Abstract
Objective
To compare computed tomography dose and noise arising from use of an automatic exposure control (AEC) system designed to maintain constant image noise as patient size varies with clinically accepted technique charts and AEC systems designed to vary image noise.
Materials and Methods
A model was developed to describe tube current modulation as a function of patient thickness. Relative dose and noise values were calculated as patient width varied for AEC settings designed to yield constant or variable noise levels and were compared to empirically derived values used by our clinical practice. Phantom experiments were performed in which tube current was measured as a function of thickness using a constant-noise-based AEC system and the results were compared with clinical technique charts.
Results
For 12-, 20-, 28-, 44-, and 50-cm patient widths, the requirement of constant noise across patient size yielded relative doses of 5%, 14%, 38%, 260%, and 549% and relative noises of 435%, 267%, 163%, 61%, and 42%, respectively, as compared with our clinically used technique chart settings at each respective width. Experimental measurements showed that a constant noise–based AEC system yielded 175% relative noise for a 30-cm phantom and 206% relative dose for a 40-cm phantom compared with our clinical technique chart.
Conclusions
Automatic exposure control systems that prescribe constant noise as patient size varies can yield excessive noise in small patients and excessive dose in obese patients compared with clinically accepted technique charts. Use of noise-level technique charts and tube current limits can mitigate these effects.
Keywords: CT, automatic exposure control, image quality, radiation dose, image noise
Introduced into clinical medicine in 1973, x-ray computed tomography (CT) has revolutionized the field of medical imaging. Its high-quality, 3-dimensional images are used to noninvasively diagnose disease or injury in every organ system and allow for both staging of disease and treatment planning. With each new scanner model came increased technical capabilities and new clinical applications, resulting in growth in CT use of approximately 10% per year since the introduction of spiral CT.1,2 It was estimated that more than 62 million CT examinations were performed in the United States in 2006.3,4 Although the dose per examination has, in many cases, fallen by a factor of 2 to 3 as technology improved,5 the continued increase in utilization has made CT one of the major contributors to the cumulative US population dose from medical uses of ionizing radiation. It is in this context that concerns have been raised regarding a potential increased risk to the US population.4,6–10
A fundamental dose management principle for CT is that the scanner output must be adjusted appropriately for both the specific patient size and the required diagnostic task.11 This is critically important for pediatric patients,12–17 where scanner radiation output must be reduced relative to adult parameters to account for the decreased attenuation of the patient. In contrast, larger patients require increased scanner radiation output to account for greater patient attenuation and yield a sufficient number of transmitted photons to render an image of diagnostic quality. This strategy can be accomplished with use of technique charts, which prescribe tube potential, tube current, rotation time, detector collimation, and pitch as a function of some measure of patient attenuation. Because x-ray attenuation is related to the total amount of tissue in the x-ray beam, local measures of patient size (eg, lateral width or perimeter at a given scan location) is a better measure of attenuation than global measures such as patient weight or body mass index. This is equally true for pediatric and adult body examinations, where age and sex can be similarly weak predictors of attenuation at a particular scan level. Computed tomographic imaging of the head is a notable exception, where age is a reasonable predictor of cranial calcification, which is the primary component of attenuation in the head.
A major advance in the appropriate management of CT dose was the introduction of automatic exposure control (AEC) systems,11,18–21 which use information obtained from the CT localizer radiograph to estimate the patient’s attenuation at each position within the scan volume. Instead of manually setting scanner parameters, which are then fixed for the entire scan, AEC automatically adjusts the tube current based on the patient attenuation, which can vary longitudinally at every z axis position and angularly for each projection. Studies have demonstrated that modulating the tube current as function of position and/or rotation can reduce patient dose by 40% to 50% without sacrificing the image quality.11,18–21 Three different tube current modulation schemes have been implemented and evaluated: (1) modulation of the tube current angularly around the patient, (2) modulation of the tube current based on the average attenuation at a given position along the long axis (ie, z axis) of the patient, and (3) the combination of these 2 approaches. In essence, AEC systems automatically adjust tube current to account for global variations in patient sizes and local thickness variations in individual patients.
