ABSTRACT
Uropathogenic Escherichia coli (UPEC) is the primary cause of community-acquired urinary tract infections (UTIs). UPEC bind the bladder using type 1 pili, encoded by the fim operon in nearly all E. coli. Assembled type 1 pili terminate in the FimH adhesin, which specifically binds to mannosylated glycoproteins on the bladder epithelium. Expression of type 1 pili is regulated in part by phase-variable inversion of the genomic element containing the fimS promoter, resulting in phase ON (expressing) and OFF (nonexpressing) orientations. Type 1 pili are essential for virulence in murine models of UTI; however, studies of urine samples from human UTI patients demonstrate variable expression of type 1 pili. We provide insight into this paradox by showing that human urine specifically inhibits both expression and function of type 1 pili. Growth in urine induces the fimS phase OFF orientation, preventing fim expression. Urine also contains inhibitors of FimH function, and this inhibition leads to a further bias in fimS orientation toward the phase OFF state. The dual effect of urine on fimS regulation and FimH binding presents a potential barrier to type 1 pilus-mediated colonization and invasion of the bladder epithelium. However, FimH-mediated attachment to human bladder cells during growth in urine reverses these effects such that fim expression remains ON and/or turns ON. Interestingly, FimH inhibitors called mannosides also induce the fimS phase OFF orientation. Thus, the transduction of FimH protein attachment or inhibition into epigenetic regulation of type 1 pilus expression has important implications for the development of therapeutics targeting FimH function.
IMPORTANCE
Urinary tract infections (UTIs) are extremely common infections, frequently caused by uropathogenic Escherichia coli (UPEC), that are treated with antibiotics but often recur. Therefore, UTI treatment both is complicated by and contributes to bacterial antibiotic resistance. Thus, it is important to understand UTI pathogenesis to devise novel strategies and targets for prevention and treatment. Based on evidence from disease epidemiology and mouse models of infection, UPEC relies heavily on type 1 pili to attach to and invade the bladder epithelium during initial stages of UTI. Here, we demonstrate that the negative effect of planktonic growth in human urine on both the function and expression of type 1 pili is overcome by attachment to bladder epithelial cells, representing a strategy to subvert this alternative innate defense mechanism. Furthermore, this dually inhibitory action of urine is a mechanism shared with recently developed anti-type 1 pilus molecules, highlighting the idea that further development of antivirulence strategies targeting pili may be particularly effective for UPEC.
INTRODUCTION
Uropathogenic Escherichia coli (UPEC) is the primary cause of community-acquired urinary tract infections (UTIs), which are extremely common and often recurrent (1). UPEC strains are thought to be distinct from other classes of E. coli, particularly commensal and intestinal pathogenic strains, due to their carriage of several virulence factors enabling specific host-pathogen interactions in the urinary tract. For example, UPEC strains typically encode a multitude of pili assembled by the chaperone-usher pathway, termed CUP pili, which are important in virulence. CUP pili terminate in specialized adhesins that recognize specific receptors with stereochemical specificity. If targeted to host receptors, such pilus adhesin interactions can determine both tissue tropism and the course of disease. For example, type 1 pili, encoded by the fim operon, terminate in the FimH adhesin, which binds specifically to mannosylated uroplakins lining the superficial bladder epithelium and mannosylated α3,β1 integrins expressed on underlying layers of epithelial cells (2–4). FimH-mediated adhesion enables UPEC colonization and invasion of bladder epithelial cells. However, UPEC can be expelled from host cells in a Tlr4-dependent exocytotic process (5). UPEC organisms that subvert this expulsion process can escape into the cytoplasm of bladder epithelial cells, where they are protected from immune detection (3) and replicate to high numbers, forming biofilm-like intracellular bacterial communities (IBCs), in a process that is also dependent on type 1 pili (6, 7). IBCs have been extensively characterized in murine models of UTIs and are found in the urine of UTI patients (8). IBCs also correlate with UTI recurrence in children (9). Because type 1 pili and FimH mediate many of the initial steps in UTI pathogenesis, UPEC strains lacking type 1 pili or the FimH adhesin are significantly attenuated in murine cystitis models (7, 10, 11).
Given the importance of type 1 pili in UTI pathogenesis (7, 10), their regulation and assembly have been studied in detail. The fimS promoter for the fim operon is encoded on a 314-bp genomic element that can be reoriented into the phase ON or OFF orientation by site-specific recombinases that cleave at specific inverted repeat sites flanking the fimS promoter, thus allowing or preventing fim expression (12, 13). Numerous regulators and environmental signals have been shown to impact the fimS phase state and thus type 1 pilus expression (14). One of these signals is the assembly status of type 1 pili themselves. Fim subunits in the periplasm require the FimC chaperone for proper folding. Each subunit has an incomplete Ig-like fold and an amino-terminal extension. FimC acts as a template for subunit folding by transiently donating a β-strand to complete the subunit’s Ig fold, a process termed donor strand complementation (15). Chaperone-subunit complexes then interact with the outer membrane usher, FimD, which catalyzes pilus assembly. In a process termed donor strand exchange, an incoming pilin subunit donates its amino-terminal extension to complete the Ig-like fold of the previously incorporated pilin subunit. Donor strand exchange displaces the chaperone β-strand and results in the noncovalent polymerization of the pilin subunits (16–18). Disruption of this assembly process can be sensed by the bacterium and results in the down-regulation of type 1 pilus expression by biasing the invertible fimS promoter toward the phase OFF orientation (19).
