Abstract
High protein secretion capacity in filamentous fungi requires an extremely efficient system for protein synthesis, folding and transport. When the folding capacity of the endoplasmic reticulum (ER) is exceeded, a pathway known as the unfolded protein response (UPR) is triggered, allowing cells to mitigate and cope with this stress. In yeast, this pathway relies on the transcription factor Hac1, which mediates the up-regulation of several genes required under these stressful conditions. In this work, we identified and characterized the ortholog of the yeast HAC1 gene in the filamentous fungus Neurospora crassa. We show that its mRNA undergoes an ER stress-dependent splicing reaction, which in N. crassa removes a 23 nt intron and leads to a change in the open reading frame. By disrupting the N. crassa hac-1 gene, we determined it to be crucial for activating UPR and for proper growth in the presence of ER stress-inducing chemical agents. Neurospora is naturally found growing on dead plant material, composed primarily by lignocellulose, and is a model organism for the study of plant cell wall deconstruction. Notably, we found that growth on cellulose, a substrate that requires secretion of numerous enzymes, imposes major demands on ER function and is dramatically impaired in the absence of hac-1, thus broadening the range of physiological functions of the UPR in filamentous fungi. Growth on hemicellulose however, another carbon source that necessitates the secretion of various enzymes for its deconstruction, is not impaired in the mutant nor is the amount of proteins secreted on this substrate, suggesting that secretion, as a whole, is unaltered in the absence of hac-1. The characterization of this signaling pathway in N. crassa will help in the study of plant cell wall deconstruction by fungi and its manipulation may result in important industrial biotechnological applications.
Introduction
The endoplasmic reticulum (ER) is crucial for the production of membrane and secreted proteins and consequently, its function is under tight control. To maintain protein folding homeostasis in the ER, the cell must balance the ER protein folding capabilities to the protein flux through the secretory pathway. When protein folding requirements exceed the ER’s folding capabilities, unfolded proteins accumulate within this organelle, a condition known as ER stress. ER resident transmembrane sensors then trigger a conserved signaling pathway known as the unfolded protein response (UPR) (reviewed in [1, 2]). Activation of these sensors lead to a major transcriptional program aimed at increasing folding capacity in the ER and adjusting the secretory pathway [3], while also mediating a decrease in ER protein load through selective mRNA degradation and translational repression [4–7], as well as a global reduction in protein synthesis [8]. These mechanisms, along with others [9], work together to revert ER stress and re-attain protein folding homeostasis in the ER [10].
In eukaryotic microorganisms such as budding yeast, where this pathway has been most extensively studied, the only identified ER stress sensor is Ire1, an ER-resident transmembrane protein that has kinase and endoribonuclease activity [11, 12]. The expression of UPR target genes is controlled by a key member of this regulatory branch, the bZIP transcription factor Hac1 [13, 14]. Upon sensing unfolded proteins on its luminal side, Ire1 oligomerizes, trans-autophosphorylates and undergoes conformational changes that ultimately lead to the activation of its cytosolic RNase domain (reviewed in [2, 15]). Once activated, this domain specifically cleaves its only known target, the HAC1 mRNA, in two sites, releasing a 252 nt intron, whereas the two resulting exons are then ligated together by the tRNA ligase Trl1/Rgl1 (reviewed in [1, 2]. This unconventional splicing reaction relieves the transcript from translational repression and leads to the generation of a potent transcriptional activator [2]. The Hac1 protein can then translocate into the nucleus and mediate a transcriptional program that ultimately affects over 5% of the Saccharomyces cerevisiae transcriptome, modulating multiple ER and secretory pathway functions, with the goal of alleviating ER stress [3]. Attenuation of the UPR is partially achieved through inactivation of Ire1 by the Ptc2 phosphatase [16]. In addition, in all fungal species evaluated so far, hac1 or ire1 deletions result in increased sensitivity to cell wall perturbing agents, suggesting a coordination between the responses to these related stress conditions [17].
Filamentous fungi have been exploited industrially for the production of economically relevant proteins due to their efficient, high-capacity protein secretion capabilities [18, 19]. The UPR has been studied in a limited number of these organisms mostly with the goal of overcoming bottlenecks encountered in the industrial production of these proteins [20, 21]. In this context, the UPR has been examined in commercially relevant filamentous fungi, including Trichoderma reesei, Aspergillus nidulans, and Aspergillus niger [22–26]. In general, the basic elements of the response are similar to those in budding yeast, with filamentous fungi exhibiting the widely conserved Ire1/Hac1 branch and regulating several aspects of ER function upon ER stress [22, 26]. One interesting difference is that the regulatory intron removed from the transcript of the Hac1 homologs in these organisms is small (20 nt long), closer to the size described in plants and animals [27]. In addition, the 5’ end of the Hac1 transcript has been reported to be truncated upon ER stress in these few evaluated filamentous fungi [22, 23, 28], another difference with the S. cerevisiae system. These findings highlight the differences that a particular (and even well characterized) process can have among different fungal species, supporting the need to further characterize it in different systems to address biodiversity and niche-associated peculiarities. Indeed, further differences can be found within fungi (this study and [24, 29]). Fungal UPR studies have gathered momentum not only due to the importance of protein secretion in industrially relevant fungi, but more recently because the UPR has been found to impact fungal lifestyle, particularly virulence, in both animal [28–31] and plant [32–34] pathogens. Whether other aspects of filamentous fungi lifestyles require a functional UPR, is largely unexplored.
Fungi are known to play an important role in the decomposition and recycling of organic material in their natural settings. To efficiently metabolize the complex polymeric substrates encountered in the wild, fungi exhibit exquisitely regulated secretion of numerous enzymes, a result of their saprobic lifestyles. Filamentous fungi such as T. reesei, A. niger and more recently Neurospora crassa, have been studied to dissect the molecular basis of plant cell deconstruction (reviewed in [35]). Importantly, the numerous molecular, genetic and biochemical tools available for N. crassa [36] have recently made this fungus an attractive system in which to study these processes and have propelled it as a platform for research in biofuel production [37].
In this work, we set out to characterize HAC1 and the unfolded protein response in N. crassa, which surprisingly, despite decades of cell biology research in this organism and the existence of a fully annotated genome [38], has not yet been studied. We show that the N. crassa hac-1 mRNA undergoes an ER stress-dependent splicing reaction, which removes a 23 nt intron which changes the open reading frame. In addition, we establish that the N. crassa gene can complement a S. cerevisiae HAC1 mutant. Disruption of hac-1 in N. crassa reveals it to be essential for growth under ER stress and for activating the UPR, but also shows that it appears to be dispensable for the response against cell wall perturbing agents, an unusual finding in fungi which suggests uncoupling between these stress responses in N. crassa. In addition, we found that growth on cellulose, the most abundant component of plant biomass and a natural substrate of Neurospora in the wild, imposes high demands on ER function and is dramatically impaired in the absence of HAC-1, pointing towards a physiological role for the UPR in this fungus. Our data thus support a specific role for HAC-1 in the UPR in N. crassa and for this transcription factor in cellulose catabolism, highlighting a basic cell signaling pathway that can be further manipulated-considering the variety of molecular tools available in N. crassa- to provide new insights into the biotechnological applications of this filamentous fungus, particularly in the context of the bioconversion of lignocellulosic material to simple sugars for biofuel production.
Results
Identification and characterization of N. crassa hac-1
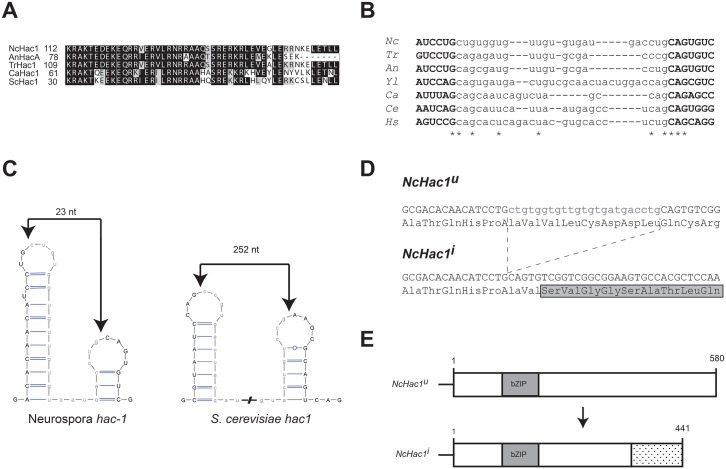
A BLASTP search of the N. crassa genome, using the S. cerevisiae Hac1 protein sequence as query, revealed NCU01856 as a putative ortholog of this gene. The predicted N. crassa protein, herein referred to as HAC-1, is composed of 580 amino acids and contains a conserved bZIP domain, highly similar to the one present in other characterized Hac1p homologs from different fungal species (Fig 1A). In addition, a small element in the 3’ UTR of the S. cerevisiae hac1 mRNA, which has been shown to be important of its splicing in vivo [39], also appears to be conserved in the identified N. crassa hac-1 gene.
