicular B cell survival15 and in generating adaptive immune responses (Figure 1B-E).
Figure 1. Method development and control immunohistochemical (IHC) stains.
(A) Western blot for total (T-) and phophorylated (P-) SYK in lysates of human peripheral blood mononuclear cells, confirming detection of SYK protein at 72kDA. (B & C) Sequential sections showing positive and negative control (NC) staining for T-SYK in human lymph node, with strong staining for SYK localised to lymphoid follicles. (D & E) Sequential sections showing positive and negative control (NC) staining for P-SYK in human lymph node, demonstrating nuclear and cytoplasmic localisation, within cells in lymphoid follicles. All sections are immunoperoxidase stains with haematoxylin counterstain, x200-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide.
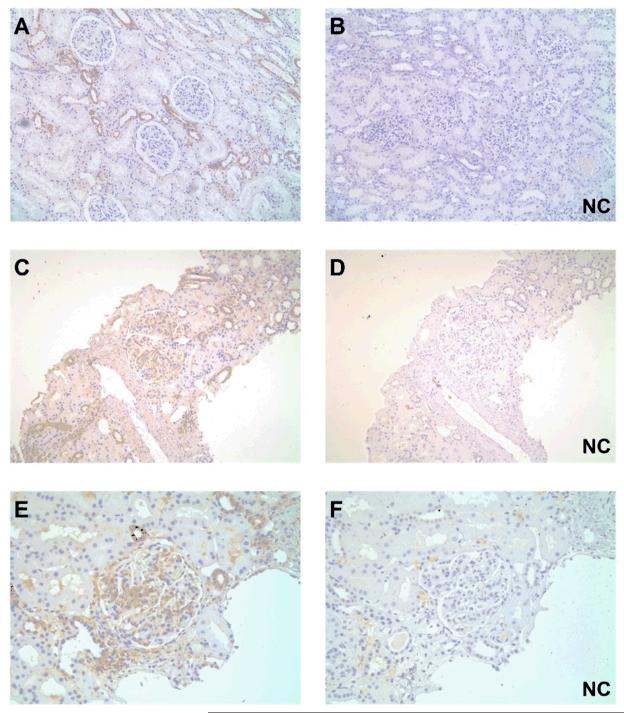
In order to define the pattern of T-SYK expression in normal kidney tissue, we examined tissue from nephrectomy specimens (n=4) and surveillance transplant biopsies with normal light micrographic findings (n=4). In all cases, we found positive staining for T-SYK within distal tubular epithelial cells (identified by their morphological features of cuboidal epithelium with little brush border and open tubular lumens). Minimal staining was observed within glomeruli (Figure 2A-B). For initial characterisation of T-SYK staining in nephritic tissue, we examined renal biopsies from patients with post-infectious diffuse proliferative glomerulonephritis. We observed positive staining of distal tubular epithelial cells, comparable to that seen in normal renal tissue. In addition, there was positive staining within nephritic glomeruli that appeared to localise to proliferating cells within the tuft (Figure 2C-F).
Figure 2. Total (T-) SYK detection in normal and nephritic renal tissue.
(A & B) Paired sections showing positive and negative control (NC) staining for T-SYK in normal renal tissue obtained from nephrectomy specimens, showing intermittent SYK expression in distal tubular epithelial cells (having thin cuboidal epithelium, little brush border and open tubular lumens). (C-F) Two pairs of sequential sections at low and high power, respectively, showing positive and negative control (NC) staining for T-SYK in diffuse proliferative post-infectious glomerulonephritis; SYK is detected within the nephritic glomerular tuft, as well as in some distal tubular epithelial cells, similar to that is seen in normal tissue. All sections are immunoperoxidase stains with haematoxylin counterstain, x100-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide.
Glomerular SYK expression levels are highest in proliferative glomerulonephritis
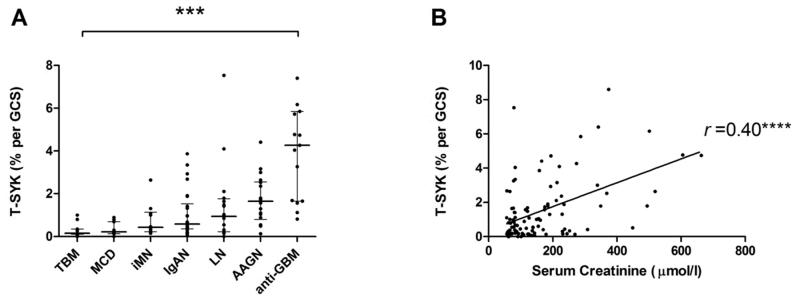
We then proceeded to perform IHC for T-SYK on renal biopsies demonstrating a range of primary glomerular lesions. In thin basement membrane lesion (TBM, n=11), minimal change disease (MCD, n=11) and idiopathic membranous nephropathy (iMN, n=10), there was negligible glomerular staining for T-SYK, with positive tubular staining as noted in normal tissue (Figure 3A; Figure 4). Glomerular T-SYK expression levels were highest in anti-glomerular basement membrane (anti-GBM) disease (n=15), and significant expression was also seen in ANCA-associated glomerulonephritis (AAGN; n=18), lupus nephritis (n=16) and IgA nephropathy (IgAN; n=26) (Figure 3A). In the entire cohort (n=107), there was a robust association between creatinine at the time of renal biopsy, and glomerular T-SYK staining (r =0.4; p<0.0001; Figure 3B). There was no association with the degree of proteinuria at the time of biopsy (r =0.03; p=0.77).
