Abstract
Reactive oxygen species (ROS)-induced oxidative stress in cells is an important pathophysiological process during myocardial ischemia/reperfusion (I/R) injury, and the transcription factor Egr-1 is a master switch for various damage pathways during reperfusion injury. An in vitro model of myocardial I/R injury and H9c2 cardiomyoblast cells hypoxia/reoxygenation (H/R) was used to assess whether there is abnormal intracellular ROS/JNK/Egr-1 signaling. We also assessed whether N-n-butyl haloperidol (F2), which exerts protective effects during myocardial I/R injury, can modulate this pathway. H/R induced ROS generation, JNK activation, and increased the expression of Egr-1 protein in H9c2 cells. The ROS scavengers edaravone (EDA) and N-acetyl-L-cysteine (NAC) reduced ROS level, downregulated JNK activation, and Egr-1 expression in H9c2 cells after H/R. The JNK inhibitor SP600125 inhibited Egr-1 overexpression in H9c2 cells caused by H/R. F2 could downregulate H/R-induced ROS level, JNK activation, and Egr-1 expression in H9c2 cells in a dose-dependent manner. The ROS donor hypoxanthine-xanthine oxidase (XO/HX) and the JNK activator ANISO antagonized the effects of F2. Therefore, H/R activates ROS/Egr-1 signaling pathway in H9c2 cells, and JNK activation plays an important role in this pathway. F2 regulates H/R-induced ROS/JNK/Egr-1 signaling, which might be an important mechanism by which it antagonizes myocardial I/R injury.
Myocardial ischemia/reperfusion (I/R) injury often occurs with angioplasty, thrombolytic therapy, ischemic heart disease, and cardiac surgery. However, its pathogenesis remains unclear. A large number of studies have shown that reactive oxygen species (ROS)-induced oxidative stress (tissue damage caused by the accumulation of ROS in cells) is an important pathophysiological process during myocardial I/R injury1. ROS are a class of chemically reactive metabolites that contain oxygen, including superoxide anion (O2•−), hydroxyl radical (·OH), and hydrogen peroxide (H2O2)2. An appropriate amount of ROS usually has a cardioprotective effect; however, excessive ROS not only directly and non-specifically oxidize biological macromolecules such as DNA, proteins, and lipids, but also damage cells by activating a redox signaling cascade that ultimately leads to cell death3,4. In recent years, ROS have generally been described as second messengers because of their key function in cellular signaling cascades and pathophysiological processes such as proliferation, gene expression, adhesion, differentiation, senescence, apoptosis, and necrosis4,5,6.
Early growth response gene -1 (Egr-1) is a transcription factor that transmits information across the nuclear membrane. It can be rapidly induced and then initiates a series of downstream target genes in all cell types, therefore, it is also known as third messengers7,8. Many studies have suggested that Egr-1 is a master switch for various pathways of reperfusion injury9. Our early in vitro and in vivo studies demonstrated that Egr-1 overexpression is an important cause of myocardial ischemia-reperfusion injury10,11.
Because ROS and Egr-1 have an obvious sequential relationship in intracellular signaling pathways and Egr-1 is considered to be I/R’s damage hub, it is possible that ROS and Egr-1 also have a sequential relationship during I/R. For example, Egr-1 might act as the “damage hub” to mediate oxidative stress and the related ROS-induced damage during I/R.
N-butyl haloperidol iodide (F2) is a novel compound synthesized by our laboratory (Chinese national invention patent, No. ZL96119098.1). Extensive research studying the efficacy and mechanism of action of F2 revealed that F2 could improve I/R or hypoxia/reoxygenation (H/R)-induced myocardial tissue or myocardial cell damage by blocking membrane L-type Ca2+ channels and inhibiting Egr-1 overexpression. Classic calcium antagonists also inhibit the expression of Egr-1, suggesting that the F2-mediated inhibition of Egr-1 expression might be related to its inhibition of Ca2+ 10,12,13,14. In addition, we found that F2 could antagonize I/R- or H/R-induced decreased SOD activity and increased malondialdehyde (MDA; an oxidative stress product) level, which suggested that F2 might play a negative regulatory role in abnormal ROS generation during myocardial I/R or H/R15. Ca2+ and ROS are related; for example, Ca2+ in cytoplasm when reperfusion can be transported to the mitochondrial matrix, reducing ATP synthesis and increasing ROS generation. Therefore, it could be speculated that high levels of ROS might lead to the overexpression of Egr-1 during myocardial I/R. As such, F2 might antagonize myocardial I/R-associated damage by decreasing the intracellular Ca2+ overload and the subsequent generation of ROS and overexpression of Egr-1.
Mitogen-activated protein kinases (MAPKs) are a protein kinase family that can phosphorylate both serine and tyrosine residues. The classic MAPK signaling pathways include extracellular signal-regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK). JNK is an important intracellular stress regulatory protein. Many studies have demonstrated that increased levels of oxidative stress/ROS activate JNK signaling, which can increase the incidence of diseases and/or the development of aging-related neurodegenerative diseases, ischemia-reperfusion injury and diabetes16,17,18. JNK might be the upstream signal that induces Erg-1 expression19; therefore, JNK might mediate the signaling between ROS and Egr-1 during myocardial I/R. In the current study, an H9c2 cardiomyoblast cell line-based H/R model was used to determine whether ROS/JNK/Egr-1 signaling pathways were active in H9c2 cells after H/R and to determine if F2 exerted any protective effects against myocardial I/R injury via these pathways.