Fig 1. Neurospora crassa has a putative Hac1 homolog.
A) Alignment of the amino acid sequence of the bZIP domain of selected fungal HAC-1 homologs. Nc: Neurospora crassa; An: Aspergillus nidulans; Tr: Trichoderma reseei; Ca: Candida albicans; Sc: Saccharomyces cerevisiae. B) Sequence alignment of the putative non-conventional intron and surrounding regions of hac-1 homologs in fungi. Asterisks denote positions conserved in all the sequences considered in the alignment. Nc: Neurospora crassa; Tr: Trichoderma reseei; An: Aspergillus nidulans; Yl: Yarrowia lipolytica; Ca: Candida albicans; Ce: Caenorhabditis elegans; Hs: Homo sapiens. C) Predicted twin stem-loop structure of the intron of the N. crassa and yeast hac-1 mRNAs. Folding prediction was made with mFOLD [76] and the structures drawn with VARNA [77]. Predicted cleavage sites are indicated by an arrow. The predicted intron sequence is shown in lowercase. D) Nucleotide and deduced amino acid sequence surrounding the putative splice sites in the N. crassa hac-1 CDS. Upon ER stress, the putative intron (shown in lowercase) is removed from the uninduced version of the hac-1 mRNA (hac-1 u), resulting in the induced version (hac-1 i). Translation of the induced version would alter the reading frame, leading to a different C-terminal region. E) Schematic representation of NcHAC-1u and NcHAC-1i.
The S. cerevisiae HAC1 mRNA undergoes a non-spliceosomal splicing reaction under ER stress, catalyzed by the kinase/RNAse Ire1p and the tRNA ligase Trl1/Rlg1p, which results in the removal of a 252 nt intron. In filamentous fungi and interestingly also in the yeast Candida albicans, shorter introns (~20 nt long) have been reported for the Hac1 homologs. By comparing the sequence of hac-1 with those in different fungal species, we predicted that under ER stress, an intron of 23 nt would be removed from the hac-1 mRNA. This is based on the known consensus splice sites for the unconventional intron in various organisms (Fig 1B). While in other filamentous fungi, the exact position of the intron could not be unambiguously determined due to the presence of a CTGCAG segment at each side of the intron [22, 23, 28], the predicted N. crassa intron sequence is asymmetrically flanked, with CTGCTG on the 5’ region and CTGCAG on the 3’ end (Fig 1B), bordering a 23 nt intron. Further supporting a 23 nt unconventional intron in the N. crassa hac-1 mRNA, is its predicted secondary structure and surrounding sequences. IRE-1 targets exhibit a similar predicted RNA secondary structure, consisting of twin hairpins in which the cleavage sites reside on the loops [40]. The lowest free energy form of hac-1 mRNA also conforms to this structure, similar to its counterparts in different organisms, with the predicted cleavage sites located on the loops and surrounding the predicted 23 nt intron (Fig 1C). Removal of this intron in the N. crassa hac-1 sequence would alter the reading frame (Fig 1D) leading to the production of a predicted shorter protein (Fig 1E). Moreover, and consistent with a phylogenetically conserved mechanism, the N. crassa genome harbors, in addition to hac-1, genes encoding for the putative homologs of Ire1p, Rlg1p and Ptc2 (S2 Table).
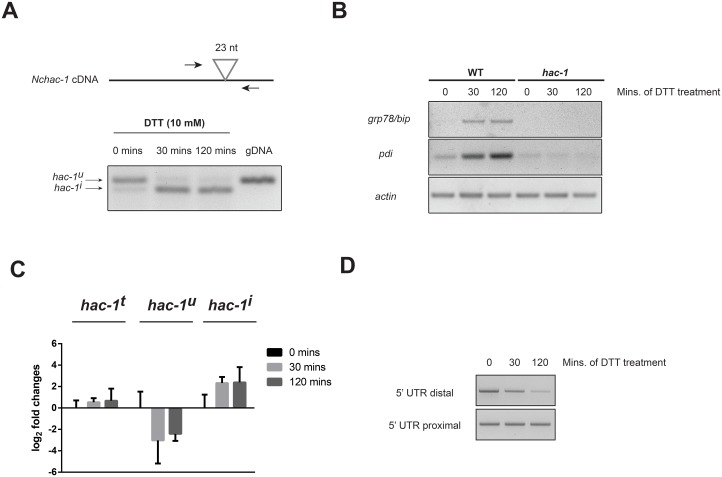
To determine whether the N. crassa hac-1 mRNA is indeed processed in response to ER stressing agents, N. crassa liquid cultures were treated with the reducing agent dithiothreitol (DTT) for 30 and 120 minutes. DTT is known to induce ER stress by altering the oxidative environment in the ER and disrupting disulfide bonds, thus leading to the accumulation of misfolded proteins. By designing primers flanking the predicted splice site (Fig 2A, top), it is therefore possible to assess whether the mRNA is processed and the predicted unconventional intron removed, in an ER stress-dependent manner in N. crassa. Indeed, as shown in Fig 2A (bottom), the hac-1 mRNA is rapidly and efficiently processed in response to DTT, whereas almost no processing is observed in untreated controls. The conditions used to detect this processing of the hac-1 mRNA indeed elicit the unfolded protein response in N. crassa, as evidenced by the induction of predicted UPR targets grp78/bip and pdi, predicted homologs of genes encoding an ER-resident class HSP70 chaperone and protein disulfide isomerase, respectively, which are known to be up-regulated in different species under ER stress (Fig 2B) [41]. Sequence comparison between hac-1 cDNA derived from untreated and DTT-treated cultures, confirmed that the observed processing (Fig 2A) corresponded exactly to the removal of the predicted unconventional intron of 23 nt (Fig 1B).
Fig 2. The N. crassa hac-1 mRNA is processed under ER stress.
A) (Top) Schematic representation of the position of the primers used to detect the processing of the unconventional intron in the N. crassa hac-1 mRNA. The position of the intron is shown as a triangle. (Bottom) RT-PCR analysis from WT strain (FGSC #988) to detect processing of the N. crassa hac-1 mRNA upon chemically-induced ER stress. Amplification using the same primers, but with genomic DNA (gDNA) as template, is shown for size comparison. B) RT-PCR analysis to detect expression of homologs of typical UPR target genes, grp78/bip (NCU03982) and pdi (NCU09223), under ER stress in both WT and Δhac-1 strains. Amplification with primers for actin mRNA was used as a loading control. C) Real-time quantitative PCR analysis of the expression levels of the distinct isoforms of hac-1 mRNA (t: total; u: uninduced; i: induced) under ER stress with DTT for the times depicted. Bars represent mean expression values +/- 95% confidence intervals, from 3 independent biological replicates. D) RT-PCR analysis for detecting changes in the length of the 5’ UTR of the N. crassa hac-1 transcript upon ER stress. Primers targeting a 5’ UTR region close to the start codon (5’ UTR proximal) and a region further upstream (5’ UTR distal) were used. Assays were performed on 3 biological replicates per condition with similar results.
Consistent with the induction of ER-stress genes, we also observed that in response to DTT, there was a rapid increase in the levels of the spliced form of hac-1 mRNA, as the levels of the unspliced one fell (Fig 2C). Total hac1 mRNA levels on the other hand, remained relatively unchanged under our conditions of chemical ER stress, suggesting that the acute response to misfolded proteins via the HAC-1 pathway operates mainly through post-transcriptional regulation of the hac-1 mRNA in N. crassa.
A truncation of the 5’ end of the hac-1 transcript has been reported to take place upon ER stress in different fungal species with short unconventional introns [22, 23, 42]. To evaluate whether such regulation exists in N. crassa, we designed an RT-PCR assay to detect ER stress-induced changes in the length of the 5’ UTR region of the hac-1 mRNA. By designing forward primers that anneal to one of two locations within the 5’ UTR (one very close to the start codon and one further upstream) and a common reverse primer within the hac-1 coding region (S1 Table), we show that the abundance of the hac-1 mRNA population that harbors a long 5’ UTR is reduced upon ER stress, while the amount of total hac-1 transcript, as assessed with the reaction targeting the proximal 5’ region, is unaffected (Fig 2D), suggesting that a truncation in the 5’ UTR region of the N. crassa hac-1 transcript takes place in the presence of ER stress in a region that is upstream of position -66.