In humans, collection of tissue biopsy specimens during infection is generally contraindicated, so there has been significant interest in profiling the UPEC physiological response to infection by examining human urine. Of note, human urine is a complex biofluid that contains over 2,000 metabolites (20), and a person’s health, as well as his/her food and drug intake, impacts the presence and concentration of the metabolites in their urine. Furthermore, urine from healthy individuals can contain low levels of erythrocytes, leukocytes, and sloughed epithelial cells from the urinary tract. Therefore, while type 1 pili are essential for infection and highly expressed in mice during UTI, their expression in human urine samples has been reported to be variable. One study found by immunofluorescence that 75% of UPEC strains from UTI patients were type 1 piliated (21). A study by Bielecki and colleagues found that while 19/21 (90%) human urine samples contained UPEC that expressed fimA, encoding the type 1 pilus major subunit, only 8 (38%) of those samples had high levels of fimA transcript (22). Hagan and colleagues used a CFT073-specific microarray to measure gene expression from the urine of eight women with E. coli bacteriuria and found that type 1 pilus genes were detectable in two of the eight samples (23). A recent study using RNA-Seq to quantify UPEC gene expression also found variable fim expression in UTI samples (24). Of note, pili that are assembled on the cell surface probably remain there for several cell divisions, likely leading to differences in pilus transcript and piliation levels, as seen in the studies cited above. There has also been extensive characterization of type 1 piliation in the murine model of UTI. In one study, UPEC from the urine of infected mice demonstrated variable fimS phase orientation, with some infected mice containing mostly phase ON UPEC and others mostly phase OFF UPEC in their urine, reflective of the data from human studies (25). In that study, the percentage of the UPEC population in the fimS phase ON state increased over the first 24 h of murine cystitis, and the fimS phase ON state positively correlated with infection burden (25). Furthermore, when the urine from mice, 24 h postinfection, was filtered through a 12-µm-pore-size filter, the UPEC organisms which were adherent to eukaryotic cells, and therefore retained on the filter, were enriched for phase ON bacteria (with a mean of 49% phase ON) relative to the planktonic UPEC organisms that passed through the filter, which were mostly fimS phase OFF (25). Further, nonpiliated UPEC organisms have been identified in murine urine, even when those in the murine bladder are piliated (26).
Thus, type 1 pilus expression in urine appears to be variable between different human patients and between mice and humans. One hypothesis that integrates these seemingly disparate results is that different external signals encountered during infection (i.e., different niches) impact type 1 pilus expression. Therefore, a more complete understanding of the effect that temporal-spatial niches within the urinary tract occupied by UPEC have on type 1 pilus expression during UTI is required. Host responses to bacteria include micturition to wash away unattached bacteria, Tlr-4 dependent exocytosis of invaded bacteria (5), and exfoliation of host cells containing IBCs or that have extracellularly attached bacteria (27). Thus, urine can contain UPEC (i) growing planktonically in urine, (ii) adherent to bladder epithelial cells, or (iii) present in biofilm-like communities inside exfoliated bladder urothelial cells. These various niches may all affect the expression of type 1 pili and regulation of fimS phase orientation during infection.
To better understand the extracellular niches, we directly measured the impact of growth in urine on fimS phase state, type 1 piliation, and type 1 pilus function. We found that planktonic growth in human urine turns UPEC fimS phase OFF, inhibiting further type 1 pilus expression. However, bacteria adherent to human bladder cells remain phase ON despite growth in human urine. We also found that urine inhibits the function of the type 1 pilus adhesin, FimH, independent of expression. This functional inhibition in turn further impacts fimS phase regulation. Therefore, niche residence within the urinary tract impacts the expression of type 1 pili. More generally, depending on the level of urothelial exfoliation and the impact of other spatial-temporal factors, genes associated with tissue-specific expression may not always correlate with the transcriptome of UPEC in the urine niche. We propose that human urine exerts an inhibitory effect on type 1 pilus expression and function, blocking UPEC colonization and thus defending against the onset of UTI, similar to an innate defense mechanism. Finally, the regulatory connection between FimH function and fimS phase state could increase the efficacy of therapeutics targeting FimH function.
RESULTS
Human urine impacts type 1 piliation and phase variation.
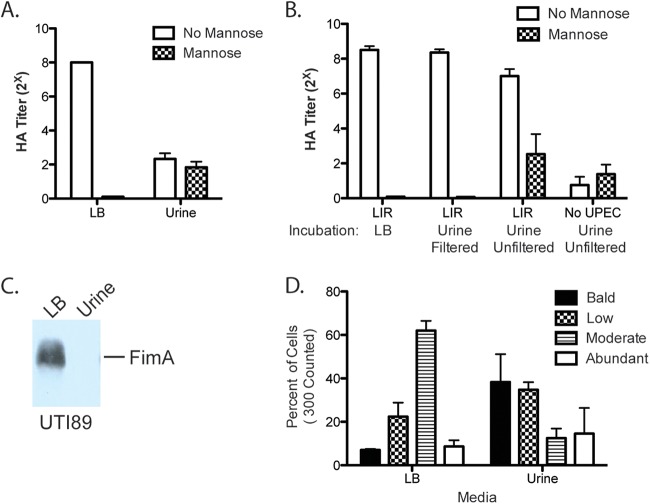
We used a hemagglutination assay (HA) to quantify the effects of growth in urine on type 1 piliation on the UPEC cell surface. In an HA, normalized bacteria are serially 2-fold diluted, and the titer indicates the maximum dilution still capable of agglutinating guinea pig erythrocytes. Mannose acts as a competitive inhibitor of type 1 pilus-mediated adhesion, abolishing agglutination by type 1 pili. Using type 1 pilus-inducing conditions (two 24-h periods of static growth at 37°C), we found that growth of the prototypical UPEC isolate UTI89 in filtered human urine compared to growth in LB decreased HA titers from 28 to 22 (Fig. 1A). This result indicates a decrease in type 1 piliation, inhibition of FimH function, or both. To distinguish between these possibilities, we used the highly piliated fimS phase-locked-ON UTI89 mutant, which has point mutations incorporated into its left inverted repeat (LIR), such that the recombinases can no longer cleave and reorient the fimS promoter element into the OFF orientation. The HA titer of the fimS phase-locked-ON strain grown in LB showed no change after a 1-h exposure to filtered human urine, indicating that filtered human urine does not contain a direct FimH inhibitor (Fig. 1B). Thus, the decreased HA titer observed after growth in filtered human urine likely reflects decreased type 1 piliation on the UPEC cell surface. We confirmed this result with immunoblot analysis, which showed that after growth in filtered human urine, FimA, the type 1 pilus major subunit, was no longer detectable (Fig. 1C). To further confirm the impact of growth in urine on CUP piliation, we quantified the extent of piliation on the surfaces of individual UTI89 cells grown statically in LB or in filtered human urine twice for 24 h each time by electron microscopy (EM). We found that growth in urine increased the proportion of nonpiliated and slightly piliated cells (Fig. 1D). The absence of any detectable FimA in the immunoblot (Fig. 1C) suggests that at least some of the pili observed by EM in Fig. 1D likely represented non-type 1 CUP pili. Together, these assays demonstrate that, relative to static growth in LB, static growth in filtered human urine decreases the level of assembled type 1 pili on the cell surface, which was reflected by a lack of FimA protein in whole-cell extracts.
