ABSTRACT
ParB proteins bind centromere-like DNA sequences called parS sites and are involved in plasmid and chromosome segregation in bacteria. We previously showed that the opportunistic human pathogen Streptococcus pneumoniae contains four parS sequences located close to the origin of replication which are bound by ParB. Using chromatin immunoprecipitation (ChIP), we found here that ParB spreads out from one of these parS sites, parS(−1.6°), for more than 5 kb and occupies the nearby comCDE operon, which drives competence development. Competence allows S. pneumoniae to take up DNA from its environment, thereby mediating horizontal gene transfer, and is also employed as a general stress response. Mutating parS(−1.6°) or deleting parB resulted in transcriptional up-regulation of comCDE and ssbB (a gene belonging to the competence regulon), demonstrating that ParB acts as a repressor of competence. However, genome-wide transcription analysis showed that ParB is not a global transcriptional regulator. Different factors, such as the composition of the growth medium and antibiotic-induced stress, can trigger the sensitive switch driving competence. This work shows that the ParB-parS chromosome segregation machinery also influences this developmental process.
IMPORTANCE
Streptococcus pneumoniae (pneumococcus) is an important human pathogen responsible for more than a million deaths each year. Like all other organisms, S. pneumoniae must be able to segregate its chromosomes properly. Not only is understanding the molecular mechanisms underlying chromosome segregation in S. pneumoniae therefore of fundamental importance, but also, this knowledge might offer new leads for ways to target this pathogen. Here, we identified a link between the pneumococcal chromosome segregation system and the competence-developmental system. Competence allows S. pneumoniae to take up and integrate exogenous DNA in its chromosome. This process plays a crucial role in successful adaptation to—and escape from—host defenses, antibiotic treatments, and vaccination strategies. We show that the chromosome segregation protein ParB acts as a repressor of competence. To the best of our knowledge, this is the first example of a ParB protein controlling bacterial competence.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is an opportunistic human pathogen with high morbidity and mortality rates which causes several invasive diseases, such as pneumonia, septicemia, and meningitis. For instance, for only the disease pneumonia, it was recently estimated that S. pneumoniae is annually responsible for more than 400,000 deaths in children under the age of 5 (1).
The evolutionary success of S. pneumoniae strongly depends on its ability to colonize its host and adapt to changing environments within the host. A crucial adaptation system in pneumococci is the competence system, which allows genetic transformation, a horizontal gene transfer mechanism in which the cells are able to take up and integrate exogenous DNA into their genome (2). During competence, cells activate expression of about 100 genes, fewer than 20 of which are required for transformation (3–8). Moreover, development of competence responds to changes in the environment and is activated by certain antibiotics (9–11). Thus, competence is considered a general stress response (6, 12). It has also been suggested that certain cell cycle cues such as DNA replication can serve as regulatory inputs for competence development (11, 13), but it remains unclear how this works under non-stressed conditions.
Chromosome segregation is an essential process during the bacterial cell cycle, and studying the molecular mechanisms underlying chromosome segregation in S. pneumoniae might offer new leads for targeting this important human pathogen. We have shown previously that chromosome segregation in S. pneumoniae is promoted by at least two proteins, ParB and SMC (structural maintenance of chromosomes) (14). ParB binds to a 16-bp DNA sequence called parS (S. pneumoniae D39 consensus sequence, tGTTTCACGtGAAACa; bases in lowercase can deviate), four of which can be found in the pneumococcal genome: parS(−19.2°), parS(−3.7°), parS(−1.6°), and parS(+2°) (the numbers indicate the relative distance in degrees from the origin of replication as a function of the circular chromosome). Interestingly, ParB recruits the conserved condensin SMC complex to these parS sites, thereby enriching SMC near the origin of replication (oriC) (14–16). In the absence of ParB or SMC, chromosome segregation is perturbed, leading to a significant fraction of anucleate cells. Time-lapse microscopy of the pneumococcal cell cycle has revealed that SMC is involved in organizing and splitting of newly replicated oriCs, and in the absence of SMC, chromosomes are frequently guillotined by the cell division machinery (17). In addition to ParB-parS and SMC, several other processes, such as transcription and replication, might also drive chromosome segregation in S. pneumoniae (17, 18). For recent reviews on chromosome segregation in other bacteria, see references 18, to ,27.
Besides having active roles in chromosome segregation, chromosomal ParB proteins were also shown to influence the bacterial cell cycle in other ways. For instance, ParB proteins affect DNA replication either directly or indirectly in a number of different bacteria, including Bacillus subtilis and Vibrio cholerae (28, 29). Moreover, plasmid ParB proteins can act as transcriptional regulators by binding parS and “spreading” into nearby plasmid promoters (30). Spreading is the ability of ParB proteins to bind nonspecific DNA, presumably via oligomerization that nucleates at a parS site, and via DNA looping, even distant sites can be bound (27). Interestingly, ParB oligomers occupying DNA near chromosomal parS sites generally do not affect gene expression (27, 31). However, there are exceptions; it was recently shown that the chromosomal ParB1 protein of V. cholerae can indeed control transcription (32), and, directly or indirectly, deletion of Pseudomonas aeruginosa parB leads to global transcriptional changes (33). Together, these observations raise the question of whether S. pneumoniae ParB also has additional roles besides recruiting SMC to oriC. In particular, we noticed that one of the parS sequences, parS(−1.6°), is located only 5 kb away from the comCDE operon (Fig. 1). The comCDE operon lies at the heart of the competence development cascade in pneumococcus (Fig. 1), and small differences in comCDE expression can activate competence because of the positive feedback loop built into the regulatory network (11, 34, 35).
FIG 1 .
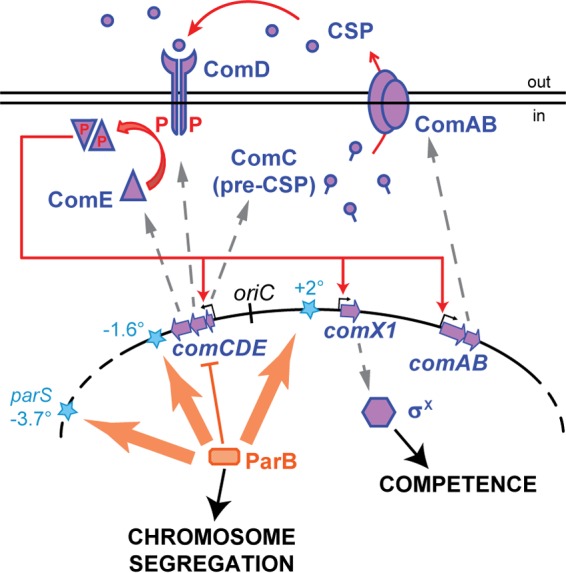
Interplay between chromosome segregation and regulation of competence in S. pneumoniae. ParB (orange rectangle) binds to parS sites (blue stars), which are located close to the origin of replication (oriC). Basal levels of comCDE transcription are required to build up the extracellular levels of competence-stimulating peptide (CSP), encoded by comC. Once a threshold level of CSP is reached and sensed by ComD, an autocatalytic loop is activated in which phosphorylated ComE (purple triangles) stimulates further expression of comCDE and comAB (encoding the CSP exporter), leading to expression of the alternative sigma factor σX and downstream competence genes. By spreading from parS and/or forming DNA loops, ParB binds the comCDE locus, effectively reducing CSP accumulation and thereby controlling competence development.