To automate tube current modulation, 2 distinct AEC methods to determine the appropriate tube current for a specific amount of patient attenuation have been implemented by scanner manufacturers. The most commonly used method adjusts the tube current so that image noise (measured as the standard deviation of the CT numbers in a uniform region of interest) is maintained across all patients sizes. The second method adjusts the tube current in a manner designed to achieve different noise levels at different patient sizes.22 For example, relative to a typical-sized adult, image noise is allowed to increase in obese patients, who inherently have increased soft tissue contrast due to increased amounts of adipose tissue layers. In small children, who typically have smaller structural details and decreased soft tissue contrast due to decreased amounts of adipose tissue, a lower level of image noise (ie, higher level of image quality) is required.11,20,23,24
The purpose of this study was to investigate relative image noise and patient dose that results from an AEC system that is predicated on a constant noise paradigm as compared with clinically accepted technique charts and AEC systems designed to vary image noise with patient size. First, a mathematical framework was developed to describe AEC-driven tube current modulation using an exponential function and was used to estimate the effect on relative noise and dose levels across patient sizes. Second, a set of experimental measurements acquired with phantoms of varied thicknesses was used to corroborate the model. Results from both the model and experiments were compared with our clinical body CT technique charts to assess the impact of each method relative to our established clinical standards. These charts were developed and used in our practice to adapt tube current and radiation dose to different sizes of patients to obtain the required level of image quality at the lowest-dose settings and are consistent with other published size adaptation schemes.25,26
MATERIALS AND METHODS
Tube Current Modulation Model
The detected x-ray intensity I after attenuation by a patient can be expressed as
| (1) |
where I0 is the incident x-ray intensity, μ is the linear attenuation coefficient of soft tissue, and t is patient thickness. This equation assumes monochromatic x-rays. For a polychromatic x-ray spectrum, μ can be considered as the linear attenuation coefficient of the mean beam energy. At a given tube potential, the incident and detected x-ray intensities are both proportional to the tube current and exposure time (gantry rotation time). Automatic exposure control systems, however, modulate the tube current only and keep the rotation time constant. Thus, tube current, instead of tube current-time product, will be used in the following analysis.
In the quantum-limited regime, image noise is determined by the photon fluence, or in this case (ie, constant gantry rotation speed), the intensity described in Eq. (1). To maintain constant noise across all patient sizes, the x-ray intensity exiting a reference patient of size tref should equal the x-ray intensity exiting a patient of different size, t. This imposes the following requirement:
| (2) |
Because I0 is proportional to tube current (mA), Eq. (2) can be expressed as
| (3) |
or
| (4) |
where the reference tube current (mAref) is defined as the lowest tube current that provides diagnostically acceptable image quality for a specific diagnostic task, tube potential, and set of reconstruction parameters.
For convenience, the attenuation coefficient of a material can be expressed in terms of its half value layer (HVL) for a given x-ray beam, which is defined as the thickness of a specified material that is required to reduce the radiation intensity by half.
| (5) |
Solving Eq. (5) for μ yields μ = ln(2)/HVL. Substituting this into Eq. (4) yields the convenient expression:
| (6) |
The human body is composed primarily of water and soft tissue, which have very similar x-ray attenuation properties. Thus, it is reasonable to use the HVL of water to estimate patient attenuation. Half value layer is dependent on the x-ray beam spectrum because attenuation changes with photon energy. For the most commonly used CT x-ray tube potential, 120 kV, the mean photon energy is approximately 70 keV.27,28 The HVL of water for such a beam is approximately 3.6 cm (linear attenuation coefficient of water at 70 keV is approximately 0.19 cm−1),29 meaning that the x-ray intensity is decreased by a factor of 2 with every additional 3.6 cm of water-equivalent material introduced into the x-ray beam. Using this value for HVL and Eq. (6), the tube current required to maintain a constant noise level in the reconstructed images can be calculated for different patient sizes. Figure 1 shows the tube current required to maintain a constant level of image noise as patient size increases. Because the tube current (for a fixed rotation time) needs to be doubled for every 3.6-cm increase in patient size, the required input x-ray intensity increases rapidly. Consequently, the tube current can easily reach a maximum value, which limits this tube current modulation strategy.