FIG 1 .
Effect of urine on type 1 piliation. (A) HA of UTI89 after growth under type 1 pilus-inducing conditions, in LB or filtered human urine. (B) HA of locked ON strain LIR grown under type 1 pilus-inducing conditions in LB and then incubated in LB or filtered or unfiltered human urine for 1 h before HA was conducted. The same batches of urine were used to compare filtered and unfiltered urine. (C) FimA immunoblot of UTI89 after growth under type 1 pilus-inducing conditions, in LB or filtered human urine. (D) Percentage of the population of each strain with abundant, moderate, low, or no piliation, as assessed by counting 300 cells on electron micrographs.
To further examine the impact of growth in urine on type 1 pilus expression, we assessed the fimS phase state. Static growth twice for 24 h each induces the fimS phase ON state in LB. To determine how urine impacts the induction and maintenance of fimS phase state, we prepared both ON and OFF initial inocula and back-diluted them 1:1,000 for two 24-h periods of static growth in LB or filtered human urine. Growth of a fimS phase ON inoculum in filtered human urine resulted in the fimS phase OFF state, while cells grown in LB remained phase ON (Fig. 2A). Alternatively, growth of a fimS phase OFF inoculum in filtered human urine maintained the fimS phase OFF state, while cells grown in LB turned from phase OFF to phase ON (Fig. 2A). Quantification of the fimS phase state indicated that growth in filtered human urine significantly impacts fimS phase orientation, favoring the phase OFF state (Fig. 2B). We also confirmed that filtered human urine induces the fimS phase OFF orientation and decreases type 1 piliation levels in another UPEC strain, CFT073, and in the K-12 strain MG1655, indicating that this is not a UTI89-specific phenotype (see Fig. S1 in the supplemental material). These E. coli isolates also grew similarly to UTI89 in human urine (see Fig. S1).
FIG 2 .
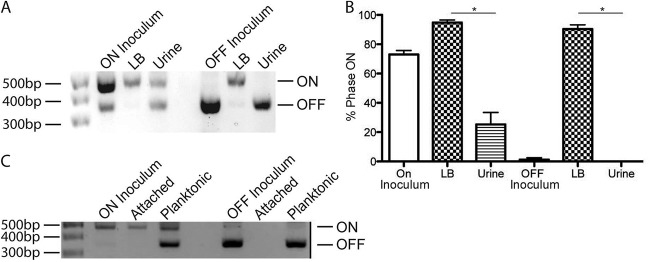
Effect of urine on fimS phase state. UTI89 was grown under type 1 pilus-inducing conditions to produce a fimS phase ON inoculum or under fimS phase OFF-inducing conditions to produce a phase OFF inoculum. Each inoculum was subcultured in LB or urine and grown twice for 24 h each time at 37°C. (A) Phase assay demonstrates the percentage of the population in the fimS phase ON or OFF orientation after two 24-h periods of growth in the indicated medium from a phase ON or phase OFF starting inoculum. (B) Graph of the ImageJ quantification of three biological replicates of the phase assay shown in panel A. (C) Phase assay of the attached or planktonic population after two 24-h growth periods with PFA-fixed 5637 bladder cells. Asterisks indicate statistical significance, as measured by the Mann-Whitney t test (P < 0.05)
UPEC colonizing the bladder can be planktonic in urine or attached to the luminal surface of the bladder epithelium. We therefore assessed the impact of UPEC attachment to bladder epithelial cells on fimS phase state. UPEC attached to paraformaldehyde (PFA)-fixed 5637 bladder cells remained phase ON, even after growth in human urine (Fig. 2C). Under these conditions, phase OFF UPEC did not convert to the phase ON orientation and could not attach to the bladder cells. Thus, growth in filtered human urine both maintains and induces the fimS phase OFF state, decreasing type 1 piliation level, an effect that was overcome by attachment to bladder epithelial cells.
Impact of pH on type 1 pilus expression.