To test whether ParB binds the comCDE operon and influences its expression, we performed chromatin immunoprecipitation (ChIP) and real-time luciferase assays. We show that ParB binds to parS(−1.6°) and from there spreads and/or loops into comCDE, thereby reducing expression of comCDE and of downstream competence genes. The data suggest that the autocatalytic nature of the competence system makes ParB regulation unique, since ParB does not influence global gene transcription in S. pneumoniae. Together, these results suggest that cell cycle events provide molecular cues for the entry into pneumococcal competence development.
RESULTS
ParB represses competence development in S. pneumoniae.
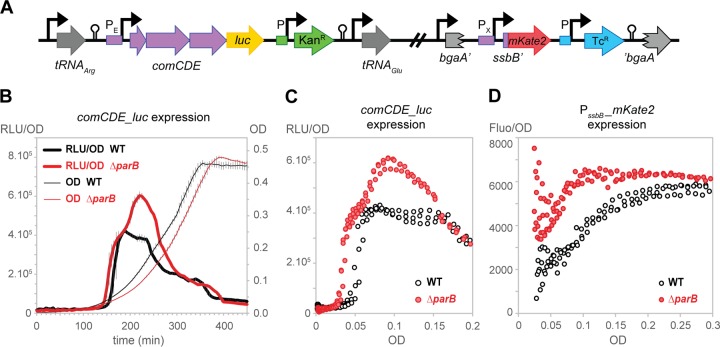
Competence is a tightly regulated physiological state in which bacteria become able to take up exogenous DNA and use it for genetic transformation. In S. pneumoniae, different factors, such as the composition of the medium, the pH of the growth medium, and certain stresses caused by antibiotics, have been shown to trigger competence (6). However, the molecular mechanisms influencing initiation of competence remains poorly understood. We recently showed that S. pneumoniae ParB binds to four parS sequences located near oriC. Intriguingly, all the operons implicated in the development of natural competence (i.e., comCDE, comAB, and comX) are also located near oriC (Fig. 1). In particular, parS(−1.6°) is located within 5 kb of the comCDE operon, suggesting that binding of ParB to this chromosomal region might influence comCDE expression and thus competence development. To test this, we first constructed a reporter strain in which we integrated the gene encoding firefly luciferase behind comCDE (Fig. 2A). This reporter (comCDE_luc) allows real-time observation of comCDE expression, since the amount of light detected (resulting from the activity of luciferase) is directly proportional to the expression level of comCDE. Moreover, as firefly luciferase is a very labile protein, this transcriptional fusion is a very sensitive reporter of any changes in expression (36). To check whether changes in comCDE expression actually alter competence development, we also introduced a second reporter (PssbB_mKate2) (Fig. 2A), where expression of the red fluorescent protein mKate2 is controlled by the promoter of the ssbB gene (PssbB). PssbB activity is dependent on the competence-specific alternative sigma factor σX and can thus serve as a reporter for competence development (Fig. 1). Wild-type and parB mutant cells were grown in C+Y medium at multiple competence-permissive pHs in microtiter plates to measure the optical density, luminescence, and fluorescence at intervals. As shown in Fig. 2 and in Fig. S1 and S2 in the supplemental material, expression of both comCDE and ssbB was increased in the absence of ParB. Specifically, their expression not only reached higher levels but also was activated at lower cell densities in the parB mutant than in the wild type (Fig. 2C and D). Although subtle, these results were highly reproducible and show that the parB mutant displays a competence-up phenotype.
FIG 2 .
ParB attenuates competence development. (A) Representation of the transcriptional fusions used to follow competence development in the double-labeled strains DLA82 (WT) and DLA84 (ΔparB). For the comCDE_luc fusion, the luc open reading frame (ORF) preceded by its ribosome binding site (and a kanamycin resistance gene with its own promoter) was inserted right after the comE ORF. A transcriptional fusion between the promoter of ssbB (late competence gene) and the mKate2 ORF was stably integrated at the nonessential bgaA locus. (B to D) The WT strain (DLA82: comCDE_luc bgaA::PssbB_mKate2) and the parB mutant (DLA84: ΔparB::spc comCDE_luc bgaA::PssbB_mKate2) were grown in C+Y medium; optical density at 595 nm, luciferase activity (in relative luminescence units [RLU]), and mKate2 production (fluorescence detection using 590 nm and 612 nm as excitation and emission wavelengths, respectively) were determined every 5 min. (B) Cell density (OD) and comCDE_luc expression (RLU/OD) as a function of time (averages of three replicates with the standard errors of the mean [SEM] are plotted). (C) Data points are from the same 3 replicates, but comCDE_luc expression (RLU/OD) is plotted as a function of OD, which allows direct comparison between strains with different growth kinetics (45). (D) Relative expression of PssbB_mKate2 (Fluo/OD) as a function of OD (see Fig. S2 in the supplemental material for the representation of Fluo/OD as a function of time).
We previously showed that competence development is promoted by certain antibiotics that perturb DNA replication (11). When these antibiotics stall replication elongation while new rounds of replication initiate, it causes an increased gene dosage for the genes located close to the origin and consequently an increased expression of comCDE. To test whether competence is activated in the absence of ParB due to an increased comCDE gene dosage, we performed quantitative real-time PCR to determine the ratio between oriC and the terminus region (ori-ter ratio). This analysis showed that the comCDE gene dosage is slightly reduced in the parB mutant (see Fig. S3 in the supplemental material), suggesting that another mechanism must be responsible for the ParB-dependent competence-up phenotype.
parS(−1.6°) contributes to the control of competence development.