Results
ROS levels and Egr-1 protein expression in H9c2 cells during H/R over time
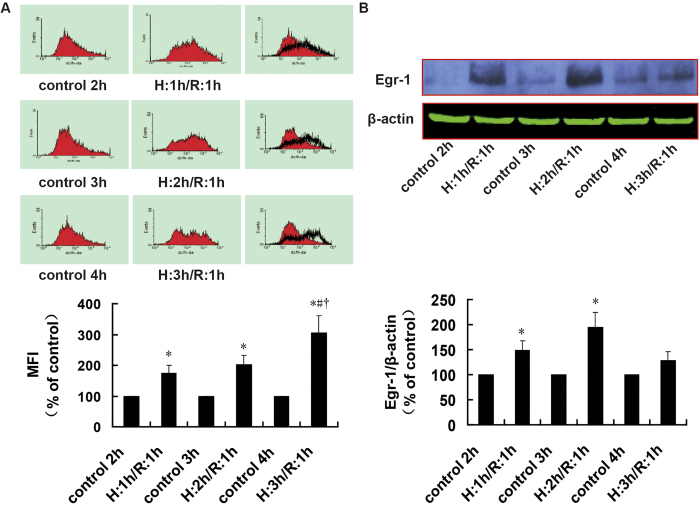
As shown in Fig. 1A, ROS levels were significantly higher in H9c2 cells than in control groups at each time point (P = 0.004, P = 0.001, and P = 0.004). In addition, the effects were time-dependent and increased with longer H/R times. Therefore there was a significant difference between the ROS levels in the H: 3 h/R: 1 h group and H: 1 h/R: 1 h or H: 2 h/R: 1 h groups (P = 0.010 and P = 0.015).
Figure 1. ROS levels and Egr-1 protein expression in H9c2 cells with different durations of H/R, as assessed using flow cytometry and western blotting.
A. ROS levels during H/R; n = 6. B. Cropped blots show protein levels of Egr-1 and β-actin; n = 4. The bands were excised from the same gel. Data are expressed as the percentages of the control group. All values are expressed as mean ± S.E.M. *P < 0.05 vs. control; #P < 0.05 vs. the H:1 h/R:1 h group; †P < 0.05 vs. the H:2 h/R:1 h group.
As shown in Fig. 1B, compared with the control groups, the expression of Egr-1 increased in the H: 1 h/R: 1 h group, H: 2 h/R: 1 h group and H: 3 h/R: 1 h groups, whereas the increase in the H: 1 h/R: 1 h and H: 2 h/R: 1 h groups was significant (P = 0.006 and P = 0.024). However, the peak expression fell in the H: 2 h/R: 1 h group. Based on the above data, subsequent experiments were performed using 2 h of hypoxia and 1 h of reoxygenation.
Relationship between ROS levels and Egr-1 expression during H/R
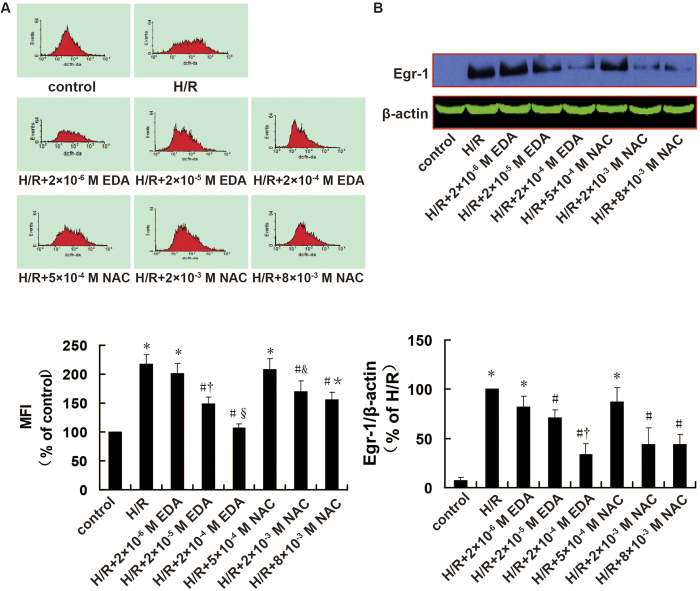
The changes in ROS level and Egr-1 expression in H9c2 cells are shown in Fig. 2. Compared with the control group, ROS levels and Egr-1 expression were increased in the H/R groups significantly (P < 0.001 and P < 0.001). Compared with the H/R groups, the ROS scavengers edaravone (EDA) and N-acetyl-L-cysteine (NAC) decreased ROS level and Egr-1 expression in H9c2 cells in a dose-dependent manner. Moderate and high concentrations of ROS scavengers (2 × 10−5 M EDA, 2 × 10−4 M EDA, 2 × 10−3 M NAC, and 8 × 10−3 M NAC) resulted in substantial decreases in H/R-induced ROS level (P = 0.003, P < 0.001, P = 0.011 and P = 0.002) and Egr-1 expression (P = 0.027, P = 0.001, P = 0.027 and P = 0.006). In contrast, low concentrations of ROS scavengers (2 × 10−6 M EDA, 5 × 10−4 M NAC) had no significant effect on ROS levels (P = 0.235 and P = 0.370) or Egr-1 expression induced by H/R (P = 0.233 and P=0.102). Linear correlation analysis between ROS level and Egr-1 expression in H9c2 cells revealed that they were positively correlated (r = 0.91, P = 0.002).
Figure 2. Effects of different doses of ROS scavengers on ROS level and Egr-1 protein expression in H9c2 cells after H/R, as assessed using flow cytometry and western blotting.
A. ROS levels; n = 6. B. Cropped blots show protein levels of Egr-1 and β-actin; n = 3. The bands were excised from the same gel. Data are expressed as the percentages of the control or H/R groups. All values are expressed as mean ± S.E.M. *P < 0.05 vs. control; #P < 0.05 vs. H/R; †P < 0.05 vs. H/R + 2 × 10−6 M EDA; §P < 0.05 vs. H/R + 2 × 10−5 M EDA; &P < 0.05 vs. H/R + 5 × 10−4 M NAC; *P < 0.05 vs. H/R + 2 × 10−3 M NAC.