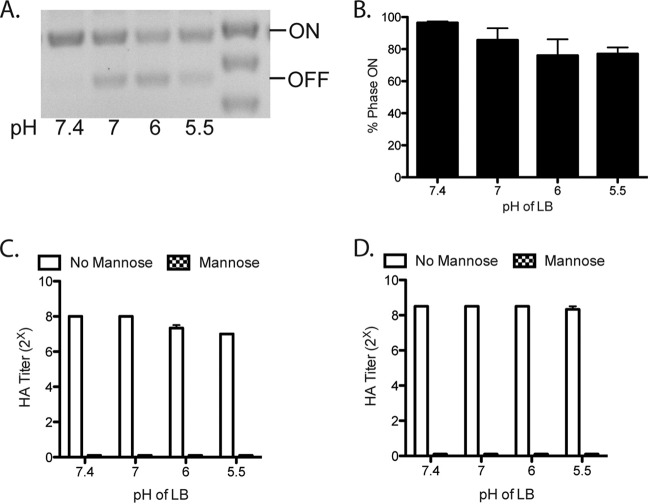
Type 1 piliation responds to a variety of environmental signals. The filtered human urine used in these experiments had pH values ranging from 5.3 to 7, which is in the normal range of urine pH but below the pH of LB (7.4). We therefore investigated whether the lower pH was responsible for the urine effect on fimS phase state and type 1 piliation level. We investigated the fimS phase state after growth in LB at different pHs. Growth in LB of pH 7, 6, or 5.5 had only a little or no effect on the fimS phase state, compared to growth in LB at pH 7.4 (Fig. 3A and B). The impact of low pH on the type 1 piliation level was quantified with HA titers of 28 after growth in LB of pH 7.4 and 27 after growth in LB at pH 5.5 (Fig. 3C). To differentiate between direct inhibition of pilus assembly versus alteration of regulation, we also grew the fimS phase-locked-ON (LIR) strain at low pH and observed no effect on type 1 piliation or function (Fig. 3D). Thus, the slight effect of pH on type 1 piliation was probably through regulation of expression, but this effect does not account for the ability of urine to induce the fimS phase OFF orientation and significantly decrease type 1 piliation. Thus, urine has a pH-independent mechanism for impacting fimS phase variation and type 1 piliation.
FIG 3 .
Impact of pH on fimS phase state and type 1 piliation. (A) Phase assay of UTI89 after two 24-h periods of static growth at 37°C in LB at the indicated pHs. (B) Quantification of three biological replicates of the phase assay shown in panel A to quantify the percentage of the population in the fimS phase ON state. (C) HA titers of UTI89 after two 24-h periods of static growth at 37°C in LB at the indicated pHs. (D) HA titers of the fimS phase-locked-ON strain LIR after two 24-h periods of static growth at 37°C in LB at the indicated pHs. These experiments were conducted in triplicate.
Whole urine inhibits FimH function.
The experiments described above were performed with pooled human urine that was filtered to remove any bacteria or eukaryotic cells. However, filtration also removed some proteins (see Fig. S2 in the supplemental material). Urine can contain mannosylated proteins, such as Tamm-Horsfall, as well as a variety of other types of proteins, carbohydrates, and small molecules that can impact FimH function. For example, previous studies have found that urine contains mannooligosaccharides and Tamm-Horsfall that can bind to type 1 pili and act as soluble inhibitors of FimH function (28). Consistent with these reports, we demonstrated that a 1-h incubation of piliated UTI89 (LIR) with unfiltered human urine decreased HA titers, indicating inhibition of FimH function (Fig. 1B). The same incubation of piliated cells with filtered urine had no impact on FimH function, indicating that a FimH inhibitor(s) in whole urine was removed by filtration.
Genetic inactivation of FimH function impacts fimS phase state but does not impede pilus assembly.
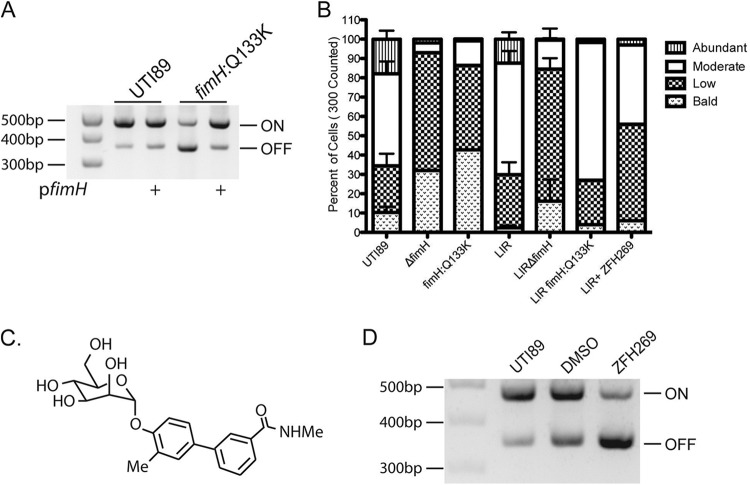
To test whether abolishing FimH function could result in further regulatory effects, we examined the fimS phase state in the point mutant UTI89-fimH Q133K, which contains a point mutation in the FimH mannose-binding pocket that abrogates mannose binding. UTI89-fimH Q133K switches to the fimS phase OFF state, even after growth under type 1 pilus-inducing conditions (29). We found that the phase defect in this mutant can be complemented by expression of wild-type fimH in trans, indicating that there were no unintended effects of this mutation on the other type 1 pilus components (Fig. 4A). Thus, inactivation of FimH function biases fimS toward the phase OFF state.
FIG 4 .
FimH inhibition. (A) Phase assay of UTI89 and fimH:Q133K with or without FimH complementation in trans by pfimH, after growth under type 1 pilus-inducing conditions. (B) Percent of the population of each strain with abundant, moderate, low, or no piliation, as assessed by counting 300 cells on electron micrographs. LIR is the fimS phase-locked-ON mutant. ZFH269 is the mannoside used. (C) Structure of mannoside ZFH269. (D) Phase assay of UTI89 grown under type 1 pilus-inducing conditions with DMSO vehicle control or 25 µM mannoside ZFH269.