FIGURE 1.
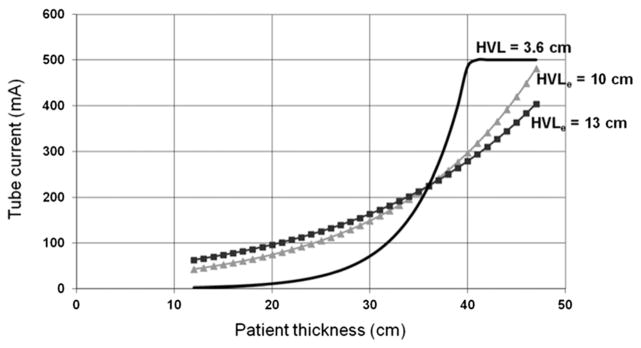
Examples of the tube current modulation schemes based on a 36-cm reference patient thickness (tref = 36 cm) and 225-mA reference tube current (mAref = 225 mA). The solid line represents the tube current required to achieve a constant noise across patient thickness variations. The scanner reaches a maximum tube current for patients at least 40 cm thick, which could lead to sacrifices in image quality through longer gantry rotation times and/or lower spiral pitch values. The dotted curve represents a less aggressive modulation of tube current, where the tube current is not increased as strongly for large patients or decreased as strongly for small patients. This maintains adequate image noise in small patients and avoids excessive radiation doses for large patients. The curves reflect the abdominal (HVLe = 10 cm; b = 0.36) and thoracic (HVLe = 13 cm; b = 0.28) technique charts used in our large clinical practice for more than 2 decades (see Eq. [7]).
Published studies, as well as our own clinical experience, have found that maintaining the same image noise across all patient sizes is not ideal in clinical practice.17,20,23,24,30,31 To this end, some AEC systems vary the image noise target with patient thickness. To model these types of AEC systems, adjustment of the HVL in Eq. (6) can be used to decrease or increase the amount of tube current modulation with patient thickness. Such an empirically determined HVL value (HVLe) can be expressed in terms of the nominal HVL of water using the following relationship:
| (7) |
where b is a parameter that can be adjusted to control the strength of the change in tube current as a function of patient size. The HVLe used in our practice for abdominal CT is 10 cm; for thoracic CT, we used an HVLe of 13 cm. A comparison of the curves relating required tube current to patient thickness for the constant noise paradigm (HVL of water = 3.6 cm) and for our empirically derived values of HVLe is shown in Figure 1.
Although we have found the use of an empirical HVL nomenclature relatively intuitive, one cannot actually adjust patient HVL, because this is a physical property related to the x-ray beam spectrum and patient composition. Using Eq. (7), we can more accurately express this adaptation of the tube current as patient size changes by rewriting Eq. (6) in terms of the physical HVL.
| (8) |
Using Eq. (8), various tube current adaptation schemes can be modeled by selecting different values for b. The case of constant noise is achieved when b = 1. The case in which the tube current changes by smaller amounts as patient size is increased or decreased (ie, relatively less noise for smaller patients and more noise for larger patients) is achieved when b < 1. Alternatively, requiring less noise in obese patients and more noise in small patients, relative to the standard patient size, is achieved when b > 1. For a given patient thickness, tube current values (for a given b value) relative to our clinical abdomen/pelvis practice (b = 0.36 and b = 0.28 for our thorax protocols) can be calculated using Eq. (8):
which can be reduced to
| (9) |
where mAclin(t) is the clinically used tube current value (b = 0.36) as a function patient thickness and mAb(t) is the modulated tube current for a given b value as a function of patient thickness.
The relative tube current values as compared with our clinical technique charts (in which b = 0.36) were calculated for each patient size as a function of tube current adaptation strength. At a given patient thickness and assuming all other scan parameters are held constant, relative changes in tube current values are equivalent to relative changes in patient dose. Thus, relative tube current values calculated here are synonymous with relative patient dose. Similarly, relative noise values were calculated for each patient size as a function of current adaptation strength. The relative image noise was calculated assuming that the system is operating in the quantum-limited region32 with the following:
| (10) |
where σ2 is the noise variance (standard deviation of CT numbers squared), the subscript b refers to the model results at a given current adaptation value, and the subscript clin refers to our clinical values (ie, b = 0.36).