Functional evaluation of hac-1
In order to evaluate the role of hac-1 in coping with ER stress and as possible regulator of the unfolded protein response in N. crassa, we proceeded to characterize a hac-1 knockout (KO) strain. Since the KO was not available from the N. crassa KO collection [36], we generated one by replacing the hac-1 coding region with a drug resistance cassette. Correct integration of the cassette and replacement of the hac-1 sequence was verified by PCR as shown in S1 Fig.
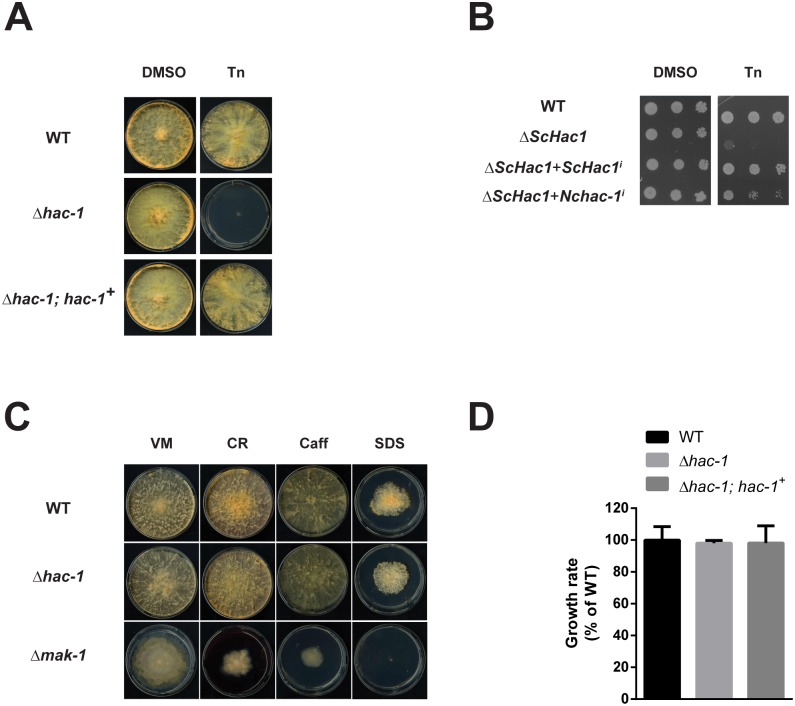
Growth of the hac-1 mutant was relatively normal under non-stressful conditions (Fig 3A), although it exhibited a slightly lower growth rate during the first day after inoculation on race tubes (a difference that was, however then lost on subsequent days, Fig 3D). We then evaluated growth of this strain under chemically-induced ER stress and as shown in Fig 3A, disruptants of hac-1 displayed enhanced sensitivity to Tunicamycin (an inhibitor of N-linked glycosylation that leads to ER stress), compared to the WT. Such sensitivity to ER stress displayed by the mutant was reverted by the reintegration of a WT copy of the hac-1 gene at the endogenous locus, thereby confirming that the HAC-1 transcription factor is required for coping with ER stress in N. crassa.
Fig 3. The hac-1 gene is necessary to cope with ER stress in N. crassa, but is dispensable for cell wall stress.
Conidia from WT (FGSC #988), Δhac-1 and Δ hac-1 complemented with a WT copy of hac-1 (Δhac-1; hac-1 +), were inoculated on solid Vogel’s media (VM) with or without Tunicamycin (Tn). Plates were placed for 3 days at 25°C in constant light. DMSO was used as a vehicle control for Tn. B) Viability assay by serial dilutions of S. cerevisiae Δhac1 strains expressing either the yeast or the N. crassa hac-1 induced versions under the control of the S. cerevisiae ADH1 promoter. Ten-fold serial dilutions of logarithmic-phase cells were spotted onto SC-leu agar plates in the presence and absence of 0.2 μg/ml Tn and the plates were incubated for 6 days at 30°C. C) Conidia from WT (FGSC #988), Δhac-1 and Δmak-1 (FGSC #11321) strains were inoculated on solid Vogel’s media with or without the chemicals shown. CR: congo red; Caff: caffeine; SDS: Sodium Dodecyl Sulfate. Plates were placed for 3 days at 34°C in constant dark and then grown for an additional 24 h in constant light at 25°C before imaging. All phenotypic assays were performed at least 3 independent times with similar results. D) Conidia from the strains described in the figure were inoculated on race tubes containing solid Vogel’s media and were then placed under constant light conditions at 25°C for 5 days. Marks were done on the tubes every 24 h and the distance between the marks was used to calculate the linear growth rate per day. Bars represent mean expression values +/- 95% confidence intervals, from 4 independent biological replicates.
As mentioned previously, up-regulation of grp78/bip and pdi are part of the typical expression signature of the unfolded protein response in several organisms. To test whether the elevated mRNA levels observed for these genes under ER stress conditions in N. crassa (Fig 2A) depend on HAC-1, we evaluated their expression in the Δhac-1 strain. As shown in Fig 2B, while the transcript levels of grp78/bip and pdi rapidly rise in response to ER stress in the WT, no induction is observed in the Δhac-1 strain, suggesting that this transcription factor is important for their up-regulation under ER stress in N. crassa. Consistent with this, analysis of the promoter region of both of these genes reveals matches to the known cis-acting unfolded protein response element cUPRE-1 (S2 Fig) [43].
The N. crassa hac-1 gene thus displays typical characteristics of a HAC1 homolog: it is required for growth under ER stress and for the induction of classic ER stress-responsive genes. We furthered our functional characterization of hac-1 by performing yeast complementation assays, to evaluate whether the N. crassa gene can functionally replace the yeast HAC1 gene. To accomplish this, we cloned the induced form of HAC1/hac-1 cDNA from both S. cerevisiae and N. crassa and placed them under control of the constitutive yeast ADH1 promoter. Constructs were then transformed into the S. cerevisiae ΔHAC1 strain and cells were spotted on solid media with or without Tunicamycin. As expected, the yeast ΔHAC1 strain displayed a growth defect when grown on media supplemented with this drug, a phenotype that is reverted by the introduction of the induced form of the WT yeast HAC1 gene (Fig 3B). Likewise, when the induced form of the N. crassa hac-1 gene was expressed in this mutant, the yeast ΔHAC1 strain recovered its ability to grow in the presence of this ER stressing agent, showing that the N. crassa hac-1 gene can functionally replace its yeast counterpart.
A number of studies have revealed a link between UPR function and cell wall homeostasis in fungi, such that hac1 or ire1 deletion strains usually display enhanced sensitivity to cell wall perturbing agents (reviewed in [17]). As part of our characterization of hac-1, we thus decided to evaluate growth of the N. crassa Δhac-1 strain under these conditions, using the Δmak-1 strain as a control for cell wall stress sensitivity, as the mak-1 gene, which encodes for a MAPK that is homologous to the yeast Slt2 MAPK, has been shown to be involved in the regulation of cell wall integrity in N. crassa [44]. As shown in Fig 3C, we observed no increased sensitivity of the mutant to the glycan-binding agent Congo Red, caffeine or the ionic detergent SDS, all of which have been used extensively to test for cell wall sensitivity in fungi [45, 46], suggesting that the connection between the UPR and cell wall integrity pathways described in some fungi, may be different in N. crassa or that other mechanisms can compensate for the absence of hac-1 for maintaining cell wall homeostasis in this fungus.
Taken together, our results indicate that N. crassa hac-1 represents a functional homolog of the yeast HAC1 gene and that it plays an important role in coping with ER stress and in mediating transcriptional responses under these conditions in N. crassa. In addition, these results highlight differences in the role of a widely conserved transcription factor in different fungal species.
The N. crassa hac-1 gene is required for growth on cellulose
We surmised that N. crassa growth under complex carbon sources, a process requiring extensive adaptation of the secretion machinery for the production of numerous enzymes, could lead to ER stress and hence, would require HAC-1. To test this idea in N. crassa, we evaluated growth of WT and Δhac-1 mutant strains on crystalline cellulose (Avicel) and hemicellulose (xylan), as degradation of these types of substrates requires the secretion of a large variety and quantity of enzymes [35, 47–50]. Interestingly, this would provide an attractive “natural” test for ER stress, considering that in its natural environment, N. crassa degrades plant biomass and has in fact emerged as a premier model system for studying plant cell wall deconstruction by filamentous fungi in the last few years [35, 37, 49, 50].