Figure 3. Quantification of Total (T-) SYK detection in a spectrum of glomerular diseases.
(A) Glomerular T-SYK expression in each form of glomerulonephritis, expressed as mean percent stain per glomerular cross section (% per GCS) in each biopsy case; negligible staining was observed in non-proliferative diseases and expression levels were significantly elevated in proliferative and crescentic forms of glomerulonephritis; TBM, thin basement membrane lesion; MCD, minimal change disease; iMN, idiopathic membranous nephropathy; IgAN, immunoglobulin A nephropathy; LN, lupus nephritis; AAGN, anti-neutrophil cytoplasm antibody-associated glomerulonephritis; anti-GBM, anti-glomerular basement membrane disease. (B) Glomerular T-SYK expression correlates with serum creatinine at the time of biopsy (n=107 cases, Spearman r =0.4, p<0.0001). *** p<0.001, ***p<0.0001.
Figure 4. Total (T-) SYK expression non-proliferative glomerulonephritides.
(A & B) Sequential sections showing positive and negative control (NC) staining for T-SYK in thin basement membrane lesion. (C) T-SYK staining in minimal change disease. (D) T-SYK staining in idiopathic membranous nephropathy. In all cases, negligible glomerular staining is seen. There is intermittent staining of distal tubular epithelial cells, comparable to that seen in normal kidney tissue. All sections are immunoperoxidase stains with haematoxylin counterstain, x100-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide.
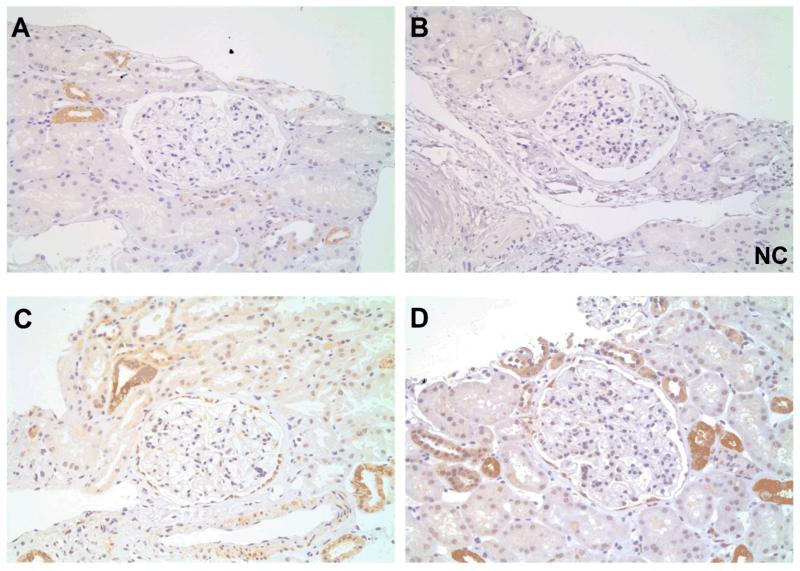
In the proliferative glomerulonephritides (anti-GBM, AAGN, lupus nephritis, IgAN), T-SYK expression localised to areas of segmental inflammation and endocapillary and extracapillary proliferation. In anti-GBM disease (Figure 5A-B) and AAGN (Figure 5D), for example, staining was observed in areas of crescent formation. No staining was observed in sclerotic lesions (Figure 5E). In IgA nephropathy (Figure 6A-B), and lupus nephritis (Figure 6D-E) staining was observed within the glomerular tuft, that morphologically localised to endocapillary cells in proliferative classes of disease. In all cases, sporadic staining within tubular epithelial cells was a common finding, which was not present in negative control sections, suggesting that SYK is expressed in these cells, although its relevance to glomerular disease is unclear.
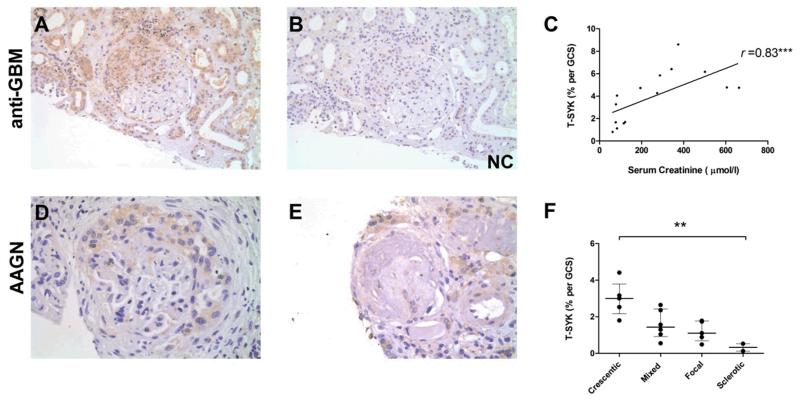
Figure 5. Total (T-) SYK expression in anti-glomerular basement membrane (anti-GBM) disease and ANCA-associated glomerulonephritis (AAGN).
(A & B) Sequential sections showing positive and negative control (NC) staining for T-SYK in a crescentic glomerulus in anti-GBM disease. (C) Glomerular T-SYK expression correlates strongly with presenting serum creatinine in anti-GBM disease (n=15 cases; Spearman r = 0.78, p=0.0006). (D) T-SYK staining in AAGN localises to areas of crescent formation. (E) Minimal glomerular staining for T-SYK in a scarred glomerulus in sclerotic-class AAGN. (F) Correlation of glomerular T-SYK staining with histologic class of AAGN, with highest levels seen in crescentic class disease. All sections are immunoperoxidase stains with haematoxylin counterstain, x200-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide. GCS, glomerular cross section. ** p<0.01, *** p<0.001.