Type 1 pili are assembled by the chaperone-usher pathway, and defects in pilus assembly are known to impact the fimS phase switch. For example, strains with deletions of fimC, the type 1 pilus chaperone, switch to the fimS phase OFF state, even after growth under type 1 pilus-inducing conditions (19). Furthermore, it was demonstrated previously that the chaperone-adhesin complex FimCH has the highest binding affinity for the FimD usher of any chaperone-subunit complex and that FimCH binding alters FimD structure (30, 31). Thus, it is proposed that the binding of the FimCH complex to the FimD usher initiates pilus assembly. To assess whether the fimH Q133K mutation prevented or slowed pilus assembly, we created a phase-locked-ON fimH Q133K mutant, LIR-fimH Q133K. We also created a LIRΔfimH strain, which encodes all the type 1 pilus components except the FimH adhesin, under the control of the fimS phase-locked-ON promoter. By electron microscopy, we quantified the percentage of cells with abundant, moderate, low, and no piliation. The LIRΔfimH strain produced very few piliated cells, confirming that FimH is needed for pilus assembly, as predicted based on the interactions of FimCH and FimD (31) (Fig. 4B). However, the LIR and LIR-fimH Q133K strains were similarly piliated, demonstrating that while the Q133K mutation in fimH results in nonpiliated cells, it does not cause a defect in pilus assembly (Fig. 4B). Thus, the fimH Q133K functionally null mutant impacts fim phase state, but not through defects in pilus assembly. This represents a novel form of type 1 pilus regulation.
Chemical inhibition of FimH affects type 1 pilus phase state.
Mannosides, small molecules derived from mannose, bind with very high affinity in the FimH mannose-binding pocket (32, 33). Growth in mannoside ZFH269 does not alter pilus assembly, as measured by EM of the fimS phase-locked-ON strain (LIR) grown in LB or LB with ZFH269 (Fig. 4B). However, growth with mannoside ZFH269 (Fig. 4C) under type 1 pilus-inducing conditions induced UTI89 cells to turn fimS phase OFF (Fig. 4D). Thus, both a genetic inactivation of FimH function in the fimH Q133K mutant and a chemical inhibition of FimH function with mannoside resulted in fimS switching to the phase OFF state. In both cases, type 1 pili are prevented from binding to a solid mannosylated surface, which may explain the effect on fimS phase. Attachment to a solid surface, such as PFA-fixed 5637 bladder epithelial cells, maintains the fimS phase ON state of UPEC even when these bacteria are grown in human urine (Fig. 2C). Possibly, physical tension on the pilus resulting from surface attachment induces UPEC to remain phase ON and continue expressing type 1 pili. Alternatively, the genetic and chemical perturbations may act via separate regulatory pathways that converge on the fimS promoter.
Recombinases control phase state in response to FimH inactivation.
To further investigate these regulatory pathways, we investigated the impact of fim recombinases on the fimS phase state during chemical and genetic manipulation of FimH. Many environmental conditions and regulators are known to impact fim expression by controlling fimB and fimE recombinase expression level or activity. While the fimH Q133K strain was 11% phase ON, a fimH Q133K ΔfimE strain was 94% phase ON after growth under type 1 pilus-inducing conditions (Fig. 5A and C). The fimH Q133K and fimH Q133K ΔfimB strains showed no significant difference in the percentage of fimS phase ON cells (Fig. 5C). Therefore, FimE, but not FimB, appears to control phase status in response to FimH functional inactivation.
FIG 5 .
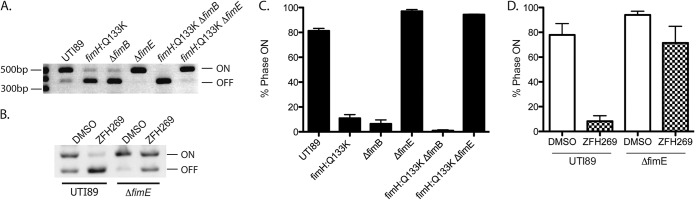
Role of fim recombinases in controlling phase state after FimH inhibition. (A) Phase assay of UTI89 and UTI89 isogenic mutants grown under type 1 pilus-inducing conditions. (B) Phase assay of UTI89 and UTI89ΔfimE isogenic mutants grown under type 1 pilus-inducing conditions with DMSO or 25 µM mannoside ZFH269. (C and D) ImageJ quantification of the phase state in represented in panels A and B, respectively, by phase assay. Graphs show quantification of three biological replicates.
We similarly found that UTI89 grown with mannoside ZFH269 was more fimS phase OFF than UTI89ΔfimE grown with mannoside (Fig. 5B and D). Therefore, fimE is at least partially required to turn UTI89 fimS phase OFF in response to both chemical and genetic FimH inactivation.
Overall, our experiments indicate that unfiltered human urine possesses two different mechanisms for inactivating type 1 pili: induction of the fimS phase OFF state and inactivation of FimH function, which itself impacts phase state.
DISCUSSION
UPEC organisms encounter several niches during the course of cystitis, including (i) urine, (ii) the luminal surface of the bladder mucosa, and (iii) the intracellular cytoplasmic space of bladder epithelial cells, where intracellular bacterial communities form. Type 1 pili mediate binding and invasion of human bladder cells (3, 4). They are necessary for the colonization and invasion of the murine bladder and thus are critical for UTIs in mice (7, 10, 11). Here, we investigated the effect of the urine niche on type 1 pilus expression and function, showing a general reduction of type 1 piliation and inhibition of type 1 pilus function by urine.