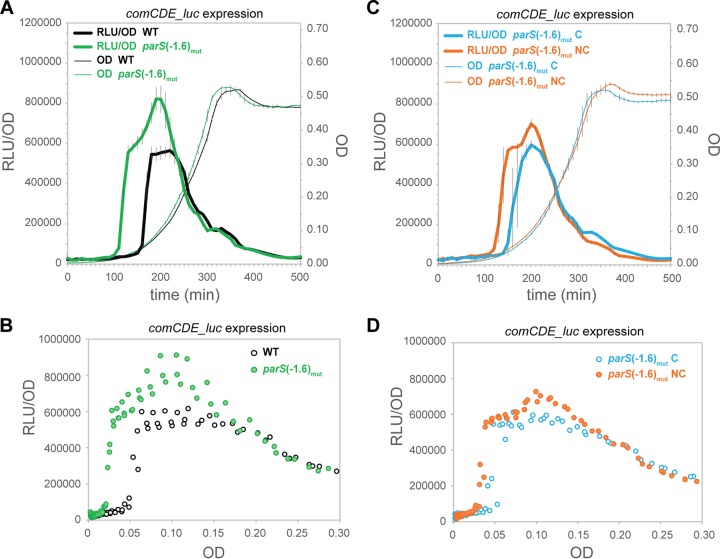
As mentioned above, parS(−1.6°) is located within 10 kb of oriC and within 5 kb of comCDE (Fig. 3A). To test whether ParB could be a direct repressor of comCDE by binding and spreading from parS(−1.6°), we mutated this site. As the parS(−1.6°) site is situated within a gene (SPD_2058), we introduced six silent point mutations to reduce homology to the parS consensus sequence [parS(−1.6°)mut] while keeping the amino acid sequence of SPD_2058 unaltered (Fig. 3B). To directly compare the impact of the presence or absence of a functional parS site at this locus, we complemented the parS(−1.6°)mut strain by reinserting the functional parS(−1.6°) sequence downstream of SPD_2058, resulting in the parS(−1.6°)mutC strain (Fig. 3C), and we also made a noncomplemented mutant derivative, the parS(−1.6°)mutNC strain (Fig. 3D).
FIG 3 .
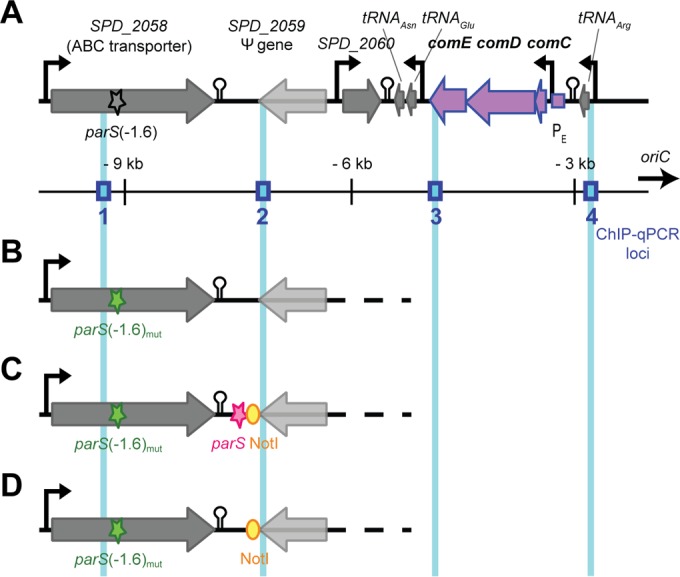
Organization of the parS(−1.6°) locus. (A) Schematic representation (proper scale is preserved, and distance from oriC is indicated) of the chromosome between parS(−1.6°) and the comCDE loci in the wild-type strain. Blue boxes show the localization of the four loci tested by qPCR after ChIP experiments (Fig. 5). (B) Chromosomal organization in the parS(−1.6°)mut strain. To be able to properly assess the influence of parS(−1.6°) on comCDE expression, a complemented (C) and a non-complemented (D) derivative of the parS(−1.6°)mut strain were constructed. In the parS(−1.6°)mutC (complemented) strain, the functional parS(−1.6°) sequence and a NotI restriction site were inserted at 1.7 kb from the parS(−1.6°) locus (between SPD_2058 and SPD_2059), whereas in the parS(−1.6°)mutNC (non-complemented) strain, only the NotI site is present.
To test the effect of the parS(−1.6°) site on competence development, we studied the expression of comCDE and ssbB by luciferase assays. As shown in Fig. 4A and B and in Fig. S4A to C in the supplemental material, comCDE and ssbB expression was upregulated and activated earlier when parS(−1.6°) was mutated. Note that mutating parS(−1.6°) did not lead to the growth defects observed in the parB mutant (Fig. 2B) (14), indicating functional redundancy of the parS sites and suggesting that the competence phenotype in parB and parS mutants is not due to a defect in ParB-dependent chromosome segregation (Fig. 4A). Our results show that reintroducing a functional parS site close to the mutated one is able to repress comCDE (and ssbB) (Fig. 4C and D; also, see Fig. S4D to F in the supplemental material). Hence, the presence of a parS sequence near comCDE is sufficient to delay competence development.
FIG 4 .
Mutating parS(−1.6°) derepresses competence development. Strains with the comCDE_luc reporter fusion were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 10 min. (A and B) The WT strain (DLA18) versus the parS(−1.6°)mut strain (DLA28). (C and D) The parS(−1.6°)mutC strain (DLA65) versus the parS(−1.6°)mutNC strain (DLA66). (A and C) Cell density (OD, right axis) and comCDE_luc expression (RLU/OD) are presented as a function of time in minutes (averages of three replicates with the SEM are plotted). (B and D) The data points are from the same 3 replicates as in panels A and C, respectively, but comCDE_luc expression (RLU/OD) is plotted as a function of OD.
ParB binds to comCDE by spreading from parS(−1.6°).
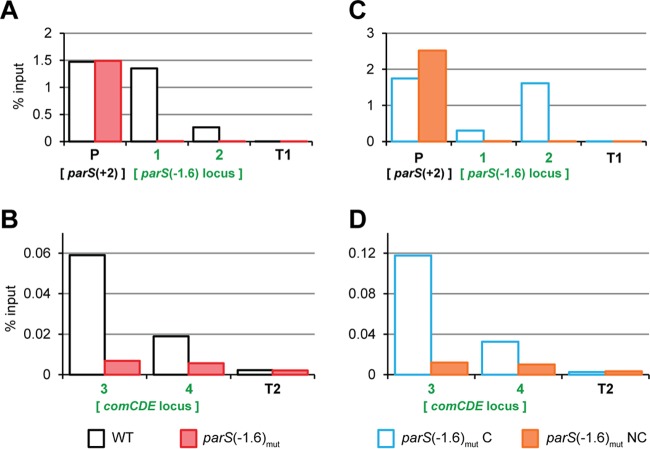
ParB proteins are thought to organize chromosomes by forming nucleoprotein complexes, nucleating from parS sites (27). To test whether S. pneumoniae ParB could spread from parS(−1.6°) into the nearby comCDE operon, and in that way control competence, we performed ChIP assays followed by quantitative real-time PCR of different loci [see Fig. 3 for the localization of the tested loci around parS(−1.6°)]. First, we replaced wild-type ParB with a fully functional green fluorescent protein (GFP)-tagged ParB that allows ChIP using anti-GFP antibodies (14). As shown in Fig. 5A, ParB-GFP was highly enriched at the two parS sites closest to oriC, parS(+2°) and parS(−1.6°), and was virtually absent from loci located close to the terminus region (T1 and T2, at −175.7° and −174.2° of the chromosome, respectively), as was established before (14). ParB-GFP was also enriched at the 3′ end of the comCDE locus and even present, although less so, at the comCDE promoter, which is 6.5 kb away from parS(−1.6°) (Fig. 5B). Importantly, mutating parS(−1.6°) led to a significant reduction of ParB-GFP at comCDE (Fig. 5B), strongly suggesting that parS(−1.6°) is the main contributor to the presence of ParB at comCDE. Introducing a parS sequence downstream of the mutated parS(−1.6°) locus restored ParB-GFP binding at comCDE (Fig. 5C and D). Together, these data show that ParB binds at parS(−1.6°) and from there spreads and/or forms DNA loops, thereby binding for more than 6.5 kb into the comCDE locus.