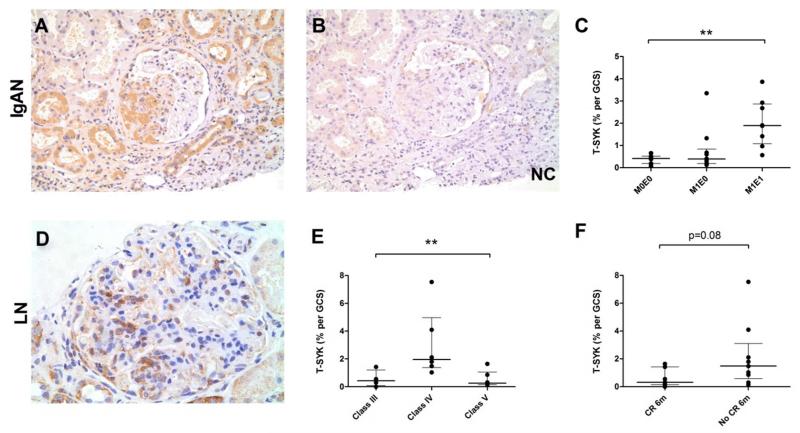
Figure 6. Total (T-) SYK expression in Immunoglobulin A nephropathy (IgAN) and lupus nephritis (LN).
(A & B) Sequential sections showing positive and negative control (NC) staining for T-SYK in IgAN, localised to a segmental area of endocapillary proliferation. (C) T-SYK detection in IgAN according to Oxford Class of disease; significant T-SYK detection was a feature of IgAN with endocapillary, and not mesangial, proliferation; M0E0, neither mesangial nor endocapillary proliferation; M1E0, mesangial proliferation without endocapillary proliferation; M1E1, both mesangial and endocapillary proliferation. (D) T-SYK detection in class IV lupus nephritis, localised to cells within the glomerular tuft. (E) T-SYK detection in LN according to ISN/RPS class of disease; SYK expression was a feature of class IV (diffuse proliferative) disease only. (F) T-SYK detection was higher in patients who failed to achieve complete remission (CR) at six months (6m) from initiating immunosuppressive treatment after this index renal biopsy. All sections are immunoperoxidase stains with haematoxylin counterstain, x200-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide. GCS, glomerular cross section. ** p<0.01
SYK expression correlates with disease activity and parameters predicting clinical outcome
In anti-GBM disease, significantly higher levels of glomerular T-SYK expression were seen in patients who presented with dialysis-dependent renal disease (median 5.46% T-SYK per glomerular cross section [GCS] in dialysis-dependent cases versus 3.28% per GCS in dialysis-independent cases; p=0.031) In addition, glomerular T-SYK expression correlated strongly with serum creatinine at presentation (r =0.82; p=0.0001; Figure 5C).
In AAGN, glomerular T-SYK expression also correlated with serum creatinine at time of biopsy (r =0.57; p=0.01). We also found that glomerular T-SYK expression correlated with the histological class of disease as recently described by Berden and colleagues16 (Figure 5F). Total SYK staining was highest in patients with crescentic class disease and minimal in patients with sclerotic class disease, suggesting that SYK expression is a feature of acute lesions that may respond to treatment.
In IgAN, highest levels of T-SYK staining were observed in biopsies with endocapillary proliferation, whereas biopsies with no proliferative features or mesangial proliferation only had negligible staining within the glomerular tuft (Figure 6C), again suggesting that SYK expression is a feature of acute inflammatory disease.
In lupus nephritis, highest SYK expression levels were seen in ISN/RPS class IV nephritis, in keeping with the observation of SYK positive cells within capillary loops in proliferative disease (Figure 6E). Minimal staining was seen in class V disease, akin to that seen in idiopathic membranous nephropathy. Notably, we found that T-SYK expression levels tended to be higher in those patients who failed to achieve complete remission by six months (median 1.48% T-SYK per GCS) versus those who did remit (median 0.31% T-SYK per GCS) following initiation of immunosuppressive treatment following the index biopsy (Figure 6F), although this difference did not achieve statistical significance (p=0.08). In lupus nephritis and IgA nephropathy, no association with serum creatinine or proteinuria was observed.
SYK is activated in pathological lesions in proliferative glomerulonephritis
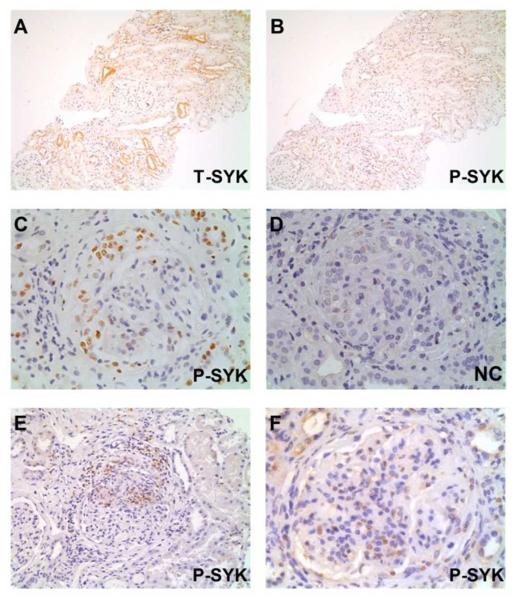
In normal renal tissue and non-proliferative GN, we did not observe significant staining for phospho-SYK in glomeruli or tubules (Figure 7A-B), suggesting that SYK is expressed but not activated in the latter in normal conditions. In those lupus nephritis, AAGN and anti-GBM biopsies that showed highest levels of T-SYK expression, we found that a pattern of phospho-SYK detection similar to that seen for T-SYK, with localisation to areas of extracapillary crescent formation and endocapillary proliferation within the glomerulus (Figure 7C-F). Notably, staining for phospho-SYK showed a predominantly nuclear pattern of expression. Nuclear staining for phospho-SYK was observed in lymphoid tissue (Figure 1D-E), and previous studies have shown that in lymphoid cells, SYK resides in both nuclear and cytoplasmic locations, although little is known about how trafficking between these compartments is regulated14.