Relationship between ROS and JNK
Xanthine oxidase (XO) is an important oxidase for ROS formation, and it releases a large number of electrons by catalyzing hypoxanthine (HX) to form xanthine (X) and uric acid. The electrons are accepted by oxygen generating O2•−, which rapidly disproportionates into H2O2. Both HX and X are XO substrates that generate ROS; therefore, XO/X and XO/HX mixtures can be used as ROS generating agents20,21,22. In the current study we used XO/HX to increase ROS levels and EDA and NAC to decrease ROS levels to assess if JNK activation or the upregulation of Egr-1 expression are mediated by ROS.
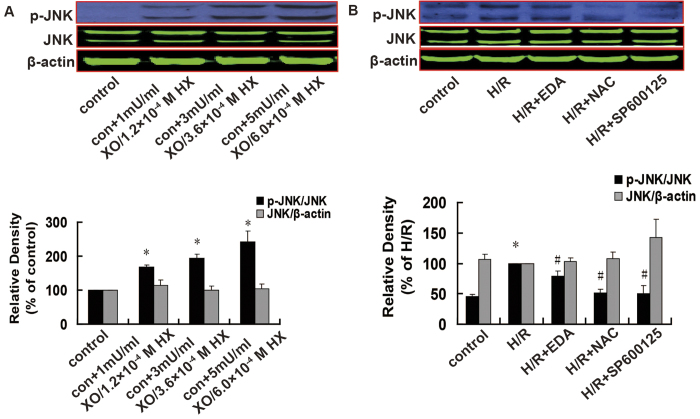
Compared with control, JNK activation was increased substantial in the XO/HX groups in a dose-dependent manner, whereas total JNK expression was unchanged (Fig. 3A). Conversely, JNK activation was decreased significantly in the presence of the ROS scavengers EDA (P = 0.027) and NAC (P < 0.001) and JNK inhibitor (P = 0.006), but total JNK expression was unchanged (Fig. 3B).
Figure 3. Effects of a ROS donor, ROS scavengers, and a JNK inhibitor on the levels of total and p-JNK expression in H9c2 cells, as assessed using western blotting.
A. Effects of a ROS donor; n = 6. B. Effects of ROS scavengers and a JNK inhibitor. n = 4. Cropped blots show protein levels of p-JNK, total JNK and β-actin. The bands were excised from different gels which were run under the same electrophoresis condition. Data are expressed as percentages of the control or H/R groups. All values are presented as mean ± S.E.M. *P < 0.05 vs. control; #P < 0.05 vs. H/R.
Relationship between JNK and Egr-1
We used the JNK inhibitor SP600125 determine which of JNK or Egr-1 was upstream from the other. As shown in Fig. 4, compared with the control group, Egr-1 expression in H9c2 cells was increased significantly in the H/R group (P < 0.001), which was inhibited by the JNK inhibitor (P = 0.02).
Figure 4. Effects of a JNK inhibitor on Egr-1 expression in H9c2 cells after H/R using western blotting. Cropped blots show protein levels of Egr-1 and β-actin.
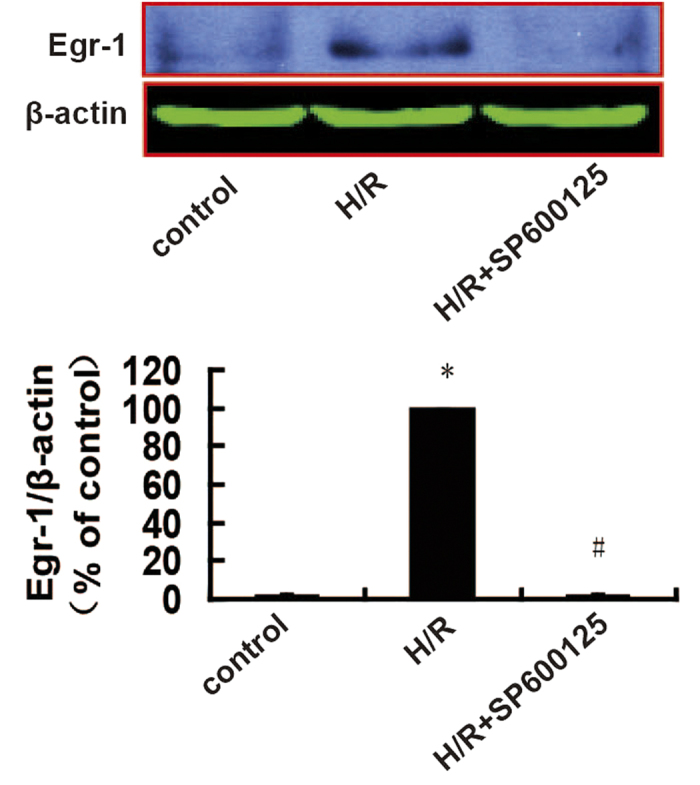
The bands were excised from the same gel. Data are expressed as percentages of the H/R group. All values are presented as mean ± S.E.M; n = 4. *P < 0.05 vs. control; #P < 0.05 vs. H/R.
