Abstract
Purpose
To evaluate the effectiveness of a policy supporting early detection and prevention of cervical cancer among low-income and uninsured women by comparing women who reported never or rarely being screened (last screen >5 years) to those who reported screening in the past ≤5 years.
Methods
We analyzed data from 1,485,251 women who received their first Pap test in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) from July 2002 through June 2012. Of these, 461,893 women (31 %) reported being never or rarely screened and 1,023,358 (69 %) reported being screened in the past 5 years. Demographic (age, race/ethnicity, residence, and region) and clinic (cytologic and histologic results) characteristics were examined for the two groups.
Results
Women who were aged ≥50 years, Asian and Pacific Islander, American Indian or Alaska Native, multiracial, living in non-metro areas, or living in the South or a territory were more likely to report being never or rarely screened. The percentage of abnormal Pap tests and the rate of precancer and cancer (combined) was higher in the never or rarely screened group compared with the screened group (abnormal percentage: 2.9 vs 2.6 %, p value < 0.01; rate of precancer and cancer: 6.9 vs 3.7 per 1,000 women, p value < 0.01).
Conclusions
The priority of reaching never or rarely screened women should continue since those women who entered the NBCCEDP not adequately screened had a greater prevalence of high-grade histological lesions and invasive cervical cancers at later stages than women screened more recently.
Keywords: Pap test, Mass screening, Cervix neoplasms
Introduction
Cervical cancer screening has resulted in well-documented declines in cervical cancer incidence and mortality in the USA [1]. However, in 2011, there were still almost 12,000 women who developed cervical cancer and 4,000 women who died from this disease [2]. It is estimated that more than half of women who develop cervical cancer were not screened or were not screened appropriately [3, 4]. In 2010, 83 % of women reported having a Pap test within the past 3 years [5], significantly lower than the Healthy People 2020 target of 93 % [6].
To improve cervical cancer screening among medically underserved women, Congress authorized the Centers for Disease Control and Prevention (CDC) to develop the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). In 1999, CDC intensified the policy to focus their efforts and resources on those who would benefit the most from screening: never or rarely screened women (last screen >5 years previously) (NR) who were at the highest risk of developing cervical cancer.
Clinical outcomes of this population have not been studied previously to evaluate the effectiveness of the NBCCEDP’s policy on early detection and prevention of cervical cancer. We describe the population of women in the NBCCEDP who reported being NR, compare them to women with prior screening, and describe patient demographics and prevalence of cervical cancer screening results by screening history across a 10-year period to assess long-term outcomes of this national cervical cancer screening program.
Methods
CDC implemented cooperative agreements with all states, the District of Columbia, American Indian/Alaska Native tribes, and territories to provide cancer screening, referral, and follow-up services to low-income, uninsured, and underinsured women; these have been described in detail elsewhere in this supplement [7]. For this study, we examined data submitted by 50 states, the District of Columbia, 12 tribes, and five territories during July 2002 through June 2012. The study was approved by CDC’s Human Subjects Committee.