Figure 7. Phophorylated (P-) SYK detection in proliferative glomerulonephritides.
(A & B) Sequential sections showing total (T-) SYK and P-SYK detection in minimal change disease; glomerular staining was not observed in either case; T-SYK staining was observed in some distal tubular epithelial cells, although minimal staining for P-SYK was observed within these cells, suggesting that SYK in not significantly activated in this cell type. (C & D) Sequential sections showing positive and negative control (NC) staining for P-SYK in anti-glomerular basement membrane disease; P-SYK is detected within cells of an extracapillary crescent, and within occasional cells in the glomerular tuft. (E) P-SYK detection in ANCA-associated glomerulonephritis, localised to an area of crescent formation and peri-glomerular inflammation; minimal tubular staining for P-SYK is seen (F) P-SYK detection in class IV lupus nephritis, showing P-SYK cells within the glomerular tuft. All sections are immunoperoxidase stains with haematoxylin counterstain, x200-400 magnification. Negative control (NC) stains were performed by pre-incubating the primary antibody with the relevant immunising peptide.
SYK localises to infiltrating leucocytes in pathological lesions
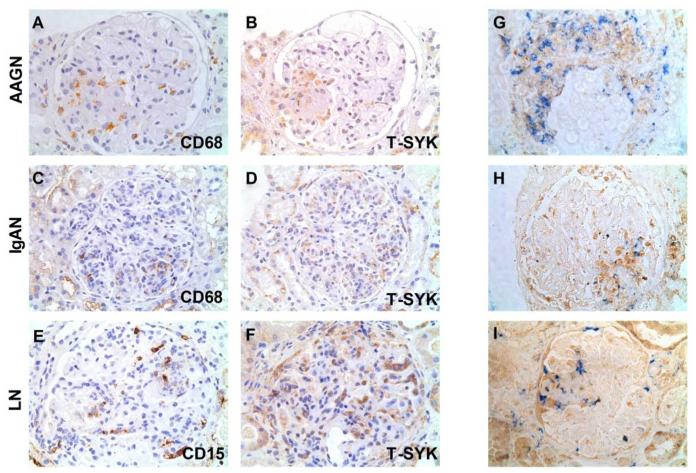
We performed staining for T-SYK and infiltrating leucocytes on paired sections of IgA, lupus and AAGN biopsies (representative images shown in Figure 8A-F). This staining demonstrated that SYK appeared to localise to infiltrating CD68+ macrophages and CD15+ neutrophils, consistent with the finding of highest SYK expression levels in biopsies showing proliferative pathology. We confirmed this observation by performing two-colour double staining on AAGN biopsies, which showed that T-SYK detection co-localised to areas of CD68+ cell infiltration (Figure 8G-I), in keeping with our current understanding of myeloid cells, and macrophages in particular, as important mediators of renal injury in a variety of renal diseases17. We observed, however, significant areas of T-SYK positivity that were negative for CD68, indirectly implying that other cell types (and in particular resident renal cells) express SYK in inflammatory lesions that may also contribute to disease pathogenesis.
Figure 8. Cellular localisation of SYK in proliferative glomerulonephritides.
(A & B) Paired sections showing staining for CD68 and total (T-) SYK, suggesting co-localisation of T-SYK and CD68+ macrophages within a segmental area of inflammation in the glomerulus (at 7 o’clock position) in a case of ANCA-associated glomerulonephritis (AAGN). (C & D) Paired sections suggesting co-localisation of T-SYK and CD68+ macrophages in the glomerulus (at 5 o’clock positition) in a case of IgA nephropathy. (E & F) Paired sections suggesting localisation of T-SYK and CD15+ neutrophils in a case of class IV lupus nephritis (LN). A-F are immunoperoxidase stains with haematoxylin counterstain, x400 magnification. (G,H & I) Two-colour double staining for CD68 (blue) and T-SYK (brown) in three cases of AAGN, showing co-localisation of T-SYK expression with CD68+ macrophage detection, within crescents (G) or segmental areas of glomerular inflammation (H & I). In addition, T-SYK+/CD68-negative cells are observed suggesting that there are additional cell types that may express T-SYK within the inflamed glomerulus. G, H & I are immunoperoxidase stain (brown; T-SYK) and immunophosphatase stain (blue; CD68), without counterstain, to enable identification of blue reporter stain.