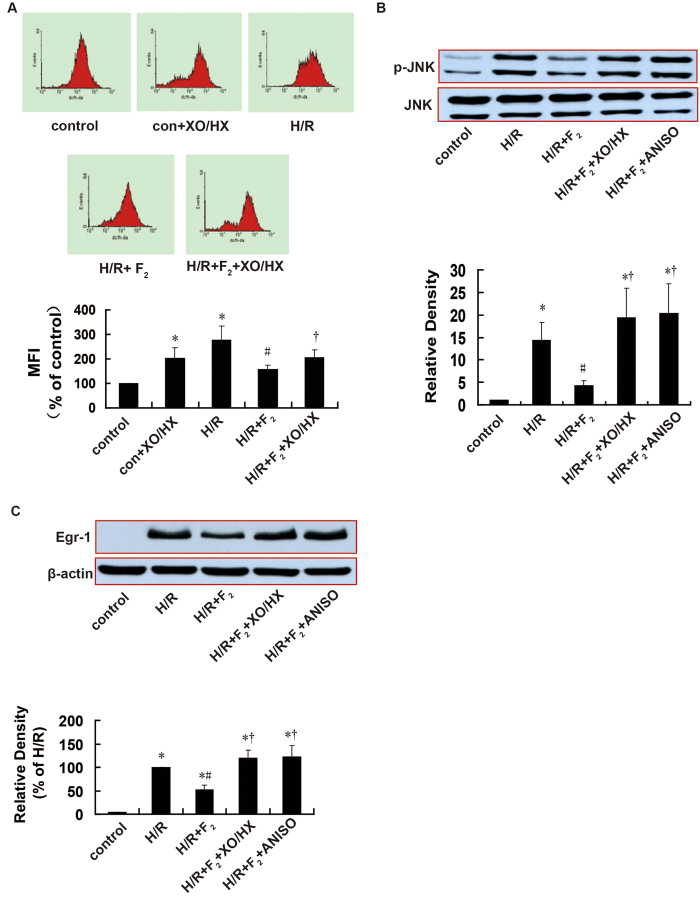
Effect of F2 on ROS level, JNK activation, and Egr-1 expression
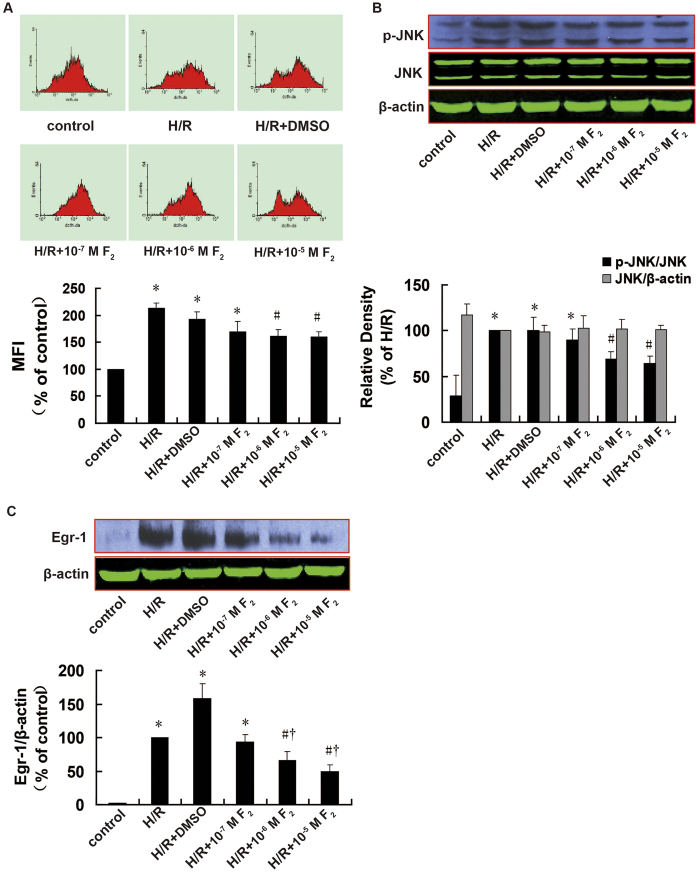
After confirming the activation of the ROS/JNK/Egr-1 signaling pathway in H9c2 cells during H/R, we next explored the effects of F2 on this pathway. As shown in Fig. 5, compared with control, ROS level (P < 0.001), JNK activation (P < 0.001), and Egr-1 expression (P < 0.001) increased significantly in the H/R group; however, these effects were inhibited by F2 in a dose-dependent manner (1 × 10−7 M, 1 × 10−6 M, 1 × 10−5 M). Compared with the H/R group, ROS levels (P = 0.021 and P = 0.006), JNK activation (P = 0.002 and P =0.002), and Egr-1 expression (P = 0.031 and P = 0.015) were significantly lower in the presence of moderate and high F2 concentrations (1 × 10−6 M, 1 × 10−5 M). In addition, moderate and high F2 concentrations had significantly stronger effects on Egr-1 protein expression compared with low concentrations (P = 0.048 and P = 0.016). These data suggest that F2 can inhibit H/R-induced ROS high-level, JNK activation, and Egr-1 expression in H9c2 cells in a dose-dependent manner. There were no differences in any measurements between the H/R and H/R+dimethyl sulfoxide (DMSO) groups, indicating that the solvent did not affect the results. There was also no change in total JNK expression among groups (P > 0.05).
Figure 5. Effects of F2 on ROS levels, JNK activation, and Egr-1 expression in H9c2 cells after H/R, as assessed using flow cytometry and western blotting.
A. ROS levels; n = 10. B. Cropped blots show total and p-JNK expressions; n = 6. The bands were excised from different gels which were run under the same electrophoresis condition. C. Cropped blots show Egr-1 and β-actin expressions; n = 6. The bands were excised from the same gel. Data are expressed as percentages of the levels of the control or H/R groups. All values are expressed as mean ± S.E.M. *P < 0.05 vs. control; #P < 0.05 vs. H/R; †P < 0.05 vs. H/R + 10−7 M F2.