Study population
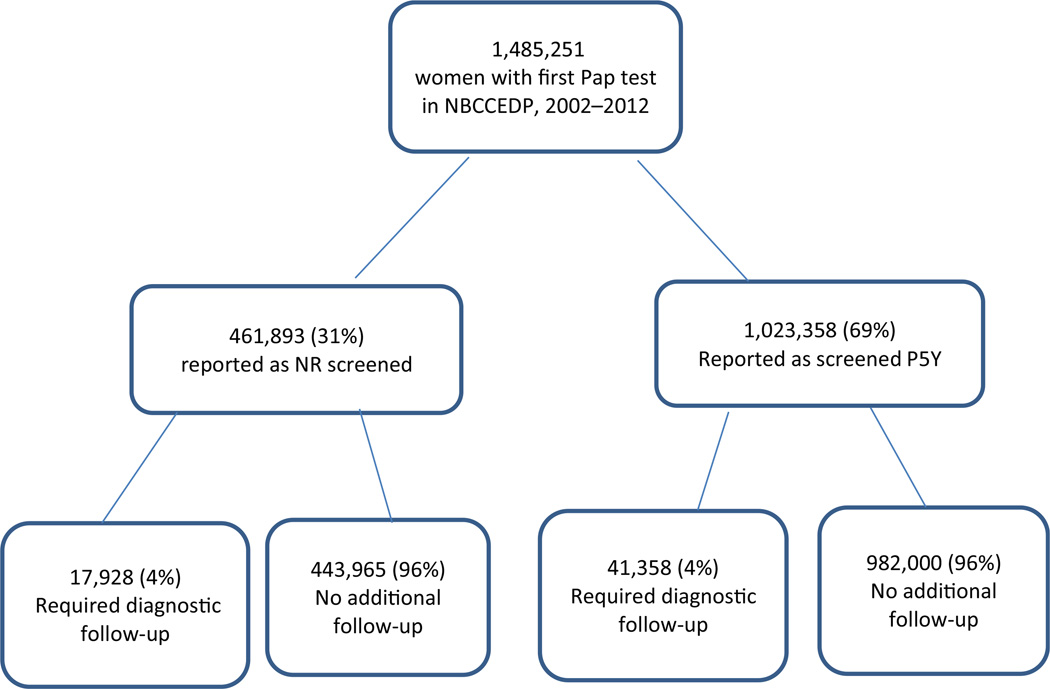
The study cohort comprised women who were newly enrolled in the NBCCEDP and had received their first NBCCEDP Pap test, and allowed at least 6 months for a diagnostic work-up to be completed after abnormal findings. Of the 1,684,272 women receiving an initial NBCCEDP Pap test, we eliminated 199,021 (12 %) from the analysis because of missing or unknown screening history, leaving a study population of 1,485,251 (Fig. 1). The women who were not included (12 %) had similar characteristics and screening results as the study population. During the study period, there were 1,023,358 women (69 %) who reported a Pap test within 5 years and 461,893 women (31 %) reporting never or rarely being screened (38 % were never screened and 62 % had their last screen >5 years defined as rarely); in both groups (screened and NR), 4 % of the Pap tests required diagnostic follow-up, including colposcopy with biopsy.
Fig. 1.
Study population of women receiving a first Pap test in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) by screening history and follow-up from 2002–2012. NBCCEDP National Breast and Cervical Cancer Early Detection Program, NR never/rarely, P5Y screened in the past ≤5 years
Study outcomes
Abnormal Pap test results that signaled the need for additional diagnostic testing included low-grade squamous intraepithelial lesions, atypical squamous cells—cannot exclude high-grade squamous intraepithelial lesion, highgrade squamous intraepithelial lesion, squamous cell cancer, and atypical glandular cells. In 2003, when the Food and Drug Administration approved the human papillomavirus (HPV) test to screen for cervical cancer in conjunction with the Pap test (known as co-testing), the NBCCEDP reimbursed for management only (known as reflex testing) [8]. However, reflex HPV testing was not systematically captured in the data system until 2009 [9]. Reimbursement for co-testing began in 2012 when the US Preventive Services Task Force recommended co-testing as an option for cervical cancer screening [10].
For the present study, we calculated age based on the birth date reported at enrollment and used six age classifications: 18–20, 21–29, 30–39, 40–49, 50–64, and ≥65 years. The age groups were selected after consideration of age-related influences on screening rates, such as Medicare coverage primarily for those aged ≥65 years and changes in the screening recommendations for younger women not to be screened (aged < 21 years) [11]. To categorize residence at the time of screening as metropolitan, urban, or rural, the rural/urban continuum codes were used [12].
We calculated the percentages of Pap test results by screening history, defined as screening in the past 5 years (P5Y) or never/rarely being screened (NR) defined as never screened or not in the past 5 years. We computed detection rates for each grade of cervical intraepithelial neoplasia (CIN) and invasive cancer as the number of cases with a final histologic diagnosis of CIN (CIN1, CIN2, CIN3/carcinoma in situ) or invasive cancer per 1,000 Pap tests performed, by screening history. To estimate the detection rate of high-grade lesions, we combined biopsy results of CIN3/carcinoma in situ, adenocarcinoma in situ, and invasive cancer (i.e., CIN3 or worse). Percentages and rates were age-adjusted by the direct method using the distribution of the population receiving a Pap test through NBCCEDP in 2000. Logistic regression models were computed to examine predictors of NR screened women compared with P5Y women, adjusting for age.