Tubulointerstitial SYK expression is upregulated in inflammatory disease
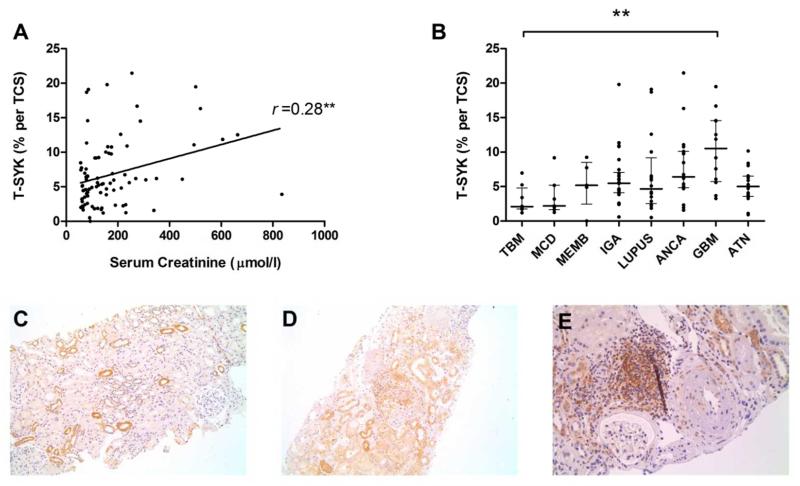
In all cases, we observed intermittent staining for T-STK in tubular epithelial cells. There was a statistically significant association between the degree of tubulointerstitial staining and serum creatinine at the time of biopsy (r =0.28; p=0.009; Figure 9A). There was no correlation with proteinuria (r =0.05, p=0.71). To examine this further, we quantified tubulointerstitial SYK expression in an additional 16 case of acute tubular necrosis (ATN; without associated glomerular pathology) and found a non-significant trend towards increased SYK expression in ATN compared to normal tissue (median 5.0% per tubular cross section [TCS] versus 2.7% per TCS; p=0.1225). Strikingly, tubular SYK expression was significantly more elevated in biopsies with severe concurrent glomerular inflammation (Figure 9B). Morphologically, increased in SYK expression was observed both within tubular epithelial cells and within inflammatory cells infiltrating the tubulointerstitium (Figure 9C-E). Collectively, these data suggest that SYK expression may be upregulated at times of injury tubulointerstitial injury, particularly in the pro-inflammatory milieu of concomitant glomerular disease.
Figure 9. Tubulointerstitial staining for total (T-) SYK in glomerulonephritis and in acute tubular necrosis (ATN).
(A) Tubulointerstitial T-SYK detection, expressed as the mean percent stain per tubular cross section (TCS) in each biopsy case, showing a statistically significant correlation with serum creatinine at the time of biopsy (Spearman r =0.28; p=0.009). (B) Tubulointerstitial SYK detection according to diagnosis in a range of glomerulonephritides and in acute tubular necrosis (ATN), demonstrating that tubular SYK expression may be upregulated at times of tubular injury. TBM, thin basement membrane lesion; MCD, minimal change disease; iMN, idiopathic membranous nephropathy; IgAN, immunoglobulin A nephropathy; LN, lupus nephritis; AAGN, anti-neutrophil cytoplasm antibody-associated glomerulonephritis; anti-GBM, anti-glomerular basement membrane disease. ** p<0.01. (C) Tubulointerstitial SYK expression in a case TBM, shown here for comparison. (D) Generalised increased in tubular SYK staining adjacent to a crescentic glomerulus in a severe case of AAGN. (E) Tubulointerstial infiltrate containing SYK positive cells, in a case AAGN with concomitant tubulointerstitial nephritis. (A-C) Immunperoxidase stain with haematoxylin counterstain, x100-200 magnification.
Discussion
This is the first systematic analysis of SYK expression in a spectrum of renal diseases, using biopsy specimens from over 120 patients. We have found that glomerular SYK expression is a feature of proliferative glomerulonephritides, that expression levels correlate with disease activity, and that SYK appears to be activated (i.e. phosphorylated) within pathological lesions in diseased glomeruli, implicating SYK in disease pathogenesis and suggesting it may be a therapeutic target.
Across the entire cohort, SYK expression levels correlated with serum creatinine at the time of biopsy and with the accepted severity of different glomerular pathologies, suggesting that SYK detection is a marker of acute, severe glomerular inflammation. Glomerular SYK expression levels were highest in anti-GBM disease, widely recognised as the most aggressive form of glomerulonephritis18. Dialysis-dependent renal failure at disease presentation is known to augur badly for future renal outcome19, and it is notable that SYK expression levels were highest in this group of patients. In those patients who were not dialysis dependent at presentation, glomerular SYK expression levels also correlated with serum creatinine, consistent with previous reports that the proportion of crescents seen on renal biopsy also correlates strongly with presenting serum creatinine19,20. Taken together, these observations suggest that SYK expression is a marker of disease severity and poor outcome in anti-GBM disease.
In other proliferative glomerulonephritides, we found that SYK expression levels correlated with histological disease activity, as defined by recognised classification systems. In AAGN, glomerular SYK expression correlated with the disease class as defined using the recently proposed histopathological classification of Berden et al16. We found that SYK expression levels were highest in crescentic class disease, and lowest in sclerotic class, suggesting that SYK expression is a feature of acute inflammatory disease that may respond to treatment. In lupus nephritis and IgA nephropathy, we observed a similar correlation of SYK expression with histological class of disease. In all three diagnoses, the respective classification systems not only describe the pathological nature of disease, they also predict prognosis. SYK expression may likewise, therefore, be a predictor of clinical outcome in proliferative glomerulonephritis. Indeed, all cases of lupus nephritis included in this study were the index biopsies from patients enrolled in the previously published open-label study of patients treated with rituximab and mycophenolate mofetil, without oral steroids21. In this cohort, no difference in response was observed between class III, IV or V disease at six months. However, we have observed that SYK expression levels were higher in patients who did not achieve complete remission at 6 months, suggesting that SYK expression in the index biopsy may indicate more resistant or aggressive disease. The number of cases used in the study, however, was not sufficient to allow meaningful multivariate analysis to correct for confounding factors.