FIG 5 .
ParB spreads from parS(−1.6°) into comCDE. In the strains of interest, ParB was replaced by a ParB-GFP fusion. Exponentially growing cells were subjected to chromatin immunoprecipitation (ChIP) using anti-GFP antibodies, and the pulled-down DNA was subsequently analyzed by qPCR. (A and B) The WT (DLA42) versus the parS(−1.6°)mut strain (DLA43). (C and D) The parS(−1.6°)mutC strain (DLA77) versus the parS(−1.6°)mutNC strain (DLA80). The loci amplified by primer pairs 1, 2, 3, and 4 are shown in blue in Fig. 3A. The primer pair P amplifies another parS site [parS(+2°)] situated +11 kb from oriC. Primer pairs T1 and T2 amplify 2 different loci in the terminus region. The graphs show pulldown efficiency (ChIP-DNA/input DNA × 100) for each primer pair. Note the different y axis scale between panels A and C and panels B and D.
ParB is not a global transcriptional regulator in S. pneumoniae.
The data so far show that S. pneumoniae ParB can bind DNA as far as 6.5 kb away from parS sequences and that it acts as a repressor for comCDE expression and in that way fine-tunes competence development. To test whether ParB affects transcription globally, we performed DNA microarray experiments under non-competence-permissive conditions (pH 7.0). Total RNA of mid-exponential-phase wild-type and parB mutant cells was isolated and used for DNA microarray experiments (see Materials and Methods for details). As shown in Table 1, expression of only 9 genes was significantly altered (>2-fold change; P < 0.005). Importantly, none of these differentially expressed genes was located close to parS sequences. Together, these data suggest that pneumococcal ParB is not a global transcriptional regulator and that, in general, transcription of genes located in close proximity to parS sites is not strongly affected.
Table 1 .
Significantly differentially regulateda genes in strain MT3 (ΔparB::spc) compared to wild-type D39
| Gene category and locus tag | Description | Fold change | P value | Nearest parS site | Distance (kb) from parSb |
|---|---|---|---|---|---|
| Upregulated genes | |||||
| SPD_0731 | Topology modulation protein | 3.6 | 0.004 | parS(+2°) | 733.4 |
| SPD_1874 | LysM domain-containing protein | 2.7 | 2.41E-08 | parS(−19.2°) | 85.2 |
| SPD_1871 | Hypothetical protein | 2.5 | 0.002 | parS(−19.2°) | 86.2 |
| SPD_0978 | Hypothetical protein | 2.3 | 0.005 | parS(+2°) | 945.9 |
| SPD_1933 |
malQ (4-alpha-glucanotransferase) |
2.2 | 0.001 | parS(−19.2°) | 31.7 |
| Downregulated genes | |||||
| SPD_2069 | parBc | −21.1 | 9.07E-11 | parS(−1.6°) | 8.6 |
| SPD_0114 | Hypothetical protein | −4.1 | 3.05E-09 | parS(+2°) | 104.4 |
| SPD_0115 | Hypothetical protein | −3.9 | 2.46E-06 | parS(+2°) | 104.9 |
| SPD_0116 | Hypothetical protein | −3.8 | 2.79E-08 | parS(+2°) | 106.4 |
| Competence genesd | |||||
| SPD_2065 | comC | 1.6 | 0.144 | parS(−1.6°) | 6.4 |
| SPD_2064 | comD | −1.2 | 0.123 | parS(−1.6°) | 6.3 |
| SPD_2063 | comE | −1.1 | 0.572 | parS(−1.6°) | 5.0 |
| SPD_0049 | comA | 1.5 | 0.157 | parS(+2°) | 30.5 |
| SPD_0050 | comB | 1.4 | 0.104 | parS(+2°) | 32.7 |
Genes were considered differentially regulated when the change was >2-fold and P was <0.005. Note that data originated from two biological replicates, one dye-swap, and three technical replicates (9 data points for each gene).
Distance from the gene to the closest parS.
Note that parB is missing in strain MT3, but due to cross-hybridization, some residual signal was detected in strain MT3.
The competence genes were not significantly differentially regulated (P > 0.005) under these non-competence-permissive conditions (pH 7.0).
Expression of a constitutive synthetic promoter is not influenced by the presence of parS.
ParB influences competence development by binding and repressing comCDE but does not significantly affect expression of other genes located close to parS sites. If regulation of comCDE by ParB is indeed unique, then transcription of a synthetic constitutive promoter should not be affected by the nearby presence of a parS site. To test this, we constructed a number of reporter constructs. First, we introduced the constitutive synthetic P32 promoter (37) fused to luciferase close to oriC at +5° of the chromosome. Next, we compared the expression of P32_luc in a wild-type strain to that in parB mutant (see Fig. S5A and B in the supplemental material) and also between the parS(−1.6°)mutNC mutant and its complemented counterpart, the parS(−1.6°)mutC strain (see Fig. S5C and D in the supplemental material). In all cases, transcription of luciferase from P32 was unaffected by the absence of ParB or by the presence of parS (see Fig. S5A to D in the supplemental material). However, the closest parS sequence in these strains is parS(+2°), approximately 18 kb away from P32_luc at 5°; thus, it could be that ParB spreading or looping is insufficient to efficiently alter transcription of the P32_luc fusion. Therefore, we introduced the P32_luc construct, together with either an intact or mutated parS sequence [parS(−1.6°)] at the ectopic bgaA locus. As shown in Fig. 6, luciferase expression from this synthetic construct is identical whether or not it is preceded by a functional parS sequence.
FIG 6 .