ROS/JNK/Egr-1 agonists antagonize the effects of F2
As shown in Fig. 6, the ROS donor XO/HX antagonized the effects of F2 on H/R-induced ROS generation (P = 0.021), JNK activation (P = 0.045), and Egr-1 expression (P = 0.014). Similarly, the JNK activator anisomycin (ANISO) antagonized the effects of F2 on H/R-induced JNK activation (P = 0.037) and Egr-1 overexpression (P = 0.027).
Figure 6. Influence of a ROS donor and JNK activator on the effects of F2 on ROS level, JNK activation, and Egr-1 expression in H9c2 cells after H/R, as assessed using flow cytometry and western blotting.
A. ROS levels; n = 6. B. Cropped blots show total and p-JNK expressions; n = 3. The bands were excised from different gels which were run under the same electrophoresis condition. C. Cropped blots show Egr-1 and β-actin expressions; n = 3. The bands were excised from the same gel. Data are expressed as percentages of the levels of the control or H/R groups. All values are expressed as means ± S.E.M. *P < 0.05 vs. control; #P < 0.05 vs. H/R; †P < 0.05 vs. H/R + 10−6 M F2.
Discussion
It is widely accepted that oxidase stress caused by ROS accumulation, calcium overload, and inflammation are important pathophysiological changes and therapeutic targets for myocardial I/R injury. In recent years, studies analyzing the signaling pathways involved in I/R injury have provided new targets for myocardial I/R injury prevention and therapy. It was suggested that calcium and ROS are second messengers during I/R, functioning to transmit information from the cell membrane to the cytoplasm. Transcription factors function as third messengers to further transmit the information to the nucleus, binding to nuclear DNA and altering downstream target gene expression, which eventually leads to I/R injury.
Egr-1 is an immediate-early gene, and a number of stimuli such as hypoxia and mechanical damage can result in its expression. It can bind to specific DNA sequences, triggering downstream target gene expression. In the year 2000, Yan et al.9 reported that Egr-1 plays roles in three pathological changes during I/R injury: inflammation, coagulation, and high vascular permeability. They subsequently proposed that Egr-1 has a central and unifying role in the pathogenesis of ischemic tissue damage9. Consistent with this, we previously used antisense oligonucleotides in the myocardial I/R model in vivo and in vitro to demonstrate that Egr-1 expression is an important cause of myocardial I/R injury11.
In recent years, studies revealed that a variety of stimuli such as drugs or glucose deprivation could activate ROS/Egr-1 pathways in different cells23,24,25,26. Aggeli et al.27 and Hartney et al.28 reported that Egr-1 expression could be induced by ROS-generating enzymes such as XO or by direct H2O2 treatment. In addition, Nozik-Grayck et al.29 found that hypoxia-induced upregulation of Egr-1 and its downstream target protein were inhibited in transgenic mice overexpressing extracellular SOD (EC-SOD). This might be related to the direct elimination of ROS by EC-SOD. Taken together, these studies demonstrated that ROS positively regulate Egr-1. Although it is known that oxidative stress and Egr-1 play roles in myocardial I/R injury, it remains unclear if there is a direct relationship between ROS (the second messenger) and Egr-1 (the third messenger) during myocardial I/R.
In the current study, H9c2 cardiomyoblasts was used as our experimental setting. This cell line derived from embryonic heart ventricle has been extensively used in studies investigating signal transduction mechanisms in cardiomyocytes because it retains properties of signaling pathways of cardiomyocytes. We used different doses of the ROS scavengers EDA and NAC during H9c2 cells H/R to demonstrate dose-dependent effects on ROS levels and Egr-1 expression. Both ROS scavengers could decrease H/R-induced high concentrations of ROS and Egr-1 overexpression. Intracellular ROS level and Egr-1 expression were highly positively correlated. The limited conditions of the experiment prevented us from detecting ROS levels and Egr-1 expression simultaneously within the same cell or group of cells. Nevertheless, we ensured that other non-experimental factors were consistent among groups (the growth state of the cells, the density of cultured cells, drug treatment, and the timing of cell synchronization). Therefore, the correlation between ROS level and Egr-1 expression is convincing, and suggests that intracellular ROS levels modulate Egr-1 expression. This supports the hypothesis that abnormal ROS/Egr-1 signaling occurs during H9c2 cells H/R. Mo et al.30 revealed that ambient ultrafine particles (UFPs) and/or cigarette smoke extract (CSE) activated NADPH oxidase, resulting in ROS generation, activation of the MAPKs p38 and ERK1/2, and the upregulation of Egr-1 in mouse pulmonary microvascular endothelial cells. Aggeli et al.27 reported that exogenous ROS (H2O2) could stimulate Egr-1 expression and nuclear translocation via ERK or JNK in H9c2 cells. The current study revealed that ROS positively regulate Egr-1 in a H9c2 cell H/R model. In addition, ROS scavengers EDA and NAC and the JNK inhibitor SP600125 were used to determine whether JNK intervenes in ROS/Egr-1 signaling. The results revealed that ROS scavengers inhibited H/R-induced JNK activation and Egr-1 overexpression substantially. Therefore, JNK and Egr-1 are downstream signaling of ROS. The JNK inhibitor SP600125 downregulated H/R-induced Egr-1 expression, suggesting that JNK is upstream signaling of Egr-1. There was no considerable difference in total JNK expression among groups, indicating that no new JNK protein was expressed during H9c2 cell H/R. These results demonstrate that Egr-1, a transcription factor sensitive to redox regulation2, can be activated by high concentrations of ROS induced by various stimuli. However, the signal-mediating MAPK can be quite different due to different cell types or pathological models. In conclusion, the current study revealed that H/R stimulus could cause ROS generation, which activated JNK, increased the expression of Egr-1, and subsequently caused H/R injury. Therefore, the ROS/JNK/Egr-1 signaling pathway mediates H9c2 cell H/R injury. These results also demonstrated that ROS-mediated oxidative stress-related damage in myocardial I/R is also associated with Egr-1, and that Egr-1 plays a key role in myocardial I/R injury pathway. In this way, this study provides a further explanation for Egr-1, acting as a master switch in I/R injury, a view proposed by Yan et al.9.