Human urine acts via at least two independent mechanisms to reduce type 1 pilus expression and function: (i) induction of the fimS phase OFF orientation, through a mechanism independent of both pH and direct FimH binding, thus preventing fim expression and further production of type 1 pili, and (ii) competitive inhibition of FimH-mediated attachment, presumably by mannosylated urine proteins, which in turn may further impact fimS phase state. Despite the fact that competitive FimH inhibition leads to the fimS phase OFF orientation, urine does mediate an independent effect on the fimS phase orientation, as growth in filtered urine, which does not inhibit FimH function, also induced the fimS phase OFF state. The combination of these urine effects leads to nearly complete loss of type 1 pilus expression (98% by HA titer; 100% by FimA Western blot analysis) during planktonic growth in urine. On a shorter time scale (1 h), unfiltered urine leads to a 50% reduction in FimH function, as measured by HA titer (Fig. 1). This dual effect of urine on type 1 pilus expression and function is especially important given that type 1 pili likely remain on the cell surface long after fim transcription is turned off. Thus, effectively inhibiting type 1 pilus-mediated attachment includes blocking both the function of pili on the cell surface and the production of new pili. We also found that UTI89 grows very poorly in filtered human urine, especially compared to growth in a rich medium such as Luria broth (LB). UTI89 grew to 11% (95% confidence interval [CI], 8.7 to 13.2) of the final bacterial culture density compared to growth in LB. Altered growth rate or metabolism in urine may directly impact type 1 pilus expression. However, it is also possible that urine acts through other upstream regulators that indirectly affect type 1 pilus expression. The combined effect of inhibition of type 1 pilus-mediated binding and physical expulsion by micturition represents a non-immune-cell-mediated mechanism to defend against UTIs.
In this study, we found that planktonic growth in urine induces the phase OFF orientation of the fimS promoter. Interestingly, fimS phase OFF UPEC organisms have been found to rapidly adopt the fimS phase ON orientation upon inoculation into the murine bladder (34). In mouse models, UPEC organisms that were adherent to sloughed bladder epithelial cells were more fimS phase ON than unattached UPEC organisms (25). We have confirmed that UPEC organisms attached to bladder epithelial cells remain phase ON, even during growth in human urine. Interestingly, binding to epithelial cells leads to UPEC invasion; once inside a bladder epithelial cell, UPEC organisms are presumably protected from traditional innate immune defenses and any inhibitory effects of urine. Thus, UPEC organisms in the urinary tract experience competing signals, which may vary with time and position, that are integrated at the fimS promoter to determine production of type 1 pili and thus UPEC binding, persistence, and infection. Similarly, it seems likely that expression of other virulence factors are also affected by different signals present in these different niches.
Previous characterization of type 1 pilus function, and the structure of the FimH adhesin, has enabled the development of mannosides, soluble mannose derivatives that bind with high affinity to FimH (32, 33). Mannosides block UPEC attachment to and invasion of bladder epithelial cells and formation of intracellular bacterial communities (IBCs), an important step in UPEC pathogenesis. Furthermore, mannosides have been shown to successfully prevent and/or to treat UTIs in a murine model, after oral delivery (32). These effects have been attributed to mannoside directly inhibiting FimH binding. We have now demonstrated that mannosides, like urine, also exert a regulatory effect by inhibiting phase variation into the fimS phase ON state. This is likely similar to the mechanism by which genetic defects in the FimH mannose-binding pocket, as in the FimH Q133K mutation, induce the fimS phase OFF state. Thus, we propose that mannosides may derive additional potency for treating UTIs from a combination of directly blocking FimH-mediated binding and invasion of bladder tissue and induction of the fimS phase OFF state, reducing overall type 1 pilus expression. This is particularly interesting given that both genetic and chemical inactivation of type 1 pilus assembly (disrupting the donor strand exchange process in the periplasm without direct effects on FimH adhesion) have also been found to induce the fimS phase OFF state (19). In summary, the fimS promoter is impacted by chemical and/or genetic interference with type 1 pilus function and assembly in order to switch to the fimS phase OFF state. This intrinsic regulatory feedback may prove beneficial in the development of therapeutics targeting type 1 pili and other adhesive organelles.
Recent transcriptional profiling studies of UTIs have been performed on bacteria from the urine of patients with UTI (23, 24). In those studies, UPEC was found to have variable expression of type 1 pili. This finding is consistent with previous work, which revealed a dichotomy between piliated tissue-associated UPEC and nonpiliated bacteria found in the urine of the same infected mouse (26). Furthermore, UPEC growth in urine is planktonic, while attachment to and invasion of the bladder epithelium involves biofilm-like communities. These niches may provide different nutrient availability, immune protection, or other benefits and challenges. Therefore, a complete understanding of UPEC virulence necessitates consideration of UPEC biology within these different niches over time. The transcriptomes of urine- and tissue-associated UPEC are thus likely to contain important differences, for example in type 1 pilus expression, in part depending on the level of exfoliated epithelial cells in the urine at the time of collection. Understanding these spatial-temporal complexities will provide insight into additional ways the host impacts UPEC physiology and virulence. Further, antivirulence compounds targeting FimH may supplement urine’s effects to block FimH function and fim expression, increasing their therapeutic potential.
MATERIALS AND METHODS
Bacterial growth conditions.