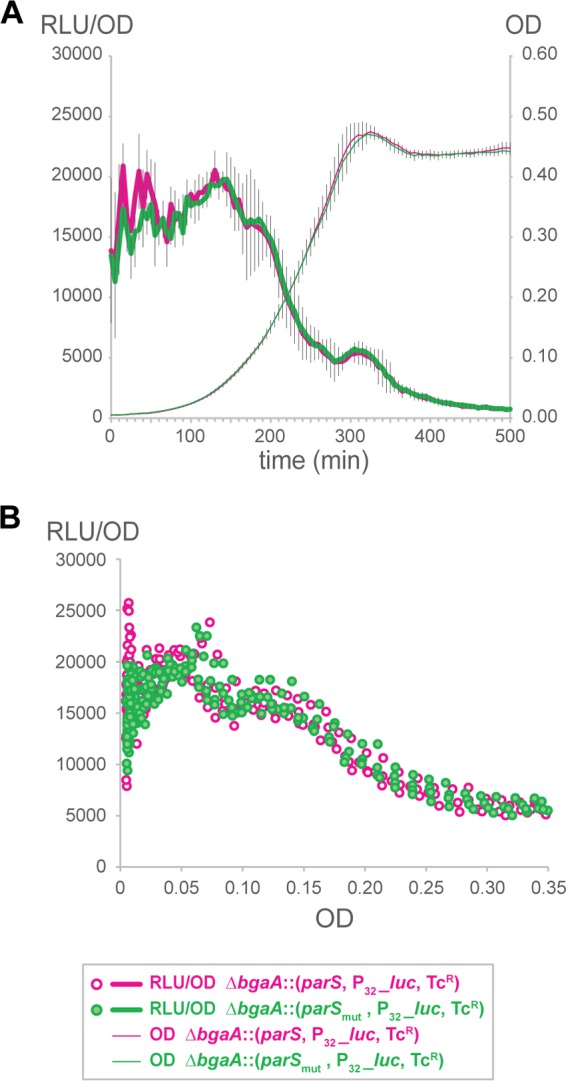
The ParB-parS repressor effect is not detected on a strong synthetic promoter. Strains were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 5 min. The P32_luc reporter fusion was inserted at the bgaA locus and preceded either by a functional parS sequence (strain DLA106) or by a mutated parS sequence (strain DLA107). (A) Cell density (OD) and P32_luc expression (RLU/OD) as a function of time (averages of three replicates with the SEM are plotted). (B) Data points are from the same 3 replicates, but P32_luc expression (RLU/OD) is plotted as a function of OD.
DISCUSSION
Competence development by the comCDE regulatory system is highly conserved in clinical isolates of S. pneumoniae (38). The system relies on the tight genetic regulation of CSP production and export, and as this regulation involves a positive feedback loop, competence activation spreads throughout the population (Fig. 1) (39). Moreover, competence development is influenced by several parameters (40). For example, the composition and pH of the medium affect induction of competence (9), and certain antibiotics promote competence (10) either by inducing the production of misfolded proteins (41) or by altering the gene dosage of comCDE (11). Here, we show that the ubiquitous chromosome segregation protein ParB also influences competence development: it binds to parS(−1.6°) and from there modulates expression of comCDE (Fig. 2, 4, and 5). Whether ParB spreads from parS(−1.6°) into comCDE or loops DNA to bind comCDE is currently not clear. Also, we cannot exclude an indirect effect of the parS-mediated ParB effect on competence. In any case, comCDE is, under our experimental conditions, the only parS-dependent ParB-regulated operon, and gene expression in general is not affected by binding of ParB (Fig. 6; Table 1). This highlights the unique sensitivity of the competence developmental system: due to its autocatalytic nature, even slight imbalances in comCDE expression can trigger competence development. The biological function of ParB-controlled competence is at this point unclear and currently under investigation by us, but it is tempting to speculate that cell cycle control of competence is crucial for the maintenance of genome integrity and for coping with stress (6, 12). While competence allows pneumococci to take up exogenous DNA and in that way rapidly acquire new traits, such as antibiotic resistance markers and altered capsule serotypes, competent cells also present a higher fitness in certain stressful conditions, even in the absence of DNA (42). Furthermore, our finding that ParB affects competence development also suggests that cell cycle events, such as chromosome segregation, can provide molecular cues for the entry into competence. Interestingly, the chromosomal location of comCDE near oriC, and thus near parS sites, is highly conserved in S. pneumoniae (11), suggesting that ParB control of competence is also conserved. It will be interesting to see if ParB proteins in other organisms can also control sensitive genetic switches and can link chromosome segregation with bacterial development.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. Growth conditions are described in detail in the supplemental material, as well as the construction of the plasmids and strains used. S. pneumoniae strains were grown as standing cultures in complex C+Y medium at 37°C. Blood agar plates were made from Columbia agar containing 4% defibrinated sheep blood (Johnny Rottier, Kloosterzade, the Netherlands). Escherichia coli EC1000 was grown at 37°C in a shaking incubator in TY broth (Bacto-tryptone [1%], Bacto-yeast extract [0.5%], and 1% NaCl).
Growth curves, luminescence, and fluorescence assays.
C+Y medium (pH adjusted with HCl) was complemented with 340 µg/ml luciferin and inoculated with mid-exponential-phase frozen cultures diluted 100 times. Cells were grown at 37°C in 96-well plates (Polystyrol, white, flat, and clear bottom; Corning) in a microtiter plate reader (Tecan Infinite F200 Pro). Throughout growth, absorbance (optical density at 595 nm [OD600]), luminescence (expressed in relative luminescence units [RLU]), and mKate2 signal (590 nm/612 nm, 50% dichroic mirror) were measured every 5 or 10 min with 3 to 5 replicates for each condition. Expression of the luc gene results in the production of luciferase and thereby in the emission of light when the medium contains luciferin (36). Due to high background fluorescence of the C+Y medium and a growth-dependent decrease in background fluorescence during bacterial growth, normalization of fluorescence values was done by using a strain identical to the one studied, except for the absence of the PssbB-mKate2 reporter in the bgaA locus (DLA18 for DLA82 and DLA20 for DLA84). The average mKate2 signal normalized to the OD600 of 3 replicates of the control strain was subtracted from the mKate2 signal normalized to the OD600 of each of the tested strain replicates to obtain the fluorescence/OD values plotted in Fig. 2, calculated as follows: [(mKate2reporter − mKate2medium)/(ODreporter − ODmedium)] − [(mKate2control − mKate2medium)/(ODcontrol − ODmedium)].
Recombinant DNA techniques and oligonucleotides.
Procedures for DNA purification, restriction, ligation, agarose gel electrophoresis and transformation of E. coli were carried out as described previously (43). The oligonucleotides used in this study are listed in Table S2 and were obtained from Biolegio (Nijmegen, the Netherlands). S. pneumoniae chromosomal DNA was isolated using a Promega Wizard genomic DNA purification kit. DNA-modifying enzymes were purchased from Roche (Mannheim, Germany), New England Biolabs (Ipswich, MA, USA), Bioline (London, United Kingdom), and Fermentas (Burlington, Canada) and used as described by the manufacturer.