Results
Table 1 shows the demographic characteristics of women who had their first Pap test in the NBCCEDP during 2002–2012, by screening history. Women in the NR screened group were older than those who reported being screened P5Y. Women in the NR screened group also were more likely to be white, Asian–Pacific Islander, American Indian/Alaskan Native, and multiracial. Geographically, women living in non-metro areas or in the South or a territory were more likely to report NR screened than to report being in the P5Y group.
Table 1.
Demographic characteristics among women receiving a first Pap test in the National Breast and Cervical Cancer Early Detection Program, by screening history, 2002–2012
| Total | Screening history (n) | Differenced (NR–P5Y %) | |
|---|---|---|---|
| NR (%) 461,893 (100 %) |
P5Y (%) 1,023,358 (100 %) |
||
| Age group (years) | |||
| 18–20 | 7,866 (1.7) | 11,890 (1.2) | 0.5 |
| 21–29 | 28,489 (6.2) | 69,523 (6.8) | −0.6 |
| 30–39 | 41,878 (9.1) | 108,966 (10.7) | −1.6 |
| 40–49 | 173,820 (37.6) | 414,599 (40.5) | −2.9 |
| 50–59 | 159,036 (34.4) | 325,157 (31.8) | 2.7 |
| 60–64 | 44,549 (9.6) | 84,537 (8.3) | 1.4 |
| ≥65 | 6,255 (1.4) | 8,686 (0.9) | 0.5 |
| Race/ethnicitya | |||
| White | 225,462 (48.8) | 465,589 (45.5) | 3.3 |
| African-American | 61,792 (13.4) | 144,648 (14.1) | −0.8 |
| Asian/Pacific Islander | 39,128 (8.5) | 47,110 (4.6) | 3.9 |
| American Indian/Alaska Native | 22,218 (4.8) | 32,495 (3.2) | 1.6 |
| Multiracial/unknownb | 11,168 (2.4) | 21,800 (2.1) | 0.3 |
| Hispanic | 102,125 (22.1) | 311,716 (30.5) | −8.4 |
| Rural–urbana | |||
| Metro | 326,163 (70.6) | 763,900 (74.6) | −4.0 |
| Urban | 110,052 (23.8) | 220,806 (21.6) | 2.2 |
| Rural | 18,187 (3.9) | 34,992 (3.4) | 0.5 |
| Unknown | 7,491 (1.6) | 3,660 (0.4) | 1.3 |
| Regiona, c | |||
| Territory | 7,502 (1.6) | 4,000 (0.4) | 1.2 |
| Northeast | 79,579 (17.2) | 203,761 (19.9) | −2.7 |
| Midwest | 94,709 (20.5) | 240,545 (23.5) | −3.0 |
| South | 159,367 (34.5) | 302,912 (29.6) | 4.9 |
| West | 120,736 (26.1) | 272,140 (26.6) | −0.5 |
NR never or rarely (not screened within 5 years) screened, P5Y screened within 5 years
Percentages age-adjusted to the 2000 National Breast and Cervical Cancer Early Detection Program
NR screened history for unknown race/ethnicity is 8,263 (1.8 %) and for P5Y is 16,589 (1.6 %)
Region: Territory (American Samoa, Guam, Commonwealth of Northern Mariana Islands, Republic of Palau, Puerto Rico, Virgin Islands); Northeast (Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont); Midwest (Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, Wisconsin); South (Alabama, Arkansas, Delaware, DC, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, West Virginia); West (Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, Wyoming)
p values computed from separate logistic models of screening history with age, race/ethnicity, rural–urban, or region as the covariate were all statistically different (<0.05)
The cytology results for women’s first Pap test in the NBCCEDP by age group and screening history are shown in Table 2. Women aged < 30 years in either screening history group had higher percentages of abnormal Pap test results (low-grade squamous intraepithelial lesions, atypical squamous cells—cannot exclude high-grade squamous intraepithelial lesions, and high-grade squamous intraepithelial lesions) than did women aged ≥30 years. Also, squamous cell carcinoma (from cytology) occurred at an earlier age (40 years old) and was more common in the NR population than in the P5Y population.
Table 2.