Phantom Measurements
To demonstrate the above principles experimentally and corroborate the model, phantom studies were performed using three semianthropomorphic thorax phantoms (Cardio CT; QRM, Mohrendorf, Germany) with lateral dimension of 30, 35, and 40 cm to simulate small, medium, and large patient sizes, respectively (Fig. 2). The phantoms were scanned using a CT system equipped with a longitudinal (z axis) AEC system (Auto-mA, LightSpeed VCT; GE Healthcare, Waukesha, WI). This system uses a constant noise paradigm to adapt the tube current modulation as patient size changes (ie, b = 1 in the proposed framework, Eq. (8)). The user prescribes a “noise index” to establish the target noise value for a standard patient size, and the system adapts the tube current as patient size changes to maintain the same noise level at each position along the z axis and each patient size. Scans were performed using noise index values of 10, 12, and 14 for each of the three phantom sizes, and the tube current prescribed by the system for each phantom size was recorded for each noise index. The results were compared with the thorax CT technique chart used in our clinic, where we change the tube current more slowly as patient size changes (ie, b = 0.28).
FIGURE 2.

Photograph of the anthropomorphic thorax phantoms used for data measurement. The lateral width of the small phantom is 30 cm. The addition of the hollow, oval tissue–equivalent material results in a net lateral thickness of 45 cm (left) and 40 cm (right).
RESULTS
Figure 3 demonstrates the influence of b, which is used to determine the strength of the tube current modulation. For our practice, we have found that b = 0.36 (HVLe = 10 cm) for abdomen and pelvis scans generate clinically acceptable image quality across the full spectrum of patient sizes. (For chest scans, we found b = 0.28 [HVLe = 13 cm] yields acceptable images for all patient sizes.)
FIGURE 3.
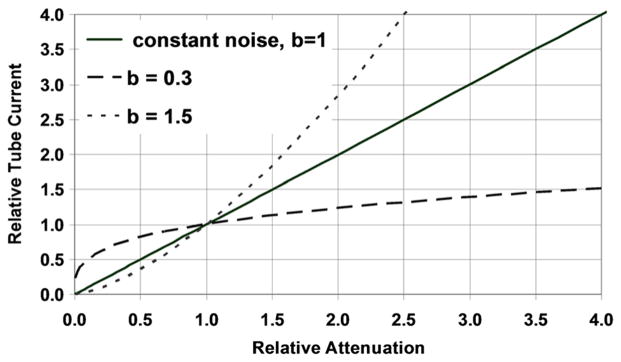
Demonstration of the effect of varying b values to adapt the strength of the tube current modulation. Relative attenuation refers to attenuation compared with the attenuation for a reference patient thickness (ie, 36 cm). Relative tube current is compared with the tube current setting for the reference patient thickness. For b = 1, the tube current is linearly increased with patient attenuation, such that image noise is held constant. For b < 1 (0.3), the tube current is neither decreased nor increased as aggressively, resulting in less noise in small patients and more noise in large patients relative to that of the reference patient. For b > 1, the tube current is very aggressively decreased for small patients and increased for large patients relative to the reference patient. This scenario is not likely to be clinically relevant.
Relative dose levels (ie, tube current), as compared with our clinical settings, for b values of 0.3, 0.5, 0.7, and 1 for patient widths from 12 to 50 cm are shown in Table 1, where a lateral thickness of 36 cm was used as our standard reference for patient size. To maintain a constant noise level (b = 1), the dose to a 12-cm-wide patient would be 5% of our typical clinical value. For a 50-cm-wide patient, the dose would be 549% of our typically administered dose. (Neither of these options is technically feasible on current CT systems.) Thus, there are large differences in prescribed tube current between the constant noise requirement (b = 1) and what was used routinely in our clinical practice (b = 0.36).