ChIP-qPCR.
The chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) protocol is detailed in reference 14 and in the supplemental material. Briefly, cells were grown to mid-exponential phase (OD600 = 0.2) in acid C+Y medium (pH = 6.8) at 37°C, and 70 ml of culture was mixed by inverting with 7 ml of fixing solution (50 mM Tris [pH 8.0], 100 mM NaCl, 0.5 mM EGTA, 1 mM EDTA, 11% [vol/vol] formaldehyde) and incubated at room temperature for 30 min. Cells were washed and sonicated, and then immunoprecipitation was performed using anti-GFP antibodies (rabbit serum, polyclonal; Invitrogen A-6455) and protein G-coupled Dynabeads (Invitrogen). The pulled-down DNA was purified and analyzed by qPCR as described in Text S1 in the supplemental material.
DNA microarrays, RNA isolation, and cDNA preparation.
S. pneumoniae D39 (wild-type) and MT3 (ΔparB::spc) cells with an OD600 of 0.2 were diluted 100-fold in 28 ml C+Y medium (pH 7.0) and grown without aeration to an OD600 of 0.2. Cells were collected by centrifugation at 8,000 × g for 5 min at 4°C. RNA was isolated using the macaloïd method and the High Pure RNA isolation kit (Roche), as previously described (44). RNA concentration and quality were determined using NanoDrop ND-1000 (Thermo Scientific) and capillary electrophoresis with an Agilent 2100 Bioanalyzer (Agilent Technology). cDNA synthesis and labeling with either DyLight550 or DyLight650 (Thermo Scientific) were performed as described in reference 11. For microarray methods and data analysis, procedures described in reference 44 were used. Genes were considered significantly affected when the absolute change was greater than 2-fold, with a P value cutoff of 0.005.
DNA microarray accession number.
The DNA microarray data have been submitted to ArrayExpress with accession no. E-MTAB-3300.
SUPPLEMENTAL MATERIAL
Supplemental information regarding the methods and experimental procedures. Download
ParB attenuates competence development. The WT (DLA18: comCDE_luc) and ΔparB (DLA20: ΔparB, comCDE_luc) strains were grown in C+Y medium at different initial pHs; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 10 min. On the left, cell density (OD, right axis) and comCDE_luc expression (RLU/OD, left axis) are presented as a function of time in minutes (averages of three replicates with the SEM are plotted). For each pH, the pink-framed zone of the first plot is represented on a larger scale in the second plot. Data points in the third graph for each pH are from the same 3 replicates, but comCDE_luc expression (RLU/OD) is plotted as a function of OD. Note that, as expected (8, 9), lowering the initial pH delayed and reduced competence development in both strains. Download
Expression of the PssbB_mKate2 reporter in WT versus ΔparB strains. The WT strain (DLA82: comCDE_luc bgaA::PssbB_mKate2) and parB mutant (DLA84: ΔparB::spc comCDE_luc bgaA::PssbB_mKate2) were grown in C+Y medium; optical density at 595 nm and mKate2 production (fluorescence detection using 590 nm and 612 nm as excitation and emission wavelengths, respectively) were determined every 5 min. The OD and the data points (from 3 replicates) of relative expression of PssbB_mKate2 (Fluo/OD) used in Fig. 2 are presented here as a function of time in minutes. Download
The ΔparB mutation does not lead to an increased ori-ter ratio. Strains DLA37 (wild type) and DLA72 (ΔparB::spc) were grown to exponential phase (OD600 = 0.2) for isolation of genomic DNA. Box plots represent ori-ter ratios as determined by real-time qPCR. The data were analyzed by Monte Carlo simulations. The whiskers represent the 10th and 90th percentiles, while the dots represent the outliers in the data from Monte Carlo simulations. Download
Mutating parS(−1.6°) derepresses competence development. Strains with the PssbB_luc reporter fusion were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 10 min. (A to C) The WT (DLA16, black and open symbols) versus the parS(−1.6°)mut strain (DLA30, green and filled symbols). (D to F) The parS(−1.6°)mutC strain (DLA59, blue and open symbols) versus the parS(−1.6°)mutNC strain (DLA60, orange and filled symbols). (A and D) Cell density (OD, right axis) and comCDE_luc expression (RLU/OD, left axis) are presented as a function of time in minutes (averages of three replicates with the SEM are plotted). (B and E) Enlargements of the times framed in pink in panels A and D, respectively. (C and F) Data points from the 3 replicates used for panels C and F, respectively, but with comCDE_luc expression (RLU/OD) plotted as a function of OD. Download
The ParB-parS repressor effect is not detected on a strong synthetic promoter. Strains were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 5 min. The P32_luc reporter fusion was inserted at +29 kb (5°) on the chromosome. (A and B) The WT (DLA100, black and empty symbols) and the parB mutant (DLA101, red and filled symbols) are compared. (C and D) The parS(−1.6°)mutC (DLA97, blue and empty symbols) and parS(−1.6°)mutNC (DLA98, orange and filled symbols) strains are compared. (A and C) Cell density (OD, right axis) and P32_luc expression (RLU/OD, left axis) are presented as a function of time (averages of three replicates with the SEM are plotted). (B and D) Data points from the 3 replicates used for panels A and C are presented, respectively, but P32_luc expression (RLU/OD) is plotted as a function of OD. Download
Plasmids and bacterial strains used in this study. For antibiotic resistance, xxx stands for the coding sequence and xxxR stands for the gene with promoter and terminator. A dash indicates a translational fusion, e.g., parB-gfp. An underscore indicates a transcriptional fusion, e.g., ssbB_luc. In DLA97, DLA98, DLA100, and DLA101, the insertion (ori::) is at 29 kb on the right arm of the chromosome, in an intergenic region between prsA and spd0034. Drug resistance is shown as follows: amp, ampicillin; ery, erythromycin; kan, kanamycin; spc, spectinomycin; tet, tetracycline.
Oligonucleotides used in this study. a, relevant restriction sites are underlined. b, the sequence in bold is the parS(−1.6°)WT sequence; the sequence in bold italics is the parS(−1.6°)mut sequence.
ACKNOWLEDGMENTS
L.A. was supported by a long-term EMBO fellowship (ALTF 611-2009). Work in the Gruber lab is supported by the Max Planck Society and the ERC starting grant 260853-DiseNtAngle. Work in the Veening lab is supported by the EMBO Young Investigator Program, a VIDI fellowship (864.12.001) from the Netherlands Organisation for Scientific Research, Earth and Life Sciences (NWO-ALW) and ERC starting grant 337399-PneumoCell.