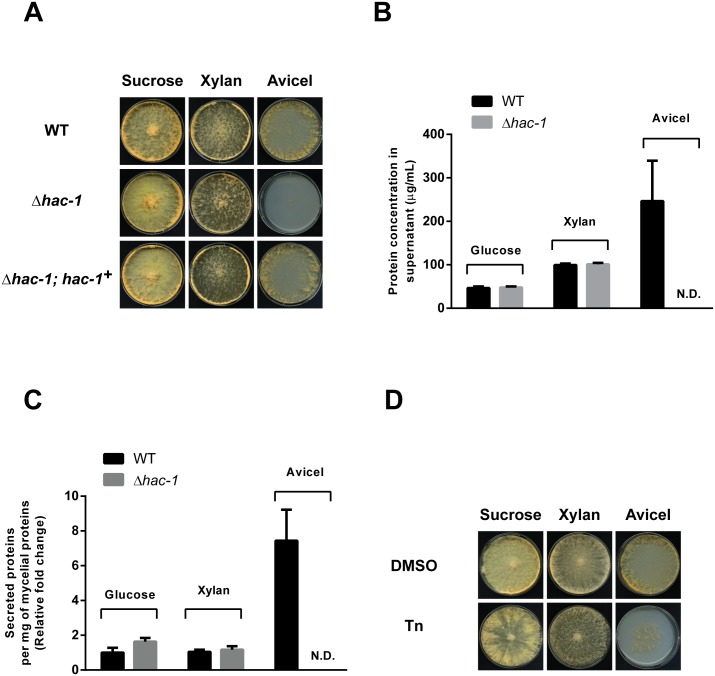
As shown in Fig 4A, growth on solid media with Avicel as the sole carbon source is dramatically impaired in the absence of hac-1, while growth on xylan appears to be normal compared to sucrose, suggesting that a particular aspect of growth, specific to growth on cellulose, requires HAC-1. Similar results were obtained under liquid culture conditions (S3 Fig). This growth phenotype exhibited by Δhac-1 is suppressed by reintroducing a WT copy of hac-1 (Fig 4A, lower panel). We reasoned that growth on cellulose may be impaired in the hac-1 mutant strain, compared to xylan (and sucrose), due to an increase in protein secretion and/or folding requirements on this carbon source over the others. Indeed, quantification of protein concentration from supernatants of the WT strain growing on Avicel, glucose or xylan, revealed that the amount of protein secreted on Avicel is higher that on the other two carbon sources (Fig 4B). This is also observed after accounting for the amount of biomass present under each condition (Fig 4C). In this scenario, growth on Avicel could then impose higher demands on ER function than growth on the other two carbon sources. Consistent with this idea, WT strains growing on Avicel display enhanced sensitivity to chemically-induced ER stress (Fig 4D), suggesting that simply growing on cellulose constitutes a basal stressful condition for the ER in N. crassa. Coping with such carbon source-related stress, a condition likely encountered by N. crassa in the wild, would require HAC-1. Quantification of the protein concentration from supernatants of the hac-1 strain growing on Avicel, glucose or xylan, shows that while no protein is detected on Avicel (which basically reflects its severe growth phenotype), the amount of protein secreted on glucose and xylan appears to be normal compared to that of the WT (Fig 4B and 4C), suggesting that a global secretion problem is unlikely to be the main cause of the growth defect of this strain on Avicel and again suggests a specific defect associated with cellulose catabolism.
Fig 4. HAC-1 is required for growth on cellulose.
A) Conidia from WT (FGSC #988), Δhac-1 and Δhac-1 complemented with a WT copy of hac-1 (Δhac-1; hac-1 +), were inoculated on solid Vogel’s media with sucrose, xylan or crystalline cellulose (Avicel) as carbon source (2% w/v). Plates were placed for 4 days at 25°C in constant light before imaging, except the Avicel plates, which were photographed after 6 days under the same conditions. B) Protein concentration from supernatants of cultures of WT and Δhac-1 strain growing on different carbon sources for 7 days, were determined. Bars represent mean expression values (+/- S.E.) from 3 independent biological replicates. N.D. not detected. C) The protein concentration from supernatants was normalized to the total amount of mycelial proteins per condition, and is expressed relative to the ratio exhibited by the WT growing on glucose. Bars represent mean expression values (+/- S.E.) from 3 independent biological replicates. D) Conidia from WT (FGSC #988) were inoculated on solid Vogel’s media with sucrose, xylan or crystalline cellulose (Avicel) as carbon source (2% w/v), with or without Tunicamycin (Tn). Plates were placed for 3 days at 25°C in constant light before imaging, except the Avicel plates, which were photographed after 4 days under the same conditions. All phenotypic assays were performed at least 3 independent times with similar results.
Taken together, our results indicate that the N. crassa hac-1 gene and hence a functional UPR, is required for growth on cellulose, highlighting a natural scenario in which the unfolded protein response is required in N. crassa in the wild.
Discussion
The unfolded protein response (UPR) signaling pathway has been intensively studied on a variety of systems since its first report over 20 years ago in S. cerevisiae [11, 12, 51]. In mammalian models, alterations in the UPR have been associated with several pathologies and syndromes [52], while UPR components have been also highlighted as a potential target for anticancer therapies [53, 54]. From a biotechnological point of view, the fungal UPR has attracted attention mainly with the goal of overcoming bottlenecks associated with the industrial production of heterologous proteins, as well as with the production of endogenous proteins of interest, particularly using T. reesei and different Aspergillus species [19–21]. In addition, in the past few years, the fungal UPR has been shown to impact virulence of several human and plant pathogens [31, 34, 55].
Despite the plethora of molecular tools available in N. crassa and its long-tradition as a model for several cell biology processes, the UPR has not been studied in this organism. Moreover, in previous compendiums of putative transcription factors encoded in the N. crassa genome [38, 56], the gene model encoding for HAC-1 (NCU01856) was not listed neither as a transcription factor nor as a putative hac-1 homolog. Therefore, we set out to characterize the UPR in N. crassa, particularly focusing on HAC-1 and its role in this signaling pathway.
We identified and characterized the ortholog of the yeast HAC1 gene in the filamentous fungus N. crassa, hac-1. We show that the N. crassa hac-1 gene can complement the growth defect exhibited by a yeast HAC1 mutant under ER stress. While complementation is not complete, such partial reversion of the mutant phenotype after introduction of a Hac1 ortholog is common [32, 33, 42] and may be due to a variety of reasons (e.g. codon usage, folding requirements, missing interacting partners, etc.). During the course of this work, we further demonstrated that NCU01856 is a sequence-specific DNA binding protein [57] and that its motif resembles the one determined for the yeast Hac1 protein, consistent with their similarity in the DNA binding domain [57, 58]. Sequence preference information is available at the Cis-BP database (http://cisbp.ccbr.utoronto.ca/).
As expected for a HAC1 ortholog, the N. crassa hac-1 mRNA has a well conserved consensus structure and it undergoes an ER stress-dependent splicing reaction, which removes a 23 nt intron. The size of this intron has recently been confirmed by another group [59]. Such intron sizes in HAC1 homologs (~20 nt) are typical among filamentous fungi and metazoans. Interestingly, most studied yeast species appear to have long ones (> 100 bases), with few exceptions [40]. In S. cerevisiae, a long intron is involved in translational attenuation of the HAC1 mRNA in the absence of ER stress via base-pairing between the intron and the 5’UTR [60, 61]. In fungal species which lack such long intron and have a short one instead, unable of such extended base-pairing, different mechanisms regulating HAC1 expression and activity have been suggested to be at play, including translational regulation via modulation of the length of the 5’ UTR. Such mechanism may similarly play a role in N. crassa, in which the length of the 5’ end of the hac-1 transcript appears to be modulated in response to ER stress, as it does in C. albicans, A. nidulans, A. niger and T. reesei [22, 23, 42]. The mechanisms regulating this change in the length of the 5’UTR (e.g. alternative transcription initiation site or post-transcriptional processing of the hac-1 transcript upon ER stress) are unknown.