Distribution (%) of cervical cancer screening results among women receiving their first Pap test in the National Breast and Cervical Cancer Early Detection Program by age and screening history, 2002–2012
| Screening history NR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR | P5Y | |||||||||||||||
| Total | 18–20 | 21–29 | 30–39 | 40–49 | 50–59 | 60–64 | 65+ | Total | 18–20 | 21–29 | 30–39 | 40–49 | 50–59 | 60–64 | 65+ | |
| Number of women with current Pap test | 461,893 | 7,866 | 28,489 | 41,878 | 173,820 | 159,036 | 44,549 | 6,255 | 1,023,358 | 11,890 | 69,523 | 108,966 | 414,599 | 325,157 | 84,537 | 8,686 |
| Cytology (%) | ||||||||||||||||
| Negative | 91.7 | 82.9 | 84.2 | 89.8 | 91.5 | 93.3 | 94.2 | 93.9 | 91.4 | 72.4 | 79.3 | 89.1 | 91.8 | 93.8 | 95.0 | 94.8 |
| ASC-US | 4.1 | 7.8 | 7.0 | 4.7 | 4.3 | 3.4 | 2.6 | 2.5 | 4.4 | 11.6 | 9.1 | 5.4 | 4.5 | 3.3 | 2.5 | 2.4 |
| LSIL | 1.7 | 7.4 | 6.1 | 2.5 | 1.5 | 1.0 | 0.6 | 0.6 | 2.0 | 12.4 | 7.9 | 2.9 | 1.6 | 1.0 | 0.7 | 0.5 |
| ASC-H | 0.3 | 0.3 | 0.5 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.7 | 0.6 | 0.4 | 0.2 | 0.2 | 0.2 | 0.1 |
| HSIL | 0.8 | 1.0 | 1.3 | 1.3 | 0.9 | 0.6 | 0.5 | 0.8 | 0.6 | 2.1 | 2.0 | 1.1 | 0.5 | 0.3 | 0.3 | 0.5 |
| Squamous cell cancer | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Atypical glandular cells | 0.3 | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | 0.0 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 |
| Unsatisfactory | 0.8 | 0.4 | 0.7 | 0.7 | 0.8 | 0.9 | 1.1 | 1.3 | 0.8 | 0.4 | 0.5 | 0.6 | 0.8 | 0.8 | 1.0 | 1.1 |
NR never or rarely (not screened within 5 years) screened, P5Y screened within 5 years, ASC-H atypical squamous cells of undetermined significance—cannot exclude HSIL, ASC-US atypical squamous cells of undetermined significance, HSIL high-grade squamous intraepithelial lesion, LSIL low-grade squamous intraepithelial lesion
Overall, there were more women with abnormal Pap test results in the NR screened group than in the P5Y group (2.9 vs 2.6 %; p value < 0.05; Table 3). The percentage with abnormal Pap tests decreased with increasing age for both screening history groups; for women aged ≥40 years, the percentage of abnormal Pap tests was higher in the NR group compared with the P5Y group. The NR group had a higher abnormal Pap test prevalence in every race/ethnicity group, rural–urban category, and region compared with the P5Y group, with significant differences in prevalence noted in the Hispanic, metro, Midwest, Northeast, South and West.
Table 3.