Experiments were conducted on the prototypical UPEC strain UTI89 or the isogenic UTI89 mutants listed in Table 1. Phase ON-inducing conditions (type 1 pilus-inducing conditions) were static incubation at 37°C for 24 h and then subculturing 1:1,000 for an additional 24-h static incubation at 37°C (30). Phase OFF-inducing conditions were shaking incubation at 25°C for 24 h and then subculturing 1:1,000 for an additional 24 h with shaking incubation at 25°C. Type 1 pilus-inducing (phase ON) and phase OFF-inducing conditions utilized growth in Luria broth (LB), unless otherwise stated. Growth was always twice for 24 h each, unless otherwise specified. Urine was collected from healthy human volunteers according to institutional review board (IRB) protocol (201207143) and filtered through a 0.22-µm filter before use, unless otherwise specified. Urine was pooled from at least two healthy volunteers for each experiment. M9 minimal medium contained 0.2% glucose as the sole carbon source and 10 µg/ml niacin.
TABLE 1 .
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| UTI89 | UPEC strain isolated from the urine of a patient with cystitis | 36 |
| MG1655 | K-12 laboratory strain | 37 |
| CFT073 | UPEC strain isolated from the blood of a patient with pyelonephritis | 38 |
| UTI89-fimH:Q133K | Q133K point mutation in fimH, Kmr | 29 |
| UTI89ΔfimH | UTI89ΔfimH | 7 |
| LIR | Mutation of the fimS left inverted repeat | 39 |
| LIR-fimH:Q133K | Mutation of the fimS left inverted repeat, Q133K point mutation in fimH, Kmr | This study |
| LIRΔfimH | Mutation of the fimS left inverted repeat, ΔfimH | This study |
| Plasmid | ||
| pfimH | pBAD33-fimH | This study |
Mannoside.
Mannoside ZFH269 was made as previously described (32).
Phase assay.
To quantify the percentage of a bacterial population in each phase state, the fimS promoter region of UTI89 and UTI89 isogenic mutants was PCR amplified out of whole bacterial cells, and then the PCR product was digested with the restriction enzyme Hinf1 at 37°C for 2 h. The resulting DNA products were resolved on a 2% agarose gel (12). DNA band intensity was quantified by ImageJ. The percent phase ON was calculated as 100 × (intensity of the top phase ON band/intensity of the top phase ON and OFF bands).
Tissue culture.
5637 bladder epithelial cells (ATCC HTB-9) were seeded into 6-well tissue culture plates and grown to confluence in RPMI 1604 medium with 10% fetal bovine serum. The cells were washed three times with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 1 h. Fixed cells were washed three times with PBS prior to inoculation with phase ON or OFF UTI89 culture diluted 1:1,000 into 3 ml pooled and filtered human urine. After two 24-h periods of growth at 37°C, culture supernatant was removed and used as the planktonic fraction for phase assays. Bladder and UTI89 cells were washed three times with PBS, scraped off the bottoms of the wells, and used as the attached fraction for phase assays.
HA.
Bacteria were grown under type 1 pilus-inducing conditions. Pilus expression was assessed by hemagglutination assays (HA) as previously described (35) using bacterial cultures normalized to an optical density at 600 nm (OD600) of 1 and guinea pig erythrocytes normalized to an OD640 of 2. The experiment was conducted in parallel with PBS–4% mannose.
Electron microscopy.
Electron micrographs were taken of UTI89 or UTI89 isogenic mutants after growth under type 1 pilus-inducing conditions, with or without 25 µM mannoside ZFH269 or dimethyl sulfoxide (DMSO) vehicle control. A total of 300 bacterial cells were counted for each condition, and piliation on those cells was classified as bald (no pili), low (1 to 20 pili/cell), moderate (20 to 200 pili/cell), or abundant (>200 pili/cell).
Bacterial culture density.
Final culture density was measured by absorbance at 600 nm.
Immunoblot analyses.
UTI89 and the LIR isogenic mutant were grown under type 1 pilus-inducing conditions in LB or filtered human urine. Cells were briefly agitated to mix the entire culture prior to sample collection by centrifugation, normalization to an OD600 of 10, and analysis by SDS-PAGE. Membranes were probed with antisera for FimA at 1:10,000.
SUPPLEMENTAL MATERIAL
Impact of urine on other E. coli strains. (A) OD600 of E. coli isolates after two 24-h periods of static growth at 37°C in filtered human urine. There was no statistically significant difference in growth as measured by the Mann-Whitney t test (P < 0.05). (B) Percentage of the population in the fimS phase ON orientation after two 24-h periods of static growth at 37°C in LB or filtered human urine, for the indicated strain. (C) HA titers of the indicated strains after two 24-h periods of static growth at 37°C in LB or filtered human urine. Download
Protein is removed from urine by filtration. Pooled human urine was analyzed by SDS-PAGE. High-molecular-weight proteins, including one with the approximate size of Tamm-Horsfall protein (L. Wai-Hoe, L. Wing-Seng, Z. Ismail, and G. Lay-Harn, Biol Proced Online 11:145–160, 2009), present in human urine are lost after filtration. Download
ACKNOWLEDGMENTS
We thank Karen Dodson for invaluable comments. We gratefully acknowledge the help and expertise of Wandy Beatty of the Imaging Facility, Washington University School of Medicine.
This work was supported by NIH grants F30 DK098870-01, R01 AI048689, R01 DK051406, and R01 AI29549.
Scott Hultgren has an ownership interest in Fimbrion and may benefit if the company is successful in marketing the mannosides that are related to this research.
Footnotes
Citation Greene SE, Hibbing ME, Janetka J, Chen S, Hultgren SJ. 2015. Human urine decreases function and expression of type 1 pili in uropathogenic Escherichia coli. mBio 6(4):e00820-15. doi:10.1128/mBio.00820-15.