It has been demonstrated previously that F2 is a novel calcium antagonist that can block L-type calcium channel in the cardiomyocyte membrane, reduce Egr-1 gene and protein expressions, and antagonize myocardial I/R and H/R injury. A previous study revealed that classical calcium antagonists such as verapamil, diltiazem, and nifedipine significantly reduced Egr-1 overexpression induced by I/R in cardiac tissue and H/R in myocardial cells15. The data show that regulating Egr-1 expression is another mechanism that, like regulating calcium channel, calcium antagonists protect I/R cardiac tissues and H/R myocardial cells. This is consistent with the hypothesis that increased calcium concentrations are a prerequisite for increasing the expression of Egr-131. ROS-induced oxidative stress and Ca2+ overload are the two main causes of myocardial I/R injury. ROS and Ca2+ are both second messengers in cellular signaling pathways that regulate a variety of physiological and pathological processes. They are also involved in the occurrence and development of myocardial I/R injury. We previously revealed that F2 and classical calcium antagonists such as verapamil, diltiazem, and nifedipine could improve cardiac tissue or cardiomyocyte SOD activity during I/R or H/R, as well as reduce MDA content15. This indirectly suggests that they regulate cardiac tissue or cardiomyocyte ROS levels during I/R or H/R. Based on the result that abnormal ROS/JNK/Egr-1 signaling pathway is active in H9c2 cells during H/R, it is here speculated that the protective effects of F2 on myocardium might be related to its regulatory role in ROS/JNK/Egr-1 signaling.
The current study is the first to reveal that F2 could antagonize the H/R-induced increased ROS level, JNK activation, and Egr-1 expression in a dose dependent manner. Then, the dose of F2 (1 × 10−6 M) was selected based on previous reports of optimal protective effects10,12. Results showed that increasing ROS level in H9c2 cells using the ROS donor XO/HX could antagonize the F2-mediated inhibition of H/R-induced intracellular JNK activation and Egr-1 overexpression. Similarly, the JNK activator ANISO inhibited the F2-mediated downregulation of JNK activation and Egr-1 overexpression, suggesting that F2 downregulates Egr-1 by inhibiting ROS high-level and JNK activation. Therefore, we demonstrated that F2 can antagonize myocardial I/R injury via regulating ROS/JNK/Egr-1 signaling. These findings provide a theoretical basis for future studies assessing the application of F2 to treat of various diseases caused by oxidative stress.
Conclusion
In summary, H/R leads to ROS/Egr-1 signaling pathway activation in H9c2 cells, and JNK activation mediates the signaling pathways between ROS and Egr-1. F2 regulates H/R-induced ROS/JNK/Egr-1 signaling, which might be an important mechanism by which it antagonizes myocardial I/R injury.
Methods
Reagent preparation
The H9c2 cell line was purchased from the Chinese Academy of Sciences (Beijing, China). Fetal bovine serum was obtained from Hyclone. F2 was dissolved in DMSO. The ROS scavenger EDA and NAC were purchased from Simcere Pharmaceuticals (Nanjing, China) and Sigma-Aldrich (United States), respectively. The ROS donors XO, HX, the JNK activator ANISO, and 2’,7’-dichlorofluorescein acetyl acetate (DCFH-DA) were purchased from Sigma-Aldrich. The JNK inhibitor SP600123 was purchased from Enzo Life Sciences (Switzerland). The p-JNK goat polyclonal antibody was purchased from Santa Cruz Biotechnology (United States), and rabbit JNK and Egr-1 monoclonal antibodies were purchased from Cell Signaling Technology (United States). Mouse β-actin antibody was purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). IRDyeTM 800CW goat anti-rabbit IgG and IRDyeTM 800CW goat anti-mouse IgG were purchased from United States LI-COR Inc. (United States). Horseradish peroxidase-labeled murine and rabbit secondary antibodies were purchased from Boster Biological Engineering Co., Ltd., (Wuhan, China). Hypoxia solution, which imitates the microenvironment of high-potassium, lactic acid accumulation and glucose depletion after myocardial ischemia in vivo, was prepared as described previously32: 137 mM NaCl, 12 mM KCl, 0.49 mM MgCl2 6H2O, 0.9 mM of CaCl2, 4 mM HEPES, and 20 mM sodium lactate.
Cell culture and establishment of H/R model
The H9c2 cells line was cultured in DMEM medium with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2, and was synchronized treatment with medium containing 0.5% FBS for 12–24 h before every experiment. To induce hypoxia, which should be oxygen-free and nutrition-free for the sake of mimicking ischemia, H9c2 cells were cultured in hypoxic solution by saturation in advance with pure nitrogen for 30 min in order to expel the air, and then pure nitrogen gas was used to fill the culture vessels and hypoxia chamber. The cells placed in hypoxia chamber were cultured at 37 °C for 1, 2, or 3 h; After that, the hypoxia solution was then replaced with fresh oxygenated culture medium and the culture vessels were transferred into normoxic incubator (5% CO2 ) at 37 °C for 1 h of reoxygenation.