TABLE 1.
Relative Radiation Dose (ie, Tube Current) as a Function of Patient Width and Modulation Strength (b) as Compared With Our Clinically Accepted Values
| Patient Width, cm | Modulation Strength (b)
|
|||
|---|---|---|---|---|
| 0.30 | 0.50 | 0.70 | 1.00 | |
| 12 | 131% | 53% | 21% | 5% |
| 20 | 120% | 65% | 36% | 14% |
| 28 | 110% | 81% | 60% | 38% |
| 36 | 100% | 100% | 100% | 100% |
| 44 | 91% | 124% | 168% | 265% |
| 50 | 85% | 145% | 247% | 549% |
Calculations were performed using Eq. (9) and a patient reference thickness of 36 cm.
The relative noise values for the same range of modulation strengths (b = 0.3, 0.5, 0.7, and 1.0) were compared with that of our clinical technique chart (b = 0.36; Table 2). A lateral width of 36 cm was again used as the reference patient size. The relative noise for patient sizes from 12 to 50 cm was calculated using Eq. (10). The values produced from a constant noise requirement greatly differed from values in our current clinical practice. Image noise for b = 1 relative to that at b = 0.36 increased with smaller patient sizes and decreased with larger patient sizes. For the 12-cm patient and b = 1, image noise was 430% of our typical clinical value. For the 50-cm patient and b = 1, image noise was 43% of our usual clinical value. Thus, using the constant noise paradigm, image noise would be unacceptably high for small patients compared with the clinical requirements reflected by our existing technique charts. In addition, the requirement of constant noise would overexpose large patients compared with what we currently use, delivering increased dose to achieve lower noise values than what we have found to be clinically necessary.
TABLE 2.
Relative Image Noise as a Function of Patient Width and Modulation Strength (b)
| Patient Width, cm | Modulation Strength (b)
|
|||
|---|---|---|---|---|
| 0.30 | 0.50 | 0.70 | 1.00 | |
| 12 | 87% | 138% | 218% | 435% |
| 20 | 91% | 124% | 168% | 267% |
| 28 | 95% | 111% | 130% | 163% |
| 36 | 100% | 100% | 100% | 100% |
| 44 | 105% | 90% | 77% | 61% |
| 50 | 109% | 83% | 63% | 42% |
Phantom Studies
In Figure 4, the required tube current values for different sizes of the anthropomorphic phantom are shown for prescribed noise index values of 10, 12, and 14, and are compared with the tube current values that we would have prescribed using our clinical technique charts (for thorax, b = 0.28). For the 35-cm-wide phantom (very close to our standard patient size of 36 cm), use of a noise index of 12 resulted in tube current values that were very similar to those that would have been prescribed using our clinical technique chart. However, as the phantom size changed, the tube current values for the constant noise paradigm were considerably different from our clinical technique charts for all noise index values. For the smaller 30-cm-wide thorax, use of noise indices of 10, 12, and 14 would result in lower tube current values and thus greater image noise than what we have accepted clinically. Conversely, for the larger 40-cm-wide thorax, even at the highest noise index level of 14, the constant noise paradigm would deliver considerably more radiation dose to the patient than would our clinical technique charts, which provide sufficient image quality for clinical diagnosis.
FIGURE 4.
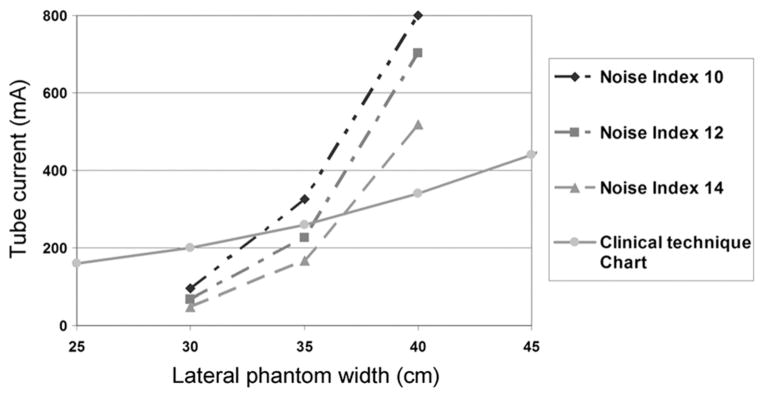
Required tube current values for different phantom sizes using noise index of 10, 12, and 14, and our clinical technique chart.