By disrupting hac-1, we were able to assess its contribution to the UPR and growth under ER stress conditions in N. crassa. We found that N. crassa HAC-1 is necessary for the up-regulation of typical UPR targets when cultures are subjected to ER stress and that HAC-1 is necessary for growth under such conditions. While HAC-1 orthologs are usually shown to be required for growth under ER stress conditions, the specific phenotype resulting from the deletion of HAC1 homologs, in the absence of such stress, depends on the organism under study. For example, in A. niger, loss of hacA leads to growth and developmental defects on rich media, in the absence of ER-stress inducing agents [62]. Similarly, loss of HAC1 has been shown to impact morphology in C. albicans [42]. Disruptants of hacA in the filamentous fungus A. fumigatus and Alternaria brassicicola on the other hand, show normal radial growth on rich media in the absence of ER-stressing agents [28, 32], highlighting that no a priori conclusions can be reached regarding the phenotype of a loss-of-function hac-1 mutant in fungi, even among closely related species. Here we show that the N. crassa hac-1 mutant exhibits relatively normal growth on media containing simple sugars as carbon source, both on liquid and solid settings, in the absence of ER-stressing agents. Notably however, in the course of characterizing the N. crassa HAC-1 transcription factor in the context of the UPR, we found that growth of the N. crassa hac-1 mutant is severely impaired on crystalline cellulose, suggesting a paramount role for HAC-1 (and possibly the UPR) in the deconstruction of this substrate found in the wild. Our study thus broadens the range of physiological functions of the UPR in fungi. Interestingly, growth on xylan, which also constitutes a rather complex carbon source, is unaffected in the mutant, suggesting a specific defect on cellulose metabolism. Notably, such a particular growth defect resembles the one described for strains lacking the cellulose-specific transcription factors CLR-1 and CLR-2 [63]: similar to Δhac-1, these mutants exhibit growth defects on Avicel, but not on xylan, suggesting that a particular aspect of growth, specific to growth on cellulose, requires HAC-1. Our findings highlight a basic, conserved and well-known signaling pathway whose manipulation can have important biotechnological implications on the industrial use of N. crassa for protein production and biofuels research, the latter of which relies on the efficient bioconversion of lignocellulosic biomass to simple sugars.
The preliminary finding herein reported of the growth defect exhibited by the hac-1 mutant on cellulose, raises the question as to why this strain is unable to deconstruct and grow on cellulose, opening an interesting area of research on its mechanistic basis, considering the growing use of N. crassa in the study of cellulose deconstruction [35]. A number of ideas can be proposed, none of which are mutually exclusive. The most intuitive explanation, considering the role of HAC-1 in the unfolded protein response, concerns a compromised secretory pathway capacity resulting from the inability of the mutant strain to mount the UPR under particular high ER function-demanding conditions. In mammals, several secretory cell types have been shown to rely on a functional UPR for differentiation and function [64]. Further, while deletion of hacA in A. fumigatus displays normal growth on rich media, this gene has been shown to be required for proper growth on a relatively complex substrate such as skim milk [28] and a similar result was also reported for the corresponding mutant in A. brassicicola [32]. Indeed, our results suggest that growth on Avicel per se imposes ER stress on N. crassa. It has been reported that N. crassa secretes large amounts of a variety of enzymes upon transfer to Avicel as a carbon source (compared to sucrose) [50] and higher secretion demands on this carbon source, with a concomitant requirement for a fully active and functional UPR, may partly explain the obvious growth defect exhibited by the hac-1 mutant growing on Avicel compared to the other two tested carbon sources. The data herein presented attest to this comparatively increased protein secretion on Avicel. Interestingly, a recent study [59] has shown that several genes likely involved in the ER stress response and the secretory pathway in N. crassa, are specifically induced by growth on Avicel (and not on other carbon sources like xylan), which is consistent with our results. Further, among the genes within the N. crassa Avicel regulon, that is, genes that specifically respond to this carbon source [63], are well characterized proteins with secretory functions, again suggesting that cellulose metabolism requires significant accommodation of the secretory pathway as a whole in this fungus. In addition, and consistent with our interpretation, it has been reported both in Trichoderma and only recently in N. crassa (while this work was in preparation), that induction of cellulase production leads to UPR activation [59, 65]. Interestingly, up-regulation of hacA has been observed in A. nidulans during growth on lignocellulose [66].
Higher demands for proper ER function on Avicel may not only be related to an increased protein flux through the ER, but they could also derive from the fact that some of the specific enzymes involved in the metabolism of this carbon source may have high folding and/or processing/modification requirements, thus demanding a functional UPR to accommodate them in large quantities [67]. It could also be proposed that growth under Avicel is impaired in the hac-1 strain due to an overall secretory pathway problem. For instance, deletion of vib-1, a gene required for extracellular protease secretion in response to carbon and nitrogen starvation in N. crassa [68], results in growth defect and reduced extracellular enzyme activity, on both cellulose and xylan [69]. In addition, loss of hacA in A. brassicicola results in an overall reduced secretion capacity [32]. Our data however, suggest that a global secretory pathway defect may not be the main cause of the growth phenotype of the Δhac-1 strain, as the amount of proteins secreted by this mutant appears to be normal on both glucose and xylan, the latter, a carbon source that induces the secretion of a variety of enzymes [49]. This is consistent with our phenotypic data, as growth on these media is unaltered in the mutant and further highlights differences in the functions of HAC-1 among fungal species. Alternatively, a general secretory problem might indeed be present, but it would only be observable when a particular threshold in ER capacity/secretory load is surpassed (integrating increased protein flux, folding requirements, etc.) and in our conditions, such a threshold would only be met under Avicel and not xylan or glucose. In any case, under non-stressing conditions, the hyphal growth rate between WT and the hac-1 strains are similar, even though hyphal growth is accompanied by the trafficking of numerous vesicles to the hyphal tip. This is noteworthy, as deletion of HAC1 has been shown to affect polarized growth in C. albicans, even in the absence of ER stress, which has been suggested to result from alteration of vesicular trafficking [42]. In addition, while an efficient secretion system is also important for cell wall biosynthesis [17], we observed that the Δhac-1 strain was not sensitive to cell wall perturbing agents, again suggesting that a general secretion problem is an unlikely explanation for the bulk of the growth defect exhibited by the hac-1 strain on Avicel. This is an unusual finding in fungi, as inactivation of this bZIP transcription factor has been shown to affect cell wall integrity in various fungal species [17, 28, 32, 42]. The observation then, that the N. crassa Δhac-1 strain is capable of normal growth under chemical cell wall stress, merits further investigation, as it highlights differences in the cellular roles of hac-1 in different species. Finally, it is also possible that hac-1 specifically regulates the expression of enzymes involved in the deconstruction of cellulose, which could explain the phenotype observed in its absence on that carbon source, an idea currently under evaluation by various laboratories, prompted by results herein presented.
We have done an initial characterization of the unfolded protein response in N. crassa, focusing on the bZIP transcription factor HAC-1. Despite decades as a research model system, such conserved regulatory pathway had not hitherto been studied in this fungus and we herein show it to display the core conserved aspects of the response. Despite this conservation and the fact that HAC-1 and the UPR have been studied in various fungal species, we report on aspects that at present, seem to be uncommon among fungi: the N. crassa hac-1 gene appears to be dispensable for growth under cell wall stress conditions and its ablation does not appear to dramatically affect global secretion. In addition, we found that HAC-1 is necessary for growth on cellulose, a substrate encountered by Neurospora in the wild, a finding that expands the range of physiological functions of the UPR known in fungi. Further studies on this system and its manipulation, considering all the molecular and genetic tools available in this model fungus, will help not only in the study of the unfolded protein response, which can now be studied on a natural setting (rather than relying on chemical insults), but will also provide new insights into the basic biology behind plant biomass deconstruction by fungi, a field that is rapidly growing [35, 37] and that has an undeniable impact in both applied and environmental biological research. Additionally, these studies, will provide not only a deeper understanding of the different mechanisms regulating protein synthesis and secretion in this organism, but also of fungal growth on a commercially relevant substrate such as cellulose, shedding light on research focused on the improvement of industrial cellulase production for biofuel generation using N. crassa [37], an area which would benefit from a better understanding of the molecular mechanisms regulating the expression of hydrolytic enzymes.
Methods
Strains
The general conditions for growth and maintenance have been previously described [70]. Wild-type strain FGSC#988 (Mat a) was used for all phenotypic and gene expression assays. A hac-1 knockout strain (Mat a) was generated by disrupting the NCU01856 ORF through targeted gene replacement [71] with a bacterial bialaphos-resistance (bar) gene (which confers resistance to Ignite), followed by homokaryonization via sexual crossing. Correct integration of the cassette was verified by PCR (S1 Fig and S1 Table). The Δhac-1; hac-1 + complemented strain was generated by transforming the aforementioned hac-1 knockout strain with a cassette, targeted at the endogenous locus (so that it would replace the KO cassette by homologous recombination) and conferring resistance to hygromycin, containing the full hac-1 gene sequence, followed by the actin gene (NCU04173) transcriptional termination sequence. Primer sequences are listed in S1 Table.