Distribution (%) of abnormal cervical cancer screening results among women receiving their first Pap test in the National Breast and Cervical Cancer Early Detection Program, by screening history, 2002–2012
| Totala | Screening history | Difference (NR–P5Y %) |
|
|---|---|---|---|
| NR | P5Y | ||
| Total abnormal Pap testsb (%) |
|||
| 2.9 | 2.6 | 0.3* | |
| Age group (years) | |||
| 18–20 | 8.8 | 15.3 | −6.5* |
| 21–29 | 8.0 | 10.8 | −2.8* |
| 30–39 | 4.6 | 4.6 | −0.1 |
| 40–49 | 3.1 | 2.6 | 0.5* |
| 50–59 | 2.3 | 1.8 | 0.5* |
| 60–64 | 1.9 | 1.4 | 0.5* |
| ≥65 | 2.1 | 1.5 | 0.6* |
| Race/ethnicitya | |||
| White | 3.3 | 3.1 | 0.3 |
| African-American | 2.6 | 2.5 | 0.1 |
| Asian/Pacific Islander | 2.1 | 1.8 | 0.3 |
| American Indian/Alaska Native | 2.0 | 2.0 | <0.1 |
| Multiracial/unknown | 2.4 | 2.3 | 0.1 |
| Hispanic | 2.6 | 2.1 | 0.5* |
| Rural–urbana | |||
| Metro | 2.8 | 2.5 | 0.3* |
| Urban | 3.1 | 2.9 | 0.2 |
| Rural | 3.1 | 2.7 | 0.3 |
| Unknown | 2.0 | 1.3 | 0.7* |
| Regiona | |||
| Territory | 1.9 | 1.6 | 0.4 |
| Northeast | 2.7 | 2.5 | 0.3* |
| Midwest | 3.5 | 3.3 | 0.2* |
| South | 3.1 | 2.8 | 0.3* |
| West | 2.4 | 2.0 | 0.4* |
NR never or rarely (not screened within 5 years) screened, P5Y screened within last 5 years
p values significantly different at <0.05 from separate nested logistic models of abnormal Pap test with age, race/ethnicity, rural–urban, or region associated with screening history
Percentages age-adjusted to the 2000 National Breast and Cervical Cancer Early Detection Program
Includes the following Pap test results: atypical glandular cells, atypical squamous cells of undetermined significance—cannot exclude high-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, and squamous cell carcinoma
Figure 2a–d shows the biopsy-confirmed histology results by age group. Younger women (aged < 30 years) had higher rates of CIN (1, 2, and 3) in the P5Y group compared with those in the NR group (Fig. 2a–c). However, the rate of invasive cancer increased with age, with higher rates in NR women compared with P5Y women, except among 21–29 years olds (Fig. 2d). The rates of CIN3 or worse were higher in the NR group than in the P5Y group overall and every characteristic group, except among younger women aged 18–29 years (Table 4). For women with invasive cervical cancer, those in the NR population had more cancers diagnosed at a late stage than did those in the P5Y group [SEER summary stage: localized 32 % NR compared with 45 % P5Y; distant 13 % NR compared with 7 % P5Y (data not shown)].
Fig. 2.
Rates of cervical precancer and invasive cancer among women receiving their first Pap test in the National Breast and Cervical Cancer Early Detection Program, by screening history and age group, 2002–2012. a Rates per 1,000 women of CIN1 by screening history and age group (years). b Rates per 1,000 women of CIN2 by screening history and age group (years). c Rates per 1,000 women of CIN3* by screening history and age group (years). d Rates per 1,000 women of invasive cancer by screening history and age group (years). CIN cervical intraepithelial neoplasia, NR never or rarely (not screened within 5 years) screened, P5Y screened within last 5 years. *CIN3 includes CIN3, carcinoma in situ, adenocarcinoma in situ
Table 4.
Rates of cervical precancer and cancer among women receiving their first Pap test in the National Breast and Cervical Cancer Early Detection Program, by screening history, 2002–2012
| Totala | Screening history | Difference (NR–P5Y %) |
|
|---|---|---|---|
| NR | P5Y | ||
| Rate of CIN3 or worseb per 1,000 women |
|||
| 6.9 | 3.7 | 3.2* | |
| Age group (years) | |||
| 18–20 | 3.7 | 12.9 | −9.2* |
| 21–29 | 8.7 | 13.5 | −4.8* |
| 30–39 | 10.7 | 8.3 | 2.4* |
| 40–49 | 7.4 | 3.7 | 3.6* |
| 50–59 | 5.9 | 2.4 | 3.5* |
| 60–64 | 6.0 | 2.3 | 3.7* |
| ≥65 | 7.4 | 4.7 | 2.6* |
| Race/ethnicitya | |||
| White | 8.6 | 4.8 | 3.9* |
| African-American | 5.3 | 3.3 | 2.0* |
| Asian/Pacific Islander | 5.5 | 2.4 | 3.1* |
| American Indian/Alaska Native | 4.4 | 2.7 | 1.7 |
| Multiracial/unknown | 4.7 | 3.8 | 0.9 |
| Hispanic | 5.4 | 2.9 | 2.5* |
| Rural–urbana | |||
| Metro | 6.4 | 3.4 | 3.0* |
| Urban | 8.2 | 4.6 | 3.6* |
| Rural | 7.9 | 4.2 | 3.6* |
| Unknown | 6.3 | 2.9 | 3.3* |
| Regiona | |||
| Territory | 6.2 | 2.7 | 3.5 |
| Northeast | 5.8 | 3.0 | 2.9* |
| Midwest | 8.7 | 5.0 | 3.7* |
| South | 7.3 | 4.0 | 3.3* |
| West | 6.4 | 3.3 | 3.1* |