DISCUSSION
Automatic exposure control systems have been widely accepted in clinical practice as an effective method of adapting radiation output to the specific patient’s size, accounting for thickness variations within a given cross section or along the patient’s longitudinal axis.11,18–21 Details of the specific implementations used by the different manufacturers, however, are absent in the scientific literature. This is a key barrier to proper use of these systems. It is essential that users understand the algorithm that decides which tube current will be prescribed as patient size varies, so that clinically acceptable image quality is obtained at the lowest reasonable dose across all patient sizes.
The tube current adaptation scheme implemented by at least 2 major manufacturers varies the tube current so that the noise level in the resulting images is constant across all patient sizes. Our results indicate that such an approach could yield excessively noisy images in children or small patients and unnecessarily high doses in large patients.
In this study, we developed a flexible model relating image noise, patient attenuation, and tube current (ie, dose) and demonstrated that the behavior predicted by the model is consistent with one commercial AEC implementation in a phantom study. Our results indicate that the requirement of constant noise across all patient sizes yields higher radiation dose for large patients, as compared with our clinical practice. This would also place unnecessarily large demands on the x-ray tube and generator, which may potentially cause tube heating problems or require the operator to increase the overall scan time. Conversely, the tube current would be dropped so dramatically for small patients that the image noise would be increased beyond what our practice would consider clinically acceptable. This may reduce the diagnostic accuracy of the examination or potentially yield examinations that are not diagnostic. In this latter scenario, a repeat examination at a higher dose level may be necessary to obtain a clinically acceptable image, wasting the radiation (and perhaps contrast media) used in the initial scan.
Although the preferred adaptation strength (b value) may differ between practices, our experience using manual technique charts has been that values close to 0.36 were appropriate for abdominal CT examinations and that values close to b = 0.28 were appropriate for thoracic examinations. These values are not necessarily the only acceptable values.
Currently, at least one manufacturer has implemented an AEC system that varies the strength of the tube current adaptation as patient size varies (ie, b value < 1). In this system, the b value used to decrease the tube current for small patient sizes is different from the b value used to increase the tube current for large patient sizes. Our practice uses a strength of b = 0.33 (“average”) for obese patients and b = 0.5 (average) for slim patients on Sensation 16 and 64 scanners (Siemens Healthcare). On Definition DS, AS+, and FLASH systems (Siemens Healthcare), we use a strength of b = 0.4 and b = 0.5 (specific b values provided by the manufacturer). For some software versions, these are global scanner settings that are independent of the specific imaging examination. For newer software versions, these can be set for each type of exam protocol. The user-prescribed reference tube current for the reference patient size determines the noise level that is acceptable for a specific diagnostic task (eg, CT colonography or CT angiography). The tube current is then decreased or increased relative to the reference values using the system’s global adaptation strengths.
Computed tomographic systems that use a constant noise AEC paradigm may result in nondiagnostic quality images or overexposure of the patient. For small patients, the dose may be decreased so much that the examination is either too noisy or nondiagnostic. For large patients, higher dose levels than are necessary may be applied. Tube current and generator power limits may be reached, potentially resulting in the need to increase the gantry rotation time or decrease the spiral pitch value. These actions would increase the overall scan times, which increases the risk of nonuniform contrast enhancement and may require a longer contrast injection or increased volume of contrast media. Motion artifacts may also occur for these longer scan times if the patient cannot hold his/her breath for the entire scan. To avoid these consequences of a constant noise requirement, a noise index technique chart can be used to manually adjust tube current modulation for patient size. Such a technique chart would prescribe different noise index values for different patient sizes, using lower noise indices for small patients and higher noise indices for obese patients. This approach has been recommended by others based on clinical experience.20,24 This approach provides sufficient image quality in pediatric patients and avoids excessive radiation doses in obese patients. In addition, some manufacturers allow the user to set both minimum and maximum tube current values. If appropriately set, these values can prevent either an excessive decrease (in small patients) or increase (in large patients) of the tube current. The noise index technique chart used in our adult abdomen CT practice for a system (GE LightSpeed VCT; GE Healthcare, Milwaukee, WI) that uses a constant noise AEC paradigm is shown in Table 3. The same approach could be adapted to build noise index technique charts for other scanners with AEC using a constant noise paradigm.22
TABLE 3.