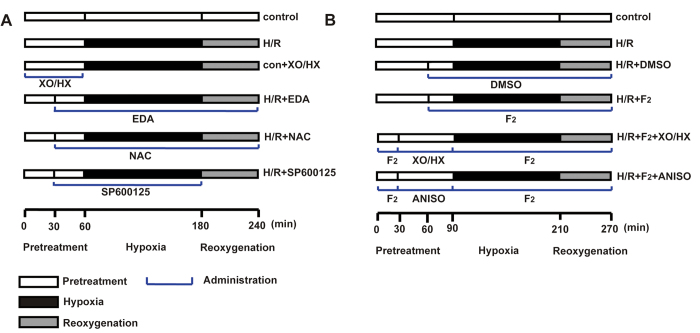
Experimental Grouping
H9c2 cells cultured for 2–3 days were grouped randomly as follows (Fig. 7): control, H/R, control + the ROS donor XO/HX (con + XO/HX), H/R + the ROS scavenger EDA (H/R + EDA), H/R + the ROS scavenger NAC (H/R + NAC), H/R + JNK inhibitor SP600125 (H/R + SP600125), H/R + different doses of F2, H/R + solvent (H/R + DMSO), H/R + F2 + XO/HX, and H/R + F2 + JNK activator ANISO (H/R + F2 + ANISO). The control group was cultured with fresh medium for 2, 3, or 4 h before the experiments. The H/R group was cultured under nitrogen-saturated hypoxia at 37 °C for 1, 2, or 3 h, and was then cultured in fresh medium under normal conditions for 1 h. XO/HX (1 mU/ml/1.2 × 10−4 M, 3 mU/ml/3.6 × 10−4 M, 5 mU/ml /6.0 × 10−4 M), EDA (2 × 10−6 M, 2 × 10−5 M, 2 × 10−4 M), NAC (5 × 10−4 M, 2 × 10−3 M, 8 × 10−3 M), SP600125 (2 × 10−5 M), ANISO (10 ng/ml), and F2 (1 × 10−7 M, 1 × 10−6 M, 1 × 10−5 M) were prepared in normal medium (for preincubation), hypoxia solution, and/or reoxygenation medium.
Figure 7. Protocol of the experimental grouping and treatments.
A. Protocol used to investigate whether ROS /JNK/Egr-1 signaling occurs in H9c2 cells after H/R. B. Protocol used to investigate the effects of F2 on ROS/JNK/Egr-1 signaling in H9c2 cells after H/R.
Western blotting
Western blotting was performed as described previously10. Briefly, total proteins were extracted and quantified. Next, 30–50 μg of each sample was separated using 10% SDS-PAGE gel electrophoresis (stacking gel 50 V, separating gel 100 V), and transferred to nitrocellulose membrane (100 V, 75 min). Membranes were then blocked and incubated with primary antibodies (Egr-1, p-JNK, JNK, and β-actin at 1:1,000, 1:200, 1:2,000, and 1:3,000 dilutions, respectively) followed by secondary antibodies (HRP-labeled rabbit anti-goat IgG, HRP-labeled goat anti-rabbit IgG, IRDyeTM 800CW goat anti-rabbit IgG, or IRDyeTM 800CW goat anti-mouse IgG at dilutions of 1:20,000, 1:30,000, 1:10,000, or 1:10,000, respectively); IRDye incubations were performed in the dark. HRP-labeled secondary antibodies were detected using chemiluminescence, and the grayscale of the protein bands was analyzed using Gel-pro Image Analysis Software (Media cybernetics, USA). IRDye-labeled secondary antibodies were analyzed using an Odyssey infrared imaging system (Odyssey LI-COR, USA). The ratio of p-JNK/JNK represented the activation of JNK, JNK/β-actin indicated the overall expression of JNK, and the ratio of Egr-1/β-actin reflected the expression of Egr-1.
Detecting ROS levels in H9c2 cells using flow cytometry
Culture medium containing 0.5% FBS was used to treat cells for 12–24 h. Cells were trypsinized with 0.125% trypsin at room temperature, and collected in a 2 ml Eppendorf tube after the indicated treatments. The cells were then centrifuged for 5 min at 5 °C and 1000 rpm, and the supernatants were discarded. The pellets were washed with serum-free medium, centrifuged again, and the supernatants were discarded. The cell pellets were mixed with 1 ml serum-free medium containing a final concentration of 10 μM DCFH-DA probe, dispersed uniformly, and then incubated in the dark at 37 °C for 30 min with gentle shaking every 5 min to fully expose the cells to the probe. The mixtures were then centrifuged for 5 min at 25 °C and 1000 rpm, the supernatants were discarded, and the pellets were washed three times with PBS. Subsequently, the cell pellets were resuspended in 500 μl PBS, mixed well, and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, USA). The green fluorescence emitted by ~10,000 cells was recorded at an excitation wavelength of 488 nm. Software WinMDI2.9 was used to analyze the mean fluorescence intensity (MFI).
Statistical analysis
Data are presented as mean ± SEM. The significance of differences was determined using one-way ANOVA followed by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Zhang, Y. et al. Effect of N-n-butyl haloperidol iodide on ROS/JNK/Egr-1 signaling in H9c2 cells after hypoxia/reoxygenation. Sci. Rep. 5, 11809; doi: 10.1038/srep11809 (2015).
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC)-Guangdong Joint Funds (No. U0932005), National Natural Science Foundations of China (No. 81473215 and No. 81072633) and The Central Government Special Funds Supporting the Development of Local Colleges and Universities.
Footnotes
Author Contributions G.G.S. and F.C.Z. supervised the overall project and helped writing the paper; Y.M.Z. designed the experiment, analyzed the data and drafted the manuscript; S.P.Z. polished the English to improve the quality of this manuscript; H.L., S.S.L., T.S., B.W., W.Q.L. and H.X. performed the experiment; F.F.G., Y.C.C. and Z.Q.H. contributed to the statistical analysis. All authors reviewed and approved the final manuscript.