Footnotes
Citation Attaiech L, Minnen A, Kjos M, Gruber S, Veening JW. 2015. The ParB-parS chromosome segregation system modulates competence development in Streptococcus pneumoniae. mBio 6(4):e00662-15. doi:10.1128/mBio.00662-15.
REFERENCES
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston C, Martin B, Fichant G, Polard P, Claverys J-P. 2014. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat Rev Microbiol 12:181–196. doi: 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- 3.Peterson S, Cline RT, Tettelin H, Sharov V, Morrison DA. 2000. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J Bacteriol 182:6192–6202. doi: 10.1128/JB.182.21.6192-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, Walling J, Li H, Mintz M, Tsegaye G, Burr PC, Do Y, Ahn S, Gilbert J, Fleischmann RD, Morrison DA. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol 51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 5.Dagkessamanskaia A, Moscoso M, Hénard V, Guiral S, Overweg K, Reuter M, Martin B, Wells J, Claverys JP. 2004. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol Microbiol 51:1071–1086. doi: 10.1111/j.1365-2958.2003.03892.x. [DOI] [PubMed] [Google Scholar]
- 6.Claverys J-P, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol 60:451–475. doi: 10.1146/annurev.micro.60.080805.142139. [DOI] [PubMed] [Google Scholar]
- 7.Weng L, Piotrowski A, Morrison DA. 2013. Exit from competence for genetic transformation in Streptococcus pneumoniae is regulated at multiple levels. PLoS One 8:e64197. doi: 10.1371/journal.pone.0064197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burghout P, Bootsma HJ, Kloosterman TG, Bijlsma JJ, de Jongh CE, Kuipers OP, Hermans PW. 2007. Search for genes essential for pneumococcal transformation: the RADA DNA repair protein plays a role in genomic recombination of donor DNA. J Bacteriol 189:6540–6550. doi: 10.1128/JB.00573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasz A, Mosser JL. 1966. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A 55:58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys JP. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313:89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- 11.Slager J, Kjos M, Attaiech L, Veening J-W. 2014. Antibiotic-induced replication stress triggers bacterial competence by increasing gene dosage near the origin. Cell 157:395–406. doi: 10.1016/j.cell.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 12.Charpentier X, Polard P, Claverys J-P. 2012. Induction of competence for genetic transformation by antibiotics: convergent evolution of stress responses in distant bacterial species lacking SOS? Curr Opin Microbiol 15:570–576. doi: 10.1016/j.mib.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Claverys JP, Prudhomme M, Mortier-Barrière I, Martin B. 2000. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol Microbiol 35:251–259. doi: 10.1046/j.1365-2958.2000.01718.x. [DOI] [PubMed] [Google Scholar]
- 14.Minnen A, Attaiech L, Thon M, Gruber S, Veening J-W. 2011. SMC is recruited to oriC by ParB and promotes chromosome segregation in Streptococcus pneumoniae. Mol Microbiol 81:676–688. doi: 10.1111/j.1365-2958.2011.07722.x. [DOI] [PubMed] [Google Scholar]
- 15.Gruber S, Errington J. 2009. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell 137:685–696. doi: 10.1016/j.cell.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan NL, Marquis KA, Rudner DZ. 2009. Recruitment of SMC by ParB-parS organizes the origin region and promotes efficient chromosome segregation. Cell 137:697–707. doi: 10.1016/j.cell.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjos M, Veening J-W. 2014. Tracking of chromosome dynamics in live Streptococcus pneumoniae reveals that transcription promotes chromosome segregation. Mol Microbiol 91:1088–1105. doi: 10.1111/mmi.12517. [DOI] [PubMed] [Google Scholar]
- 18.Pinho MG, Kjos M, Veening J-W. 2013. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. Nat Rev Microbiol 11:601–614. doi: 10.1038/nrmicro3088. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Lamothe R, Nicolas E, Sherratt DJ. 2012. Chromosome replication and segregation in bacteria. Annu Rev Genet 46:121–143. doi: 10.1146/annurev-genet-110711-155421. [DOI] [PubMed] [Google Scholar]
- 20.Mierzejewska J, Jagura-Burdzy G. 2012. Prokaryotic ParA-ParB-parS system links bacterial chromosome segregation with the cell cycle. Plasmid 67:1–14. doi: 10.1016/j.plasmid.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Montero Llopis P, Rudner DZ. 2013. Organization and segregation of bacterial chromosomes. Nat Rev Genet 14:191–203. doi: 10.1038/nrg3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Vernaza MA, Leach DR. 2013. Symmetries and asymmetries associated with non-random segregation of sister DNA strands in Escherichia coli. Semin Cell Dev Biol 24:610–617. doi: 10.1016/j.semcdb.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Kuzminov A. 2014. The precarious prokaryotic chromosome. J Bacteriol 196:1793–1806. doi: 10.1128/JB.00022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouet J-Y, Stouf M, Lebailly E, Cornet F. 2014. Mechanisms for chromosome segregation. Curr Opin Microbiol 22:60–65. doi: 10.1016/j.mib.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Le TB, Laub MT. 2014. New approaches to understanding the spatial organization of bacterial genomes. Curr Opin Microbiol 22:15–21. doi: 10.1016/j.mib.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Rudner DZ. 2014. Spatial organization of bacterial chromosomes. Curr Opin Microbiol 22:66–72. doi: 10.1016/j.mib.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song D, Loparo JJ. 2015. Building bridges within the bacterial chromosome. Trends Genet 31:164–173. doi: 10.1016/j.tig.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Kadoya R, Baek JH, Sarker A, Chattoraj DK. 2011. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J Bacteriol 193:1504–1514. doi: 10.1128/JB.01067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray H, Errington J. 2008. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell 135:74–84. doi: 10.1016/j.cell.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes K, Howard M, Szardenings F. 2010. Pushing and pulling in prokaryotic DNA segregation. Cell 141:927–942. doi: 10.1016/j.cell.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 31.Breier AM, Grossman AD. 2007. Whole-genome analysis of the chromosome partitioning and sporulation protein Spo0J (ParB) reveals spreading and origin-distal sites on the Bacillus subtilis chromosome. Mol Microbiol 64:703–718. doi: 10.1111/j.1365-2958.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- 32.Baek JH, Rajagopala SV, Chattoraj DK. 2014. Chromosome segregation proteins of Vibrio cholerae as transcription regulators. mBio 5:e01061-14. doi: 10.1128/mBio.01061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartosik AA, Glabski K, Jecz P, Mikulska S, Fogtman A, Koblowska M, Jagura-Burdzy G. 2014. Transcriptional profiling of ParA and ParB mutants in actively dividing cells of an opportunistic human pathogen Pseudomonas aeruginosa. PLoS One 9:e87276. doi: 10.1371/journal.pone.0087276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alloing G, Martin B, Granadel C, Claverys JP. 1998. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol Microbiol 29:75–83. doi: 10.1046/j.1365-2958.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 35.Pestova EV, Håvarstein LS, Morrison DA. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol 21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 36.Prudhomme M, Claverys J-P. 2005. There will be a light: the use of luc transcriptional fusions in living pneumococcal cells, p 519–524. In Hakenbeck R, Chhatwal GS (ed), The molecular biology of streptococci. Horizon Scientific Press, Norfolk, United Kingdom. [Google Scholar]