Noise Index Technique Chart Used in Our Adult Abdominal CT Practice for an AEC System That Uses the Constant Noise Paradigm (VCT; GE Healthcare)
| Lateral Patient Width, cm | Noise Index | Rotation Time, s | Minimum Tube Current, mA | Maximum Tube Current, mA |
|---|---|---|---|---|
| 22.1–30 | 9 | 0.5 | 150 | 280 |
| 30.1–40 | 11.5 | 0.5 | 220 | 500 |
| 40.1–45 | 14.5 | 0.5 | 400 | 720 |
| 45.1–50+ | 17 | 0.7 | 450 | 770 |
These values are for 5-mm slice thicknesses. A gantry rotation of 0.5 seconds is used until patient size exceeds 45 cm, when a gantry rotation of 0.7 seconds is required to achieve sufficient tube output.
Acknowledgments
Cynthia McCollough is the recipient of a research grant from Siemens Healthcare. No grant support was used to fund this work.
Footnotes
The other authors declare no conflict of interest.
References
- 1.Crawford CR, King KF. Computed tomography scanning with simultaneous patient translation. Med Phys. 1990;17:967–982. doi: 10.1118/1.596464. [DOI] [PubMed] [Google Scholar]
- 2.Kalender WA, Seissler W, Klotz E, et al. Spiral volumetric CTwith single-breath-hold technique, continuous transport, and continuous scanner rotation. Radiology. 1990;176:181–183. doi: 10.1148/radiology.176.1.2353088. [DOI] [PubMed] [Google Scholar]
- 3.IMV 2006 CT Market Summary Report. Des, Plains, IL: IMV Medical Information Division; 2006. [Google Scholar]
- 4.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 5.McCollough CH, Guimaraes L, Fletcher JG. In defense of body CT. AJR Am J Roentgenol. 2009;193:29–39. doi: 10.2214/AJR.09.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody AS, Frush DP, Huda W, et al. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 8.Rice HE, Frush DP, Farmer D, et al. Review of radiation risks from computed tomography: essentials for the pediatric surgeon. J Pediatr Surg. 2007;42:603–607. doi: 10.1016/j.jpedsurg.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Nickoloff EL, Alderson PO. Radiation exposures to patients from CT: reality, public perception, and policy. AJR Am J Roentgenol. 2001;177:285–287. doi: 10.2214/ajr.177.2.1770285. [DOI] [PubMed] [Google Scholar]
- 10.Schauer DA, Linton OW. NCRP Report No. 160, ionizing radiation exposure of the population of the United States, medical exposure—are we doing less with more, and is there a role for health physicists? Health Phys. 2009;97:1–5. doi: 10.1097/01.HP.0000356672.44380.b7. [DOI] [PubMed] [Google Scholar]
- 11.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26:503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 12.Frush DP. Strategies of dose reduction. Pediatr Radiol. 2002;32:293–297. doi: 10.1007/s00247-002-0684-9. [DOI] [PubMed] [Google Scholar]
- 13.Huda W, Bushong SC. In x-ray computed tomography, technique factors should be selected appropriate to patient size. Med Phys. 2001;28:1543–1545. doi: 10.1118/1.1388903. [DOI] [PubMed] [Google Scholar]
- 14.Huda W, Lieberman KA, Chang J, et al. Patient size and x-ray technique factors in head computed tomography examinations. II. Image quality. Med Phys. 2004;31:595–601. doi: 10.1118/1.1646233. [DOI] [PubMed] [Google Scholar]
- 15.Huda W, Lieberman KA, Chang J, et al. Patient size and x-ray technique factors in head computed tomography examinations. I. Radiation doses. Med Phys. 2004;31:588–594. doi: 10.1118/1.1646232. [DOI] [PubMed] [Google Scholar]