Our study has other limitations, such as retrospective selection of cases to include a spectrum of histological activity, and thus it remains to be seen if SYK staining in unselected cases will be informative. Whilst our data strongly suggests that SYK is expressed within infiltrating leucocytes, SYK was also observed in cells negative for leucocyte markers, most likely resident renal cells, such as mesangial cells, parietal epithelial cells and podocytes. Future work should aim to establish the identity of these cells and their contribution to glomerular inflammation. We also observed significant SYK staining outwith glomeruli in tubular epithelial and interstitial cells, the significance of which is not clear. Tubulointerstitial SYK expression tended to increase with tubular injury, particularly with concomitant glomerulonephritis, suggesting that SYK upregulation in tubular cells may occur as a response to ischaemic or inflammatory injury. Notably, SYK inhibition has shown efficacy in non-renal models is ischaemia-reperfusion injury22, and the relevance of SYK in the pathogenesis of acute tubular injury (and potentially delayed renal allograft function) warrants further investigation. Finally, staining for phospho-SYK, which may have more relevance to disease pathogenesis, could not be quantified, due to variable detection within tissue sections. We believe this may be due to rapid dephosphorylation of SYK prior to adequate tissue fixation, a recognised difficulty in immunostaining for phosphorylated proteins23, resulting in loss of phospho-SYK signal in a proportion of biopsies. Since this study was performed on surplus sections from routine clinical biopsies that were not specifically processed for research purposes, this problem count not be resolved and hence it was not possible to reliably quantify the degree of P-SYK detection in our cohort. We believe, however, that our observations provide evidence that SYK is, in principle, often activated within proliferative glomerular lesions.
Several other studies have suggested a role for SYK in the pathogenesis of glomerulonephritis. We and others have previously reported that SYK inhibition using selective kinase inhibitors is remarkably effective in both preventing and treating disease in neutrophil- and macrophage-dependent rodent models of anti-GBM disease12,24,25. Similarly, SYK inhibitor treatment using fostamatinib has shown efficacy in three distinct murine models of systemic lupus erythematosus26,27. In particular, treatment of the lupus-prone NZB/NZW and MRL/lpr mouse strains reduced kidney disease as determined by both proteinuria and renal histology. Higher levels of SYK expression and activity have been reported in T cells from SLE patients versus normal controls28, and it was recently reported that silencing of SYK expression in SLE T cells, or forced over-expression of SYK in normal T cells, normalised their gene expression signature, or recapitulated an SLE expression signature, respectively29. SYK has also been shown to have an important role in ANCA-mediated activation of primed neutrophils in vitro30, suggesting it may contribute to the development of AAGN. Finally, SYK is also implicated in the pathogenesis of IgA nephropathy, the most common primary glomerulonephritis for which there is still no universally accepted treatment. We previously reported that IgA1 isolated from patients with IgA nephropathy induced proliferation and pro-inflammatory cytokine production by human renal mesangial cells in vitro, and that this could be inhibited by pharmacological inhibition using R406, the active metabolite of fostamatinib, or genetic ‘knock-down’ using siRNA techniques14.
Collectively, these findings suggest that SYK inhibition may be an appropriate therapeutic target in a range of glomerulonephritides. Indeed, the findings reported here, along with our previous in vivo and in vitro studies, were critical in informing the design of a Phase II clinical study examining the safety and efficacy of treating IgA nephropathy with fostamatinib, (NCT02112838), which opened for enrolment in November 2014, and in which recruitment will be stratified for the finding of endocapillary proliferation, following its observed association with glomerular SYK expression. SYK may also represent a potential target in other glomerular diseases, including AAGN, lupus nephritis and anti-GBM disease, and the results of this study may likewise inform future trial design in these other conditions.
Methods
Study approval
This study was conducted under local ethics committee approval: 04/Q0406/25 Hammersmith and Queen Charlotte’s & Chelsea Hospitals Research Ethics Committee. Renal biopsy specimens were obtained from patients after informed consent, and only spare material surplus to clinical need was used. Lymph node and nephrectomy tissue specimens were provided the Imperial College NHS Trust Tissue Bank.
Identification of cases
Cases of thin basement membrane lesion (n=11), minimal change disease (n=11), idiopathic membranous nephropathy (n=), IgA nephropathy (n=26), anti-glomerular basement membrane (anti-GBM) disease (n=15), and acute tubular necrosis (N=16) were identified using a central renal pathology database at Imperial College Renal and Transplant Centre. Cases of IgA nephropathy were selected to include a mix of histological classes of disease. All anti-GBM cases with available biopsy material were included in the study. Cases of lupus nephritis (n=16) and anti-neutrophil cytoplasm antibody (ANCA) associated glomerulonephritis (AAGN; n=18) were identified using well described clinical cohorts from our centre21,31. Biopsies were selected to include a mix of histological disease classes. All biopsies were reported by an experienced renal histopathologist, and where appropriate these were prospectively classified according to recognised classification systems: the Oxford Classification for IgAN32,33; the International Society of Nephrology/Renal Pathology Society (ISN/RPS) Classification for lupus nephritis34; and the recently proposed histopathological classification for AAGN16.