CIN cervical intraepithelial neoplasia, NR never or rarely (not screened within 5 years) screened, P5Y screened within last 5 years
p values significantly different at <0.05 from separate nested logistic models of CIN3 or worse, with age, race/ethnicity, residence, or region associated with screening history
Percentages age-adjusted to the 2000 National Breast and Cervical Cancer Early Detection Program
CIN3 or worse includes CIN3, carcinoma in situ, adenocarcinoma in situ, and invasive cervical cancer
Discussion
The National Breast and Cervical Cancer Early Detection Program targeted never and rarely screened women for outreach as these women were presumed to benefit the most from screening, since they are at the highest risk of developing invasive disease. Our study demonstrated that this was an effective strategy for screening, because more NR women developed invasive cancers at younger ages and at later stages than did women screened more frequently.
Our data showed that younger women had higher rates of abnormal Pap tests and high-grade lesions (CIN2 or 3), yet older women had higher rates of invasive disease. These findings are consistent with those of other population- based studies [13] and with the natural history of the disease [14]. However, percentages and rates of abnormal test results in the younger age groups in our study population were higher than in other studies (especially among P5Y women compared with NR women) [15] Of note is the higher rate of CIN3 or worse among younger women, especially women aged < 30 years in the P5Y screened group (12.9 per 1,000 Pap tests among those aged 18–20 and 13.5 per 1,000 Pap tests among those aged 21–29; Table 4). It is likely that the younger women in the P5Y group of our study population were referred to the NBCCEDP after obtaining an abnormal screening result elsewhere. Additionally prior to the 2012 recommendations, which included most of the time period for this study, women were screened depending on age and sexual activity [16] and thus (women ≤30 years) may represent a group at higher risk of HPV exposure [17]. Since the 2012 recommendations against screening adolescents, the NBCCEDP has targeted women aged ≥30 years who reported being NR. We analyzed these data without including younger women aged < 30 years and found a much larger difference between the percentages of abnormal Pap tests and the rates of CIN and invasive disease among the women in the NR group compared with the P5Y group (data not shown).
Researchers at CDC conducted a review of the scientific literature, professional organization guidelines related to cervical cancer, and NBCCEDP data on Pap screening outcomes and collaborated on the development of the new cervical cancer screening policy. In 1999, NBCCEDP recommended that women who had three normal Pap tests could wait 3 years before their next routine screening. This policy recommendation was made before the national recommendations were changed in 2002 to wait 3 years between screenings [18]. In 2005, NBCCEDP policy was incorporated as a core indicator within a CDC-administered performance management system to prioritize funding resources for NR women, with a goal that at least 20 % of women newly enrolled in the NBCCEDP for cervical screening should be NR [19]. Our data indicated that most NR women who came to the NBCCEDP were aged ≥50 years, white, Asian–Pacific Islander, American Indian/Alaskan Native, or multiracial, and were living in nonmetro areas and in the South or a territory. Similarly, other studies have found that older women, racial minorities, and women living in rural areas in the South have a higher disease burden [20–23]. Therefore, more targeted efforts to screen these specific groups may be warranted to reach those at the highest risk of cervical cancer.
Many changes in the early detection and prevention of cervical cancer have occurred in the 10 years of this study period, including the introduction of the HPV vaccine [24], the HPV test for screening [25], updates in reporting systems [26], and updates in recommendations for screening [10] and management [27]. Therefore, some interpretations of findings for both cytology and histology may differ depending on the year of outcome. Additionally, this study may be limited because of the lack of information on whether women had previously experienced abnormal results and been referred to NBCCEDP prior to 2009, when data collection was expanded to include the reason for the visit.