Culture conditions for gene expression assays
Conidia from WT and hac-1 KO strains (105 conidia/mL) were inoculated into flasks containing liquid Vogel’s medium (pH 5.8) with 2% (wt/vol) glucose, 0.5% arginine and 50 ng/mL biotin. Flasks were kept in incubators (Percival Scientific) in constant light at 25°C and were shaken at 125 rpm. After 2 days, a 1M stock solution of dithiothreitol (DTT) was added directly to each culture, to a final concentration of 10 mM for the times described in the Figure legends. Mycelia was harvested, dried and then wrapped in aluminium foil. Mycelia was then rapidly frozen in liquid nitrogen and stored at -80°C.
RNA extraction and RT-PCR
Total RNA was prepared essentially as described by Kramer [72]. The concentration of each RNA sample was measured using the Nanodrop 2000 Spectrophotometer (Thermo Scientific). All of the RNA samples had a 260/280 ratio and a 260/230 ratio of ≥2. RNA integrity was verified on 1% agarose gel with ethidium bromide staining. Prior to cDNA synthesis, RNA samples were treated with RQ1 RNase-free DNase (Promega) according to manufacturer’s instructions. For end-point RT-PCR, 0.25 μg of DNAse-treated RNA was reverse-transcribed using M-MLV (Promega) and oligo-dT, according to manufacturer’s instructions. For RT-quantitative PCR, reverse transcription was performed on 0.5 μg of DNAse-treated RNA using SuperScript III (Invitrogen) and anchored oligo-dT, according to manufacturer’s instructions. The reaction was subsequently diluted 10 times with nuclease-free water (Life Technologies) and used for real-time quantitative PCR (qPCR).
Real-time PCR
Real-time quantitative PCR was performed in the StepOnePlus system (Applied Biosystems) in a 96-well plate format. Each qPCR reaction contained the SensiMix SYBR Hi-ROX mix (Bioline Inc. USA) (6.25 μL), 0.25 μL of a mix of specific forward and reverse primers (10 μM each), 1 μL nuclease-free water and 5 μL of cDNA (2.5 ng/μL of RNA equivalents). The cycling conditions were as follows: 10 min at 95°C and 40 cycles of 15 s at 95°C, 15 s at a 66°C (which was determined as an optimal annealing temperature for all the primer pairs used) and 15 s at 72°C, followed by melt curve analysis (ran from 60°C to 95°C with 0.3°C increments). Primer specificity was evaluated by both melt curve and agarose gel analyses. Primer sequences for the quantification of the different hac-1 isoforms are listed in S1 Table. Actin was used as a reference gene for normalization. All reaction efficiencies were between 90–100%. Three independent biological replicates per conditions were used.
Phenotypic assays
For chemically-induced ER stress assays, conidia (106) from WT (FGSC#988), Δhac-1 and Δhac-1; hac-1 +, were inoculated on plates containing solid Vogel’s media (1X Vogel’s salts, 2% sucrose, 1.5% agar), supplemented with 0.4 μg/mL Tunicamycin or vehicle (DMSO). Plates were grown in constant light at 25°C for the number of days described in the corresponding Figure legends before imaging. For growth assays under different carbon sources, conidia were inoculated on solid media containing 1X Vogel’s salts, 0.5% arginine, 50 ng/uL biotin and 1.5% agar, supplemented with either 2% sucrose, 2% Avicel PH-101 (Sigma-Aldrich 11365) or 2% Xylan from beechwood (Sigma-Aldrich X4252). To test for hypersensitivity to chemical ER-stressing agents under these carbon sources, the aforementioned media was supplemented with 0.4 μg/mL Tunicamycin or vehicle (DMSO). Plates were incubated in constant light at 25°C for the number of days mentioned in the corresponding Figure legends before imaging. Imaging of Avicel plates was always performed later than for the other media, as strains grow relatively slower on this carbon source. For cell wall sensitivity assays, conidia from WT (FGSC #988), Δhac-1 and Δmak-1 (FGSC #11321) were inoculated on solid Vogel’s media supplemented with Congo Red (200 μg/mL), caffeine (5 mM) and SDS (0.01% w/v), which are known fungal cell wall stress inducers [45, 46]. All phenotypic assays were repeated at least 3 independent times.
Yeast complementation assay
The full coding sequence of the yeast hac1 gene and the N. crassa hac-1 gene was amplified from cDNA obtained under ER stress conditions and cloned by yeast recombinational cloning [73], together with the in-frame sequence for a C-terminal V5 tag, into the integrative plasmid pGAD424 (Clonetech), after its linearization with KpnI and BglII,. This placed the cDNA sequences under control of the yeast ADH1 promoter. Yeast were transformed using the LiAc/SS carrier DNA/PEG method [74] and selected on SC-leu media. For the phenotypic assay, logarithmic-phase cells were adjusted to OD600 0.4 and 2 μl of serial 10-fold dilutions were spotted onto SC-leu agar plates in the presence and absence of 0.2 μg/ml Tunicamycin and the plates were incubated for 6 days at 30°C. Assays were repeated 3 independent times.
Protein quantification
Conidia from WT (FGSC#988) and Δhac-1 strains were inoculated into flasks containing liquid Vogel’s medium (pH 5.8) and grown as described above, with 2% (wt/vol) of the carbon source of interest (glucose, xylan or Avicel) for 7 days. After that time, the amount of secreted proteins per condition was measured using the Bradford assay (BioRad), using 100 μL of culture supernatant. The amount of mycelial proteins was determined as in [75]. The assay was performed 3 independent times.
Supporting Information
A) Schematic representation of the hac-1 (NCU01856) gene replacement event by a bialaphos-resistance (bar) cassette through homologous recombination. B) PCR was used to check for the presence of the hac-1 gene in the WT strain and C) to evaluate the correct integration of the cassette used for hac1 gene replacement in the homokaryon strain. D) Gel analysis of the PCR reactions depicted in B and C.
(DOC)
An alignment of known and putative HAC1 target promoters containing cUPRE-1 variants from different species is shown. The orange box marks the putative cUPRE-1 cis element. Conserved flanking nucleotides are shown in orange, using the known cUPRE-1 region from the yeast KAR2 gene as the consensus sequence, as in [43]. For the N. crassa genes, the search for matches to the cUPRE-1 sequences was restricted to the first 1000 bp upstream of the start codon.
(DOC)
Conidia from WT (FGSC#988) and Δhac-1 strains were inoculated into flasks containing liquid Vogel’s medium (pH 5.8) with 2% (wt/vol) of the carbon source of interest (glucose, xylan or Avicel). Pictures were taken after 7 days of growth at 25°C in constant light conditions.
(DOC)
When primers are described with both upper and lower case, the former denotes a region used for recombinational cloning and the latter, the specific target sequence.
(DOC)
(DOC)
Acknowledgments
We thank Consuelo Olivares-Yañez for her help with the protein secretion work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Millennium Nucleus for Fungal Integrative and Synthetic Biology to LFL (URL: http://www.iniciativamilenio.cl/centros/nucleos_det.php?id=134), Grant Number: NC120043; Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) to LFL, (URL: http://w1.conicyt.cl/bases/fondecyt/personas/3/0/30225.html) Grant number: 1131030.