Immunohistochemistry
All immunohistochemistry was performed on formalin-fixed paraffin-embedded tissues. Sections were subject to heat-induced antigen retrieval in 0.1M citrate buffer (pH 6) and then sequentially blocked in 0.3% hydrogen peroxide and 20% goat serum (Dako Ltd, Cambridgeshire UK). Sections were then incubated with the following primary antibodies either for one hour at room temperature or overnight at 4°C: total SYK N-19 (Santa Cruz Biotechnology, Dallas TX; dilution 1:500); phosphorylated SYK Tyr 525/526 (Cell Signaling Technology, Danvers MA; dilution 1:20); CD68 (Serotec, Oxford UK; dilution 1:500). Negative controls for total and phosphorylated SYK were performed using primary antibodies that were pre-incubated with the relevant immunising peptide (provided by the respective manufacturer) overnight at 4°C. After washing, sections were incubated in a secondary polymer-HRP system for either mouse or rabbit primary antibodies as appropriate (EnVision+; Dako) and developed in 3,3-diaminobenzidine (DAB), according to the manufacturer’s instructions. The sections were then rinsed, counterstained with filtered Harris’ haematoxylin (CellPath, Powys UK), dehydrated and mounted with DPX mounting medium (Fisher Scientific, Leicester UK).
For double staining for total SYK and CD68, sections were first stained for SYK as described above. Following development in DAB, the sections were sequentially rinsed, re-subjected to antigen retrieval, blocked with 20% rabbit serum, then incubated with the primary antibody for CD68 at room temperature for one hour, then incubated with a biotinylated rabbit anti-mouse immunoglobulin secondary antibody (Sigma-Aldrich, Poole, UK; dilution 1:100) for one hour, then ALP-conjugated streptavidin (Roche Diagnostics; dilution 1:100) for 30 minutes, before final development using the Vector Blue Substrate Kit III (Vector Labs, Peterborough, UK) according to the manufacturer’s specifications. Slides were mounted in AquaPerm (ThermoFisher Scientific) without counterstaining.
Western Blot
Peripheral blood mononuclear cells (PBMC) were obtained by ficoll density gradient separation of whole blood samples obtained from healthy volunteers. The cells were lysed in ice-cold lysis buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, pH 7.8, 1% digitonin), containing protease inhibitor mixture (Sigma) with 1 mM DTT, 10 mM NaF, 100 mM sodium orthovanadate). The nuclei and cell debris were removed by centrifugation. Samples were resolved on 10% SDS-PAGE gels (Criterion). After electrotransfer on to nitrocellulose membrane, the membranes were blocked in 2% BSA/TBS/Tween (0.05%) and probed with the appropriate antibodies at 4 °C overnight followed by HRP-conjugated secondary antibody. Blots were developed using ECL (Amersham Biosciences).
Quantification and Statistical Analysis
Quantification of total SYK staining was conducted by an observer blinded to the histological class of disease, using automated image analysis software (ImagePro Plus, Media Cybernetics, Bethesda MD), using an algorithm that measured all immunoperoxidase stain above a threshold detectable from background haematoxylin stain. For glomerular scoring, the proportion of T-SYK staining in each glomerulus was measured, and a mean result per glomerular cross section (GCS) calculated for each biopsy case. For tubulointerstitial scoring, the mean proportion of T-SYK staining in five high power fields was calculated for each biopsy, and expressed as the mean result per tubular cross section (TCS). Statistical analysis was conducted using GraphPad Prism 5.0 (GraphPad Software, San Diego CA). All results are expressed as median with interquartile range per group. Comparison between groups was by Mann-Whitney U test or Kruskal-Wallis test with Dunn’s multiple comparison. Correlation analysis was by Spearman’s Rank Order correlation. In the reporting of results, * signifies p value <0.05, **p<0.01, ***p<0.0001, ****P<0.00001.
Acknowledgements
We would like to thank Dr Anisha Tanna, Dr Lina Nikolopoulou and Dr Liz Lightstone for providing clinical data on the AAGN, membranous nephropathy and lupus nephritis cohorts, respectively.
This work was presented in abstract form at the American Society of Nephrology Renal Week Meeting in Atlanta, GA in November 2013, and at the UK Renal Association meeting in Glasgow, Scotland, in May 2014.
SPM is in receipt of Clinical Research Training Fellowship funded by the UK Medical Research Council (Grant Number: G0901997/1). FWKT is supported by the Diamond Fund from Imperial College Healthcare Charity. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Support: This work was funded by the UK Medical Research Council (Grant Number G0901997)
Footnotes
Disclosure Statement
FWKT has received research project grants from AstraZeneca Limited, and has a consultancy agreement with Rigel Pharmaceuticals. CDP has received a research project grant from GlaxoSmithKline and has a consultancy agreement with Genzyme. The remaining authors have no financial conflict of interest to declare.