REFERENCES
- 1.Hooton TM, Stamm WE. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 11:551–581. doi: 10.1016/S0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 2.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. 2007. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog 3:e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 5.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. 2009. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A 106:14966–14971. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. 2004. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol 12:424–430. doi: 10.1016/j.tim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Wright KJ, Seed PC, Hultgren SJ. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol 9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Pírez MC, Vignoli R. 2014. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin Infect Dis 59:e158–e164. doi: 10.1093/cid/ciu634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell I, Agace W, Klemm P, Schembri M, Mărild S, Svanborg C. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A 93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol 45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 12.Abraham JM, Freitag CS, Clements JR, Eisenstein BI. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A 82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemm P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J 5:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwan WR. 2011. Regulation of genes in uropathogenic. World J Clin Infect Dis 1:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer FG, Fütterer K, Pinkner JS, Dodson KW, Hultgren SJ, Waksman G. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058–1061. doi: 10.1126/science.285.5430.1058. [DOI] [PubMed] [Google Scholar]
- 16.Remaut H, Rose RJ, Hannan TJ, Hultgren SJ, Radford SE, Ashcroft AE, Waksman G. 2006. Donor-strand exchange in chaperone-assisted pilus assembly proceeds through a concerted beta strand displacement mechanism. Mol Cell 22:831–842. doi: 10.1016/j.molcel.2006.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remaut H, Tang C, Henderson NS, Pinkner JS, Wang T, Hultgren SJ, Thanassi DG, Waksman G, Li H. 2008. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133:640–652. doi: 10.1016/j.cell.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose RJ, Welsh TS, Waksman G, Ashcroft AE, Radford SE, Paci E. 2008. Donor-strand exchange in chaperone-assisted pilus assembly revealed in atomic detail by molecular dynamics. J Mol Biol 375:908–919. doi: 10.1016/j.jmb.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 19.Greene SE, Pinkner JS, Chorell E, Dodson KW, Shaffer CL, Conover MS, Livny J, Hadjifrangiskou M, Almqvist F, Hultgren SJ. 2014. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5:e02038-14. doi: 10.1128/mBio.02038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, Dame ZT, Poelzer J, Huynh J, Yallou FS, Psychogios N, Dong E, Bogumil R, Roehring C, Wishart DS. 2013. The human urine metabolome. PLoS One 8:e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisielius PV, Schwan WR, Amundsen SK, Duncan JL, Schaeffer AJ. 1989. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect Immun 57:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielecki P, Muthukumarasamy U, Eckweiler D, Bielecka A, Pohl S, Schanz A, Niemeyer U, Oumeraci T, von Neuhoff N, Ghigo JM, Häussler S. 2014. In vivo mRNA profiling of uropathogenic Escherichia coli from diverse phylogroups reveals common and group-specific gene expression profiles. mBio 5:e01075-14. doi: 10.1128/mBio.01075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6:e1001187. doi: 10.1371/journal.ppat.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunther NW, Lockatell V, Johnson DE, Mobley HL. 2001. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun 69:2838–2846. doi: 10.1128/IAI.69.5.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hultgren SJ, Porter TN, Schaeffer AJ, Duncan JL. 1985. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun 50:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkkinen J, Virkola R, Korhonen TK. 1988. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect Immun 56:2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ. 2009. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci U S A 106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkan E, Ford BA, Pinkner JS, Dodson KW, Henderson NS, Thanassi DG, Waksman G, Hultgren SJ. 2012. Domain activities of PapC usher reveal the mechanism of action of an Escherichia coli molecular machine. Proc Natl Acad Sci U S A 109:9563–9568. doi: 10.1073/pnas.1207085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saulino ET, Thanassi DG, Pinkner JS, Hultgren SJ. 1998. Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J 17:2177–2185. doi: 10.1093/emboj/17.8.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cusumano CK, Pinkner JS, Han Z, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ. 2011. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 3:109ra115. doi: 10.1126/scitranslmed.3003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Z, Pinkner JS, Ford B, Chorell E, Crowley JM, Cusumano CK, Campbell S, Henderson JP, Hultgren SJ, Janetka JW. 2012. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem 55:3945–3959. doi: 10.1021/jm300165m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannan TJ, Mysorekar IU, Chen SL, Walker JN, Jones JM, Pinkner JS, Hultgren SJ, Seed PC. 2008. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol Microbiol 67:116–128. doi: 10.1111/j.1365-2958.2007.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hultgren SJ, Schwan WR, Schaeffer AJ, Duncan JL. 1986. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun 54:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103:5977–5982. doi: 10.1073/pnas.0600938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalf WW, Steed PM, Wanner BL. 1990. Identification of phosphate starvation-inducible genes in Escherichia coli K-12 by DNA sequence analysis of psi::lacZ(Mu d1) transcriptional fusions. J Bacteriol 172:3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kostakioti M, Hadjifrangiskou M, Cusumano CK, Hannan TJ, Janetka JW, Hultgren SJ. 2012. Distinguishing the contribution of type 1 pili from that of other QseB-misregulated factors when QseC is absent during urinary tract infection. Infect Immun 80:2826–2834. doi: 10.1128/IAI.00283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impact of urine on other E. coli strains. (A) OD600 of E. coli isolates after two 24-h periods of static growth at 37°C in filtered human urine. There was no statistically significant difference in growth as measured by the Mann-Whitney t test (P < 0.05). (B) Percentage of the population in the fimS phase ON orientation after two 24-h periods of static growth at 37°C in LB or filtered human urine, for the indicated strain. (C) HA titers of the indicated strains after two 24-h periods of static growth at 37°C in LB or filtered human urine. Download
Protein is removed from urine by filtration. Pooled human urine was analyzed by SDS-PAGE. High-molecular-weight proteins, including one with the approximate size of Tamm-Horsfall protein (L. Wai-Hoe, L. Wing-Seng, Z. Ismail, and G. Lay-Harn, Biol Proced Online 11:145–160, 2009), present in human urine are lost after filtration. Download