The NBCCEDP is the only national screening program for cervical cancer in the USA, and ours is the first analysis focusing on women who had never or rarely been screened. Our findings of high-grade lesions and invasive cancers beginning at younger ages for NR women suggest that reaching this group before progression to invasive disease is essential to preventing and reducing cervical cancer incidence and mortality and that screening this group should continue to be a priority.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
V. B. Benard, Email: vdb9@cdc.gov, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), 4770 Buford Highway NE, Mailstop F-76, Atlanta, GA 30341, USA.
J. Royalty, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), 4770 Buford Highway NE, Mailstop F-76, Atlanta, GA 30341, USA
M. Saraiya, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), 4770 Buford Highway NE, Mailstop F-76, Atlanta, GA 30341, USA
T. Rockwell, Information Management Services, Inc., 6110 Executive Blvd., Suite 310, Rockville, MD 20852, USA
W. Helsel, Information Management Services, Inc., 6110 Executive Blvd., Suite 310, Rockville, MD 20852, USA
References
- 1.Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute CfDCa- PaNC, editor. Group UCSW. United States cancer statistics: 1999–2010 incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 3.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 4.Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000;88:2283–2289. [PubMed] [Google Scholar]
- 5.Cancer screening—United States, 2010. MMWR. Morbidity and mortality weekly report. 2012;61:41–45. [PubMed] [Google Scholar]
- 6.Brown ML, Klabunde CN, Cronin KA, White MC, Richardson LC, McNeel TS. Challenges in meeting healthy people 2020 objectives for cancer-related preventive services, National Health Interview Survey, 2008 and 2010. Prev Chron Dis. 2014;11:E29. doi: 10.5888/pcd11.130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangka F. Cancer Causes Control. 2014 [Google Scholar]
- 8.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 9.Watson M. Cancer Causes and Control. 2015 [Google Scholar]
- 10.Moyer VA. Screening for cervical cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;156(880–91):W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 11.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 12.USDA Rural–Urban Continuum Codes
- 13.Flagg EW, Datta SD, Saraiya M, et al. Population-based surveillance for cervical cancer precursors in three central cancer registries, United States 2009. Cancer Causes Control. 2014;25:571–581. doi: 10.1007/s10552-014-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomark Prev. 2013;22:553–560. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(46):e1–e11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Bulletin AP. Clinical management of guidelines for obstetrician– gynaecologists. Number 45. Obstet Gynecol. 2003;102:417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 17.Benard VB, Howe W, Saraiya M, Helsel W, Lawson HW. Assessment of follow-up for low-grade cytological abnormalities in the National Breast and Cervical Cancer Early Detection Program, 2000–2005. J Low Genit Tract Dis. 2008;12:300–306. doi: 10.1097/LGT.0b013e31817e308e. [DOI] [PubMed] [Google Scholar]
- 18.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 19.Degroff A, Royalty JE, Howe W, Buckman DW, Gardner J, Poister T, Hayes N. When performance management works: a study of the National Breast And Cervical Cancer Early Detection Program. Cancer. 2014;120(Suppl 16):2566–2574. doi: 10.1002/cncr.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benard VB, Coughlin SS, Thompson T, Richardson LC. Cervical cancer incidence in the United States by area of residence, 1998–2001. Obstet Gynecol. 2007;110:681–686. doi: 10.1097/01.AOG.0000279449.74780.81. [DOI] [PubMed] [Google Scholar]
- 21.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113:2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 22.Chen HY, Kessler CL, Mori N, Chauhan SP. Cervical cancer screening in the United States, 1993–2010: characteristics of women who are never screened. J Womens Health (Larchmt) 2012;21:1132–1138. doi: 10.1089/jwh.2011.3418. [DOI] [PubMed] [Google Scholar]
- 23.Stanley SL, Thomas CC, King JB, Richardson LC. Predictors of never being screened for cervical cancer by metropolitan area. J Community Health. 2014;39:400–408. doi: 10.1007/s10900-013-9778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP) MMWR. Recommendations and reports: morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 2007;56:1–24. [PubMed] [Google Scholar]
- 25.Murphy J, Kennedy EB, Dunn S, et al. HPV testing in primary cervical screening: a systematic review and meta-analysis. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2012;34:443–452. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- 26.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 27.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829–846. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]