References
- 1. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. Epub 2011/11/26. 10.1126/science.1209038 334/6059/1081 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2. Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169 Epub 2013/02/08. 10.1101/cshperspect.a013169 a013169 [pii] cshperspect. a013169 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–58. Epub 2000/06/10. doi: S0092-8674(00)80835-1 [pii] [DOI] [PubMed] [Google Scholar]
- 4. Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–31. Epub 2009/08/05. doi: jcb.200903014 [pii] 10.1083/jcb.200903014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–7. Epub 2006/07/11. doi: 313/5783/104 [pii] 10.1126/science.1129631 . [DOI] [PubMed] [Google Scholar]
- 6. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–4. Epub 1999/02/04. 10.1038/16729 . [DOI] [PubMed] [Google Scholar]
- 7. Kimmig P, Diaz M, Zheng J, Williams CC, Lang A, Aragon T, et al. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. Elife. 2012;1:e00048 Epub 2012/10/16. 10.7554/eLife.00048 00048 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pincus D, Aranda-Diaz A, Zuleta IA, Walter P, El-Samad H. Delayed Ras/PKA signaling augments the unfolded protein response. Proc Natl Acad Sci U S A. 2014;111(41):14800–5. Epub 2014/10/03. 10.1073/pnas.1409588111 1409588111 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thibault G, Ng DT. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harb Perspect Biol. 2012;4(12). Epub 2012/12/05. 10.1101/cshperspect.a013193 a013193 [pii] 4/12/a013193 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. Epub 2007/06/15. doi: nrm2199 [pii] 10.1038/nrm2199 . [DOI] [PubMed] [Google Scholar]
- 11. Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–206. Epub 1993/06/18. . [DOI] [PubMed] [Google Scholar]
- 12. Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74(4):743–56. Epub 1993/08/27. . [DOI] [PubMed] [Google Scholar]
- 13. Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87(3):391–404. Epub 1996/11/01. doi: S0092-8674(00)81360-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 14. Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells. 1996;1(9):803–17. Epub 1996/09/01. . [DOI] [PubMed] [Google Scholar]
- 15. Korennykh A, Walter P. Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol. 2012;28:251–77. Epub 2012/10/13. 10.1146/annurev-cellbio-101011-155826 . [DOI] [PubMed] [Google Scholar]
- 16. Welihinda AA, Tirasophon W, Green SR, Kaufman RJ. Protein serine/threonine phosphatase Ptc2p negatively regulates the unfolded-protein response by dephosphorylating Ire1p kinase. Mol Cell Biol. 1998;18(4):1967–77. Epub 1998/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malavazi I, Goldman GH, Brown NA. The importance of connections between the cell wall integrity pathway and the unfolded protein response in filamentous fungi. Brief Funct Genomics. 2014. Epub 2014/07/26. doi: elu027 [pii] 10.1093/bfgp/elu027 . [DOI] [PubMed] [Google Scholar]
- 18. Nevalainen KMH, Te'o VSJ, Bergquist PL. Heterologous protein expression in filamentous fungi. Trends in Biotechnology. 2005;23(9):468–74. 10.1016/j.tibtech.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 19. Saloheimo M, Pakula TM. The cargo and the transport system: secreted proteins and protein secretion in Trichoderma reesei (Hypocrea jecorina) . Microbiology. 2012;158(Pt 1):46–57. Epub 2011/11/05. 10.1099/mic.0.053132-0 . [DOI] [PubMed] [Google Scholar]
- 20. Conesa A, Punt PJ, van Luijk N, van den Hondel CA. The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol. 2001;33(3):155–71. Epub 2001/08/10. 10.1006/fgbi.2001.1276 S1087-1845(01)91276-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21. Archer DB, Turner G. Genomics of Protein Secretion and Hyphal Growth in Aspergillus In: Brown AP, editor. Fungal Genomics. The Mycota. 13: Springer; Berlin Heidelberg; 2006. p. 75–96. [Google Scholar]
- 22. Saloheimo M, Valkonen M, Penttila M. Activation mechanisms of the HAC1-mediated unfolded protein response in filamentous fungi. Mol Microbiol. 2003;47(4):1149–61. Epub 2003/02/13. doi: 3363 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23. Mulder HJ, Saloheimo M, Penttila M, Madrid SM. The transcription factor HACA mediates the unfolded protein response in Aspergillus niger, and up-regulates its own transcription. Mol Genet Genomics. 2004;271(2):130–40. Epub 2004/01/20. 10.1007/s00438-003-0965-5 . [DOI] [PubMed] [Google Scholar]
- 24. Arvas M, Pakula T, Lanthaler K, Saloheimo M, Valkonen M, Suortti T, et al. Common features and interesting differences in transcriptional responses to secretion stress in the fungi Trichoderma reesei and Saccharomyces cerevisiae. BMC Genomics. 2006;7:32. Epub 2006/03/01. doi: 1471-2164-7-32 [pii] 10.1186/1471-2164-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sims AH, Gent ME, Lanthaler K, Dunn-Coleman NS, Oliver SG, Robson GD. Transcriptome analysis of recombinant protein secretion by Aspergillus nidulans and the unfolded-protein response in vivo. Appl Environ Microbiol. 2005;71(5):2737–47. Epub 2005/05/05. doi: 71/5/2737 [pii] 10.1128/AEM.71.5.2737-2747.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guillemette T, van Peij N, Goosen T, Lanthaler K, Robson GD, van den Hondel CA, et al. Genomic analysis of the secretion stress response in the enzyme-producing cell factory Aspergillus niger. BMC Genomics. 2007;8:158. Epub 2007/06/15. doi: 1471-2164-8-158 [pii] 10.1186/1471-2164-8-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollien J. Evolution of the unfolded protein response. Biochim Biophys Acta. 2013;1833(11):2458–63. Epub 2013/02/02. 10.1016/j.bbamcr.2013.01.016 S0167-4889(13)00031-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28. Richie DL, Hartl L, Aimanianda V, Winters MS, Fuller KK, Miley MD, et al. A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 2009;5(1):e1000258 Epub 2009/01/10. 10.1371/journal.ppat.1000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheon SA, Jung KW, Chen YL, Heitman J, Bahn YS, Kang HA. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 2011;7(8):e1002177 Epub 2011/08/20. 10.1371/journal.ppat.1002177 PPATHOGENS-D-11-00144 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyazaki T, Kohno S. ER stress response mechanisms in the pathogenic yeast Candida glabrata and their roles in virulence. Virulence. 2014;5(2):365–70. Epub 2013/12/18. 10.4161/viru.27373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheon SA, Jung KW, Bahn YS, Kang HA. The unfolded protein response (UPR) pathway in Cryptococcus. Virulence. 2014;5(2):341–50. Epub 2014/02/08. 10.4161/viru.26774 26774 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joubert A, Simoneau P, Campion C, Bataille-Simoneau N, Iacomi-Vasilescu B, Poupard P, et al. Impact of the unfolded protein response on the pathogenicity of the necrotrophic fungus Alternaria brassicicola. Mol Microbiol. 2011;79(5):1305–24. Epub 2011/01/22. 10.1111/j.1365-2958.2010.07522.x . [DOI] [PubMed] [Google Scholar]
- 33. Heimel K, Freitag J, Hampel M, Ast J, Bolker M, Kamper J. Crosstalk between the unfolded protein response and pathways that regulate pathogenic development in Ustilago maydis. Plant Cell. 2013;25(10):4262–77. Epub 2013/11/02. doi: 10.1105/tpc.113.115899 tpc.113.115899 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guillemette T, Calmes B, Simoneau P. Impact of the UPR on the virulence of the plant fungal pathogen A. brassicicola. Virulence. 2014;5(2):357–64. Epub 2013/11/06. 10.4161/viru.26772 26772 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glass NL, Schmoll M, Cate JH, Coradetti S. Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol. 2013;67:477–98. Epub 2013/07/03. 10.1146/annurev-micro-092611-150044 . [DOI] [PubMed] [Google Scholar]
- 36. Dunlap JC, Borkovich KA, Henn MR, Turner GE, Sachs MS, Glass NL, et al. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv Genet. 2007;57:49–96. Epub 2007/03/14. doi: S0065-2660(06)57002-6 [pii] 10.1016/S0065-2660(06)57002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Znameroski EA, Glass NL. Using a model filamentous fungus to unravel mechanisms of lignocellulose deconstruction. Biotechnol Biofuels. 2013;6(1):6 Epub 2013/01/24. 10.1186/1754-6834-6-6 1754-6834-6-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, et al. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev. 2004;68(1):1–108. Epub 2004/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aragon T, van Anken E, Pincus D, Serafimova IM, Korennykh AV, Rubio CA, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457(7230):736–40. Epub 2008/12/17. 10.1038/nature07641 nature07641 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hooks KB, Griffiths-Jones S. Conserved RNA structures in the non-canonical Hac1/Xbp1 intron. RNA Biol. 2011;8(4):552–6. Epub 2011/05/20. doi: 15396 [pii] 10.4161/rna.8.4.15396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nishikawa S, Brodsky JL, Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD). J Biochem. 2005;137(5):551–5. Epub 2005/06/10. doi: 137/5/551 [pii] 10.1093/jb/mvi068 . [DOI] [PubMed] [Google Scholar]