- 16.McCollough CH. CT dose: how to measure, how to reduce. Health Phys. 2008;95:508–517. doi: 10.1097/01.HP.0000326343.35884.03. [DOI] [PubMed] [Google Scholar]
- 17.Nyman U, Ahl TL, Kristiansson M, et al. Patient-circumference–adapted dose regulation in body computed tomography. A practical and flexible formula. Acta Radiol. 2005;46:396–406. doi: 10.1080/02841850510021193. [DOI] [PubMed] [Google Scholar]
- 18.Gies M, Kalender WA, Wolf H, et al. Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies. Med Phys. 1999;26:2235–2247. doi: 10.1118/1.598779. [DOI] [PubMed] [Google Scholar]
- 19.Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements. Med Phys. 1999;26:2248–2253. doi: 10.1118/1.598738. [DOI] [PubMed] [Google Scholar]
- 20.Kalra MK, Maher MM, Toth TL, et al. Techniques and applications of automatic tube current modulation for CT. Radiology. 2004;233:649–657. doi: 10.1148/radiol.2333031150. [DOI] [PubMed] [Google Scholar]
- 21.Kalra MK, Naz N, Rizzo SM, et al. Computed tomography radiation dose optimization: scanning protocols and clinical applications of automatic exposure control. Curr Probl Diagn Radiol. 2005;34:171–181. doi: 10.1067/j.cpradiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Goo JM, Ye HJ, et al. Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics. 2008;28:1451–1459. doi: 10.1148/rg.285075075. [DOI] [PubMed] [Google Scholar]
- 23.Wilting JE, Zwartkruis A, van Leeuwen MS, et al. A rational approach to dose reduction in CT: individualized scan protocols. Eur Radiol. 2001;11:2627–2632. doi: 10.1007/s003300101039. [DOI] [PubMed] [Google Scholar]
- 24.Kalra MK, Maher MM, D’Souza RV, et al. Detection of urinary tract stones at low-radiation-dose CT with z axis automatic tube current modulation: phantom and clinical studies. Radiology. 2005;235:523–529. doi: 10.1148/radiol.2352040331. [DOI] [PubMed] [Google Scholar]
- 25.American Association of Physicists in Medicine. Report #96. College Park, MD: American Association of Physicists in Medicine; 2008. The measurement, reporting and management of radiation dose in CT. [Google Scholar]
- 26. [Accessed March 2015];Image Gently Development of Pediatric CT Protocols 2014. Available at: http://www.imagegently.org/Portals/6/Procedures/IG%20CT%20Protocols%20111714.pdf.
- 27.Boone JM, Seibert JA. An accurate method for computer-generating tungsten anode x-ray spectra from 30 to 140 kV. Med Phys. 1997;24:1661–1670. doi: 10.1118/1.597953. [DOI] [PubMed] [Google Scholar]
- 28.Siewerdsen JH, Waese AM, Moseley DJ, et al. Spektr: a computational tool for x-ray spectral analysis and imaging system optimization. Med Phys. 2004;31:3057–3067. doi: 10.1118/1.1758350. [DOI] [PubMed] [Google Scholar]
- 29.Hubbell JH, Seltzer SM. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest*. [Accessed July 2010];NIST. Available at: http://www.nist.gov/physlab/data/xraycoef/index.cfm.
- 30.McCollough CH. Automatic exposure control in CT: are we done yet? Radiology. 2005;237:755–756. doi: 10.1148/radiol.2373051151. [DOI] [PubMed] [Google Scholar]
- 31.McCollough CH, Zink FE, Kofler J, et al. Dose optimization in CT: creation, implementation and clinical acceptance of size-based technique charts. Radiology. 2002;225(P):591. [Google Scholar]
- 32.Kalender WA. Computed Tomography: Fundamentals, System Technology, Image Quality, Applications. 2. Erlangen: Publicis Corporate Publishing; 2005. [Google Scholar]