- 37.Yuzenkova Y, Gamba P, Herber M, Attaiech L, Shafeeq S, Kuipers OP, Klumpp S, Zenkin N, Veening J-W. 2014. Control of transcription elongation by GreA determines rate of gene expression in Streptococcus pneumoniae. Nucleic Acids Res 42:10987–10999. doi: 10.1093/nar/gku790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez M, Morrison DA, Tomasz A. 1997. Ubiquitous distribution of the competence related genes comA and comC among isolates of Streptococcus pneumoniae. Microb Drug Resist 3:39–52. doi: 10.1089/mdr.1997.3.39. [DOI] [PubMed] [Google Scholar]
- 39.Martin B, Granadel C, Campo N, Hénard V, Prudhomme M, Claverys J-P. 2010. Expression and maintenance of ComD-ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Mol Microbiol 75:1513–1528. doi: 10.1111/j.1365-2958.2010.07071.x. [DOI] [PubMed] [Google Scholar]
- 40.Claverys J-P, Havarstein LS. 2002. Extracellular-peptide control of competence for genetic transformation in Streptococcus pneumoniae. Front Biosci 7:d1798–d1814. doi: 10.2741/claverys. [DOI] [PubMed] [Google Scholar]
- 41.Stevens KE, Chang D, Zwack EE, Sebert ME. 2011. Competence in Streptococcus pneumoniae is regulated by the rate of ribosomal decoding errors. mBio 2:e00071-11. doi: 10.1128/mBio.00071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelmoer DJ, Rozen DE. 2011. Competence increases survival during stress in Streptococcus pneumoniae. Evolution 65:3475–3485. doi: 10.1111/j.1558-5646.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 44.Van Hijum SA, de Jong A, Baerends RJ, Karsens HA, Kramer NE, Larsen R, den Hengst CD, Albers CJ, Kok J, Kuipers OP. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77. doi: 10.1186/1471-2164-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorg RA, Kuipers OP, Veening J-W. 2015. Gene expression platform for synthetic biology in the human pathogen Streptococcus pneumoniae. ACS Synth Biol 4:228–239. doi: 10.1021/sb500229s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information regarding the methods and experimental procedures. Download
ParB attenuates competence development. The WT (DLA18: comCDE_luc) and ΔparB (DLA20: ΔparB, comCDE_luc) strains were grown in C+Y medium at different initial pHs; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 10 min. On the left, cell density (OD, right axis) and comCDE_luc expression (RLU/OD, left axis) are presented as a function of time in minutes (averages of three replicates with the SEM are plotted). For each pH, the pink-framed zone of the first plot is represented on a larger scale in the second plot. Data points in the third graph for each pH are from the same 3 replicates, but comCDE_luc expression (RLU/OD) is plotted as a function of OD. Note that, as expected (8, 9), lowering the initial pH delayed and reduced competence development in both strains. Download
Expression of the PssbB_mKate2 reporter in WT versus ΔparB strains. The WT strain (DLA82: comCDE_luc bgaA::PssbB_mKate2) and parB mutant (DLA84: ΔparB::spc comCDE_luc bgaA::PssbB_mKate2) were grown in C+Y medium; optical density at 595 nm and mKate2 production (fluorescence detection using 590 nm and 612 nm as excitation and emission wavelengths, respectively) were determined every 5 min. The OD and the data points (from 3 replicates) of relative expression of PssbB_mKate2 (Fluo/OD) used in Fig. 2 are presented here as a function of time in minutes. Download
The ΔparB mutation does not lead to an increased ori-ter ratio. Strains DLA37 (wild type) and DLA72 (ΔparB::spc) were grown to exponential phase (OD600 = 0.2) for isolation of genomic DNA. Box plots represent ori-ter ratios as determined by real-time qPCR. The data were analyzed by Monte Carlo simulations. The whiskers represent the 10th and 90th percentiles, while the dots represent the outliers in the data from Monte Carlo simulations. Download
Mutating parS(−1.6°) derepresses competence development. Strains with the PssbB_luc reporter fusion were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 10 min. (A to C) The WT (DLA16, black and open symbols) versus the parS(−1.6°)mut strain (DLA30, green and filled symbols). (D to F) The parS(−1.6°)mutC strain (DLA59, blue and open symbols) versus the parS(−1.6°)mutNC strain (DLA60, orange and filled symbols). (A and D) Cell density (OD, right axis) and comCDE_luc expression (RLU/OD, left axis) are presented as a function of time in minutes (averages of three replicates with the SEM are plotted). (B and E) Enlargements of the times framed in pink in panels A and D, respectively. (C and F) Data points from the 3 replicates used for panels C and F, respectively, but with comCDE_luc expression (RLU/OD) plotted as a function of OD. Download
The ParB-parS repressor effect is not detected on a strong synthetic promoter. Strains were grown in C+Y medium; optical density at 595 nm and luciferase activity (in relative luminescence units [RLU]) were determined every 5 min. The P32_luc reporter fusion was inserted at +29 kb (5°) on the chromosome. (A and B) The WT (DLA100, black and empty symbols) and the parB mutant (DLA101, red and filled symbols) are compared. (C and D) The parS(−1.6°)mutC (DLA97, blue and empty symbols) and parS(−1.6°)mutNC (DLA98, orange and filled symbols) strains are compared. (A and C) Cell density (OD, right axis) and P32_luc expression (RLU/OD, left axis) are presented as a function of time (averages of three replicates with the SEM are plotted). (B and D) Data points from the 3 replicates used for panels A and C are presented, respectively, but P32_luc expression (RLU/OD) is plotted as a function of OD. Download
Plasmids and bacterial strains used in this study. For antibiotic resistance, xxx stands for the coding sequence and xxxR stands for the gene with promoter and terminator. A dash indicates a translational fusion, e.g., parB-gfp. An underscore indicates a transcriptional fusion, e.g., ssbB_luc. In DLA97, DLA98, DLA100, and DLA101, the insertion (ori::) is at 29 kb on the right arm of the chromosome, in an intergenic region between prsA and spd0034. Drug resistance is shown as follows: amp, ampicillin; ery, erythromycin; kan, kanamycin; spc, spectinomycin; tet, tetracycline.
Oligonucleotides used in this study. a, relevant restriction sites are underlined. b, the sequence in bold is the parS(−1.6°)WT sequence; the sequence in bold italics is the parS(−1.6°)mut sequence.