- 42. Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, et al. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet Biol. 2008;45(9):1235–47. Epub 2008/07/08. 10.1016/j.fgb.2008.06.001 S1087-1845(08)00102-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 43. Fordyce PM, Pincus D, Kimmig P, Nelson CS, El-Samad H, Walter P, et al. Basic leucine zipper transcription factor Hac1 binds DNA in two distinct modes as revealed by microfluidic analyses. Proc Natl Acad Sci U S A. 2012;109(45):E3084–93. Epub 2012/10/12. 10.1073/pnas.1212457109 1212457109 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park G, Pan S, Borkovich KA. Mitogen-activated protein kinase cascade required for regulation of development and secondary metabolism in Neurospora crassa. Eukaryot Cell. 2008;7(12):2113–22. Epub 2008/10/14. 10.1128/EC.00466-07 EC.00466-07 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arias P, Diez-Muniz S, Garcia R, Nombela C, Rodriguez-Pena JM, Arroyo J. Genome-wide survey of yeast mutations leading to activation of the yeast cell integrity MAPK pathway: novel insights into diverse MAPK outcomes. BMC Genomics. 2011;12:390 Epub 2011/08/04. 10.1186/1471-2164-12-390 1471-2164-12-390 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hill TW, Loprete DM, Momany M, Ha Y, Harsch LM, Livesay JA, et al. Isolation of cell wall mutants in Aspergillus nidulans by screening for hypersensitivity to Calcofluor White. Mycologia. 2006;98(3):399–409. Epub 2006/10/17. . [DOI] [PubMed] [Google Scholar]
- 47. Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66(3):506–77. Epub 2002/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bouws H, Wattenberg A, Zorn H. Fungal secretomes--nature's toolbox for white biotechnology. Appl Microbiol Biotechnol. 2008;80(3):381–8. Epub 2008/07/19. 10.1007/s00253-008-1572-5 . [DOI] [PubMed] [Google Scholar]
- 49. Sun J, Tian C, Diamond S, Glass NL. Deciphering transcriptional regulatory mechanisms associated with hemicellulose degradation in Neurospora crassa. Eukaryot Cell. 2012;11(4):482–93. Epub 2012/02/22. 10.1128/EC.05327-11 EC.05327-11 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian C, Beeson WT, Iavarone AT, Sun J, Marletta MA, Cate JH, et al. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 2009;106(52):22157–62. Epub 2009/12/19. 10.1073/pnas.0906810106 0906810106 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mori K, Sant A, Kohno K, Normington K, Gething MJ, Sambrook JF. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. Embo J. 1992;11(7):2583–93. Epub 1992/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cornejo VH, Pihan P, Vidal RL, Hetz C. Role of the unfolded protein response in organ physiology: lessons from mouse models. IUBMB Life. 2013;65(12):962–75. Epub 2013/11/15. 10.1002/iub.1224 . [DOI] [PubMed] [Google Scholar]
- 53. Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–97. Epub 2014/08/26. 10.1038/nrc3800 nrc3800 [pii]. . [DOI] [PubMed] [Google Scholar]
- 54. Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–19. Epub 2013/08/31. 10.1038/nrd3976 nrd3976 [pii]. . [DOI] [PubMed] [Google Scholar]
- 55. Krishnan K, Askew DS. The fungal UPR: a regulatory hub for virulence traits in the mold pathogen Aspergillus fumigatus. Virulence. 2014;5(2):334–40. Epub 2013/11/06. 10.4161/viru.26571 26571 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tian C, Li J, Glass NL. Exploring the bZIP transcription factor regulatory network in Neurospora crassa. Microbiology. 2011;157(Pt 3):747–59. Epub 2010/11/18. doi: 10.1099/mic.0.045468–0 mic.0.045468-0 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weirauch MT, Yang A, Albu M, Cote A, Montenegro-Montero A, Drewe P, et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 2014;158 10.1016/j.cell.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bernard B, Thorsson V, Rovira H, Shmulevich I. Increasing coverage of transcription factor position weight matrices through domain-level homology. PLoS One. 2012;7(8):e42779 Epub 2012/09/07. 10.1371/journal.pone.0042779 PONE-D-11-25476 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benz JP, Chau BH, Zheng D, Bauer S, Glass NL, Somerville CR. A comparative systems analysis of polysaccharide-elicited responses in Neurospora crassa reveals carbon source-specific cellular adaptations. Mol Microbiol. 2014;91(2):275–99. Epub 2013/11/15. 10.1111/mmi.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chapman RE, Walter P. Translational attenuation mediated by an mRNA intron. Curr Biol. 1997;7(11):850–9. Epub 1998/02/28. doi: S0960-9822(06)00373-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 61. Ruegsegger U, Leber JH, Walter P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell. 2001;107(1):103–14. Epub 2001/10/12. doi: S0092-8674(01)00505-0 [pii]. . [DOI] [PubMed] [Google Scholar]
- 62. Mulder HJ, Nikolaev I. HacA-dependent transcriptional switch releases hacA mRNA from a translational block upon endoplasmic reticulum stress. Eukaryot Cell. 2009;8(4):665–75. Epub 2009/02/03. doi: EC.00131-08 [pii] 10.1128/EC.00131-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coradetti ST, Craig JP, Xiong Y, Shock T, Tian C, Glass NL. Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc Natl Acad Sci U S A. 2012;109(19):7397–402. Epub 2012/04/26. 10.1073/pnas.1200785109 1200785109 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moore KA, Hollien J. The unfolded protein response in secretory cell function. Annu Rev Genet. 2012;46:165–83. Epub 2012/09/01. 10.1146/annurev-genet-110711-155644 . [DOI] [PubMed] [Google Scholar]
- 65. Collen A, Saloheimo M, Bailey M, Penttila M, Pakula TM. Protein production and induction of the unfolded protein response in Trichoderma reesei strain Rut-C30 and its transformant expressing endoglucanase I with a hydrophobic tag. Biotechnol Bioeng. 2005;89(3):335–44. Epub 2004/12/25. 10.1002/bit.20350 . [DOI] [PubMed] [Google Scholar]
- 66. Brown NA, de Gouvea PF, Krohn NG, Savoldi M, Goldman GH. Functional characterisation of the non-essential protein kinases and phosphatases regulating Aspergillus nidulans hydrolytic enzyme production. Biotechnol Biofuels. 2013;6(1):91 Epub 2013/06/27. 10.1186/1754-6834-6-91 1754-6834-6-91 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ilmen M, den Haan R, Brevnova E, McBride J, Wiswall E, Froehlich A, et al. High level secretion of cellobiohydrolases by Saccharomyces cerevisiae. Biotechnol Biofuels. 2011;4:30 Epub 2011/09/14. 10.1186/1754-6834-4-30 1754-6834-4-30 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dementhon K, Iyer G, Glass NL. VIB-1 is required for expression of genes necessary for programmed cell death in Neurospora crassa. Eukaryot Cell. 2006;5(12):2161–73. Epub 2006/10/03. doi: EC.00253-06 [pii] 10.1128/EC.00253-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiong Y, Sun J, Glass NL. VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by Neurospora crassa. PLoS Genet. 2014;10(8):e1004500 Epub 2014/08/22. 10.1371/journal.pgen.1004500 PGENETICS-D-13-03538 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davis RH, de Serres FJ. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970;17A:79–143. [Google Scholar]
- 71. Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A. 2006;103(27):10352–7. Epub 2006/06/28. doi: 0601456103 [pii] 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kramer C. Isolation of total RNA from Neurospora mycelium. Methods Mol Biol. 2007;362:291–303. Epub 2007/04/10. doi: 1-59745-257-2:291 [pii] 10.1007/978-1-59745-257-1_19 . [DOI] [PubMed] [Google Scholar]
- 73. Oldenburg KR, Vo KT, Michaelis S, Paddon C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997;25(2):451–2. Epub 1997/01/15. doi: gka088 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. Epub 2002/06/21. . [DOI] [PubMed] [Google Scholar]
- 75. Larrondo LF, Olivares-Yanez C, Baker CL, Loros JJ, Dunlap JC. Circadian rhythms. Decoupling circadian clock protein turnover from circadian period determination. Science. 2015;347(6221):1257277 Epub 2015/01/31. 10.1126/science.1257277 1257277 [pii] 347/6221/1257277 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–15. Epub 2003/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Darty K, Denise A, Ponty Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009;25(15):1974–5. Epub 2009/04/29. 10.1093/bioinformatics/btp250 btp250 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Schematic representation of the hac-1 (NCU01856) gene replacement event by a bialaphos-resistance (bar) cassette through homologous recombination. B) PCR was used to check for the presence of the hac-1 gene in the WT strain and C) to evaluate the correct integration of the cassette used for hac1 gene replacement in the homokaryon strain. D) Gel analysis of the PCR reactions depicted in B and C.
(DOC)
An alignment of known and putative HAC1 target promoters containing cUPRE-1 variants from different species is shown. The orange box marks the putative cUPRE-1 cis element. Conserved flanking nucleotides are shown in orange, using the known cUPRE-1 region from the yeast KAR2 gene as the consensus sequence, as in [43]. For the N. crassa genes, the search for matches to the cUPRE-1 sequences was restricted to the first 1000 bp upstream of the start codon.
(DOC)
Conidia from WT (FGSC#988) and Δhac-1 strains were inoculated into flasks containing liquid Vogel’s medium (pH 5.8) with 2% (wt/vol) of the carbon source of interest (glucose, xylan or Avicel). Pictures were taken after 7 days of growth at 25°C in constant light conditions.
(DOC)
When primers are described with both upper and lower case, the former denotes a region used for recombinational cloning and the latter, the specific target sequence.
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.