References
- 1.Geahlen RL. Syk and pTyr’d: Signaling through the B cell antigen receptor. Biochimica et biophysica acta. 2009 Jul;1793(7):1115–1127. doi: 10.1016/j.bbamcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley MT, Costello PS, Fitzer-Attas CJ, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. The Journal of experimental medicine. 1997 Oct 6;186(7):1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiefer F, Brumell J, Al-Alawi N, et al. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Molecular and cellular biology. 1998 Jul;18(7):4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Castro RO. Regulation and function of syk tyrosine kinase in mast cell signaling and beyond. Journal of signal transduction. 2011;2011:507291. doi: 10.1155/2011/507291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nature reviews. Immunology. 2010 Jun;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. Journal of biochemistry. 2001 Aug;130(2):177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki N, Suzuki S, Ishida M, et al. Syk-dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti-collagen antibody-induced arthritis. International immunology. 2012 Sep;24(9):539–550. doi: 10.1093/intimm/dxs078. [DOI] [PubMed] [Google Scholar]
- 8.Pine PR, Chang B, Schoettler N, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clinical immunology. 2007 Sep;124(3):244–257. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 9.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis and rheumatism. 2008 Nov;58(11):3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 10.Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. The New England journal of medicine. 2010 Sep 30;363(14):1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis and rheumatism. 2011 Feb;63(2):337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 12.Smith J, McDaid JP, Bhangal G, et al. A spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritis. Journal of the American Society of Nephrology: JASN. 2010 Feb;21(2):231–236. doi: 10.1681/ASN.2009030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAdoo SP, Reynolds J, Bhangal G, et al. Spleen Tyrosine Kinase Inhibition Attenuates Autoantibody Production and Reverses Experimental Autoimmune GN. Journal of the American Society of Nephrology: JASN. 2014 Oct;25(10):2291–2302. doi: 10.1681/ASN.2013090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, McDaid JP, McAdoo SP, et al. Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. Journal of immunology. 2012 Oct 1;189(7):3751–3758. doi: 10.4049/jimmunol.1102603. [DOI] [PubMed] [Google Scholar]
- 15.Schweighoffer E, Vanes L, Nys J, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013 Mar 21;38(3):475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. Journal of the American Society of Nephrology: JASN. 2010 Oct;21(10):1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 17.Duffield JS. Macrophages and immunologic inflammation of the kidney. Seminars in nephrology. 2010 May;30(3):234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney international. 2003 Mar;63(3):1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 19.Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Annals of internal medicine. 2001 Jun 5;134(11):1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- 20.Fischer EG, Lager DJ. Anti-glomerular basement membrane glomerulonephritis: a morphologic study of 80 cases. American journal of clinical pathology. 2006 Mar;125(3):445–450. doi: 10.1309/nptp-4ukv-7ju3-elmq. [DOI] [PubMed] [Google Scholar]
- 21.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Annals of the rheumatic diseases. 2013 Aug;72(8):1280–1286. doi: 10.1136/annrheumdis-2012-202844. [DOI] [PubMed] [Google Scholar]
- 22.Pamuk ON, Lapchak PH, Rani P, Pine P, Dalle Lucca JJ, Tsokos GC. Spleen tyrosine kinase inhibition prevents tissue damage after ischemia-reperfusion. American journal of physiology. Gastrointestinal and liver physiology. 2010 Aug;299(2):G391–399. doi: 10.1152/ajpgi.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer TR, Fulford AD, Arkins AM, et al. Ischemic time impacts biological integrity of phospho-proteins in PI3K/Akt, Erk/MAPK, and p38 MAPK signaling networks. Anticancer research. 2011 Jun;31(6):2073–2081. [PubMed] [Google Scholar]
- 24.McAdoo SP, Reynolds J, Bhangal G, et al. Spleen Tyrosine Kinase Inhibition Attenuates Autoantibody Production and Reverses Experimental Autoimmune GN. Journal of the American Society of Nephrology: JASN. 2014 Apr 3; doi: 10.1681/ASN.2013090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan J, Ma FY, Kanellis J, Delgado M, Blease K, Nikolic-Paterson DJ. Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis. Laboratory investigation; a journal of technical methods and pathology. 2011 Dec;91(12):1727–1738. doi: 10.1038/labinvest.2011.137. [DOI] [PubMed] [Google Scholar]
- 26.Bahjat FR, Pine PR, Reitsma A, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis and rheumatism. 2008 May;58(5):1433–1444. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]
- 27.Deng GM, Liu L, Bahjat FR, Pine PR, Tsokos GC. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis and rheumatism. 2010 Jul;62(7):2086–2092. doi: 10.1002/art.27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan S, Juang YT, Chowdhury B, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. Journal of immunology. 2008 Dec 1;181(11):8145–8152. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grammatikos AP, Ghosh D, Devlin A, Kyttaris VC, Tsokos GC. Spleen tyrosine kinase (Syk) regulates systemic lupus erythematosus (SLE) T cell signaling. PloS one. 2013;8(8):e74550. doi: 10.1371/journal.pone.0074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewins P, Williams JM, Wakelam MJ, Savage CO. Activation of Syk in neutrophils by antineutrophil cytoplasm antibodies occurs via Fcgamma receptors and CD18. Journal of the American Society of Nephrology: JASN. 2004 Mar;15(3):796–808. doi: 10.1097/01.asn.0000113241.98702.77. [DOI] [PubMed] [Google Scholar]
- 31.Tanna A, Guarino L, Tam FW, et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 Jul 12; doi: 10.1093/ndt/gfu237. [DOI] [PubMed] [Google Scholar]
- 32.Working Group of the International Ig ANN, the Renal Pathology S. Cattran DC, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney international. 2009 Sep;76(5):534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 33.Working Group of the International Ig ANN, the Renal Pathology S. Roberts IS, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney international. 2009 Sep;76(5):546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 34.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology: JASN. 2004 Feb;15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]