References
- Murphy E. & Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 88, 581–609 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel J. I. & Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int J Mol Sci. 13, 13104–13117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T. Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J. 76, 1074–1082 (2012). [DOI] [PubMed] [Google Scholar]
- Madureira P. A. & Waisman D. M. Annexin A2: the importance of being redox sensitive. Int J Mol Sci. 14, 3568–3594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino-Gomes D., Rocco-Machado N. & Meyer-Fernandes J. R. Cell Signaling through Protein Kinase C Oxidation and Activation. Int J Mol Sci. 13, 10697–10721 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S., Panda P., Sahoo L. & Panda S. K. Reactive oxygen species signaling in plants under abiotic stress. Plant signal Beha. 8, e23681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K. & Thiel G. Epidermal growth factor and thrombin induced proliferation of immortalized human keratinocytes is coupled to the synthesis of Egr-1, a zinc finger transcriptional regulator. J Cell Biochem. 85, 381–391 (2002). [DOI] [PubMed] [Google Scholar]
- Grohe C., Nouskas J., Vetter H. & Neyses L. Effects of nisoldipine on endothelin-1- and angiotensin II-induced immediate/early gene expression and protein synthesis in adult rat ventricular cardiomyocytes. J Cardiovas Pharmacol. 24, 13–16 (1994). [DOI] [PubMed] [Google Scholar]
- Yan S. F. et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 6, 1355–1361 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. The protective effects of N-n-butyl haloperidol iodide on myocardial ischemia-reperfusion injury in rats by inhibiting Egr-1 overexpression. Cell Physiol Biochemi. 20, 639–648 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. The protective effect of Egr-1 antisense oligodeoxyribonucleotide on myocardial injury induced by ischemia-reperfusion and hypoxia-reoxygenation. Cell Physiol Biochemi. 22, 645–652 (2008). [DOI] [PubMed] [Google Scholar]
- Wang J. Z. et al. N-n-Butyl haloperidol iodide protects against hypoxia/reoxygenation-induced cardiomyocyte injury by modulating protein kinase C activity. Biochem Pharmacol. 79, 1428–1436 (2010). [DOI] [PubMed] [Google Scholar]
- Gao F. F. et al. Cardiac electrophysiological and antiarrhythmic effects of N-n-butyl haloperidol iodide. Cell Physiol Biochemi. 25, 433–442 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. N-n-butyl haloperidol iodide ameliorates cardiomyocytes hypoxia/reoxygenation injury by extracellular calcium-dependent and -independent mechanisms. Oxid Med Cell Longevi. 2013, 912310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. et al. Egr-1, the potential target of calcium channel blockers in cardioprotection with ischemia/reperfusion injury in rats. Cell Physiol Biochemi. 24, 17–24 (2009). [DOI] [PubMed] [Google Scholar]
- Lamb R. E. & Goldstein B. J. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract. 62, 1087–1095 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkaddem S. B., Bens M. & Vandewalle A. Differential activation of Toll-like receptor-mediated apoptosis induced by hypoxia. Oncotarget 1, 741–750 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K. H., Lee H. G., Smith M. A. & Shah K. Direct and indirect roles of cyclin-dependent kinase 5 as an upstream regulator in the c-Jun NH2-terminal kinase cascade: relevance to neurotoxic insults in Alzheimer’s disease. Mol Biol Cell. 20, 4611–4619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saben J. et al. Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: role in maternal obesity. Am J Physiol Endocrinol Metab. 305, E1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Na H. Y., Kim J. A., Cho S. E. & Suh S. H. Contradictory Effects of Superoxide and Hydrogen Peroxide on KCa3.1 in Human Endothelial Cells. Korean J Physiol Pharmacol. 17, 181–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley E. E. et al. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 48, 493–498 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Shuvaev V. V. & Muzykantov V. R. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Pharmacol Exp Ther. 338, 82–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. H. et al. ZnO nanoparticles induce TNF-alpha expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J Dermatol Sci. 72, 263–273 (2013). [DOI] [PubMed] [Google Scholar]
- Han M. H. et al. Apoptosis induction of human bladder cancer cells by sanguinarine through reactive oxygen species-mediated up-regulation of early growth response gene-1. PloS one 8, e63425 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M. H., Kim G. Y., Yoo Y. H. & Choi Y. H. Sanguinarine induces apoptosis in human colorectal cancer HCT-116 cells through ROS-mediated Egr-1 activation and mitochondrial dysfunction. Toxicol Lett. 220, 157–166 (2013). [DOI] [PubMed] [Google Scholar]
- Jeon H. M. et al. Early growth response 1 regulates glucose deprivation-induced necrosis. Oncol Rep. 29, 669–675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeli I. K., Beis I. & Gaitanaki C. ERKs and JNKs mediate hydrogen peroxide-induced Egr-1 expression and nuclear accumulation in H9c2 cells. Physiol Res. 59, 443–454 (2010). [DOI] [PubMed] [Google Scholar]
- Hartney T. et al. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PloS one 6, e27531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozik-Grayck E. et al. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung cell Mol Physiol. 295, L422–430 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y. et al. Combination effects of cigarette smoke extract and ambient ultrafine particles on endothelial cells. Toxicol In vitro. 26, 295–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., Mayer S. I., Muller I., Stefano L. & Rossler O. G. Egr-1-A Ca(2+)-regulated transcription factor. Cell calcium 47, 397–403 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang Y. M. et al. Effects of N-n-butyl haloperidol iodide on myocardial ischemia/reperfusion injury and Egr-1 expression in rat. Acta Biochim Biophys Sin. 38, 435–441 (2006). [DOI] [PubMed] [Google Scholar]